Abstract
Light provides a uniquely powerful stimulus to help visualize and/or perturb biological systems. The use of tissue penetrant near-IR wavelengths enables in vivo applications, however the design of molecules that function in this range remains a substantial challenge. Heptamethine cyanine fluorophores are already important tools for near-IR optical imaging. These molecules are susceptible to photobleaching through a photooxidative cleavage reaction. This review details efforts to define the mechanism of this reaction and two emerging fields closely tied to this process. In the first, efforts that slow photooxidation enable the creation of photobleaching resistant fluorophores. In the second, cyanine photooxidation has recently been employed as the cornerstone of a near-IR uncaging strategy. This review seeks to highlight the utility of mechanistic organic chemistry insights to help tailor cyanine scaffolds for new, and previously intractable, biological applications.
Introduction
Optical methods that probe and/or alter biological processes are central to biomedicine. Near-IR light (~650–900 nm) is dramatically more tissue penetrant than visible light, facilitating in vivo applications [1]. Nevertheless, improved chemical tools are required to fully realize the benefits of these wavelengths. In the context of imaging, there is a significant need for long wavelength fluorophores with enhanced chemical/photochemical stability and improved optical properties [2,3]. With regard to altering biology and treating disease, existing near-IR methods typically rely on the local generation of toxic levels of reactive oxygen species (ROS) through ‘photodynamic’ approaches [4]. By analogy to the significant utility of existing photocaging approaches for cell-based studies, many in vivo biomedical applications would be possible if diverse chemotypes could be site-specifically released with near-IR light [5•,6].
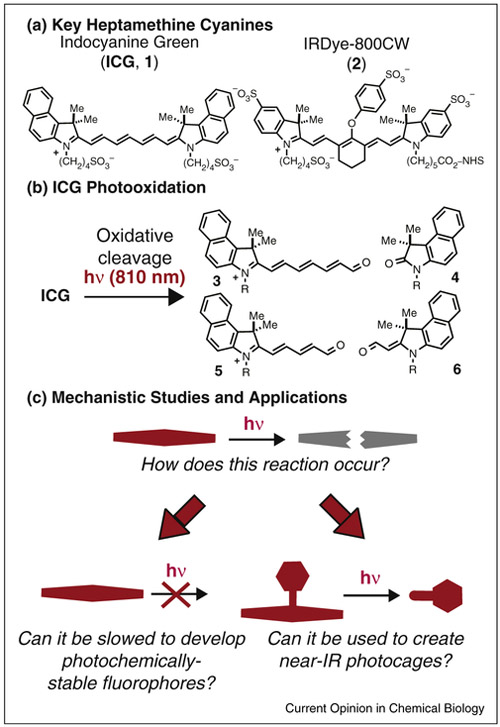
Indocyanine fluorophores, often referred to as ‘Cy’ dyes, are used for a variety of fluorescence-based applications. Heptamethine variants form the basis of many, if not most, clinical near-IR imaging efforts, as well as many preclinical studies. One example, indocyanine green (ICG, 1, Figure 1a), is an FDA-approved diagnostic agent used in a variety of clinical contexts [7,8]. Another example, the bioconjugatable IRDye-800CW (2), is in clinical trials as an optical beacon to guide the surgical resection of squamous cell carcinoma [9]. As with many fluorophores, cyanines are prone to light-dependent decomposition, or photobleaching. Somewhat unusually, cyanine photobleaching derives from a well-defined photooxidative cleavage reaction, which is exemplified by the conversion of ICG to carbonyls 3 to 6 in Figure 1b [10]. This review describes efforts to define the mechanism of this photooxidative reaction and two fields where this process plays a central role (Figure 1c). In the first, chemists seek to develop molecules with improved photochemical stability by blocking, or at least slowing, photooxidation. In the second, photooxidative reactions have been used to create near-IR photocaging strategies. In both cases, organic chemistry provides critical insights, and the molecular entities, to enable biological advances
Figure 1.
Overview. (a) Heptamethine cyanines that have been used extensively for in vivo fluorescence imaging. (b) Photooxidation of ICG to carbonyl products 3-6. (c) Understanding the mechanism of cyanine photobleaching enables efforts to generate photochemically stable near-IR fluorophores and near-IR uncaging strategies.
