Abstract
Objective: The present study was to evaluate the feasibility of using the multi-biomarker strategy for the prediction of sepsis-induced myocardial dysfunction (SIMD) and mortality in septic patients. Methods: Brain natriuretic peptide (BNP), cardiac troponin I (cTnI), and heart-type fatty acid-binding protein (h-FABP) in 147 septic patients were assayed within 6 h after admission. We also determined the plasma levels of myeloperoxidase (MPO) and pregnancy-associated plasma protein-A (PAPP-A). The receiver operating characteristic (ROC) curve was used to assess the best cutoff values of various single-biomarkers for the diagnosis of SIMD and the prediction of mortality. Also, the ROC curve, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) indices were used to evaluate the feasibility of using multi-biomarkers to predict SIMD and mortality. Results: Our statistics revealed that only h-FABP independently predicted SIMD (P<0.05). The addition of MPO and cTnI to h-FABP for SIMD prediction provided an NRI of 18.7% (P=0.025) and IDI of 3.3% (P=0.033). However, the addition of MPO or cTnI to h-FABP did not significantly improve the predictive ability of h-FABP to SIMD, as evidenced by the area under the curve (AUC), NRI, and IDI (all P>0.05). A history of shock and MPO were independent predictors of mortality in septic patients (both P<0.05). The addition of PAPP-A and h-FABP to MPO resulted in a mortality prediction with NRI of 25.5% (P=0.013) and IDI of 2.9% (P=0.045). However, this study revealed that the addition of h-FABP or PAPP-A to MPO did not significantly improve the ability to predict mortality, as evidenced by the AUC, NRI, and IDI (all P>0.05). Conclusions: The findings of this study indicate that a sensitive and specific strategy for early diagnosis of SIMD and mortality prediction in sepsis should incorporate three biomarkers.
Keywords: Multi-biomarker, Myocardial dysfunction, Sepsis, Mortality
1. Introduction
Sepsis refers to a life-threatening organ dysfunction caused by a dysregulated host response to bacterial, fungal, or viral infection (Singer et al., 2016; Huber et al., 2018). Sepsis affects approximately 19 million patients in the world each year, of which 6 million succumb to the disease. The mortality rate due to sepsis is more than 1/4 of all intensive care unit (ICU) deaths, and about 3 million of the surviving patients develop cognitive dysfunction (Perner et al., 2018). Sepsis-induced myocardial dysfunction (SIMD) occurs in 25% to 50% of all adult septic shock patients (Jardin et al., 1999). First described by Parker et al. (1984), SIMD refers to a reversible myocardial injury. SIMD encompasses peripheral vascular dysfunction and myocardial dysfunction, and is characterized by left ventricular dilatation, lower ejection fraction, and myocardial damage (Sato and Nasu, 2015).
Traditional biomarkers of myocardial dysfunction including brain natriuretic peptide (BNP) and cardiac troponin (cTn) appear to be associated with SIMD and prognosis in septic patients (Post et al., 2008; Kim et al., 2019). Other studies demonstrate that BNP and cTn alone were positively correlated with the severity of sepsis (Charpentier et al., 2004; Klouche et al., 2014; Papanikolaou et al., 2014), regardless of whether cardiac function was damaged. However, this relation could be disappeared after attenuation of the severity of sepsis (Berdal et al., 2008; Yucel et al., 2008). In recent years, novel biomarkers, such as heart-type fatty acid-binding protein (h-FABP) and pregnancy-associated plasma protein-A (PAPP-A), were identified to predict the myocardial dysfunction and poor outcome in patients with sepsis (Zhang et al., 2012, 2014), but the predictive values of PAPP-A and h-FABP alone were limited. Additionally, myeloperoxidase (MPO) was also considered as a potential biomarker for cardiovascular disease and sepsis (Schrijver et al., 2017; Avaliani et al., 2019).
We hypothesized that the use of multi-biomarker is more valuable than use of single-biomarker in the early diagnosis of cardiac dysfunction and the prognosis of septic patients. Therefore, a prospective observational study was conducted to compare the efficiency of multi-biomarker with single-biomarker in predicting SIMD and mortality in patients with sepsis.
2. Patients and methods
2.1. Participants
The present study involved 147 septic patients admitted to the ICUs (emergency ICU, neurological ICU, and medical ICU) at the Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China), from April 2012 to December 2016. The diagnostic criteria of severe sepsis and septic shock were defined according to the 2001 International Sepsis Definitions Conference (Levy et al., 2003). Patients who met the diagnostic criteria were treated with 6-h resuscitation and 24-h management bundles detailed in the Surviving Sepsis Campaign guidelines (Dellinger et al., 2008). The exclusion criteria were pregnancy, life expectancy less than 24 h, preexisting left ventricular dysfunction, dilated cardiomyopathy, valvular heart disease, chronic renal failure, acute coronary ischemia, and cardiogenic or hemorrhagic shock.
Both clinical and biologic data were collected during the follow-up period. The data included demographic characteristics such as age, gender, and comorbidities including chronic obstructive pulmonary disease (COPD), stroke, and trauma. Details of both peripheral blood biochemistry panel and arterial blood gas analysis were taken at admission. Three clinical scores were recorded: the initial severity was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score (range 0 to 71), the Sequential Organ Failure Assessment (SOFA) score (range 0 to 24), and the Multiple Organ Dysfunction Syndrome (MODS) score (range 0 to 24) within 24 h after admission. Both the need for and the timing of inotropic support defined as a continuous infusion of inotropes (milrinone or dobutamine) or vasopressors (dopamine, epinephrine, norepinephrine, or vasopressin) based on the professional guidelines were also recorded (Dellinger et al., 2008).
