Abstract
Pacemaking dysfunction has become a significant disease that may contribute to heart rhythm disorders, syncope, and even death. Up to now, the best way to treat it is to implant electronic pacemakers. However, these have many disadvantages such as limited battery life, infection, and fixed pacing rate. There is an urgent need for a biological pacemaker (bio-pacemaker). This is expected to replace electronic devices because of its low risk of complications and the ability to respond to emotion. Here we survey the contemporary development of the bio-pacemaker by both experimental and computational approaches. The former mainly includes gene therapy and cell therapy, whilst the latter involves the use of multi-scale computer models of the heart, ranging from the single cell to the tissue slice. Up to now, a bio-pacemaker has been successfully applied in big mammals, but it still has a long way from clinical uses for the treatment of human heart diseases. It is hoped that the use of the computational model of a bio-pacemaker may accelerate this process. Finally, we propose potential research directions for generating a bio-pacemaker based on cardiac computational modeling.
Keywords: Biological pacemaker, Gene therapy, Cell therapy, Cardiac simulation, Computational modeling
1. Introduction
Cardiovascular disease has become a severe health problem all over the world. In China, cardiac diseases account for about 40% of total mortality (Chen et al., 2018). Particularly, the pacemaking dysfunction, such as sick sinus syndrome and atrioventricular heart-block, is one of the most serious cardiac diseases, and may result in heart failure and even death. Currently, the electronic pacemaker is the only treatment for pacing dysfunction. Since the clinical use of the electronic pacemaker from 1958, it has saved more than three million lives all over the world. However, there are still several shortcomings of the device. For instance, the batteries and leads have limited lives and therefore need to be changed periodically; the device may suffer interference from electromagnetics which may obstruct the normal life of the patients; open-chest surgery during implanting a pacemaker may induce infection; an electronic pacemaker cannot response to emotion; the device must be changed with the growth of a pediatric patient (Cohen et al., 2005; Rosen et al., 2011). What is more, the long-term use of electronic pacemakers will increase the risk of heart failure (Freudenberger et al., 2005). Therefore, a biological pacemaker (bio-pacemaker) was proposed as a substitute for the electrical pacemaker (Starzl et al., 1963; Mayer et al., 1967; Morishita et al., 1981).
A bio-pacemaker is potentially a new treatment for pacing dysfunction. It is developed to conquer the defects of electronic pacemakers, sustaining a robust pacing activity in the whole heart. Bio-pacemaker implementation can be performed by injecting pacemaker cells into the heart, which then initiates and controls the spontaneous rhythms in the whole heart. This approach avoids the risk of repeated surgery and the rhythm can respond to emotion (Shlapakova et al., 2010). We compared the performances of electronic pacemakers and bio-pacemakers (Table 1). Actually, several biological experiments have provided some promising evidence for the practicability of the bio-pacemaker. Quiescent heart-muscle cells have been successfully converted into pacemaker cells using viral gene transfer (Miake et al., 2002). Also, human embryonic stem cells (hESCs) have been successfully differentiated into cardiomyocytes (CMs) which have the ability to activate quiescent ventricular myocytes (VMs) (Xue et al., 2005). Indeed, human embryonic stem-derived CMs were transplanted to the hearts of swine with complete atrioventricular block, and successfully drove the whole heart (Kehat et al., 2004). These approaches provide an encouraging perspective on arrhythmia treatment.
Table 1.
Comparison between electronic pacemaker and bio-pacemaker
| Pacemaker | Extra power | Fixed size | Electromagnetic interference | Transplant surgery | Emotion response | Risk of heart failure |
| Electronic pacemaker | Yes | Yes | Yes | Yes | No | Yes |
| Biological pacemaker | No | No | No | No | Yes | Maybe |
Theoretically, the construction of a bio-pacemaker is based on the differences in cellular electrophysiology between pacemaking sinoatrial node (SAN) cells and non-autorhythmic cardiac cells. SAN is the origin of intrinsic automaticity. The pacing ability of SAN cells results from a low expression of the inward rectifier potassium channel current (I K1) and a high expression of hyperpolarization-activated channel current (funny current (I f)) as compared with atrial myocytes (AMs) and VMs. I K1 is an outward current, repolarizing cellular action potential (AP) during the terminal phase of the AP. Thus, the low expression of I K1 could reduce the level of hyperpolarization and elevate the maximum diastolic potential, which makes depolarization easier. I f is generally regarded as the “pacemaker current,” playing a dominant role in initiating the diastolic depolarization and pacing activity (Ravagli et al., 2016). In non-autorhythmic cells, I K1 is highly expressed (Miake et al., 2002; Azene et al., 2005; Cho et al., 2007), but I f expression is low or even absent (Yu et al., 1993). The high expression of I K1 and absence of I f jointly result in the loss of auto-rhythm in non-autorhythmic cells. In contrast, overexpressing I f and inhibiting I K1 in non-autorhythmic cells are possible approaches to turn non-autorhythmic cells into autorhythmic cells, creating a bio-pacemaker. At the high-dimensional level, the intercellular coupling between SAN cells and neighboring cells is weak, which helps to conduct the spontaneous electrical signal to the neighboring cells. When producing the bio-pacemaker at the two-dimensional or three-dimensional level, reducing the intercellular coupling should be taken into consideration.
After over five decades’ studies of bio-pacemaker, there has been considerable progress. However, limitations such as funding and moral issues are limiting experimental research. Thus, the computational simulation of a bio-pacemaker based on the cardiac computational model is a necessity.
In this review, we first introduce the progress in the development of a bio-pacemaker aiming for gene therapy and cell therapy. Then, we summarize current progress of autologous therapy and highlight the obstacles in producing a bio-pacemaker. Finally, we propose possible future trends in bio-pacemaker research—computational simulation. This review provides a systematic view on generating a bio-pacemaker from biological experiment to computational simulation. This may be helpful for the better design of a future generation of stable and robust bio-pacemakers.
2. Biological experiments for producing biological pacemakers
In the heart, there are three aspects to causing auto-rhythmicity: trigger cells with spontaneous beats, substrate cells with the function of delivering signal, and trigger–substrate connection (Rosen et al., 2011). In the cases of SAN cell failure, a possible treatment could be inducing rhythm in other positions, such as AMs or VMs, and making them take over the function of SAN. To realize this scenario, first, autonomic pacemaker cells should be developed. Generally, some methods have been used to create pacemaker cells: expressing a specific gene which controls pacemaking-related ionic channels by cell fusion (called gene therapy), and differentiating ESCs into pacemaker cells (called cell therapy) (Munshi and Olson, 2014; Rosen, 2014). Cell therapy is always accompanied by gene therapy so that cardiac myocytes transformed from stem cells could produce a pacemaking function, which is called hybrid therapy. The experiments by hybrid therapy are described in the section of “Cell therapy.” Then, a certain amount of pacemaker cells should be injected into specific locations of the heart in vivo to induce heartbeats. The number and location of pacemaker cells should ensure the ability and stability of pacing activity.