Cyanine photooxidation: mechanistic studies
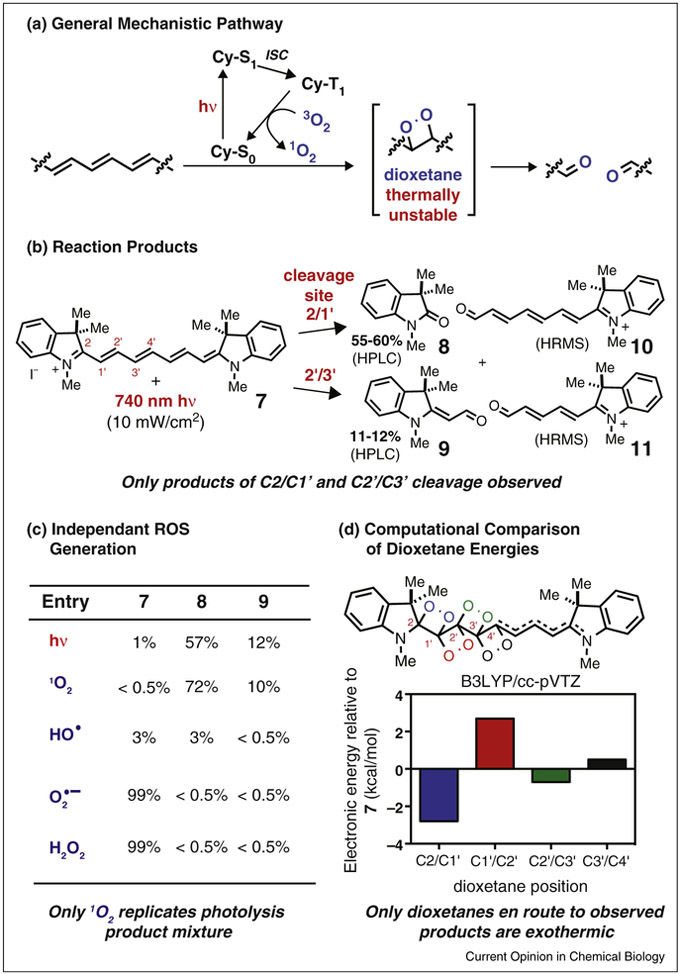
Building on seminal studies from the Kodak laboratories, various reports have described the oxidative cleavage products that derive from cyanine photolysis [11,12,13•]. Although the products are well defined, key mechanistic details have been described disparately. For example, the relevant ROS have been variably assigned as singlet oxygen (1O2), hydrogen peroxide (H2O2), or hydroxy radical (•OH) [13•]. In general, we have been guided by the notion — first suggested in the Kodak study and invoked elsewhere — that the formation of carbonyl products is best understood through the 1O2-mediated dioxetane formation/cycloreversion mechanism shown in Figure 2a.
Figure 2.
Probing the mechanism of cyanine photooxidation. (a) General reaction pathway. (b) Carbonyl products 8-11 resulting from regioselective formation and then cleavage of dioxetanes at the C2/C1′ and C2′/C3′ positions on the polyene. (c) Relative product mixture resulting from exposure of 7 to either 740 nm light or candidate ROS. Only 1O2 produces product ratios similar to those obtained with direct photolysis. (d) Energies of possible dioxetane intermediates en route to carbonyl products.
Seeking further insights for the complex and relatively minimally studied case of heptamethine cyanines, we recently carried out a set of mechanistic studies using compound 7 [14•]. The major reaction pathway was analyzed following either photolysis or exposure to candidate ROS. These studies revealed that only 1O2 (generated either chemically or through independent photosensitization), and not other candidate ROS, is capable of inducing C-C cleavage at 2 of the 4 feasible reaction sites along the cyanine polyene. These results nearly match those obtained by direct photolysis (Figure 2b,c). Moreover, MS/MS fragmentation of a near-IR light-dependent [7 + O2]+ adduct — the putative dioxetane intermediate — provides ions corresponding to carbonyls 8-11. Finally, a quantum mechanical analysis of the photooxidative pathway indicated that the relative ground state energy of the intermediate dioxetanes correlates closely with the observed product distribution (Figure 2d). Together, these studies provide strong evidence that the major photooxidation pathway of heptamethine cyanines entails 1O2-mediated cleavage of the polyene via dioxetane intermediates. Despite these insights, a number of questions remain. In particular, an understanding of the dramatic differences in photo-chemical stability between cyanines would be quite useful. For example, trimethine and pentamethine cyanines are substantially less susceptible to photodegradation than the heptamethine variants described above [15,16].
Slowing photooxidation to create stable fluorophores
The creation of photobleaching resistant fluorophores will broaden the time interval over which phenomena of interest can be observed [17,18]. For example, signal stability is crucial for single molecule microscopy [19••]. This is also true for fluorescence-guided surgical interventions, where significant light exposure over long timeframes is inescapable [20,21]. Three different strategies have been pursued with cyanine derivatives: (1) altering electron density, (2) encapsulation, and (3) the use of triplet-state quenchers (TSQs).
As 1O2 is a highly electrophilic species, a reasonable strategy to reduce photooxidation involves removing electron density from the cyanine polyene. Two different strategies have been explored. In the first, a cyano functional group was attached directly to the polyene of a merocyanine derivative [22]. In the second, the aromatic rings of a bisbenzothiazole pentamethine cyanine were perfluorinated [23]. In both cases, the resulting fluorophores were significantly more photostable than the corresponding compounds lacking these modifications. Further efforts with this particularly direct approach are likely to be productive.
The concept of chromophore encapsulation garners inspiration from Green Fluorescent Protein (GFP), where the positioning of the chromophoric element inside a β-barrel motif provides needed rigidity and improved stability. Cyanine encapsulation was first explored by Anderson and coworkers using irreversibly rotaxane encapsulated hepta- and pentamethine cyanines [24,25,26]. Elegant work by Smith and coworkers has also explored this concept with squarine-based dyes [27,28]. In both instances, the encapsulated dyes are more resistant to photochemical and chemical degradation than the free molecules, and, in the case of the latter, the complexes have been applied in a variety of imaging contexts. An alternative approach to encapsulate chromophoric elements entails the combination of exogenously provided small molecules and genetically encoded protein hosts. Armitage and coworkers have realized this concept using single-chain variable fragment (scFv) antibodies that were evolved to bind a family of cyanines [29,30]. These molecules are nearly non-fluorescent initially, but, upon binding to the expressed protein host, become dramatically more fluorescent with significant improvements in photo-stability.
