Overview
Intracellular pathogens represent a special challenge for the host. Because they reside within the host cell, elimination of the infection inevitably involves a balance between maximizing pathogen destruction while minimizing host cell damage. This balance between pathogen destruction and host cell preservation involves a spectrum of host strategies that range from direct pathogen killing with host cell survival to more suicidal strategies in which the infected host cell is sacrificed to facilitate pathogen killing. Intracellular pathogens have evolved to modify these host strategies to enable pathogenesis, leading to an arms race of host and pathogen defenses. In this issue, we will explore the diversity of cell intrinsic and cell extrinsic mechanisms deployed to control intracellular pathogens, with a particular emphasis on bacterial pathogens, and the mechanisms by which pathogens counteract these strategies.
Brewer, Brubaker, Monack [1] review the broad role of the inflammasome, and the pathogen countermeasures that neutralize the inflammasome, in host defense. They present a conceptual framework to understand the varied inflammasomes by outlining the distinct sensors, adaptors, and effector proteins that assemble into inflammasome complexes, ultimately leading to cleavage or pro-IL1B, pro-IL-18, and Gasdermin D. The diverse pathogen strategies for neutralizing this potent host defense mechanism are comprehensively summarized.
Mitchell, Sandstrom, and Vance [2] focus on new insights into the mechanism of pathogen sensing by NLRP1. In contrast to the more prevalent model of NLR activation, in which pathogen-derived molecules are directly sensed by the inflammasome, NLRP1 appears to be activated by the enzymatic activity of pathogen effectors by undergoing “functional degradation” by the proteasome, resulting in inflammasome activation. The authors highlight the novelty of this mechanism and the possibility that additional pathogen effectors are sensed in this manner, and that additional NLR proteins may share this activation strategy.
Escoll and Buchrieser [3] describe how intracellular pathogens and the host cells they infect battle for the limited metabolic resources available within the cell during infection. To adapt and meet their metabolic needs, innate immune cells reprogram their metabolism upon activation by pathogen-derived molecules, which has implications for metabolic homeostasis, nutrient availability for the pathogen, and metabolic regulation of immune and inflammatory responses. In a countermeasure, intracellular bacterial pathogens modulate host metabolism to promote their own replication and survival. Escoll and Buchrieser highlight in detail the induction of Warburg-like metabolism and the manipulation of the catabolic process of autophagy by multiple different intracellular bacterial pathogens and the implications of this for host-pathogen interactions.
Knight and Stanley [4] describe the central role for hypoxia-inducible transcription factor-1α (HIF-1α) in regulating cellular responses to infection. Infection induces changes in host cell metabolism and activates HIF-1α, coupling shifts in cellular metabolism to transcriptional regulation of host responses. HIF-1α in turn reprograms cellular metabolism to trigger an immune response that involves cell intrinsic immune defenses as well as regulation of inflammation through the expression of specific inflammatory cytokines. Knight and Stanley discuss roles for HIF-1α in controlling bacterial, fungal, and protozoan intracellular pathogens as well as how some pathogens benefit from HIF-1α activation, thus exploiting this host defense.
Nauseef [5] discusses the role of the phagocyte NADPH oxidase in governing innate immune responses to intracellular pathogens. The NADPH oxidase is a multicomponent complex that in stimulated phagocytes translocates to assemble a functional enzyme complex at plasma or phagosomal membranes. In this review, Nauseef describes the mechanistic detail of how NADPH oxidase shuttles electrons to molecular oxygen in phagosomes or the extracellular space to produce oxidants that confer antimicrobial properties and signaling capabilities to regulate inflammation and host responses. Also highlighted are the gaps in knowledge that still exist in this process, including the mechanism of oxidant-dependent killing.
Vazquez-Torres and Fang [6] discuss the many roles of nitric oxide (NO) in host-pathogen interactions, including direct bacterial killing and regulation of inflammation. The authors highlight recent discoveries regarding mechanisms of NO production by human macrophages, as well as how some pathogenic bacteria resist killing by NO, produce NO as a defense mechanism, and exploit nitrate derived from host nitric oxide synthesis to use as an electron acceptor and compete with the microbiome. Vazquez-Torres and Fang also discuss recent evidence of the therapeutic potential of NO in treating bacterial infections in the lungs of cystic fibrosis patients.