2.1. Gene therapy
Up to now, bio-pacemaker gene therapy usually controls the expression of hyperpolarization-activated cyclic nucleotide-gated (HCN) gene family (Santoro and Tibbs, 1999), Kir2.1 and Kir2.2 genes (Zaritsky et al., 2001), and transcription factor T-box 18 (TBX18) (Kapoor et al., 2013). The AP characteristics and I f density of bio-pacemaker cells created by gene therapy are shown in Table 2.
Table 2.
Review of gene therapy experiments
| Reference | Gene type | Subject | CL (ms) | APD50 (ms) | APD90 (ms) | MDP (mV) | V 1/2 (mV) | I f density at −70 mV (pA/pF) |
| Qu et al., 2003 | HCN2 | Canine left atrium | −94.00 | −3.2 | ||||
| Plotnikov et al., 2004 | HCN2 | Canine left bundle-branch | 340 | 100 | 180 | −74.7 | −31.0 | |
| Qu et al., 2001 | HCN2 | Neonatal rat VMs | 681 | 170 | 255 | −42.5±1.6# (n=11) | −78.00 | −41.3 |
| Boink et al., 2013 | HCN2/SkM1 | Canine left bundle-branch | 625 | 195±5# (n=6) | 213±5# (n=6) | −85.0±2.0# (n=6) | ||
| Valiunas et al., 2009 | Mouse HCN2 | Canine VMs | 648 | 266 | 479 | −66.0 | ||
| Cho et al., 2007 | HCN1 | Guinea pig VMs | 279 | 113 | 155 | −70.0 | −12.5 | |
| Miake et al., 2002 | Kir2.1-AAA | Guinea pig VMs | 598 | 230 | 295 | −63.0 | ||
| Zaritsky et al., 2001 | Kir2.1 −/− | Neonatal mouse VMs | Non* | −11.7±1.2# (n=8) | ||||
| Kapoor et al., 2013 | TBX18 | Neonatal rat VMs | 631 | 242 | 320 | −47.0±10.0# (n=6) | −3.46 | −8.0 |
| Guinea pig VMs | 443 | 137 | 189 | −55.8±6.0# (n=5) | ||||
| Hu et al., 2014 | TBX18 | Porcine VMs | 730 | |||||
| Bakker et al., 2012 | TBX3 | Neonatal rat VMs | 580 |
HCN: hyperpolarization-activated cyclic nucleotide-gated; HCN1/2: HCN channel 1/2; SkM1: skeletal muscle sodium channel 1; TBX18: transcription factor T-box 18; VMs: ventricular myocytes; CL: cycle length; APD50: action potential duration at 50% repolarization; APD90: action potential duration at 90% repolarization; MDP: maximum diastolic potential; V 1/2: average half-maximal activation voltage of funny current’s activation gate; I f: funny current; pA/pF: unit of current density, (10−12 A)/(10−12 F).
No auto-rhythm was observed;
Data are expressed as mean±standard error of the mean (SEM)
HCN gene family controls the expression of I f. Overexpressing the HCN gene into non-autorhythmic cells could promote the expression of I f in these cells, promoting depolarization during the diastolic interval, hence inducing spontaneous beats. In the mammalian heart, injecting HCN channel 2 (HCN2) into the canine left atrium (LA) (Qu et al., 2003) and the left bundle-branch (LBB) (Plotnikov et al., 2004) via adenoviral constructs has successfully produced escape beats. That experiment also indicated that the LBB was a preferable location when creating pacemaker cells because it was proximal to the ventricular conducting system and easy to conduct the electrical signal into the ventricle (Plotnikov et al., 2004). As for rodent animals, highly expressing HCN2 in neonatal rat VMs caused spontaneous beats. However, in adult rat VMs, it did not work, because the I K1 density was higher in adult rat VMs than in neonatal rat cells (Qu et al., 2001), which inhibited the depolarization. In addition to single gene expression, a dual gene therapy, which co-expressed skeletal muscle sodium channel 1 (SkM1) with HCN2 in the canine left bundle branch, showed a more efficient pacemaking activity than single gene expression. SkM1 could maximize the opening of a sodium current channel, which led to a quick depolarization and a more positive AP (Boink et al., 2013). This is the first report that cardiac auto-rhythms were induced by bio-pacemaker in large animals. An experiment in a two-cell system verified that an HCN2-expressing cell could propagate spontaneous electrical signals to a quiescent VM cell (Valiunas et al., 2009). This provides evidence for the possibility of creating a bio-pacemaker by overexpressing HCN2. HCN1 was also used to induce spontaneous rhythms. Spontaneous oscillations could be initiated in guinea pig VMs fused with HCN1-expressing fibroblasts under inhibition of I K1 (Cho et al., 2007). HCN1 currents needed a long period (approximately 3000 ms) to reach equilibrium (Azene et al., 2005), but the pacing cycle is only 250 ms in guinea pigs and 1000 ms in humans, which would be a potential problem for the stability of HCN1-induced bio-pacemaker cells. Also, some experiments indicated that acute expression of HCN gene suppresses cardiac automaticity (Azene et al., 2005; Cho et al., 2007; Lieu et al., 2008; Sun et al., 2017) and the expression of the HCN gene in VMs may raise the risk of arrhythmicity (Kuwabara et al., 2013). Thus, the stability and robustness of HCN gene therapy need to be proved.
Kir2.1 and Kir2.2 encode I K1 in many cell types, including CMs. Knocking out the Kir2.1 and Kir2.2 could depress I K1 and initiate spontaneous beats in isolated CMs. Removing Kir2.1 and Kir2.2 in newborn murine VMs could elevate the resting potential and promote spontaneous rhythms (Zaritsky et al., 2001). Replacing three amino-acid residues in the pore structure of Kir2.1 produced a dominant negative construct called Kir2.1-AAA, which could inhibit I K1. Miake et al. (2002) transduced Kir2.1-AAA in the left ventricular cavity of guinea-pigs, discovering that destroying Kir2.1 caused an 80% decline of I K1. They first verified that infecting Kir2.1-AAA in VMs produced more rapid beats than native pacing activity. After this study, they analyzed the role of I K1 in the excitability of VM via adenoviral gene transfection (Miake et al., 2003). They reported that Kir2.1-AAA caused a 50%–90% decline of I K1 and only a greater than 80% suppression of I K1 resulted in pacemaking activity, contributing to a longer AP duration, a slow phase 3 repolarization, and a more positive resting membrane potential.