Another approach to slow photobleaching is to reduce the generation of 1O2 and other ROS. In principle, lowering the quantum yield of intersystem crossing (ΦISC) and/or shortening the triplet state lifetime (τT1) should achieve this goal. Efforts in this area draw from the development of antifade buffers for microscopy. These buffers often contain redox active small molecules, for example quinones, thiols, nitroaryls, or, most recently, Ni(0) salts, and are frequently used in combination with enzymatic oxygen exclusion [31,32,33,34•]. The requirement of high concentrations (mM) of the redox active molecule(s) imposes certain limitations, including rendering this approach incompatible with many live-cell and tissue imaging applications. Thus, intramolecular tethering is a reasonable tactic to increase the effective concentration of the redox partner. Recently, Blanchard and coworkers dramatically illustrated this concept through generation of Cy3, Cy5, and Cy7 modified with the putative TSQs nitrobenzene, trolox, and 1,3,5,7-cyclooctatetraene (COT) [35•,36]. These conjugates exhibit improvements in stability as measured in bulk solution and at the single molecule level. Recent mechanistic studies have shown that the impact on τT1 varies and is highly dependent on the identity of the TSQ and even the linker, although some of these issues may be solvent dependant [37•,38]. This concept has also been explored through recent studies by Cordes and coworkers using both DNA tethering strategies and direct covalent attachment [39,40,41]. In total, these studies reveal that various features of cyanine photooxidation, including kinetic parameters, are subject to modification. Future efforts based on mechanistic insights may provide molecules functionally nearly immune to photobleaching.
Cyanine photooxidation for photocaging
Uncaging reactions that use near-IR light will enable the site-specific delivery of bioactive compounds in complex physiological settings. This is a challenging chemical problem because the modest photonic energy of these wavelengths is not easily translated into bond cleavage [42•]. While two-photon methods are useful, the scope of possible applications is somewhat limited because uncaging only occurs in the small focal volumes excited by pulsed laser sources (nanoliters to picoliters) [43,44]. The first single-photon uncaging methods used metallic nanoparticles through uncaging mechanisms that rely on localized heating or upconversion [45,46]. Recently, strategies that release payloads from custom-built liposomal formulations have also emerged [47••,48,49]. Also desirable are small molecule approaches, which, along with their bioconjugates, still comprise the vast majority of clinical modalities. Only recently have such methods started to appear. Exciting0 progress has been achieved with the use of 1O2-cleavable olefinic linkers in combination with porphyrin and phthalocyanine-based photo-sensitizers and with contact quenching-induced scission of Co-C bonds [50•,51,52,53,54,55•]. Long wavelength uncaging cleavage strategies using the BOPIDY scaffold have also recently appeared [56•,57,58,59].
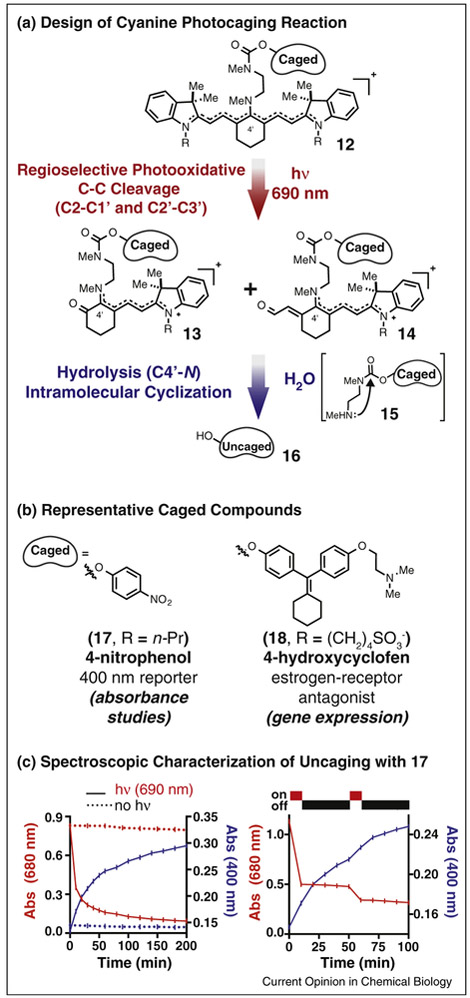
Inspired by the well-defined photooxidation chemistry described above, we set out to employ the heptamethine cyanine scaffold as the chemical backbone of a near-IR uncaging strategy. We reasoned that this ubiquitous, albeit typically deleterious, photobleaching reaction could be adapted for this new use. Also motivating this effort was the long-standing observation that cyanines are quite non-toxic, even after protracted irradiation. The chemical design of our first cyanine photooxidation-dependent uncaging strategy is shown in Figure 3a [60•]. The uncaging reaction sequence entails photooxidative cleavage of 12 at the C2/C1’ and C2’/C3’ bonds to afford 13 and 14, which then spontaneously hydrolyze to release 15. These steps liberate a secondary amine, which cyclizes onto a pendant carbamate to release phenol-containing payloads (16). The mechanistic premise of this strategy is that photooxidation products 13 and 14 are much more susceptible to hydrolysis under physiological conditions than starting 12 (perhaps due to increased iminium character in the key C4′-N bond).