Mortality was defined as patients who died within 28 d after enrollment (ACCP/SCCM, 1992). All clinical events and outcomes were followed throughout the hospitalization period. Patient follow-up was limited to the period of hospitalization.
2.2. Blood sampling
Central venous blood was sampled within 6 h after admission. For detection of complete blood count, conventional biochemical assay, and levels of cTnI, h-FABP, MPO, PAPP-A, and procalcitonin (PCT), serum samples were collected after 15-min centrifugation at 3000g (4 °C) and stored at −40 °C until the assay was done. For BNP measurement, plasma samples were isolated from 4 mL ethylenediamine tetraacetic acid (EDTA) whole blood after 10-min centrifugation at 3000g (4 °C) and stored at −40 °C until the assay was done. Besides, 0.5 mL of heparinized arterial blood was collected for an instant blood gas analysis. Lactate detection was done using an automated analyzer (ABL800; Radiometer Co., Denmark) with the matched reagent.
2.3. Biochemical assays
Complete blood count was assessed from serum samples using an automated system (XN9000; Sysmex Co., Japan). Liver function, renal function, and electrolytes were assessed for serum samples using a chemistry analyzer (AU 5800; Beckman Coulter Inc., USA) with a commercially available reagent. Serum PCT and cTnI levels were determined using immunoluminometric assay (Cobas E601, Roche, Switzerland). All samples were analyzed in triplicate. Plasma samples for detection of BNP levels were run in an immunoassay analyzer (Triage, Biosite Inc., USA) using the appropriate kit. Serum PAPP-A, h-FABP, and MPO levels were detected by a sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Kangchen Bioscience, Shanghai, China).
2.4. Transthoracic echocardiographic detection
Early recognition of SIMD was ensured by conducting transthoracic echocardiography (Sonos-550, Philips, USA) on all participants within 24 h after enrollment. An experienced cardiologist evaluated the left ventricular ejection fraction (LVEF). SIMD was defined as an LVEF less than 50% or the need of inotropes (milrinone or dobutamine) or vasopressors (dopamine, epinephrine, norepinephrine, or vasopressin) or both.
2.5. Statistical analysis
Continuous variables and categorical variables were described through mean±standard deviation (SD) and percentage (%), respectively. PCT, h-FABP, cTnI, BNP, PAPP-A, and MPO levels were presented as the median (25th/75th percentile) because they were skewed. Furthermore, h-FABP, cTnI, BNP, PAPP-A, and MPO levels were logarithmically normalized using the natural logarithm to the base e (presented as ln h-FABP, ln cTnI, ln BNP, ln PAPP-A, and ln MPO), where e is an irrational constant approximately equal to 2.72 to account for the skewed distribution. Baseline characteristics between groups (patients with SIMD and without SIMD; survivals and non-survivals) were compared using either the Chi-square for binary variables or categorical variables, Student’s t-test for non-skewed continuous variables, or the Mann-Whitney test for skewed continuous variables. Odds ratios (ORs) for SIMD and mortality were assessed using logistic regression analysis at a 95% confidence interval (CI). Predictive indicators that were significant in univariate analysis were included in a multivariate model using a forward selection procedure.
The receiver operating characteristic (ROC) curve and calculation of the area under the curve (AUC) were conducted to compare the predictive abilities of different variables. The best cutoff values were determined using the Youden index. ROC curve was also constructed using a combination of two or three variables for predicting SIMD and mortality applying the Mackinnon and Mulligan (1998)’s weighted sum rule. The differences between AUCs were tested by Hanley-McNeil methods to examine whether the addition of one or two of the biomarkers improved the bias of the model (Hanley and McNeil, 1983).
An improvement in risk prediction for biomarkers is assessed by the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices (Pencina et al., 2008). NRI was calculated from the net increase versus decrease in risk categories among case patients, minus that among control patients. The NRI determined whether a priori meaningful risk existed among the categories. We used <10%, 10% to <30%, and 30% to 50%, and >50% for the risk stratifications of SIMD and septic death (Cook and Ridker, 2009). IDI is the difference in Yates slopes between models, where the Yates slope is the average difference in predicted probabilities between case participants and control patients (Cook and Ridker, 2009). Statistical analyses were done using MedCalc 15 software and SPSS (Version 23.0 for Windows; IBM Corp., Armonk, NY, USA). A P value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics according to SIMD
A total of 147 patients were divided into the SIMD group (n=70) and the non-SIMD group (n=77) depending on whether the left heart function was impaired. The baseline characteristics and laboratory indicators of patients are represented in Table 1. The mean age of patients was 66.0 years, and men accounted for 81.6% of the patients. According to the APACHE-II, SOFA, and MODS scores, there were no significant differences in the severity of organ dysfunction between the two groups. The APACHE-II scores for the non-SIMD and SIMD groups were 18.9±3.2 and 19.4±2.8 (P=0.193), respectively, the SOFA scores were 10.4±2.5 and 10.3±2.0 (P=0.871), and the MODS scores were 11.4±2.5 and 11.4±2.2 (P=0.825), respectively. The cTnI, h-FABP, and MPO levels were significantly higher in patients with SIMD as compared to patients without SIMD (P=0.038, P<0.0005, and P=0.011, respectively). There were no significant differences in PAPP-A or BNP between the two groups (P=0.359 and P=0.267, respectively). Besides, there was no significant difference in whether the patients had a history of shock, stroke, or trauma (P=0.914, P=0.251, or P=0.136, respectively). However, the number of patients with COPD in the SIMD group was significantly higher than that in the non-SIMD group (P=0.007).
Table 1.