Sheldon and Skaar [7] review the role of metals in host pathogen interactions by highlighting the diverse, metal specific effects of transition metals. In some cases, the host uses metal limitation (e.g. iron) as an antimicrobial strategy, a strategy that pathogens counter by elaborating high affinity metal chelators or adapting to the low metal environment. Conversely, the toxic effects of metal excess are exploited by host cells to neutralize pathogens (e.g. Cu/Zn); a toxicity that pathogens evade either by sequestering the metal or neutralizing its toxic effects.
Upadhyay and Phillips [8] highlight an important mechanism of pathogen limitation, which is based not on elaboration of chemical or protein effectors, but rather on the intracellular routing of pathogens in the endosomal network. This review highlights the expanding appreciation that the route of phagosomal uptake and trafficking can have dramatic effects on infectious outcome. LC3 associated phagocytosis, or LAP, is a recently identified pathway that sequesters pathogens in an LC3 positive vacuole. Some pathogens are able to avoid LAP or neutralize the effectors delivered to the LAP vacuole, countermeasures that are detailed in this review.
Huang, Meng, Maminska, and MacMicking [9] review the diverse roles of interferon inducible guanylate binding proteins (GBPs) in host defense. They highlight that these proteins have diverse functions but can be understood as a scaffolding system that assembles anti-pathogen effector proteins at the site of subcellular infection. The specifics of each GBP and their recruited complexes are diverse and ever expanding, suggesting that our understanding of this protein family in host defense is still rudimentary. Of particular interest will be whether pathogen effector molecules specifically target GBPs as an avoidance strategy, a topic that has received relatively little attention to date.
Briken and Behar [10] discuss how host cell death impacts immune responses to intracellular bacterial pathogens. Regulated cell death via apoptosis is an important innate defense mechanism whereby pathogen spread is restricted, and inflammation is controlled. In addition, the engulfment of apoptotic infected cells, a process known as efferocytosis, can lead to killing of the intracellular bacteria and the acquisition of antigen for presentation to adaptive immune cells. In contrast, other more inflammatory cell death pathways, such as necroptosis, are generally thought to promote bacterial spread. Briken and Behar describe evidence that some bacteria counteract the host by manipulating cell death pathways and efferocytosis to facilitate their pathogenesis.
Allen and Criss [11] discuss how neutrophils, the most abundant white blood cell in humans, defend the host from intracellular infections and how pathogens fight back. Neutrophils respond quickly to infections by migrating to the infected tissue and phagocytosing the invading pathogens. Once the pathogen is engulfed, the phagosome matures by fusing to various granule subsets that contain an arsenal of toxic molecules and enzymes that kill the pathogen. In addition, neutrophils communicate with other innate immune cells to coordinate host responses and resolve inflammation to repair host tissue damage. However, some pathogens have evolved to evade or subvert the defenses imparted by neutrophils. This review also highlights new insight into neutrophil heterogeneity and how this may impact disease outcomes.
Dotiwala and Lieberman [12] focus on the emerging role of granule proteins (granulysin, perforin, and granzymes) of killer lymphocytes (cytotoxic T-cells, MAIT cells, and NK cells) in killing intracellular microbes. These proteins represent a complex cooperative strategy that surmounts the cell biologic challenge of delivering microbicidal proteins across multiple membranes to the site of intracellular infection. The differential pore forming ability of perforin and granulysin on mammalian and bacterial membranes, respectively, allows delivery of granzymes to the bacterial cytoplasm, thereby causing microbial cell death via complex metabolic disruption.
Hoffman and Aballay [13] highlight the emerging roles of neurons in immune defense against pathogens. It has become clear from studies in nematodes and mammals that molecules produced by neurons directly influence the function and trafficking of immune cells, including neutrophils, macrophages, T cells, and ILCs. Neurons also play important roles in pathogen sensing and avoidance and thereby integrate behavioral defense with immune sensing and effector function. It is clear that the field of immune-pathogen-neuronal circuits is in its infancy and will be an area of intense interest for years to come. It seems inevitable that pathogens have evolved mechanisms to counter the neuronal arm of the immune response, but this fascinating possibility is largely unexplored.
Future Perspective
We hope that the assembled set of reviews will provide a timely and state of the art discussion of our understanding of the innate immune responses to intracellular pathogens and how the pathogens counteract these defenses. Taken as a whole, these reviews highlight a striking diversity of host effector strategies and a similarly striking set of pathogen countermeasures. When we look to the future, we are certain that additional pathways, both host and bacterial, will be discovered by the vibrant pathogenesis community. In addition, we anticipate that our field will begin to consider the idea that each pathway outlined above occurs not as a monolithic defense strategy, but within the context of multiple simultaneously applied host effector pathways. These host defense strategies are applied in various combinations and with differing kinetics, requiring pathogen responses that are tuned over time and space. For example, the pathogen may need to manipulate LAP trafficking, but also may simultaneously resist NO, scavenge iron, and respond to Granzymes. Such complex responses require not only distinct response pathways, but integration of these pathways into a temporally coordinated response. How the host and pathogen integrate the application and response to such combinatorial conditions will be a fascinating topic of future study.