Figure 3.
Using cyanine photooxidation for near-IR uncaging. (a) General reaction sequence for uncaging of phenol-containing payloads from 12 using 690 nm light. Compound 12 is converted to hydrolytically labile 13 and 14 via 1O2-mediated photooxidation of the polyene. Subsequent hydrolysis at the C4′ position provides amine 15, which spontaneously cyclizes to uncage phenol 16. (b) Representative phenols successfully uncaged using the strategy shown in (a). (c) Spectroscopic characterization of uncaging using 17. Absorbance traces at 400 nm (blue) and 680 nm (red) with (solid line) or without (dashed line) 1 mW/cm2 690 nm irradiation of a 50 μM solution of 7 (HEPES buffer). Irradiation with 690 nm light leads to cyanine photooxidation (indicated by a decrease in cyanine absorption at 680 nm, left) and uncaging of 4-nitrophenolate (indicated by an increase in its absorption at 400 nm). No such effects are observed in the absence of irradiation. In intermittent irradiation experiments (right), cyanine absorption decreases only upon exposure to 690 nm light, while 4-nitrophenolate absorption increases in interim dark periods due to accumulation and then release from intermediates 13 and 14.
Compound 17 (Figure 3b), which releases the absorbance reporter 4-nitrophenolate, was used to spectroscopically characterize the uncaging reaction. Irradiation with modest flux of 690 nm light (1 mW/cm2 from an LED source) decreases the cyanine absorption (t1/2 = 8.5 min) with concommitant appearance of the 4-nitrophenolate signal (t1/2 = 40 min) (Figure 3c, left). The effect of intermittent irradiation was also examined. The profile of cyanine absorption decrease correlates directly with irradiation, while the nitrophenolate signal increases in interim periods (Figure 3c, right). Time course studies using mass spectrometry showed that intermediates corresponding to 13 and 14 accumulate upon irradiation and then decrease in a light-independent manner. In total, these results are consistent with the proposed mechanism; photooxidation of the cyanine initiates the process and subsequent light-independent steps, hydrolysis and cyclization, are needed prior to phenol uncaging. Irradiation of compound 18 provides a useful yield of the estrogen receptor antagonist 4-hydroxycyclofen (59%), while displaying excellent stability in the dark. Caged 18 was used to control gene expression in combination with a Cre-ERT transgenic cell line. In line with prior observations, we demonstrated that cyanines that release only non-toxic payloads have little effect on mammalian cell viability upon irradiation. This absence of significant phototoxicity may initially seem surprising, given that the photooxidative reaction described above involves 1O2. However, the modest toxicity is consistent with the observation that heptamethine cyanines are weak 1O2 generators (Φ∆ ≈ 0.01–0.001) [61,62]. Moreover, generated 1O2 rapidly destroys the chromophoric element through the photooxidation reaction, limiting the potential to accumulate toxic ROS levels.
Applications in drug delivery
Near-IR drug delivery strategies could complement, or improve upon, existing light-based therapeutic methods that rely on photosensitizer-dependent mechanisms. In particular, the ability to target highly potent molecules specifically to disease sites could enable novel therapeutic strategies. Our efforts to date have focused on antibodydrug conjugates (ADCs). ADCs provide numerous benefits, including excellent pharmacological properties Using cyanine photooxidation for near-IR uncaging. (a) General reaction sequence for uncaging of phenol-containing payloads from 12 using 690 nm light. Compound 12 is converted to hydrolytically labile 13 and 14 via 1O2-mediated photooxidation of the polyene. Subsequent hydrolysis at the C4′ position provides amine 15, which spontaneously cyclizes to uncage phenol 16. (b) Representative phenols successfully uncaged using the strategy shown in (a). (c) Spectroscopic characterization of uncaging using 17. Absorbance traces at 400 nm (blue) and 680 nm (red) with (solid line) or without (dashed line) 1 mW/cm2 690 nm irradiation of a 50 μM solution of 7 (HEPES buffer). Irradiation with 690 nm light leads to cyanine photooxidation (indicated by a decrease in cyanine absorption at (even with quite complex payloads) and antigen targeting. While the recent clinical progress of ADC strategies is remarkable, cleavage approaches using endogenous cellular processes, e.g. disulfide or peptidic linkers, have little inherent tumor selectivity [63,64]. As a consequence, undesirable release, either in circulation or in benign tissue, is often a significant issue [65,66,67].