Baseline characteristics of the SIMD and non-SIMD groups
| Characteristics | All patients (n=147) | Patients with SIMD (n=70) | Patients without SIMD (n=77) | P |
| Age (year) | 66.0±15.5 | 65.2±14.1 | 66.8±16.6 | 0.592 |
| Sex, male | 120 (81.6%) | 57 (81.4%) | 63 (81.8%) | 0.879 |
| Treatment day in ICU | 22.3±9.1 | 22.8±9.0 | 21.8±9.1 | 0.285 |
| Stroke | 82 (55.8%) | 43 (61.4%) | 39 (51.0%) | 0.251 |
| Trauma | 86 (58.5%) | 36 (51.4%) | 50 (64.9%) | 0.136 |
| Shock | 50 (34.0%) | 23 (32.9%) | 27 (35.1%) | 0.914 |
| COPD | 72 (49.0%) | 43 (61.4%) | 29 (37.7%) | 0.007 |
| cTnI (μg/L) | 0.91 (0.13/1.37) | 0.97 (0.22/1.86) | 0.83 (0.08/1.24) | 0.038 |
| BNP (pg/mL) | 637.0 (398.5/1010.0) | 678.0 (405.8/1080.0) | 610.0 (347.0/887.5) | 0.267 |
| h-FABP (ng/mL) | 9.59 (5.94/12.06) | 11.35 (9.01/14.13) | 8.06 (5.47/10.16) | <0.0005 |
| PAPP-A (ng/mL) | 4.49 (2.38/6.61) | 4.57 (2.51/6.71) | 4.44 (2.11/6.44) | 0.359 |
| MPO (ng/mL) | 13.96 (10.13/22.99) | 17.62 (11.29/23.38) | 12.11 (8.62/17.23) | 0.011 |
| PCT (μg/L) | 3.90 (1.99/5.35) | 3.18 (2.00/5.35) | 3.50 (1.87/5.35) | 0.756 |
| APACHE-II score (points) | 19.2±3.0 | 19.4±2.8 | 18.9±3.2 | 0.193 |
| SOFA score (points) | 10.3±2.3 | 10.3±2.0 | 10.4±2.5 | 0.871 |
| MODS score (points) | 11.4±2.3 | 11.4±2.2 | 11.4±2.5 | 0.825 |
Data are expressed as mean±standard deviation (SD), number (percentage), or median (25th/75th percentile). SIMD: sepsis-induced myocardial dysfunction; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; cTnI: cardiac troponin I; BNP: brain natriuretic peptide; h-FABP: heart-type fatty acid-binding protein; PAPP-A: pregnancy-associated plasma protein-A; MPO: myeloperoxidase; PCT: procalcitonin; APACHE-II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; MODS: Multiple Organ Dysfunction Syndrome
3.2. Predictive ability to SIMD
Univariate logistic regression analysis demonstrated that higher plasma concentrations of cTnI, h-FABP, and MPO (P=0.027, P=0.000, and P=0.001, respectively) indicated a higher risk of developing SIMD (Table 2). The BNP and PAPP-A concentrations, MODS, SOFA and APACHE-II scores, and history of shock, stroke, and trauma did not accurately predict SIMD. However, a history of COPD accurately predicted SIMD (OR=2.636, P=0.004). A multivariate analysis using a model with COPD disease history, cTnI, h-FABP, and MPO found that only h-FABP independently predicted SIMD (P=0.000).
Table 2.
Univariate and multivariate odds ratios of predictors of SIMD
| Predictor | OR | 95% CI | P |
| Univariate analysis | |||
| Age | 0.993 | 0.937 to 1.014 | 0.537 |
| COPD | 2.636 | 1.354 to 5.133 | 0.004 |
| Shock | 0.906 | 0.457 to 1.796 | 0.778 |
| Stroke | 1.522 | 0.805 to 2.992 | 0.190 |
| Trauma | 0.572 | 0.295 to 1.109 | 0.098 |
| ln BNP | 1.215 | 0.821 to 1.792 | 0.330 |
| ln h-FABP | 8.900 | 3.406 to 23.257 | 0.000 |
| ln PAPP-A | 1.258 | 0.796 to 1.990 | 0.326 |
| ln cTnI | 1.299 | 1.030 to 1.638 | 0.027 |
| ln MPO | 2.922 | 1.550 to 5.510 | 0.001 |
| ln PCT | 1.013 | 0.725 to 1.416 | 0.939 |
| APACHE-II score | 1.054 | 0.946 to 1.173 | 0.339 |
| SOFA score | 0.968 | 0.840 to 1.115 | 0.651 |
| MODS score | 0.999 | 0.870 to 1.148 | 0.992 |
| Multivariate analysis | |||
| ln h-FABP | 8.900 | 3.406 to 23.256 | 0.000 |
SIMD: sepsis-induced myocardial dysfunction; OR: odds ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease; BNP: brain natriuretic peptide; h-FABP: heart-type fatty acid-binding protein; PAPP-A: pregnancy-associated plasma protein-A; cTnI: cardiac troponin I; MPO: myeloperoxidase; PCT: procalcitonin; APACHE-II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; MODS: Multiple Organ Dysfunction Syndrome
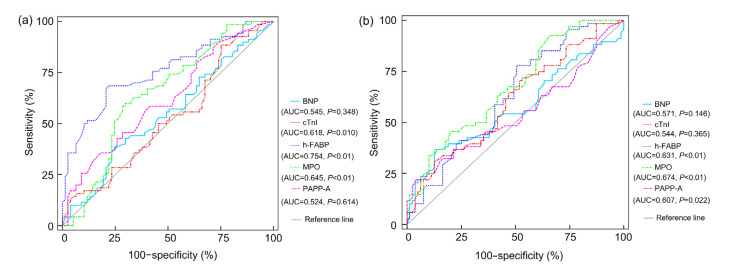
An ROC curve (Fig. 1a) was established to evaluate the predictive value of the above single index for SIMD. The AUC of h-FABP was 0.754 (P<0.01), MPO was 0.645 (P<0.01), cTnI was 0.618 (P=0.010), BNP was 0.545 (P=0.348), and PAPP-A was 0.524 (P=0.614). The sensitivity and specificity of h-FABP at the optimal cutoff value of 10.16 ng/mL were 68.6% and 77.9%, respectively. The optimal cutoff value of MPO was 14.27 ng/mL, which gave a sensitivity of 70.1% and a specificity of 60.0%. The cTnI was 1.27 μg/L, and it provided a sensitivity of 81.8% and a specificity of 35.7%.