This experiment provided a possible direction for pacemaker creation. However, an unfavorable finding was that decreased I K1 caused a long-QT phenotype and produced arrhythmia (Miake et al., 2003). Also, Kir2.1-knockout was readily reported to cause death in the adult animals, so that the experimental subjects were limited to the newborn animals (Zaritsky et al., 2001; Miake et al., 2003). As a result, developing a pacemaker by I K1-knocking-out seems not to be an excellent approach.
Sun et al. (2017) showed that neither I f overexpression nor I K1 suppression alone could initiate automaticity rhythm in CMs. As a result, the gene that codes I K1 and I f at the same time may be a promising candidate of bio-pacemaker gene therapy. The embryonic transcription factor TBX18 has effects on the expression of several pacemaking-related genes such as the HCN gene family and Cx43 gene family as well as encodes the activation of I K1 (Choudhury et al., 2018). Cx43 encodes electrical propagation between cells and downregulating intercellular coupling facilitates the transmission of automatic rhythms between cardiac cells. All these facts imply that TBX18 has tremendous potential for producing a bio-pacemaker. In rodent animals, VMs, which were infected with TBX18 via adenoviral vectors, showed pacemaker cell morphology and appropriate autonomic responses in the induced SAN (iSAN) (Kapoor et al., 2013). The expression of TBX18 caused a 78% reduction in the density of I K1, a 1.4-fold increase in the HCN4 expression level as well as the downregulation of Cx43, which produced a reduction of coupling between cells (Kapoor et al., 2013). These factors caused the auto-rhythmicity jointly. Experiment in large animals exhibited similar results. Hu et al. (2014) created iSAN cells in porcine VMs by reprogramming TBX18, which was the second report of a bio-pacemaker created in the large animals. Indeed, the injected site showed a downregulated Kir2.1, a promoted HCN4, and a sparse Cx43, which had the result that the iSAN cells had the ability to support both heart rate and physical activity. Although this was just a transient bio-pacemaker, an excellent result was that TBX18 expression did not increase the risk of arrhythmia. Apart from TBX18, TBX3 could also reduce I K1 and intercellular coupling in the mice atrium (Bakker et al., 2012). In the rat right atrium, overexpressing TBX3 caused upregulation of HCN1, HCN2, and RYR2, but downregulation of HCN4 was witnessed (Bakker et al., 2012). There was no evidence that overexpressing TBX3 could create a bio-pacemaker.
2.2. Cell therapy
Generally, four kinds of cells were used to differentiate into bio-pacemakers: ESCs, bone marrow stem cells (BMSCs), adipose-derived stem cells (ADSCs) and induced pluripotent stem cells (iPSCs). Other types of cells were also deemed as candidates in cell therapy, such as SAN (Zhang et al., 2008) and CM progenitor cells (Végh et al., 2019). To create preferable autonomic pacing CMs, pacing-related genes are usually expressed into induced CMs. This is a hybrid therapy. In this paper, we consider hybrid therapy as a kind of cell therapy, which is introduced in this section. The cycle length (CL) and I f density of bio-pacemaker cells created by cell therapy as well as the CL of co-cultured cells are presented in Table 3.
Table 3.
Review of cell therapy experiments
| Reference | Cell type | Gene type | CL1 (ms) | I f density at −70 mV (pA/pF) | Co-cultured subject | CL2(ms) |
| Kehat et al., 2004 | Human ESCs | 1351 | ||||
| Xue et al., 2005 | Human ESCs | Neonatal rat VMs | 1224 | |||
| Adult guinea pig heart | 740 | |||||
| Saito et al., 2015 | Mouse ESCs | HCN4 | 724 | |||
| Ionta et al., 2015 | Mouse ESCs | Shox2 | 192 | −1.80 | Rat heart with complete heart block | 1416 |
| Potapova et al., 2004 | Human MSCs | Mouse HCN2 | −7.90 | Canine VMs | 983 | |
| Neonatal rat VMs | 372 | |||||
| Plotnikov et al., 2007 | Human MSCs | Mouse HCN2 | −7.12 | Canine heart | 1153 | |
| Bruzauskaite et al., 2016 | Human BMSCs | Mouse HCN2 | Non* | −3.15 | ||
| Zhou et al., 2007 | Rabbit BMSCs | Human HCN2 | Cardiac myocytes | 402 | ||
| Zhou et al., 2013 | Rabbit MSCs | Human HCN1 | Neonatal rabbit VMs | 465 | ||
| Zhang et al., 2013 | Porcine BMSCs | Human HCN4 | −1.26 | Porcine VMs | 1224 | |
| Planat-Benard et al., 2004 | Mouse ADSCs | 909 | ||||
| Yang et al., 2016 | ADSCs | TBX18 | Non* | −5.30 | Neonatal rat VMs | 800 |
| Chen et al., 2017 | ADSCs | TBX18 | 181 | |||
| Ruhparwar et al., 2002 | Fetal atrial CMs | Canine left VMs | 710±30# (n=2) | |||
| Zhang et al., 2011 | Sino atrial nodes | Canine right ventricle | 1200 | |||
| Chauveau et al., 2017 | Human iPSCs | 435 | −24.50 | Canine VMs | 1395 |
ESCs: embryonic stem cells; MSCs: mesenchymal stem cells; BMSCs: bone marrow stem cells; ADSCs: adipose-derived stem cells; CMs: cardiac myocytes; iPSCs: induced pluripotent stem cells; HCN: hyperpolarization-activated cyclic nucleotide-gated; HCN1/2/4: HCN channel 1/2/4; TBX18: transcription factor T-box 18; CL: cycle length; I f: funny current; pA/pF: unit of current density, (10−12 A)/(10−12 F); VMs: ventricular myocytes.
CL of pacemaker cell;
CL of co-cultured cell.