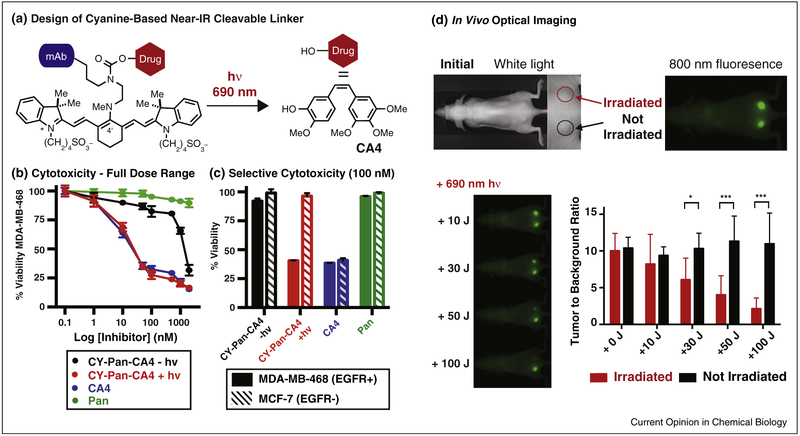
Cyanine-based ADC linkers could enable small molecule delivery with high precision through the combination of antibody targeting and near-IR light mediated release. Light provides an external stimulus to precisely time and target the critical small molecule release event, in principle ameliorating dose-limiting toxicities arising from ‘off-target’ cleavage. We have prepared and characterized first generation cyanine photocaged conjugates of combretastatin A4 (CA4), a potent tubulin polymerization inhibitor, and panitumumab (Pan), a clinically used anti-EGFR antibody (Figure 4) [68•]. This approach appends a bioconjugatable linker to the carbamate functional group (Figure 4a). The key NHS ester was prepared through a 7-step sequence and conjugated to Pan through lysine labeling. Pan was chosen because several near-IR light-accessible tumor types, including head and neck, ovarian, and bladder, are often EGFR+. We validated that these conjugates efficiently release CA4 upon irradiation, display high dark stability, and maintain EGFR binding.
Figure 4.
Cyanine uncaging applied to drug delivery. (a) Design of a near-IR light-cleavable ADC via conjugation of a cyanine-caged drug to a monoclonal antibody (CY-Pan-CA4). (b) Light-dependent growth inhibition of EGFR+ cells is observed upon exposure to ADC and 690 nm irradiation. Significantly diminished (~70-fold) CA4 effects are observed in the absence of irradiation, indicating high dark stability. (c) Incubation of EGFR+ and EGFR− cells with conjugate followed by a media exchange prior to photolysis leads to CA4 effects only in the receptor-positive and irradiated cell line. (d) Selective tumor localization is obtained in an EGFR+ double xenograft model. Irradiation of one tumor with a 690 nm laser ablates the cyanine fluorescence signal after 100 J (~3 min), with no effect on the signal of the unirradiated tumor.
This construct enabled in vitro and in vivo characterization, which was carried out partly through a collaboration with our colleague at the National Cancer Institute, Dr. Kobayashi. The growth inhibitory activity of the conjugate was highly light dependent (irradiated IC50 = 16 nM vs. unirradiated IC50 = 1.1 μM, a ~70-fold window, Figure 4b). Furthermore, no inhibitory effects were observed using a version of the antibody conjugate that releases only biologically inactive phenol, indicating that the observed cytotoxicity is solely a consequence of drug release. We also evaluated the internalized and cell surface bound antibody fraction. A significant reduction in cell viability was observed only upon irradiation in the EGFR+ cell line, with little effect in either the EGFR– cell line or in the absence of irradiation (Figure 4c). Mouse imaging studies using the near-IR fluorescence of the cyanine conjugate were also carried out. Selective tumor accumulation was observed, with high tumor-to-background ratios obtained at 1 day post-injection. Moreover, significant signal is still observed 7 days post-injection, suggesting high stability of the cyanine component.
As a prelude to in vivo drug delivery studies, we assessed if tumor irradiation could be used to deplete the fluorescence signal. Encouragingly, the cyanine fluorescence signal, which was stable under typical imaging conditions, can be depleted using external irradiation from a 690 nm PDT laser (Figure 4d). Future efforts that investigate more potent payloads are likely needed to overcome the modest cell-surface concentrations of relevant tumor-associated antigens, such as EGFR [69].
Conclusions
Indocyanines are already essential tools for a variety of fluorescence-based applications. Controlling the photo-chemical oxidative cleavage process of this privileged scaffold will enable currently intractable applications in imaging and drug delivery. Given the centrality of fluorescence imaging to biomedical research, the generation of photobleaching-resistant fluorophores will have a significant impact in many fields. In the context of drug delivery, cyanine-based approaches will allow bioactive molecules to be delivered to sites of interest in complex physiological settings. In this latter area, the development of novel, biologically useful bond cleavage strategies complements complements the significant progress in bioorthogonal bond forming reactions [70,71•]. Chemists’ capacity to create and then deploy precisely controlled molecular entities for imaging and drug delivery presents a variety of opportunities. The integration of mechanistic organic chemistry insights, complex molecule synthesis, and advanced imaging and biomedical techniques will be needed.