Fig. 1.
ROC curves of BNP, cTnI, h-FABP, MPO, and PAPP-A to predict SIMD (a) and mortality (b)
ROC: receiver operating characteristic; BNP: brain natriuretic peptide; cTnI: cardiac troponin I; h-FABP: heart-type fatty acid-binding protein; MPO: myeloperoxidase; PAPP-A: pregnancy-associated plasma protein-A; SIMD: sepsis-induced myocardial dysfunction; AUC: area under the curve
3.3. Combination of biomarkers to predict SIMD
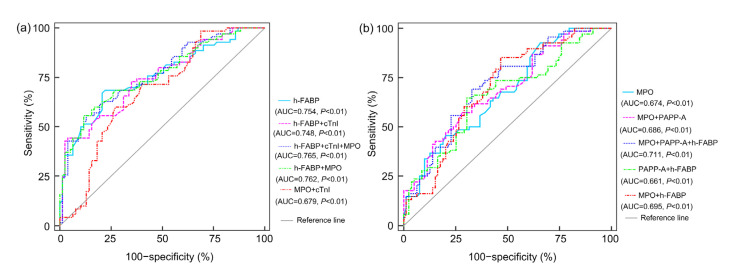
An ROC curve was developed to evaluate whether MPO and cTnI, both singly and in combination with h-FABP, improve the prediction level of SIMD (Fig. 2a). MPO alone (AUC=0.762) or cTnI alone (AUC=0.748) or both (AUC=0.765), in combination with h-FABP, did not significantly increase the AUC of h-FABP by Hanley-McNeil periods (P=0.494, P=0.548, and P=0.482, respectively). The combination of cTnI and MPO (AUC=0.679) was inferior to h-FABP in predicting SIMD. NRI and IDI were more sensitive tests for improving model discrimination than ROC curves (Pencina et al., 2008). In Table 3, the addition of MPO and cTnI to h-FABP provided an NRI of 18.7% (P=0.025) and IDI of 3.3% (P=0.033). The NRI and IDI of MPO combined with h-FABP were 9.2% (P=0.212) and 1.8% (P=0.108), respectively, and the NRI and IDI of cTnI added to h-FABP were 9.5% (P=0.103) and 0.8% (P=0.353), respectively.
Fig. 2.
ROC curves for the combination of two or three significant variables to predict SIMD (a) and mortality (b)
ROC: receiver operating characteristic; SIMD: sepsis-induced myocardial dysfunction; h-FABP: heart-type fatty acid-binding protein; cTnI: cardiac troponin I; MPO: myeloperoxidase; PAPP-A: pregnancy-associated plasma protein-A; AUC: area under the curve
Table 3.
NRI and IDI for evaluating improvement to predict SIMD after adding biomarkers to h-FABP
| Logarithm of the variable | NRI (%) | P value for NRI | IDI (%) | P value for IDI |
| ln h-FABP+ln MPO+ln cTnI | 18.7 | 0.025 | 3.3 | 0.033 |
| ln h-FABP+ln MPO | 9.2 | 0.212 | 1.8 | 0.108 |
| ln h-FABP+ln cTnI | 9.5 | 0.103 | 0.8 | 0.353 |
NRI: net reclassification improvement; IDI: integrated discrimination improvement; SIMD: sepsis-induced myocardial dysfunction; h-FABP: heart-type fatty acid-binding protein; MPO: myeloperoxidase; cTnI: cardiac troponin I
3.4. Characteristics of patients according to mortality
The comparisons of patients’ characteristics based on the mortality data are shown in Table 4. Comparison of the non-survival and survival groups revealed that there were no differences in age, sex, MODS score, SOFA score, APACHE-II score, or a history of COPD, stroke and trauma. However, patients with a history of shock had a significantly higher probability of death within 28 d than survival (P=0.000). Plasma levels of h-FABP, PAPP-A, BNP, and MPO in the dead group were significantly higher than those in the survival group (P=0.03, P=0.002, P=0.018, and P<0.0005, respectively). However, PCT and cTnI levels were similar in the two groups (P=0.838 and P=0.063, respectively). Data from the APACHE-II, SOFA, and MODS scores indicated that the degree of sepsis was not significantly different between the two groups (Table 4).
Table 4.
