Abstract
Mitochondrial disorders (MIDs) are a heterogeneous group of genetic metabolic diseases due to mutations in the mitochondrial DNA (mtDNA) or in the nuclear DNA (nDNA) (Rahman and Rahman, 2018). Some affected genes encode proteins with various functions, or structural RNAs such as transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs). MIDs may also be caused by mutations in non-coding regions (e.g., D-loop of mtDNA) (Rahman and Rahman, 2018). Proteins involved in MIDs include enzymes, assembling factors, transport proteins, signaling proteins, pore proteins, and fusion/fission proteins (Gorman et al., 2016). The pathways most frequently affected by mutations in “mitochondrial genes” are the respiratory chain and the oxidative phosphorylation. Dysfunction of many other pathways (e.g., β-oxidation, pyruvate-dehydrogenase complex, and heme synthesis) may also manifest as MIDs (Hu et al., 2019). The estimated prevalence of MIDs is at least 1:5000 (Ng and Turnbull, 2016).
Keywords: Mitochondrial disorder, nDNA, Multiple mtDNA deletions, Phenotype, Multisystem involvement
Mitochondrial disorders (MIDs) are a heterogeneous group of genetic metabolic diseases due to mutations in the mitochondrial DNA (mtDNA) or in the nuclear DNA (nDNA) (Rahman and Rahman, 2018). Some affected genes encode proteins with various functions, or structural RNAs such as transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs). MIDs may also be caused by mutations in non-coding regions (e.g., D-loop of mtDNA) (Rahman and Rahman, 2018). Proteins involved in MIDs include enzymes, assembling factors, transport proteins, signaling proteins, pore proteins, and fusion/fission proteins (Gorman et al., 2016). The pathways most frequently affected by mutations in “mitochondrial genes” are the respiratory chain and the oxidative phosphorylation. Dysfunction of many other pathways (e.g., β-oxidation, pyruvate-dehydrogenase complex, and heme synthesis) may also manifest as MIDs (Hu et al., 2019). The estimated prevalence of MIDs is at least 1:5000 (Ng and Turnbull, 2016).
Because of the strong genetic heterogeneity and highly variable pathogenetic background of MIDs, a myriad of phenotypes ensue, which can vary even with a single mutation (phenotypic heterogeneity) (Finsterer, 2018). Phenotypic heterogeneity of MIDs results from the broad genetic background of these disorders, which is further complicated by the unique characteristics of mitochondrial genetics (e.g., heteroplasmy, copy number variation, threshold effect, and mitotic segregation) (Rahman, 2020). MIDs may manifest in any organ or tissue, but more frequently affect those with high energy demand (Rahman, 2020). These organs include brain, eyes, ears, endocrine organs, heart, intestines, kidneys, muscle, and nerves (Finsterer, 2012). Rarely, MIDs manifest in lungs, immune system, skin, bones, arteries, or bone marrow (Finsterer, 2012). MIDs either manifest as a syndrome, defined as a collection of characteristic symptoms (syndromic MIDs), or non-syndrome (Finsterer, 2012). Mitochondrial syndromes are frequently expressed as acronyms, of which over 50 are currently known. The best known syndromic MIDs are MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes), MERRF (myoclonus epilepsy with ragged-red fibers), LHON (Leber hereditary optic neuropathy), CPEO (chronic progressive external ophthalmoplegia), NARP (neuropathy, ataxia and retinitis pigmentosa), KSS (Kearns-Sayre syndrome), and LBSL (leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation) (Finsterer, 2012). MIDs are diagnosed based on the individual and family history, clinical exam, muscle biopsy, immunohistological analysis, biochemical studies, polarography, cybrid studies, and genetic tests.
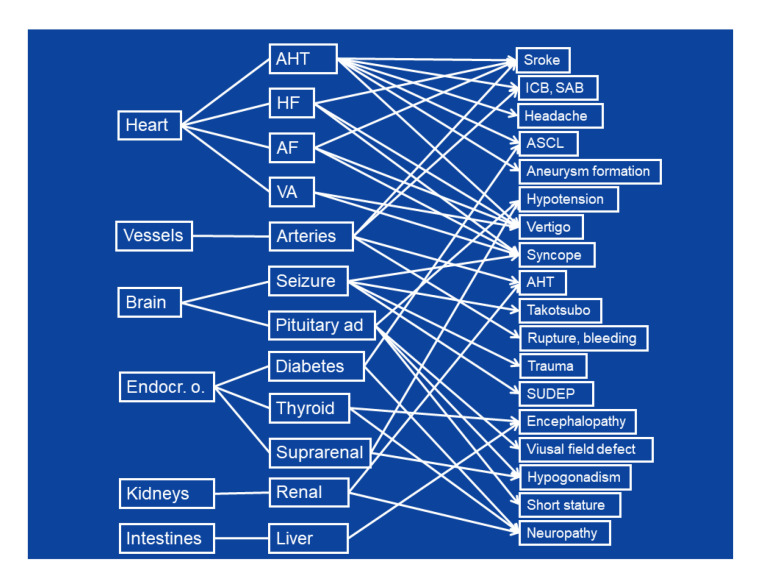
Primary manifestations of MIDs in organs may lead to secondary disorders in an organ/tissue/system that are not primarily affected (Fig. 1). Recognizing secondary complications is crucial as they may strongly influence the clinical presentation and outcome of an MID. Moreover, these secondary diseases should be recognized as they are usually more accessible to treatment than the primary metabolic defects. Secondary diseases may originate from any organ at a variable frequency, but most frequently involve heart, arteries, and brain (Fig. 1). Secondary complications may be transmitted to another organ via arteries, nerves, or endocrine signaling.