Acknowledgements
Dr. Roger Nani is thanked for helpful discussions and comments. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hilderbrand SA, Weissleder R: Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol 2010, 14:71–79. [DOI] [PubMed] [Google Scholar]
- 2.Escobedo JO, Rusin O, Lim S, Strongin RM: NIR dyes for bioimaging applications. Curr Opin Chem Biol 2010, 14:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nani RR, Shaum JB, Gorka AP, Schnermann MJ: Electrophile-integrating Smiles rearrangement provides previously inaccessible C4′-O-alkyl heptamethine cyanine fluorophores. Org Lett 2015, 17:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor AE, Gallagher WM, Byrne AT: Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol 2009, 85:1053–1074. [DOI] [PubMed] [Google Scholar]
- 5.Lee HM, Larson DR, Lawrence DS: Illuminating the chemistry of life: design, synthesis, and applications of ‘caged’ and related photoresponsive compounds. ACS Chem Biol 2009, 4:409–427.• An excellent overview of modern photocaging approaches and applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J: Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem Rev 2013, 113:119–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, Kobayashi H, Choyke PL: Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging 2009, 8:341–354. [PubMed] [Google Scholar]
- 8.Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA: Indocyanine green: historical context, current applications, and future considerations. Surg Innov 2016, 23:166–175. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G et al. : Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 2015, 21:3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel E, Schraml R, Maisch T, Kobuch K, Koenig B, Szeimies RM, Hillenkamp J, Baumler W, Vasold R: Light-induced decomposition of indocyanine green. Invest Ophthalmol Vis Sci 2008, 49:1777–1783. [DOI] [PubMed] [Google Scholar]
- 11.Byers GW, Gross S, Henrichs PM: Direct and sensitized photooxidation of cyanine dyes. Photochem Photobiol 1976, 23:37–43. [DOI] [PubMed] [Google Scholar]
- 12.Henary M, Mojzych M: Stability and Reactivity of Polymethine Dyes in Solution. Heterocyclic Polymethine Dyes: Synthesis, Properties and Applications In Topics in Heterocyclic Chemistry 14 Edited by Strekowski L Berlin: Springer-Verlag; 2008:221–238. [Google Scholar]
- 13.Gorka AP, Nani RR, Schnermann MJ: Cyanine polyene reactivity: scope and biomedical applications. Org Biomol Chem 2015, 13:7584–7598.• A broad review of cyanine polyene reactivity and modifications that have enabled advances across diverse biomedical fields, including optical imaging, sensing, and super resolution microscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nani RR, Kelley JA, Ivanic J, Schnermann MJ: Reactive species involved in the regioselective photooxidation of heptamethine cyanines. Chem Sci 2015, 6:6556–6563.• The major photooxidation mechanism of heptamethine cyanines is investigated. Computational, chromatographic, and mass spectrometry analyses provide a comprehensive accounting of cyanine photooxidation products that arise from direct photolysis or reaction with candidate ROS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P, Sun SQ, Hu YF, Qian ZG, Zheng DS: Structure and solvent effect on the photostability of indolenine cyanine dyes. Dyes Pigments 1999, 41:227–231. [Google Scholar]
- 16.Ballou B, Ernst LA, Waggoner AS: Fluorescence imaging of tumors in vivo. Curr Med Chem 2005, 12:795–805. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Zhu W: Stability enhancement of fluorophores for lighting up practical application in bioimaging. Chem Soc Rev 2015, 44:4179–4184. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Q, Juette MF, Jockusch S, Wasserman MR, Zhou Z, Altman RB, Blanchard SC: Ultra-stable organic fluorophores for single-molecule research. Chem Soc Rev 2014, 43:1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitus M, Ranjit S: Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments. Q Rev Biophys 2011, 44:123–151.•• An important overview of our current understanding of cyanine photophysics, with a particular focus on issues related to single molecule spectroscopy. [DOI] [PubMed] [Google Scholar]
- 20.Gioux S, Choi HS, Frangioni JV: Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 2010, 9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen QT, Tsien RY: Fluorescence-guided surgery with live molecular navigation – a new cutting edge. Nat Rev Cancer 2013, 13:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toutchkine A, Nguyen DV, Hahn KM: Merocyanine dyes with improved photostability. Org Lett 2007, 9:2775–2777. [DOI] [PubMed] [Google Scholar]
- 23.Renikuntla BR, Rose HC, Eldo J, Waggoner AS, Armitage BA: Improved photostability and fluorescence properties through polyfluorination of a cyanine dye. Org Lett 2004, 6:909–912. [DOI] [PubMed] [Google Scholar]
- 24.Yau CM, Pascu SI, Odom SA, Warren JE, Klotz EJ, Frampton MJ, Williams CC, Coropceanu V, Kuimova MK, Phillips D et al. : Stabilisation of a heptamethine cyanine dye by rotaxane encapsulation. Chem Commun 2008:2897–2899. [DOI] [PubMed] [Google Scholar]