Comparison of the characteristics of survivals and non-survivals
| Characteristics | 28-d survivals (n=79) | 28-d non-survivals (n=68) | P |
| Age (year) | 63.9±15.8 | 68.5±14.7 | 0.086 |
| Sex, male | 66 (83.5%) | 54 (79.4%) | 0.666 |
| Treatment day in ICU | 18.4±8.2 | 25.7±8.5 | 0.000 |
| Stroke | 41 (51.9%) | 34 (50.0%) | 0.392 |
| Trauma | 47 (59.5%) | 39 (57.4%) | 0.925 |
| Shock | 16 (20.3%) | 34 (50.0%) | 0.000 |
| COPD | 40 (50.6%) | 32 (47.1%) | 0.790 |
| cTnI (μg/L) | 0.93 (0.14/1.25) | 0.91 (0.12/1.76) | 0.063 |
| BNP (pg/mL) | 626.0 (346.5/870.0) | 707.5 (409.5/1305.5) | 0.018 |
| h-FABP (ng/mL) | 8.44 (4.96/10.99) | 10.16 (8.40/12.20) | 0.030 |
| PAPP-A (ng/mL) | 3.57 (1.99/6.36) | 4.72 (2.92/7.42) | 0.002 |
| MPO (ng/mL) | 12.62 (8.37/17.80) | 15.65 (10.59/26.15) | <0.0005 |
| PCT (μg/L) | 3.50 (1.89/5.65) | 3.30 (1.99/5.30) | 0.838 |
| APACHE-II score (points) | 19.1±3.0 | 19.2±3.0 | 0.791 |
| SOFA score (points) | 10.4±2.4 | 10.2±2.2 | 0.470 |
| MODS score (points) | 11.1±2.3 | 11.8±2.3 | 0.109 |
Data are expressed as mean±standard deviation (SD), number (percentage), or median (25th/75th percentile). ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; cTnI: cardiac troponin I; BNP: brain natriuretic peptide; h-FABP: heart-type fatty acid-binding protein; PAPP-A: pregnancy-associated plasma protein-A; MPO: myeloperoxidase; PCT: procalcitonin; APACHE-II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; MODS: Multiple Organ Dysfunction Syndrome
3.5. Predictive values of biomarkers for mortality
As shown in Table 5, univariate logistic regression analysis showed that PAPP-A, h-FABP, MPO, and a history of shock indicated a higher risker of mortality (P=0.021, P=0.003, P=0.000, and P=0.000, respectively). Multivariate analysis demonstrated that MPO and a history of shock were associated with mortality (P=0.001 and P=0.002, respectively). According to the Fig. 1b, the calculated AUC for MPO was 0.674 (P<0.01), h-FABP was 0.631 (P<0.01), PAPP-A was 0.607 (P=0.022), BNP was 0.571 (P=0.146), and cTnI was 0.544 (P=0.365). The best cutoff value of MPO was 10.05 ng/mL, which provided a sensitivity of 92.7% and a specificity of 34.1%. The best cutoff value for h-FABP was 8.01 ng/mL (77.9% sensitivity and 49.4% specificity), and PAPP-A was 3.23 ng/mL (72.1% sensitivity and 46.8% specificity).
Table 5.
Univariate and multivariate odds ratios of predictors of mortality
| Predictor | OR | 95% CI | P |
| Univariate analysis | |||
| Age | 1.019 | 0.998 to 1.042 | 0.077 |
| COPD | 0.807 | 0.453 to 1.659 | 0.666 |
| Shock | 3.937 | 1.905 to 8.138 | 0.000 |
| Stroke | 1.407 | 0.730 to 2.713 | 0.307 |
| Trauma | 0.916 | 0.474 to 1.768 | 0.793 |
| ln BNP | 1.408 | 0.944 to 2.099 | 0.093 |
| ln h-FABP | 3.271 | 1.482 to 7.221 | 0.003 |
| ln PAPP-A | 1.767 | 1.091 to 2.863 | 0.021 |
| ln cTnI | 1.068 | 0.854 to 1.336 | 0.563 |
| ln MPO | 3.683 | 1.874 to 7.236 | 0.000 |
| ln PCT | 1.083 | 0.774 to 1.516 | 0.641 |
| APACHE II score | 1.027 | 0.923 to 1.143 | 0.627 |
| SOFA score | 0.966 | 0.839 to 1.113 | 0.635 |
| MODS score | 1.140 | 0.988 to 1.315 | 0.072 |
| Multivariate analysis | |||
| Shock | 3.193 | 1.506 to 6.769 | 0.002 |
| ln MPO | 3.238 | 1.594 to 6.577 | 0.001 |
OR: odds ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease; BNP: brain natriuretic peptide; h-FABP: heart-type fatty acid-binding protein; PAPP-A: pregnancy-associated plasma protein-A; cTnI: cardiac troponin I; MPO: myeloperoxidase; PCT: procalcitonin; APACHE-II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; MODS: Multiple Organ Dysfunction Syndrome
3.6. Combination of biomarkers to predict mortality
The addition of either PAPP-A alone (AUC=0.686) or h-FABP alone (AUC=0.695) or both (AUC=0.711) to MPO did not significantly improve the predictive ability of MPO by Hanley-McNeil periods (P=0.611, P=0.369, and P=0.224, respectively; Fig. 2b). Besides, the combination of h-FABP and PAPP-A (AUC=0.661) was lower than MPO alone for predicting prognosis of septic patients. According to Table 6, the addition of PAPP-A and h-FABP to MPO yielded an NRI of 25.5% (P=0.013) and IDI of 2.9% (P=0.045) as compared with MPO. NRI and IDI for MPO combined with h-FABP to predict mortality were 13.7% (P=0.051) and 1.7% (P=0.131), respectively, and MPO combined with PAPP-A were 8.9% (P=0.211) and 1.1% (P=0.200), respectively.
Table 6.