No auto-rhythm was observed on that occasion;
Data are expressed as mean±standard error of the mean (SEM)
Kehat et al. (2001) reported that hESCs could differentiate into CMs. Then, they verified that rat CMs derived from hESCs could generate embryonic-like AP and pacing activity. They first demonstrated that hESCs-induced CMs (hESC-CMs) could successfully create electromechanical integration and drive the heart in the pig with complete atrioventricular block (Kehat et al., 2004). Then, researchers began focusing on the pacing function of CMs differentiated from ESCs. In vivo, under atrioventricular nodal cryoablation condition, the hESC-CMs-injected adult guinea pig heart presented induced automaticity, and no evidence showed that hESC-CMs led to tumor or arrhythmias (Xue et al., 2005). To enhance the ability of pacing, pacing-related genes were injected into the ESCs in hybrid therapy. Overexpressing HCN4 in mouse ESCs (mESCs) generated considerably larger I f currents, and hence a faster spontaneous rate of (87.4±11.9) beats/min (bpm) than the control (Saito et al., 2015). Actually, in human iPSCs (hiPSCs) co-cultured with HCN4-overexpressing mESC-CMs, there was a higher beating rate at (82.8±4.8) bpm than that in the control condition (hiPSCs co-cultured with enhanced green fluorescent protein with a beating rate of (55.2±13.8) bpm) (Saito et al., 2015). It meant that overexpressing HCN4 promoted the pacing ability of mESC-CMs in co-culture system with other excitable cells in vitro. Shox2 is a homeodomain transcription factor that controls the development and function of the native cardiac pacemaker, specifically expressing in the SAN but not in the atrium or ventricle. In the single cell, expressing Shox2 caused a lager I f, a shorter AP duration, and a faster spontaneous pacing rate (Ionta et al., 2015). In vitro, under the overexpression of Shox2, the proportion of mESCs with spontaneous beats increased and its beating rate was considerably faster than that under the control condition (Ionta et al., 2015). Meanwhile, in vivo, injecting Shox2-mESCs into the left ventricular apex of the rat heart contributed to a higher rate (at (44±3) bpm) than the control (Ionta et al., 2015). After that, this team established an ESC-based cardiac differentiation model using Shox2 as a molecular tool (Hoffmann et al., 2017), which provides a fundamental basis for the investigation of molecular pathways. These studies indicate that ESCs have great potential to differentiate into sinoatrial-like cells.
Compared with ESCs, mesenchymal stem cells (MSCs) are easier to achieve and culture. Some experiments verified that implanting MSCs loaded with the pacemaker gene in the myocardium could induce pacemaker function. For instance, the expression of mouse HCN2 (mHCN2) in human MSCs (hMSCs) could create I f-like current and initiate spontaneous rates in rat VMs at (93±16) bpm (Potapova et al., 2004). mHCN2-induced hMSCs could also generate electrical coupling in canine VMs and drive the canine heart with idioventricular rates at (61±5) bpm during sinus arrest (Potapova et al., 2004). This team also investigated the influence of the size and location of mHCN2-hMSCs on the pacing function, concluding that injecting 70 000 hMSCs into the conducting system could drive the ventricle (Plotnikov et al., 2007). The MSCs are usually extracted from bone marrow and adipose tissues; these are called BMSCs and ADSCs, respectively. Both BMSCs (Zhou et al., 2007, 2013; Huang et al., 2010, 2012; Zhang et al., 2013; Bruzauskaite et al., 2016) and ADSCs (Rangappa et al., 2003; Planat-Benard et al., 2004; Choi et al., 2010; Yang J et al., 2012; Zhao et al., 2012; Yang M et al., 2016; Chen et al., 2017) show the ability to differentiate into CMs. This provides another option for creating a bio-pacemaker by cell therapy.
Experiments showed that fluid shear stress (Huang et al., 2010) and cyclic strain (Huang et al., 2012) could transdifferentiate BMSCs into CMs. Except for physiotherapy, BMSCs are ideal candidates in hybrid therapy. Transfecting human HCN2 (hHCN2) into rabbit BMSCs could express I f current (Zhou et al., 2007). Rabbit CMs co-cultured with hHCN2-transfected MSCs showed spontaneous beats at (149±14) bpm, which was significantly higher than the control at (87±11) bpm (Zhou et al., 2007). High cell viability and spontaneous beating activity were witnessed in mHCN2-transfected hMSCs, but the proliferative activity was somewhat low (Bruzauskaite et al., 2016). In terms of other kinds of HCN gene, injecting HCN1 into BMSCs can initiate spontaneous automaticity in co-cultured neonatal rabbit VMs (Zhou et al., 2013). Also, HCN4-expressed BMSCs can drive the porcine ventricle (Zhang et al., 2013). In addition, infecting TBX18 into rat BMSCs can make them differentiate into a phenotypic and functional pacemaker. More specifically, the TBX18-BMSCs expressed structural proteins—α-actin, Ca2+ dynamic proteins—cTnI, HCN4 gene and repressed Cx43 (Li et al., 2018). The vector used to transform the pacemaking-related gene is also a vital factor in long-term gene expression. Adenovirus is the most frequently used vector in gene transfection, but the gene expression by adenovirus vector showed a decline in one month (Zhang et al., 2013) and the adenovirus vector easily induced an immune reaction (Li et al., 2018). To create a durable pacemaker, lentiviral vector (LentiV) was used to transfect hHCN2 into rabbit BMSCs because of its function of generating persistent transgene expression. Indeed, the rabbit CMs co-cultured with LentiV-transfected MSCs produced a persistent gene expression (Zhou et al., 2007).
Compared with BMSCs, ADSCs appear to be a superior source to be used to differentiate into a sinus node-like cell, because the sample is available, easy to culture and reproduce. Also, ADSCs present more attractive pacing properties than BMSCs, such as a superior ability to differentiate into CMs as well as readily expressing the HCN gene (Yang et al., 2012). In the rat, 5-azacytidine (5-aza) chemical treatment can cause spontaneous beats in rabbit ADSCs at three weeks’ culture (Rangappa et al., 2003). Also, adult mouse ADSCs can be induced into pacemaker cells with CM-like morphology and AP with a pacing rate of 66 bpm (Planat-Benard et al., 2004). However, 5-aza could not differentiate human ADSCs into pacemaker cells (Choi et al., 2010). Except for chemical substances like 5-aza, some genes could also influence the differentiation of ADSCs into CMs. For example, the TBX18 gene is helpful in differentiating ADSCs into a pacemaker-like cell. An experiment indicated that silencing TBX18 inhibited the expression of TBX3 and Shox2, and terminated the differentiation of ADSCs into a pacemaker phenotype (Chen et al., 2017). Not surprisingly, expressing TBX18 into ADSCs promoted the expression of HCN4 and hence increased the expression of I f (Yang et al., 2016). TBX18-ADSC showed a similar morphology to pacemaker cells (Zhang and Huang, 2019). The neonatal rat VMs co-cultured with TBX18-transfected ADSCs presented autonomous synchronized beatings (Yang et al., 2016). Nkx2.5 is an essential transcription factor that regulates CM differentiation. It has been used to affect the differentiation of ADSCs into a pacemaker, although the results were not satisfactory. In mouse ADSCs, the overexpression of Nkx2.5 and cardiac α-actin can increase the expression of cardiac-related proteins but inhibit beating function (Zhao et al., 2012). Dramatically, an experiment successfully differentiated ADSCs into beating CMs by direct cell–cell interaction with rat CMs (Choi et al., 2010), but the reason for this is not clear. Although further experiments are required to ensure the safety of ADSCs-induced bio-pacemakers, the work provides a new research direction for clinical bio-pacemakers.