- 25.Buston JEH, Young JR, Anderson HL: Rotaxane-encapsulated cyanine dyes: enhanced fluorescence efficiency and photostability. Chem Commun 2000:905–906. [Google Scholar]
- 26.Buston JEH, Marken F, Anderson HL: Enhanced chemical reversibility of redox processes in cyanine dye rotaxanes. Chem Commun 2001:1046–1047. [Google Scholar]
- 27.Arunkumar E, Forbes CC, Noll BC, Smith BD: Squaraine-derived rotaxanes: sterically protected fluorescent near-IR dyes. J Am Chem Soc 2005, 127:3288–3289. [DOI] [PubMed] [Google Scholar]
- 28.Gassensmith JJ, Baumes JM, Smith BD: Discovery and early development of squaraine rotaxanes. Chem Commun 2009:6329–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozhalici-Unal H, Pow CL, Marks SA, Jesper LD, Silva GL, Shank NI, Jones EW, Burnette JM 3rd, Berget PB, Armitage BA: A rainbow of fluoromodules: a promiscuous scFv protein binds to and activates a diverse set of fluorogenic cyanine dyes. J Am Chem Soc 2008, 130:12620–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shank NI, Zanotti KJ, Lanni F, Berget PB, Armitage BA: Enhanced photostability of genetically encodable fluoromodules based on fluorogenic cyanine dyes and a promiscuous protein partner. J Am Chem Soc 2009, 131:12960–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widengren J, Chmyrov A, Eggeling C, Lofdahl PA, Seidel CA: Strategies to improve photostabilities in ultrasensitive fluorescence spectroscopy. J Phys Chem A 2007, 111:429–440. [DOI] [PubMed] [Google Scholar]
- 32.Vogelsang J, Kasper R, Steinhauer C, Person B, Heilemann M, Sauer M, Tinnefeld P: A reducing and oxidizing system minimizes photobleaching and blinking of fluorescent dyes. Angew Chem Int Ed Engl 2008, 47:5465–5469. [DOI] [PubMed] [Google Scholar]
- 33.Dave R, Terry DS, Munro JB, Blanchard SC: Mitigating unwanted photophysical processes for improved single-molecule fluorescence imaging. Biophys J 2009, 96:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glembockyte V, Lincoln R, Cosa G: Cy3 photoprotection mediated by Ni2+ for extended single-molecule imaging: old tricks for new techniques. J Am Chem Soc 2015, 137:1116–1122.• The addition of Ni2+ in combination with O2 scavenging provides photoprotective effects that exceed those of existing organic small molecule approaches. [DOI] [PubMed] [Google Scholar]
- 35.Altman RB, Terry DS, Zhou Z, Zheng Q, Geggier P, Kolster RA, Zhao Y, Javitch JA, Warren JD, Blanchard SC: Cyanine fluorophore derivatives with enhanced photostability. Nat Methods 2011, 9:68–71.• Key demonstration that appending triplet quenchers to cyanine fluorophores can extend photostabilty at both the single molecule and bulk solution level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman RB, Zheng Q, Zhou Z, Terry DS, Warren JD, Blanchard SC: Enhanced photostability of cyanine fluorophores across the visible spectrum. Nat Methods 2012, 9:428–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng QS, Jockusch S, Zhou Z, Altman RB, Warren JD, Turro NJ, Blanchard SC: On the mechanisms of cyanine fluorophore photostabilization. J Phys Chem Lett 2012, 3:2200–2203.• Mechanistic studies on the basis of using intramolecular tethering towards slowing photooxidation. Triplet lifetime measurements as function of quencher and linker are reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Q, Jockusch S, Zhou Z, Blanchard SC: The contribution of reactive oxygen species to the photobleaching of organic fluorophores. Photochem Photobiol 2014, 90:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Velde JH, Oelerich J, Huang J, Smit JH, Aminian Jazi A, Galiani S, Kolmakov K, Guoridis G, Eggeling C, Herrmann A et al. : A simple and versatile design concept for fluorophore derivatives with intramolecular photostabilization. Nat Commun 2016, 7:10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Velde JH, Ploetz E, Hiermaier M, Oelerich J, de Vries JW, Roelfes G, Cordes T: Mechanism of intramolecular photostabilization in self-healing cyanine fluorophores. ChemPhysChem 2013, 14:4084–4093. [DOI] [PubMed] [Google Scholar]
- 41.van der Velde JH, Oelerich J, Huang J, Smit JH, Hiermaier M, Ploetz E, Herrmann A, Roelfes G, Cordes T: The power of two: covalent coupling of photostabilizers for fluorescence applications. J Phys Chem Lett 2014, 5:3792–3798. [DOI] [PubMed] [Google Scholar]
- 42.Solomek T, Wirz J, Klan P: Searching for improved photoreleasing abilities of organic molecules. Acc Chem Res 2015, 48:3064–3072.• An excellent and very recent photochemistry-oriented review that provides the current state-of-the-art in designing and probing the mechanism of uncaging strategies. A particular focus is the use of Huückel molecular orbital theory methods to understand and predict C-X cleavage reactions. [DOI] [PubMed] [Google Scholar]
- 43.Dore TM, Wilson HC: Chromophores for the delivery of bioactive molecules with two-photon excitation. Photosensitive Molecules for Controlling Biological Function In Neuromethods, Vol. 55 Edited by Chambers JJ, Kramer RH. New York: Humana Press; 2011:57–92. [Google Scholar]