Mechanisms of antimicrobial defense highlighted in Host Pathogens 2019.
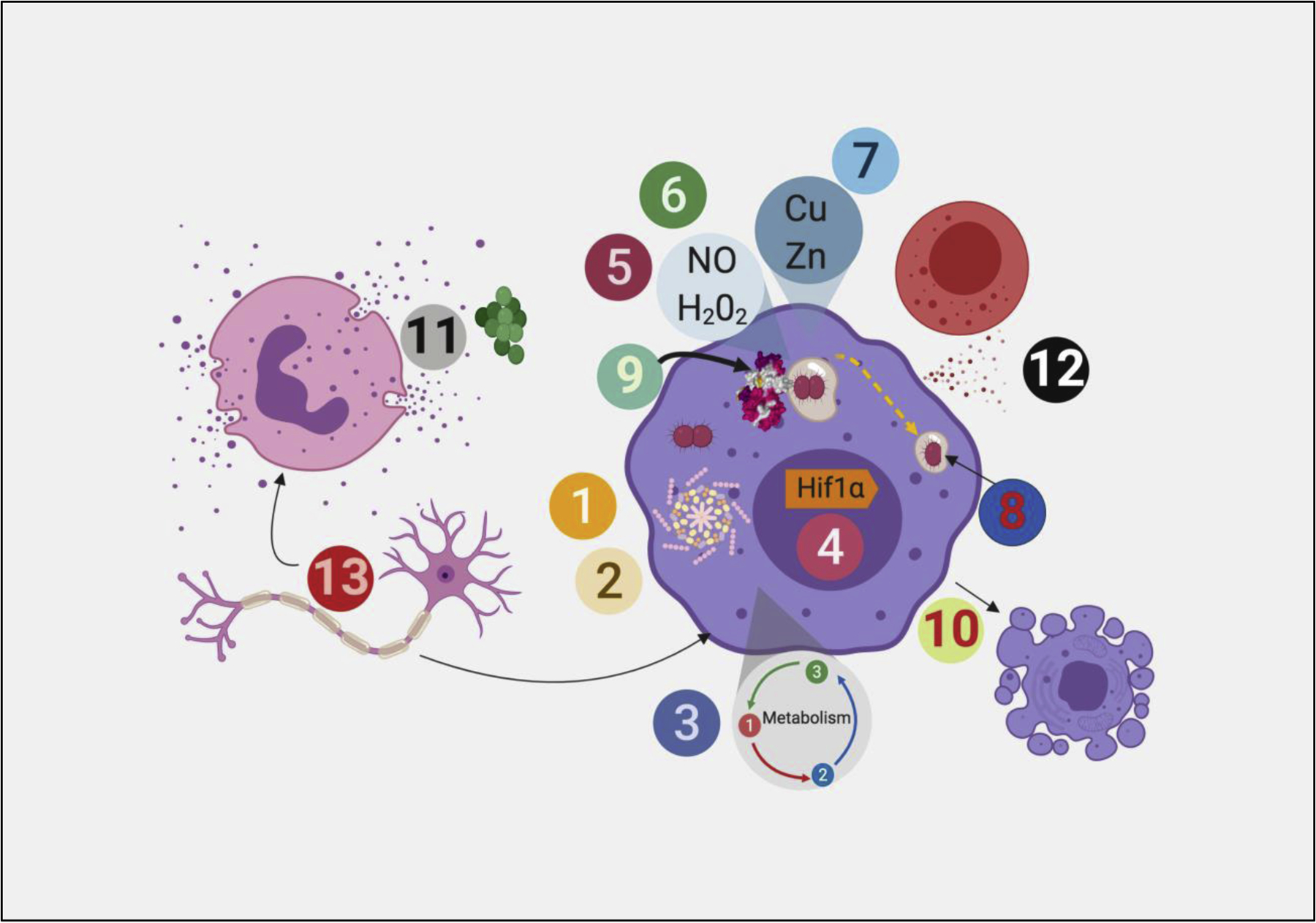
Four cell types are pictured: phagocyte (purple), neutrophil (pink), neuron, and T cell (red). Each number refers to a distinct pathway of antibacterial defense highlighted in the issue, as follows: 1: Host Inflammasome and bacterial evasion 2: NLRP1 Inflammasome 3: Metabolic reprogramming as a cellular defense mechanism 4: Hif1α in cellular resistance to intracellular pathogens 5: Reactive Oxygen 6: Reactive Nitrogen Intermediates 7: Metals as antimicrobial effectors 8: LC3 associated phagocytosis 9: IFN regulated GTPases 10: Apoptosis as cellular defense mechanism 11: Neutrophil effector mechanisms 12: Perforin-Granzyme mediated killing of intracellular pathogens 13:. Neurons in immune defense
Acknowledgments
We thank all of the contributors and reviewers for making this issue possible and apologize to those whose work was not cited due to space constraints. MSG was supported by P30 CA 008748 and U19 AI 11143. CLS was supported by NIH NIAID R01 AI132653, R01 AI132697, U19 AI142784, and a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award.