Another candidate for creating CMs is iPSC. Human iPSCs can be generated from various types of somatic cells, particularly fibroblasts (Novak et al., 2010; Chauveau et al., 2017) and can be induced into CMs by the LentiV (Novak et al., 2010) and chemical means (Burridge et al., 2014). Zhang et al. (2009) indicated that hiPSC could differentiate into nodal, atrial, and ventricular phenotypes. iPSC from human hair follicle keratinocytes also had potential to differentiate into functional CMs and this kind of iPSC-CMs showed intracellular calcium concentration ([Ca2+]i) transient and contraction amplitudes under the action of β-adrenergic (Novak et al., 2010). In addition, iPSC from human foreskin fibroblast presented [Ca2+]i handling and excitation–contraction coupling (Germanguz et al., 2011). Moreover, the iPSC-CMs can respond to ivabradine and iPSC-CMs-injected canine heart can display rhythms with the infusion of epinephrine (Chauveau et al., 2017). These experiments suggest the possibility of creating a biological pacemaker cell based on iPSC. Based on these facts, it is hoped that the iPSC-CM can produce automaticity. Gorabi et al. (2019b) used a hybrid therapy to produce a pacemaker cell based on iPSC. They successfully transfected TBX18 into hiPSC-CMs by a LentiV and TBX18-hiPSC-CMs appeared to present more pacemaker-like traits than the control (8% vs. 2%). A further study, which transferred TBX18 lentiviruses or TBX18-hiPSC-pacemakers into the rat ventricle, indicated that the function of TBX18-transduced VMs (cell therapy) was better than that of TBX18-hiPSC-pacemakers (hybrid therapy) on some measures, such as heart rate (Gorabi et al., 2019a). The TBX18-hiPSC-pacemaker injection seemed to be a safer therapy because the coupling between pacemaker cells and native myocytes could be controlled and optimized.
2.3. Autologous therapy
Up to now, most bio-pacemaker experiments are xenograft, which has the risk of delayed rejection and ethical issues. Also the function of pacemakers may be damaged by their low durability (Gorabi et al., 2019a). Zhang et al. (2013) showed that autograft HCN4-transferred porcine BMSCs could create a pacemaking function in the porcine heart with complete heart blocks. Moreover, 5-aza chemical treatment is potentially an autologous therapy for a bio-pacemaker (Rangappa et al., 2003). In addition, human somatic cells such as hair follicle keratinocytes or skin cells have been verified to be reprogrammed to iPSC and differentiated into CMs (Zhang et al., 2009), and this provides a possible approach for creating an autograft human pacemaker. Apart from autograft stem cell therapy and chemical treatment, autologous-SAN-injection is also a hopeful therapy for a bio-pacemaker. Fetal canine atrial CMs were implanted into the adult canine left ventricle, and an escape rhythm was observed in the site where the atrial CMs were injected (Ruhparwar et al., 2002). Zhang et al. (2008) showed that injecting autologous SAN cells in the canine right ventricle could create spontaneous beats with a rate of approximately 50 bpm. However, its biological pacing properties are distinct from native SAN. This may relate to the difference of substrate (Zhang et al., 2011). The mortality of the SAN cells in the injected site is somewhat high, and stable pacing is difficult to ensure. Therefore, the dose of SAN cells for injection is a research interest for the future. It is hoped that these approaches will eliminate the immunosuppression of the xenograft.
2.4. Obstacles to producing biological pacemakers
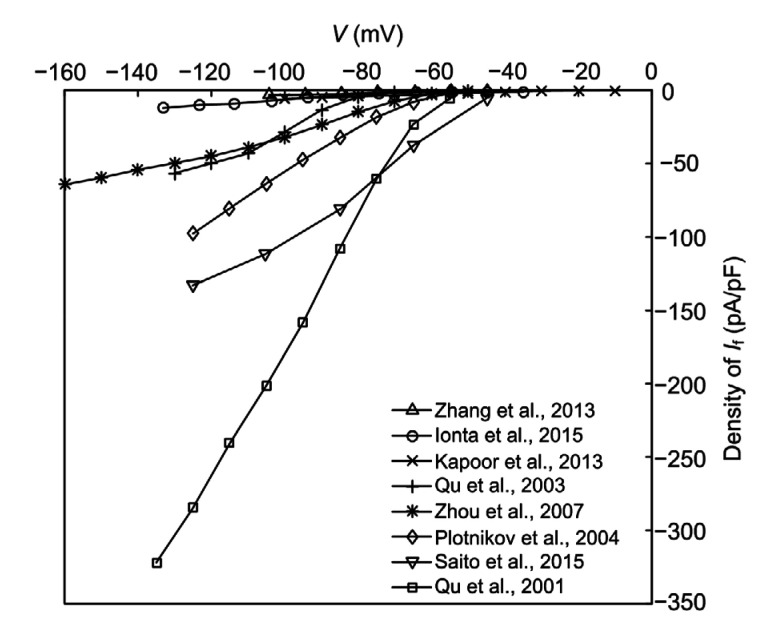
Research on creating a bio-pacemaker by gene and cell therapies has provided many useful results. However, biological experiments have to overcome several obstacles in producing a bio-pacemaker. For instance, it is not easy to explain the synchronous mechanism of bio-pacemaker by biological experiment. The molecular mechanisms of how a bio-pacemaker works in healthy and diseased conditions are barely understood. In addition, experiments are limited by errors caused by experimental condition and by species difference. The current–voltage (I–V) curves of I f in different bio-pacemaker experiments are shown in Fig. 1. The current density shows a great difference because it is not easy to control in experiments. In addition, considering the great effects of the location and size of bio-pacemaker cells on the cardiac electrical and mechanical dynamics, pacemaker cells should be injected into a certain position and in a certain pattern, which is not easy to accurately control. Also, culturing materials and subjects needs a lot of time and expenditure. Last but not least, biological experiments have ethical and moral issues, such as with the xenograft (Zhang et al., 2008). All these shortcomings prompt us to build a computational model of a bio-pacemaker to simulate the process and results of bio-experiments. This is a more feasible approach for studying the bio-pacemaker mechanism.
Fig. 1.