NRI and IDI for evaluating improvement to predict mortality after adding biomarkers to MPO
| Logarithm of the variable | NRI (%) | P value for NRI | IDI (%) | P value for IDI |
| ln MPO+ln PAPP-A+ln h-FABP | 25.5 | 0.013 | 2.9 | 0.045 |
| ln MPO+ln PAPP-A | 8.9 | 0.211 | 1.1 | 0.200 |
| ln MPO+ln h-FABP | 13.7 | 0.051 | 1.7 | 0.131 |
NRI: net reclassification improvement; IDI: integrated discrimination improvement; MPO: myeloperoxidase; PAPP-A: pregnancy-associated plasma protein-A; h-FABP: heart-type fatty acid-binding protein
4. Discussion
SIMD is the primary determinant of the outcome of septic shock and increases the mortality of septic patients (Blanco et al., 2008). Therefore, early diagnosis and aggressive supportive therapy for SIMD are recommended (Annane et al., 2005). It is imperative to develop biomarkers that effectively help to monitor and treat sepsis (Riedemann et al., 2003). The multi-biomarker strategy may provide a better predictive value for SIMD and prognosis of sepsis. Our study used biomarkers with predictive value to evaluate the ability of multi-biomarkers to predict SIMD and outcome in septic patients.
h-FABP is a small cytoplasmic protein found within cardiomyocytes that have recently emerged as a cardiac biomarker (Alhadi and Fox, 2010). The h-FABP is rapidly released from cardiomyocytes into blood circulation shortly after the occurrence of an ischemic episode (Glatz et al., 1988; Alhadi and Fox, 2010). Some studies (Nakata et al., 2003; O'Donoghue et al., 2006) have reported that h-FABP is a promising biomarker for acute coronary syndrome (ACS) and cardiac dysfunction (Arimoto et al., 2005; Kadowaki et al., 2017). It has also been reported that h-FABP is a biomarker for mortality in patients with sepsis and septic shock (Jo et al., 2012) and is associated with SIMD (Zhang et al., 2012). In our study, h-FABP had good diagnostic value for SIMD and was significantly higher in non-survivors than in survivors. The capability of h-FABP to diagnose SIMD was higher than that of cTnI and MPO (AUC: 0.754 vs. 0.618 vs. 0.645, OR: 8.900 vs. 1.299 vs. 2.922). However, the accuracy of h-FABP for the early diagnosis of SIMD, as quantified by the AUC, was not significantly high. Although h-FABP provided predictive value to evaluate the outcome of sepsis, its AUC was relatively low (AUC<0.70). The exact mechanism leading to the elevated h-FABP levels in septic patients is uncertain. It is hypothesized that h-FABP is released from cardiomyocytes following cardiac dysfunction (Glatz et al., 1988). It is also speculated that the catabolism of glycogen and lipids during severe sepsis and septic shock increases the free fatty acid level, which subsequently elevates h-FABP concentrations (Yan et al., 2009).
MPO is a heme-containing peroxidase mostly secreted by activated neutrophils and has strongly oxidative activity (Kothari et al., 2011). MPO catalyzes nitric oxide (NO) consumption and therefore reduces the bioavailability of NO and impairs its vasodilation and anti-inflammatory functions (Podrez et al., 2000; Fu et al., 2001). MPO is considered both a marker and mediator of vascular inflammation, and is associated with increased cardiovascular events (Baldus et al., 2003) and increased risk of mortality in patients with ACS (Kolodziej et al., 2019). Besides, recent studies (Ng et al., 2006; Avaliani et al., 2019) indicated that MPO had diagnostic and prognostic values in chronic heart failure (HF). As a novel biomarker, MPO could also aid in the prognosis of septic patients (Schrijver et al., 2017) and hence, is a potential biomarker for SIMD and mortality in sepsis. The present study revealed that MPO is also an independent predictor of mortality in septic patients. The predictive mortality ability of MPO was superior to that of PAPP-A and h-FABP, as manifested from the AUC (0.674 vs. 0.607 vs. 0.631). The association between MPO and mortality could be due to that MPO directly damages extracellular collateral and is an inflammatory marker, which causes organ failure (Schrijver et al., 2017). Besides, elevated MPO concentrations could detect SIMD in septic patients, as evidenced by the calculation of AUC. The mechanism might be that leukocyte-mediated oxidation reactions play a critical role in heart remodeling processes. The activation of polymorphonuclear cells is associated with increased plasma concentrations of MPO (Askari et al., 2003; Melenovsky et al., 2014). However, our study reveals that the accuracy of MPO in predicting SIMD and septic mortality is relatively low, as demonstrated by the AUC (all AUC<0.70).
BNP is a neurohormone secreted by cardiac ventricles in response to pressure overload. This neurohormone promotes natriuresis and diuresis, diastolic blood vessel, and antagonizes the vasoconstriction effects of the renin-angiotensin-aldosterone system. Besides, the secretion of BNP depends on the filling pressure of the left ventricle and extension of the myocardial wall. The increase in serum concentrations of BNP is valuable in the diagnosis of SIMD and prognosis of septic patients (Witthaut et al., 2003; Post et al., 2008). However, previous research (Charpentier et al., 2004) and recent observational study (Papanikolaou et al., 2014) demonstrated that the severity of illness, rather than SIMD, is the primary cause of increased BNP in septic patients. The use of BNP alone to predict SIMD may be, therefore, not recommended. In our research, patient’s BNP levels at the point of admission did not accurately predict SIMD or adverse outcomes in septic patients.
As pathological markers of acute myocardial infarction, the assay of cTn is the gold standard for the diagnosis of acute myocardial injury (Park et al., 2017). Serum levels of cTnI are also increased in blood flow-restricted coronary artery diseases, including pulmonary embolism, sepsis, myocarditis, and acute stroke (Fromm, 2007). Several studies (Mehta et al., 2004; Bessière et al., 2013; Kim et al., 2019) have shown that cTnI is associated with myocardial injury in patients with sepsis and can predict the outcome of patients. In the current study, increased serum cTnI levels at admission were found to be a predictor of SIMD, but it was not associated with mortality.