Fig. 1.
Secondary manifestations of mitochondrial disorders
AF: atrial fibrillation; AHT: arterial hypertension; ASCL: atherosclerosis; HF: heart failure; ICB: intracerebral bleeding; VA: ventricular arrhythmia; SAB: subarachnoid bleeding; SUDEP: sudden unexplained death in epilepsy; Endocr. o.: endocrine organs
Cardiac involvement in MIDs is most frequently associated with complications in the brain. Primary mitochondrial arterial hypertension (Lin et al., 2019) may lead to ischemic stroke or intracerebral bleeding (ICB), headache, atherosclerosis (ASCL), vertigo, or aneurysm formation. Heart failure caused by cardiomyopathy (Mima et al., 2011) may lead to ischemic stroke (due to intra-cardiac thrombus formation), vertigo, or syncope (due to arterial hypotension). Atrial fibrillation (Malfatti et al., 2013) may be associated with cardio-embolic events, vertigo, or syncope (Fig. 1). Ventricular arrhythmias may lead to arterial hypotension, vertigo, or syncope (Fig. 1).
Involvement of the arteries (Finsterer and Zarrouk-Mahjoub, 2016) may lead to arterial hypertension or ischemia due to dissection and consequent occlusion of an artery, or to aneurysm formation and consequent spontaneous rupture and bleeding (Kalashnikova et al., 2010; Sun et al., 2018). Rupture of intracerebral aneurysms may lead to subarachnoid bleeding. Spontaneous rupture of the aorta may lead to shock or even death (Tay et al., 2006).
Moreover, involvement of the central nervous system (CNS) may cause severe complications in other organs (Fig. 1). Seizures may lead to multiple CNS manifestations: traumas, including traumatic brain injury during falls (Rahman, 2019); syncope, confusion, or psychosis; Takotsubo syndrome or sudden unexplained death in epilepsy (SUDEP) (Nandal et al., 2019); and severe ventricular arrhythmias (Kennedy et al., 2016). Pituitary adenoma (macro-adenoma) (Varanasi et al., 1999) may be associated with arterial hypotension or visual field defects.
Every endocrine organ can be affected in an MID. Diabetes, hyperthyroidism, or hypothyroidism that occurs in an MID may be associated with encephalopathy (Finsterer et al., 2019). Pituitary adenoma may be associated with hypocorticism, arterial hypotension, hypogonadism, or short stature. Hypoparathyroidism may be complicated by neuropathy, encephalopathy, or visual impairment (Kytövuori et al., 2016). Diabetes may be responsible for ASCL. Hypoaldosteronism may be associated with hyponatremia or arterial hypotension.
Involvement of the kidneys (Emma and Salviati, 2017) may lead to arterial hypertension. Liver involvement may lead to lactic acidosis or hepatic encephalopathy. If the cellular immune system is affected by the MID, recurrent infectious disease may occur (Galassi et al., 2017).
In conclusion, the broad spectrum of MID phenotypes is further complicated by secondary manifestations in organs that are not primarily affected. Differentiating secondary from primary manifestations is crucial for targeted treatment of secondary complications. Secondary manifestations not only complicate the clinical presentations, but also largely determine the outcome of MID patients.
Footnotes
Compliance with ethics guidelines: Josef FINSTERER declare that he has no conflict of interest.
This article does not contain any studies with human or animal subjects performed by the author.