I–V curves of I f measured in biological pacemaker experiments
I–V: current–voltage; I f: funny current; pA/pF: unit of current density, (10−12 A)/(10−12 F)
3. Computational simulations of biological pacemaker
Although there have been plenty of experiments on the bio-pacemaker, up to now, few bio-pacemaker computational models have been published. To build a bio-pacemaker computational model, first, an appropriate chamber of the heart should be chosen as the initiation of heartbeats. It has been verified that VMs can be induced as pacemaker cells via adenoviral gene transfection (Hu et al., 2014), which makes VMs a candidate for a pacemaking origin. A series of simulations have been done to examine the effect of different ion currents on the ventricular automaticity. Kurata et al. (2005) elucidated the roles of individual ionic currents in pacemaking by bifurcation analyses based on the first human VM model (PB model (Priebe and Beuckelmann, 1998)). They claimed that L-type calcium current (I CaL) is responsible for equilibrium point instability, whereas the deactivation of Na+/Ca2+ exchanger current (I NaCa) and rapid delayed rectifier current (I Kr) contributes to phase 4 depolarization. Another simulation (Zhang et al., 2014) demonstrated that I CaL and I NaCa play a positive role in the initiation of automatic depolarization, while I Kr and Na+/K+ pump current (I NaK) have a contrary effect.
Although these ion channels show a degree of influence on cardiac pacing, I K1, I f, and cell coupling are still the crucial factors that induce automatic depolarization. Inhibiting I K1, overexpressing I f, and downregulating the diffusion constant are the main approaches to causing automatic activity in the VM single cell and tissue model. There has been some research on the role of I K1 in induced pacing. For example, Silva and Rudy (2003) clarified the mechanism of pacing activity in I K1-downregulated VMs by adjusting conductance of I K1 (G K1) in a guinea pig VM model (Luo-Rudy model (Luo and Rudy, 1991)). They verified that it was I NaCa that carried the pacemaking current, and this process depended on the oscillation of [Ca2+]i. Then, Tong and Holden (2005) used a continuation algorithm to analyze the stability of induced pacemaker activity by downregulating G K1 in a human VM model (TP04 model (ten Tusscher et al., 2004)) and found that the stable periodic oscillations appeared when G K1 was 0.02–0.05 times the value of original G K1. Based on the result of the single cell model, the number of bio-pacemaker cells, which are needed for successful pacing, was investigated in a two-dimensional (2D) model. The result was that adjacent VMs suppressed the depolarization of automatic cells. Therefore, Purkinje fiber was added as a medium for propagating electric excitation from pacemakers to VMs. In this model, only 500 automatic cells were needed to drive the tissue slice (Zhang et al., 2016). As for cell coupling, biological experiments showed that overexpressing TBX18 in the rat (Cho et al., 2007) and the pig (Azene et al., 2005) VMs could downregulate Cx43, and then diminish intercellular coupling, thus inducing auto-rhythms in VMs. A simulation verified that decreasing coupling conductance could increase ventricular pacing activity (Zhang et al., 2015), which was consistent with the experimental result. According to the experiment by Qu et al. (2001), increasing the density of I f is helpful to enhance the CL and give rise to pacemaker activity. It is hoped that computational simulation will reveal the effect of I f on pacing activity in a non-autorhythmic CM model. In addition, an experiment has preliminarily explored the synergetic actions of I K1 and I f. A simulation (Sun et al., 2017) showed that it was the ratio of I K1 and I f that regulated the frequency of rhythmic oscillation. However, this model included I K1 and I f only, which was not a whole CM model.
Computational study based on CM model provides a fundamental basis for the investigation of molecular pathways for creating a bio-pacemaker under physiological conditions. Some publications (Azene et al., 2005; Sun et al., 2017) have combined the biological experiments and computational approaches, setting an example for guiding biological research by computational simulation. The conclusions of simulation could be verified by biological experiments, i.e., establishing the feasibility of model forecasts. The computational approach will be a trend for bio-pacemaker research.
4. Conclusions
The bio-pacemaker is an attractive therapy for cardiac rhythm disturbances. Because of its biological properties, it has shown many advantages over electrical pacemakers. For example, the bio-pacemaker needs no open-chest surgery, and this could reduce the risk of complications. Also, the pacing frequency of a bio-pacemaker can respond to emotion rather than remaining a fixed rhythm. However, some problems still need to be solved, such as the approaches of producing pacemaker cells, the magnitude of pacing cells, as well as the location for injection of the pacing cells. Fortunately, the computational model could be used to simulate the production of pacemaker cells and its effect on the cardiac pacemaking activity. The computational approach could avoid the high cost and moral constraints of clinical experiments. The development of bio-pacemaker simulation began from modifying I K1 and I f channel formulations at the subcellular level and should end at establishing pacemaker–CM-coupling tissue models at the multi-dimensional level. Many problems in bio-pacemaker simulation still need to be solved, such as the interaction mechanism between ionic channels across the cell membrane, the space structures of the pacemaker, and so on.
Although further experiments are required to assess safety and efficacy before implementation in clinical practice, the bio-pacemaker is still a discernible overall trend for the clinical therapy of arrhythmia and computational simulation would accelerate its development.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 61572152, 61601143, and 81770328), the Science Technology and Innovation Commission of Shenzhen Municipality (Nos. JCYJ20151029173639477 and JSGG20160229125049615), and the China Postdoctoral Science Foundation (No. 2015M581448)
Contributors: Yacong LI performed the literature research, wrote and edited the manuscript. Kuanquan WANG participated in the conceptualization, and reviewed and edited the manuscript. Qince LI investigated and collected the experiment data, and wrote the manuscript. Henggui ZHANG participated in the conceptualization, and writing and editing of the manuscript. All authors have read and approved the final manuscript.