PAPP-A is in the metzincin superfamily of metalloproteinases. The PAPP-A glycoprotein is produced by the placental syncytiotrophoblast during pregnancy and fibroblasts, osteoblasts, and vascular smooth muscle cells (Consuegra-Sanchez et al., 2009). High serum concentrations of PAPP-A are associated with HF (Funayama et al., 2011). Besides, a study indicated that elevated levels of plasma PAPP-A could predict myocardial dysfunction and death in patients with severe sepsis (Zhang et al., 2014). According to our results, high levels of PAPP-A predict the risk of death in patients with sepsis, but its predictive value is relatively low (AUC=0.607). Therefore, PAPP-A was not feasible in the early diagnosis of SIMD. Systemic inflammation, sepsis-related vascular damages, and the administration of heparin and insulin to patients may be related to the elevated serum PAPP-A concentrations (Pellitero et al., 2007; Wittfooth et al., 2011).
Although h-FABP and MPO were good indicators for SIMD and mortality, the calculation of AUC indicated that their predictive value was unsatisfactory. To improve the prediction accuracy of SIMD and mortality in septic patients, this study, therefore, combined two or three biomarkers. The result revealed that the addition of cTnI or MPO or both to h-FABP did not significantly improve the predictive ability for SIMD. The contribution of new biomarkers based on the ROC curve is immense, but this method has been highly criticized because it is insensitive in comparing models (Pepe et al., 2004) and has little direct clinical relevance (Janes et al., 2008). Several new techniques have, therefore, been proposed to evaluate and compare predictive risk models (Cook and Ridker, 2009). The present study used a more sensitive test to improve model discrimination (Pencina et al., 2008). We found that the addition of cTnI and MPO to h-FABP significantly improved the early diagnosis capacity of SIMD by the IDI (18.7% P=0.025) and NRI (3.3%, P=0.033) indices. However, the SIMD diagnosis capacities of IDI and NRI indices did not significantly increase through the addition of cTnI alone or MPO alone to h-FABP. The reason for choosing h-FABP, MPO, and cTnI combined to predict SIMD was that h-FABP, MPO, and cTnI were predictive indicators for SIMD through the AUC in our study. Furthermore, h-FABP and cTnI have been proved as sensitive biomarkers for myocardial injury (Arimoto et al., 2005; Kadowaki et al., 2017; Park et al., 2017). The addition of h-FABP or PAPP-A to MPO slightly improved its mortality predictive ability, as demonstrated by AUC, NRI, and IDI. Although the AUC showed that the addition of PAPP-A and h-FABP to MPO did not significantly improve the predictive ability, NRI (13.7%, P=0.051) and IDI (1.7%, P=0.131) were statistically significant. Inflammation is the pathophysiological basis of sepsis and MPO and PAPP-A are the indicators of inflammation, which may explain that the combination of MPO, PAPP-A, and h-FABP significantly improve the ability to predict outcome of sepsis.
Our current study had several limitations. First, it was a retrospective cohort study of prospectively collected data from one center. Second, the number of patients in our study was insufficient and may influence the outcome. Third, NRI depends on the specific categories of risk stratification used (Cook and Ridker, 2009). Our study used <10%, 10% to <30%, 30% to 50%, and >50% risk stratifications for SIMD and mortality. The NRI is affected by risk stratification, while risk stratification is currently not well recognized.
5. Conclusions
Our study revealed that MPO and h-FABP were good predictors of myocardial dysfunction and outcome in septic patients. PAPP-A can serve as a useful biomarker for prognosis in septic patients. Also, this study revealed that cTnI provided predictive value for SIMD. Through the calculated IDI and NRI indices, the addition of MPO and cTnI to h-FABP could significantly improve the ability to predict mortality and the combination of MPO, PAPP-A, and h-FABP significantly improved the predictive ability to SIMD in septic patients.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ16H020003) and the National Natural Science Foundation of China (Nos. 81971860 and 81772110)
Contributors: Fa-chao CHEN performed the experimental research and data analysis, and wrote and edited the manuscript. Yin-chuan XU and Zhao-cai ZHANG participated in the study design, data analysis, and writing and editing of the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Fa-chao CHEN, Yin-chuan XU, and Zhao-cai ZHANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Written informed consent was required from all participants or their legal proxies before registration for being included in the study.
References
- 1.ACCP/ SCCM. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 2.Alhadi HA, Fox KAA. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction: the potential for influencing patient management. Sultan Qaboos Univ Med J. 2010;10(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- 3.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/s0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 4.Arimoto T, Takeishi Y, Shiga R, et al. Prognostic value of elevated circulating heart-type fatty acid binding protein in patients with congestive heart failure. J Card Fail. 2005;11(1):56–60. doi: 10.1016/j.cardfail.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Askari AT, Brennan ML, Zhou XR, et al. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197(5):615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avaliani T, Talakvadze T, Tabagari S. Prognostic value of plasma myeloperoxidase level’s and echocardiographic determinants in chronic heart failure patients. Georgian Med News. 2019;(288):55–60. [PubMed] [Google Scholar]
- 7.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–1445. doi: 10.1161/01.cir.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 8.Berdal JE, Stavem K, Omland T, et al. Prognostic merit of N-terminal-proBNP and N-terminal-proANP in mechanically ventilated critically ill patients. Acta Anaesthesiol Scand. 2008;52(9):1265–1272. doi: 10.1111/j.1399-6576.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 9.Bessière F, Khenifer S, Dubourg J, et al. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39(7):1181–1189. doi: 10.1007/s00134-013-2902-3. [DOI] [PubMed] [Google Scholar]
- 10.Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12(6):R158. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32(3):660–665. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 12.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis. 2009;203(2):346–352. doi: 10.1016/j.atherosclerosis.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 13.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150(11):795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fromm RE., Jr Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med. 2007;35(2):584–588. doi: 10.1097/01.ccm.0000254349.10953.be. [DOI] [PubMed] [Google Scholar]