Biographies
Michael S. Glickman MD is a Member in the Immunology Program of the Sloan Kettering Institute, an Attending Physician in Infectious Diseases at Memorial Hospital, and an Alfred Sloan Chair at MSKCC. A microbiologist and immunologist, Dr. Glickman’s research focuses of the pathogenesis of mycobacterial infections with an emphasis on Mtb cell wall biosynthesis, DNA damage responses, and signal transduction.
Michael S. Glickman

Christina L. Stallings is an Associate Professor in the Department of Molecular Microbiology at Washington University in St. Louis, School of Medicine. She also serves as the Director for the Molecular Microbiology and Microbial Pathogenesis Graduate Program at Washington University. She received her Ph.D. with distinction from Columbia University College of Physicians and Surgeons where she performed her thesis work on alphaherpesviruses in the laboratory of Dr. Saul Silverstein. She then transitioned to another fascinating and chronic pathogen, Mycobacterium tuberculosis, for her postdoctoral research in Dr. Michael Glickman’s Laboratory at the Sloan-Kettering Institute. She started her faculty position at Washington University in St. Louis in 2010 and research in her laboratory seeks to dissect the molecular mechanisms involved in M. tuberculosis pathogenesis, from the perspective of both the host and the pathogen. She has been recognized for her accomplishments in these areas by being awarded a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award, an Arnold and Mabel Beckman Foundation Young Investigator Award, and an American Lung Association Young Investigator Award, under which she was designated a TB Scholar. She also serves as an Editor for the American Society for Microbiology mBio and mSphere Journals as well as PLOS Biology.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
MG reports receiving consulting fees and equity from Vedanta Biosciences and receiving consulting fees from Takeda.
References
- 1.Brewer SM, Brubaker SW, Monack DM: Host inflammasome defense mechanisms and bacterial pathogen evasion strategies. Curr Opin Immunol 2019, 60:63–70. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell PS, Sandstrom A, Vance RE: The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries. Curr Opin Immunol 2019, 60:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escoll P, Buchrieser C: Metabolic reprogramming: an innate cellular defence mechanism against intracellular bacteria? Curr Opin Immunol 2019, 60:117–123. [DOI] [PubMed] [Google Scholar]
- 4.Knight M, Stanley S: HIF-1α as a central mediator of cellular resistance to intracellular pathogens. Curr Opin Immunol 2019, 60:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauseef WM: The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr Opin Immunol 2019, 60:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang FC, Vázquez-Torres A: Reactive nitrogen species in host–bacterial interactions. Current Opinion in Immunology 2019, 60:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldon JR, Skaar EP: Metals as phagocyte antimicrobial effectors. Curr Opin Immunol 2019, 60:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay S, Philips JA: LC3-associated phagocytosis: host defense and microbial response. Curr Opin Immunol 2019, 60:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Meng Q, Maminska A, MacMicking JD: Cell-autonomous immunity by IFN-induced GBPs in animals and plants. Curr Opin Immunol 2019, 60:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behar SM, Briken V: Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Curr Opin Immunol 2019, 60:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen L-AH, Criss AK: Cell intrinsic functions of neutrophils and their manipulation by pathogens. Curr Opin Immunol 2019, 60:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotiwala F, Lieberman J: Granulysin: killer lymphocyte safeguard against microbes. Curr Opin Immunol 2019, 60:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman C, Aballay A: Role of neurons in the control of immune defense. Current Opinion in Immunology 2019, 60:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