Compliance with ethics guidelines: Yacong LI, Kuanquan WANG, Qince LI, and Henggui ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Azene EM, Xue T, Marbán E, et al. Non-equilibrium behavior of HCN channels: insights into the role of HCN channels in native and engineered pacemakers. Cardiovasc Res. 2005;67(2):263–273. doi: 10.1016/j.cardiores.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bakker ML, Boink GJJ, Boukens BJ, et al. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res. 2012;94(3):439–449. doi: 10.1093/cvr/cvs120. [DOI] [PubMed] [Google Scholar]
- 3.Boink GJJ, Duan L, Nearing BD, et al. HCN2/SkM1 gene transfer into canine left bundle branch induces stable, autonomically responsive biological pacing at physiological heart rates. J Am Coll Cardiol. 2013;61(11):1192–1201. doi: 10.1016/j.jacc.2012.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruzauskaite I, Bironaite D, Bagdonas E, et al. Relevance of HCN2-expressing human mesenchymal stem cells for the generation of biological pacemakers. Stem Cell Res Ther, 7:67. 2016 doi: 10.1186/s13287-016-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauveau S, Anyukhovsky EP, Ben-Ari M, et al. Induced pluripotent stem cell-derived cardiomyocytes provide in vivo biological pacemaker function. Circ Arrhythm Electrophysiol. 2017;10(5):e004508. doi: 10.1161/CIRCEP.116.004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Deng ZJ, Zhou JS, et al. Tbx18-dependent differentiation of brown adipose tissue-derived stem cells toward cardiac pacemaker cells. Mol Cell Biochem. 2017;433(1-2):61–77. doi: 10.1007/s11010-017-3016-y. [DOI] [PubMed] [Google Scholar]
- 8.Chen WQ, Gao RL, Liu LS, et al. Chinese cardiovascular disease report essentials. Chin Circ J. 2018;33(1):1–8. (in Chinese) [Google Scholar]
- 9.Cho HC, Kashiwakura Y, Marban E. Creation of a biological pacemaker by cell fusion. Circ Res. 2007;100(8):1112–1115. doi: 10.1161/01.Res.0000265845.04439.78. [DOI] [PubMed] [Google Scholar]
- 10.Choi YS, Dusting GJ, Stubbs S, et al. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14(4):878–889. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhury M, Black N, Alghamdi A, et al. TBX18 overexpression enhances pacemaker function in a rat subsidiary atrial pacemaker model of sick sinus syndrome. J Physiol. 2018;596(24):6141–6155. doi: 10.1113/JP276508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen IS, Brink PR, Robinson RB, et al. The why, what, how and when of biological pacemakers. Nat Clin Pract Cardiovasc Med. 2005;2(8):374–375. doi: 10.1038/ncpcardio0276. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberger RS, Wilson AC, Lawrence-Nelson J, et al. Permanent pacing is a risk factor for the development of heart failure. Am J Cardiol. 2005;95(5):671–674. doi: 10.1016/j.amjcard.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Germanguz I, Sedan O, Zeevi-Levin N, et al. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15(1):38–51. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorabi AM, Hajighasemi S, Khori V, et al. Functional biological pacemaker generation by T-Box18 protein expression via stem cell and viral delivery approaches in a murine model of complete heart block. Pharmacol Res. 2019;141:443–450. doi: 10.1016/j.phrs.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Gorabi AM, Hajighasemi S, Tafti HA, et al. TBX18 transcription factor overexpression in human-induced pluripotent stem cells increases their differentiation into pacemaker-like cells. J Cell Physiol. 2019;234(2):1534–1546. doi: 10.1002/jcp.27018. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann S, Schmitteckert S, Griesbeck A, et al. Comparative expression analysis of Shox2-deficient embryonic stem cell-derived sinoatrial node-like cells. Stem Cell Res. 2017;21:51–57. doi: 10.1016/j.scr.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Hu YF, Dawkins JF, Cho HC, et al. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med. 2014;6(245):245ra94. doi: 10.1126/scitranslmed.3008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Jia XL, Bai K, et al. Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch Med Res. 2010;41(7):497–505. doi: 10.1016/j.arcmed.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Zheng LS, Gong XH, et al. Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PLoS ONE. 2012;7(4):e34960. doi: 10.1371/journal.pone.0034960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ionta V, Liang WB, Kim EH, et al. SHOX2 overexpression favors differentiation of embryonic stem cells into cardiac pacemaker cells, improving biological pacing ability. Stem Cell Rep. 2015;4(1):129–142. doi: 10.1016/j.stemcr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapoor N, Liang WB, Marbán E, et al. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18 . Nat Biotechnol. 2013;31(1):54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI200112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22(10):1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 25.Kurata Y, Hisatome I, Matsuda H, et al. Dynamical mechanisms of pacemaker generation in I K1-downregulated human ventricular myocytes: insights from bifurcation analyses of a mathematical model. Biophys J. 2005;89(4):2865–2887. doi: 10.1529/biophysj.105.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwabara Y, Kuwahara K, Takano M, et al. Increased expression of HCN channels in the ventricular myocardium contributes to enhanced arrhythmicity in mouse failing hearts. J Am Heart Assoc. 2013;2(3):e000150. doi: 10.1161/Jaha.113.000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YJ, Yang M, Zhang GG, et al. Transcription factor TBX18 promotes adult rat bone mesenchymal stem cell differentiation to biological pacemaker cells. Int J Mol Med. 2018;41(2):845–851. doi: 10.3892/ijmm.2017.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieu DK, Chan YC, Lau CP, et al. Overexpression of HCN-encoded pacemaker current silences bioartificial pacemakers. Heart Rhythm. 2008;5(9):1310–1317. doi: 10.1016/j.hrthm.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res. 1991;68(6):1501–1526. doi: 10.1161/01.RES.68.6.1501. [DOI] [PubMed] [Google Scholar]
- 30.Mayer JH 3rd, Almond CH, Anido H, et al. Complete heart block. Treatment by pedicle grafting of the sinoauricular node to the right ventricle. Arch Surg. 1967;94(1):90–95. doi: 10.1001/archsurg.1967.01330070092019. [DOI] [PubMed] [Google Scholar]
- 31.Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419(6903):132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 32.Miake J, Marban E, Nuss HB. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003;111(10):1529–1536. doi: 10.1172/Jci200317959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morishita Y, Poirier RA, Rohner RF. Sino-atrial node transplantation in the dog. Vasc Surg. 1981;15(6):388–393. doi: 10.1177/153857448101500603. [DOI] [Google Scholar]
- 34.Munshi NV, Olson EN. Improving cardiac rhythm with a biological pacemaker. Science. 2014;345(6194):268–269. doi: 10.1126/science.1257976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak A, Shtrichman R, Germanguz I, et al. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogram. 2010;12(6):665–678. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 36.Planat-Benard V, Menard C, André M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94(2):223–229. doi: 10.1161/01.Res.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 37.Plotnikov AN, Sosunov EA, Qu JH, et al. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109(4):506–512. doi: 10.1161/01.Cir.0000114527.10764.Cc. [DOI] [PubMed] [Google Scholar]