- 16.Fu XY, Kassim SY, Parks WC, et al. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 17.Funayama A, Shishido T, Netsu S, et al. Serum pregnancy-associated plasma protein a in patients with heart failure. J Card Fail. 2011;17(10):819–826. doi: 10.1016/j.cardfail.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Glatz JFC, van Bilsen M, Paulussen RJA, et al. Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988;961(1):148–152. doi: 10.1016/0005-2760(88)90141-5. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 20.Huber W, Mayr U, Umgelter A, et al. Mandatory criteria for the application of variability-based parameters of fluid responsiveness: a prospective study in different groups of ICU patients. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(7):515–524. doi: 10.1631/jzus.B1700243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008;149(10):751–760. doi: 10.7326/0003-4819-149-10-200811180-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardin F, Fourme T, Page B, et al. Persistent preload defect in severe sepsis despite fluid loading: a longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354–1359. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 23.Jo YH, Kim K, Lee JH, et al. Heart-type fatty acid-binding protein as a prognostic factor in patients with severe sepsis and septic shock. Am J Emerg Med. 2012;30(9):1749–1755. doi: 10.1016/j.ajem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki S, Watanabe T, Otaki Y, et al. Combined assessment of myocardial damage and electrical disturbance in chronic heart failure. World J Cardiol. 2017;9(5):457–465. doi: 10.4330/wjc.v9.i5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JS, Kim M, Kim YJ, et al. Troponin testing for assessing sepsis-induced myocardial dysfunction in patients with septic shock. J Clin Med. 2019;8(2):239. doi: 10.3390/jcm8020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klouche K, Pommet S, Amigues L, et al. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: relationships with systolic myocardial dysfunction and intensive care unit mortality. J Intensive Care Med. 2014;29(4):229–237. doi: 10.1177/0885066612471621. [DOI] [PubMed] [Google Scholar]
- 27.Kolodziej AR, Abo-Aly M, Elsawalhy E, et al. Prognostic role of elevated myeloperoxidase in patients with acute coronary syndrome: a systemic review and meta-analysis. Mediat Inflamm, 2019:2872607. 2019 doi: 10.1155/2019/2872607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kothari N, Keshari RS, Bogra J, et al. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care. 2011;26(4):435.e1–435e7. doi: 10.1016/j.jcrc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon A, Mulligan R. Combining cognitive testing and informant report to increase accuracy in screening for dementia. Am J Psychiatry. 1998;155(11):1529–1535. doi: 10.1176/ajp.155.11.1529. [DOI] [PubMed] [Google Scholar]
- 31.Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95(1):13–17. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakata T, Hashimoto A, Hase M, et al. Human heart-type fatty acid-binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology. 2003;99(2):96–104. doi: 10.1159/000069726. [DOI] [PubMed] [Google Scholar]
- 34.Ng LL, Pathik B, Loke IW, et al. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J. 2006;152(1):94–101. doi: 10.1016/j.ahj.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 35.O'Donoghue M, de Lemos JA, Morrow DA, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114(6):550–557. doi: 10.1161/circulationaha.106.641936. [DOI] [PubMed] [Google Scholar]
- 36.Papanikolaou J, Makris D, Mpaka M, et al. New insights into the mechanisms involved in B-type natriuretic peptide elevation and its prognostic value in septic patients. Crit Care. 2014;18(3):R94. doi: 10.1186/cc13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708–1718. doi: 10.1093/cvr/cvx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 39.Pellitero S, Reverter JL, Pizarro E, et al. Pregnancy-associated plasma protein-A levels are related to glycemic control but not to lipid profile or hemostatic parameters in type 2 diabetes. Diabetes Care. 2007;30(12):3083–3085. doi: 10.2337/dc07-1092. [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D'Agostino RB, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 41.Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 42.Perner A, Cecconi M, Cronhjort M, et al. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med. 2018;44(6):791–798. doi: 10.1007/s00134-018-5177-x. [DOI] [PubMed] [Google Scholar]
- 43.Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105(8):1095–1108. doi: 10.1172/jci8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Post F, Weilemann LS, Messow CM, et al. B-type natriuretic peptide as a marker for sepsis-induced myocardial depression in intensive care patients. Crit Care Med. 2008;36(11):3030–3037. doi: 10.1097/CCM.0b013e31818b9153. [DOI] [PubMed] [Google Scholar]
- 45.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 46.Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care, 3:48. 2015 doi: 10.1186/s40560-015-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrijver IT, Kemperman H, Roest M, et al. Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intensive Care Med Exp, 5:43. 2017 doi: 10.1186/s40635-017-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittfooth S, Tertti R, Lepäntalo M, et al. Studies on the effects of heparin products on pregnancy-associated plasma protein A. Clin Chim Acta. 2011;412(3-4):376–381. doi: 10.1016/j.cca.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29(10):1696–1702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 51.Yan GT, Lin J, Hao XH, et al. Heart-type fatty acid-binding protein is a useful marker for organ dysfunction and leptin alleviates sepsis-induced organ injuries by restraining its tissue levels. Eur J Pharmacol. 2009;616(1-3):244–250. doi: 10.1016/j.ejphar.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 52.Yucel T, Memiş D, Karamanlioglu B, et al. The prognostic value of atrial and brain natriuretic peptides, troponin I and C-reactive protein in patients with sepsis. Exp Clin Cardiol. 2008;13(4):183–188. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang ZC, Dai HW, Yu YH, et al. Usefulness of heart-type fatty acid-binding protein in patients with severe sepsis. J Crit Care. 2012;27(4):415.e13–415e18. doi: 10.1016/j.jcrc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhang ZC, Dai HW, Yu YH, et al. Elevated pregnancy-associated plasma protein A predicts myocardial dysfunction and death in severe sepsis. Ann Clin Biochem. 2014;51(1):22–29. doi: 10.1177/0004563213489275. [DOI] [PubMed] [Google Scholar]