References
- 1.Emma F, Salviati L. Mitochondrial cytopathies and the kidney. Néphrol Thér. 2017;13(S1):S23–S28. doi: 10.1016/j.nephro.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Finsterer J. Inherited mitochondrial disorders. In: Scatena R Bottoni P, Giardina B , editors. Advances in Mitochondrial Medicine. Springer, Dordrecht; 2012. pp. 187–213. [DOI] [PubMed] [Google Scholar]
- 3.Finsterer J. Clinical perspectives of mitochondrial disorders. Pediatr Endocrinol Rev. 2018;16(1):203–208. doi: 10.17458/per.vol16.2018.f.mitochondrialdisorders. [DOI] [PubMed] [Google Scholar]
- 4.Finsterer J, Zarrouk-Mahjoub S. Mitochondrial vasculopathy. World J Cardiol. 2016;8(5):333–339. doi: 10.4330/wjc.v8.i5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finsterer J, Melichart-Kotig M, Woehrer A. Mitochondrial disorder mimicking rheumatoid disease. Z Rheumatol. 2019;78(9):875–880. doi: 10.1007/s00393-018-0551-1. [DOI] [PubMed] [Google Scholar]
- 6.Galassi G, Maggi L, Lamantea E, et al. C10ORF2 mutation associated with progressive external ophthalmoplegia and clinically isolated syndrome. Acta Neurol Belg. 2017;117(4):947–949. doi: 10.1007/s13760-017-0793-8. [DOI] [PubMed] [Google Scholar]
- 7.Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2(1):16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 8.Hu SY, Zhuang QQ, Qiu Y, et al. Cell models and drug discovery for mitochondrial diseases. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2019;20(5):449–456. doi: 10.1631/jzus.B1900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalashnikova LA, Sakharova AV, Dobrynina LA, et al. Mitochondrial arteriopathy as a cause of spontaneous dissection of cerebral arteries. Zh Nevrol Psikhiatr Im S S Korsakova. 2010;110(4 Suppl 2):3–11. (in Russian) [PubMed] [Google Scholar]
- 10.Kennedy H, Haack TB, Hartill V, et al. Sudden cardiac death due to deficiency of the mitochondrial inorganic pyrophosphatase PPA2. Am J Hum Genet. 2016;99(3):674–682. doi: 10.1016/j.ajhg.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kytövuori L, Lipponen J, Rusanen H, et al. A novel mutation m.8561C>G in MT-ATP6/8 causing a mitochondrial syndrome with ataxia, peripheral neuropathy, diabetes mellitus, and hypergonadotropic hypogonadism. J Neurol. 2016;263(11):2188–2195. doi: 10.1007/s00415-016-8249-2. [DOI] [PubMed] [Google Scholar]
- 12.Lin L, Cui P, Qiu ZP, et al. The mitochondrial tRNAAla 5587T>C and tRNALeu(CUN) 12280A>G mutations may be associated with hypertension in a Chinese family. Exp Ther Med. 2019;17(3):1855–1862. doi: 10.3892/etm.2018.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malfatti E, Laforêt P, Jardel C, et al. High risk of severe cardiac adverse events in patients with mitochondrial m.3243A>G mutation. Neurology. 2013;80(1):100–105. doi: 10.1212/WNL.0b013e31827b1a2f. [DOI] [PubMed] [Google Scholar]
- 14.Mima A, Shiota F, Matsubara T, et al. An autopsy case of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) with intestinal bleeding in chronic renal failure. Ren Fail. 2011;33(6):622–625. doi: 10.3109/0886022X.2011.585730. [DOI] [PubMed] [Google Scholar]
- 15.Nandal S, Castles A, Asrar ul Haq M, et al. Takotsubo cardiomyopathy triggered by status epilepticus: case report and literature review. BMJ Case Rep. 2019;12(1):e225924. doi: 10.1136/bcr-2018-225924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng YS, Turnbull DM. Mitochondrial disease: genetics and management. J Neurol. 2016;263:179–191. doi: 10.1007/s00415-015-7884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet. 2018;391(10139):2560–2574. doi: 10.1016/S0140-6736(18)30727-X. [DOI] [PubMed] [Google Scholar]
- 18.Rahman S. Advances in the treatment of mitochondrial epilepsies. Epilepsy Behav, 101:106546. 2019 doi: 10.1016/j.yebeh.2019.106546. [DOI] [PubMed] [Google Scholar]
- 19.Rahman S. Mitochondrial disease in children. J Intern Med. 2020;287:609–633. doi: 10.1111/joim.13054. [DOI] [PubMed] [Google Scholar]
- 20.Sun XR, Jiang GH, Ju XY, et al. MELAS and macroangiopathy: a case report and literature review. Medicine. 2018;97(52):e13866. doi: 10.1097/MD.0000000000013866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tay SHK, Nordli DR, Jr, Bonilla E, et al. Aortic rupture in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes. Arch Neurol. 2006;63(2):281–283. doi: 10.1001/archneur.63.2.281. [DOI] [PubMed] [Google Scholar]
- 22.Varanasi SS, Francis RM, Berger CEM, et al. Mitochondrial DNA deletion associated oxidative stress and severe male osteoporosis. Osteoporos Int. 1999;10(2):143–149. doi: 10.1007/s001980050209. [DOI] [PubMed] [Google Scholar]