- 38.Plotnikov AN, Shlapakova I, Szabolcs MJ, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116(7):706–713. doi: 10.1161/Circulationaha.107.703231. [DOI] [PubMed] [Google Scholar]
- 39.Potapova I, Plotnikov A, Lu ZJ, et al. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circu Res. 2004;94(7):952–959. doi: 10.1161/01.Res.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 40.Priebe L, Beuckelmann D. Simulation study of cellular electric properties in heart failure. Circu Res. 1998;82(11):1206–1223. doi: 10.1161/01.RES.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 41.Qu JH, Barbuti A, Protas L, et al. HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circu Res. 2001;89(1):e8–e14. doi: 10.1161/hh1301.094395. [DOI] [PubMed] [Google Scholar]
- 42.Qu JH, Plotnikov AN, Danilo P, Jr, et al. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107(8):1106–1109. doi: 10.1161/01.Cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 43.Rangappa S, Fen C, Lee EH, et al. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75(3):775–779. doi: 10.1016/S0003-4975(02)04568-X. [DOI] [PubMed] [Google Scholar]
- 44.Ravagli E, Bucchi A, Bartolucci C, et al. Cell-specific dynamic clamp analysis of the role of funny I f current in cardiac pacemaking. Prog Biophys Mol Biol. 2016;120(1-3):50–66. doi: 10.1016/j.pbiomolbio.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Rosen MR. Gene therapy and biological pacing. N Engl J Med. 2014;371(12):1158–1159. doi: 10.1056/Nejmcibr1408897. [DOI] [PubMed] [Google Scholar]
- 46.Rosen MR, Robinson RB, Brink PR, et al. The road to biological pacing. Nat Rev Cardiol. 2011;8(11):656–666. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 47.Ruhparwar A, Tebbenjohanns J, Niehaus M, et al. Transplanted fetal cardiomyocytes as cardiac pacemaker. Eur J Cardio-Thorac Surg. 2002;21(5):853–857. doi: 10.1016/S1010-7940(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 48.Saito Y, Nakamura K, Yoshida M, et al. Enhancement of spontaneous activity by HCN4 overexpression in mouse embryonic stem cell-derived cardiomyocytes–a possible biological pacemaker. PLoS ONE. 2015;10(9):e0138193. doi: 10.1371/journal.pone.0138193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- 50.Shlapakova IN, Nearing BD, Lau DH, et al. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm. 2010;7(12):1835–1840. doi: 10.1016/j.hrthm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Silva J, Rudy Y. Mechanism of pacemaking in I K1-downregulated myocytes. Circu Res. 2003;92(3):261–263. doi: 10.1161/01.Res.0000057996.20414.C6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starzl TE, Hermann G, Axtell HK, et al. Failure of sino-atrial nodal transplantation for the treatment of experimental complete heart block in dogs. J Thorac Cardiovasc Surg. 1963;46:201–205. [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Y, Timofeyev V, Dennis A, et al. A singular role of I K1 promoting the development of cardiac automaticity during cardiomyocyte differentiation by I K1-induced activation of pacemaker current. Stem Cell Rev Rep. 2017;13(5):631–643. doi: 10.1007/s12015-017-9745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ten Tusscher KHWJ, Noble D, Noble PJ, et al. A model for human ventricular tissue. Am J Physiol-Heart Circ Physiol. 2004;286(4):H1573–H1589. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 55.Tong WC, Holden AV. Induced pacemaker activity in virtual mammalian ventricular cells. In: Frangi AF Radeva PI, Santos A et al., editors. Functional Imaging and Modeling of the Heart. FIMH 2005, Lecture Notes in Computer Science, Vol. 3504. Springer, Berlin, Heidelberg; 2005. pp. 226–235. [DOI] [Google Scholar]
- 56.Valiunas V, Kanaporis G, Valiuniene L, et al. Coupling an HCN2-expressing cell to a myocyte creates a two-cell pacing unit. J Physiol. 2009;587(21):5211–5226. doi: 10.1113/jphysiol.2009.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Végh AMD, den Haan AD, Cócera Ortega L, et al. Cardiomyocyte progenitor cells as a functional gene delivery vehicle for long-term biological pacing. Molecules. 2019;24(1):181. doi: 10.3390/molecules24010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111(1):11–20. doi: 10.1161/01.Cir.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Song T, Wu P, et al. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol Med Rep. 2012;5(1):108–113. doi: 10.3892/mmr.2011.611. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Zhang GG, Wang T, et al. TBX18 gene induces adipose-derived stem cells to differentiate into pacemaker-like cells in the myocardial microenvironment. Int J Mol Med. 2016;38(5):1403–1410. doi: 10.3892/ijmm.2016.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu H, Chang F, Cohen IS. Pacemaker current exists in ventricular myocytes. Circ Res. 1993;72(1):232–236. doi: 10.1161/01.RES.72.1.232. [DOI] [PubMed] [Google Scholar]
- 62.Zaritsky JJ, Redell JB, Tempel BL, et al. The consequences of disrupting cardiac inwardly rectifying K+ current (I K1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533(3):697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Shlapakova IN, Zhao X, et al. Biological pacing by implantation of autologous sinoatrial node cells into the canine right ventricle. Circulation. 2008;118:S427–S428. [Google Scholar]
- 64.Zhang H, Lau DH, Shlapakova IN, et al. Implantation of sinoatrial node cells into canine right ventricle: biological pacing appears limited by the substrate. Cell Transplant. 2011;20(11-12):1907–1914. doi: 10.3727/096368911x565038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Li SC, Qu D, et al. Autologous biological pacing function with adrenergic-responsiveness in porcine of complete heart block. Int J Cardiol. 2013;168(4):3747–3751. doi: 10.1016/j.ijcard.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Huang CX. A new combination of transcription factors increases the harvesting efficiency of pacemaker-like cells. Mol Med Rep. 2019;19(5):3584–3592. doi: 10.3892/mmr.2019.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JH, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Wang KQ, Zhang HG, et al. Simulation of ventricular automaticity induced by reducing inward-rectifier K+ current. 2014 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Belfast, UK. IEEE; 2014. pp. 458–462. [DOI] [Google Scholar]
- 69.Zhang Y, Wang KQ, Zhang HG, et al. Simulation of effects of TBX18 on the pacemaker activity of human ventricular cells; 2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Washington, DC, USA. IEEE, p.1548-1551; 2015. [DOI] [Google Scholar]
- 70.Zhang Y, Wang KQ, Li QC, et al. Pacemaker created in human ventricle by depressing inward-rectifier K+ current: a simulation study. Biomed Res Int, 2016:3830682. 2016 doi: 10.1155/2016/3830682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao LL, Ju DP, Gao Q, et al. Over-expression of Nkx2.5 and/or cardiac α-actin inhibit the contraction ability of ADSCs-derived cardiomyocytes. Mol Biol Rep. 2012;39(3):2585–2595. doi: 10.1007/s11033-011-1011-z. [DOI] [PubMed] [Google Scholar]
- 72.Zhou YF, Yang XJ, Li HX, et al. Mesenchymal stem cells transfected with HCN2 genes by LentiV can be modified to be cardiac pacemaker cells. Med Hypotheses. 2007;69(5):1093–1097. doi: 10.1016/j.mehy.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 73.Zhou YF, Yang XJ, Li HX, et al. Genetically-engineered mesenchymal stem cells transfected with human HCN1 gene to create cardiac pacemaker cells. J Int Med Res. 2013;41(5):1570–1576. doi: 10.1177/0300060513501123. [DOI] [PubMed] [Google Scholar]