- 44.Warther D, Gug S, Specht A, Bolze F, Nicoud JF, Mourot A, Goeldner M: Two-photon uncaging: New prospects in neuroscience and cellular biology. Bioorg Med Chem 2010, 18:7753–7758. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Shao Q, Deng R, Wang C, Teng X, Cheng K, Cheng Z, Huang L, Liu Z, Liu X et al. : In vitro and in vivo uncaging and bioluminescence imaging by using photocaged upconversion nanoparticles. Angew Chem Int Ed Engl 2012, 51:3125–3129. [DOI] [PubMed] [Google Scholar]
- 46.Viger ML, Sheng W, Dore K, Alhasan AH, Carling CJ, Lux J, de Gracia Lux C, Grossman M, Malinow R, Almutairi A: Near-infrared-induced heating of confined water in polymeric particles for efficient payload release. ACS Nano 2014, 8:4815–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olejniczak J, Carling CJ, Almutairi A: Photocontrolled release using one-photon absorption of visible or NIR light. J Control Release 2015, 219:18–30.• Provides an excellent broad description of one-photon drug delivery strategies using near-IR light with an emphasis on nanoparticle and liposomal formulations. [DOI] [PubMed] [Google Scholar]
- 48.Helmy S, Leibfarth FA, Oh S, Poelma JE, Hawker CJ, Read de Alaniz J: Photoswitching using visible light: a new class of organic photochromic molecules. J Am Chem Soc 2014, 136:8169–8172. [DOI] [PubMed] [Google Scholar]
- 49.Carling CJ, Olejniczak J, Foucault-Collet A, Collet G, Viger ML, Huu VAN, Duggan BM, Almutairi A: Efficient red light photo-uncaging of active molecules in water upon assembly into nanoparticles. Chem Sci 2016, 7:2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shell TA, Shell JR, Rodgers ZL, Lawrence DS: Tunable visible and near-IR photoactivation of light-responsive compounds by using fluorophores as light-capturing antennas. Angew Chem Int Ed Engl 2014, 53:875–878.• An novel strategy for rapid single-photon near-IR uncaging at wave-lengths up to 800 nm. This approach utilizes cyanine fluorophores as chromophoric elements that induce Co-C bond scission in the corrin ring of Vitamin B12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith WJ, Oien NP, Hughes RM, Marvin CM, Rodgers ZL, Lee J, Lawrence DS: Cell-mediated assembly of phototherapeutics. Angew Chem Int Ed Engl 2014, 53:10945–10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang MY, Dolphin D: Site-specific prodrug release using visible light. J Am Chem Soc 2008, 130:4236–4237. [DOI] [PubMed] [Google Scholar]
- 53.Rotaru A, Mokhir A: Nucleic acid binders activated by light of selectable wavelength. Angew Chem Int Ed Engl 2007, 46:6180–6183. [DOI] [PubMed] [Google Scholar]
- 54.Bio M, Rajaputra P, Nkepang G, Awuah SG, Hossion AM, You Y: Site-specific and far-red-light-activatable prodrug of combretastatin A-4 using photo-unclick chemistry. J Med Chem 2013, 56:3936–3942. [DOI] [PubMed] [Google Scholar]
- 55.Nkepang G, Bio M, Rajaputra P, Awuah SG, You Y: Folate receptor-mediated enhanced and specific delivery of far-red light-activatable prodrugs of combretastatin A-4 to FR-positive tumor. Bioconjug Chem 2014, 25:2175–2188.• A near-IR uncaging strategy using a diaxially-substitued silicon phthalo-cyanine containing a folate targeting ligand and a 1O2-cleavable small molecule therapuetic. Likely the current state-of-the-art for in vivo appli-cations, this report includes molecular targeting, in vivo imaging, and tumor burden reduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umeda N, Takahashi H, Kamiya M, Ueno T, Komatsu T, Terai T, Hanaoka K, Nagano T, Urano Y: Boron dipyrromethene as a fluorescent caging group for single-photon uncaging with long-wavelength visible light. ACS Chem Biol 2014, 9:2242–2246.• Single-photon uncaging of phenols from the BODIPY scaffold using 500 nm light suggests the potential for future single-photon uncaging in the near-IR range. This approach was shown to be effective for stimulating intracellular calcium production via histamine uncaging. [DOI] [PubMed] [Google Scholar]
- 57.Rubinstein N, Liu P, Miller EW, Weinstain R: meso-Methylhydroxy BODIPY: a scaffold for photo-labile protecting groups. Chem Commun 2015, 51:6369–6372. [DOI] [PubMed] [Google Scholar]
- 58.Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R, Smith EA, Winter AH: BODIPY-derived photoremovable protecting groups unmasked with green light. J Am Chem Soc 2015, 137:3783–3786. [DOI] [PubMed] [Google Scholar]
- 59.Palao E, Slanina T, Muchova L, Solomek T, Vitek L, Klan P: Transition-metal-free CO-releasing BODIPY derivatives activatable by visible to NIR light as promising bioactive molecules. J Am Chem Soc 2016, 138:126–133. [DOI] [PubMed] [Google Scholar]
- 60.Gorka AP, Nani RR, Zhu J, Mackem S, Schnermann MJ: A near-IR uncaging strategy based on cyanine photochemistry. J Am Chem Soc 2014, 136:14153–14159.• First use of the heptamethine cyanine scaffold for near-IR uncaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delaey E, van Laar F, De Vos D, Kamuhabwa A, Jacobs P, de Witte P: A comparative study of the photosensitizing characteristics of some cyanine dyes. J Photochem Photobiol B 2000, 55:27–36. [DOI] [PubMed] [Google Scholar]
- 62.Redmond RW, Gamlin JN: A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol 1999, 70:391–475. [PubMed] [Google Scholar]
- 63.Casi G, Neri D: Antibody-drug conjugates: basic concepts, examples and future perspectives. J Control Release 2012, 161:422–428. [DOI] [PubMed] [Google Scholar]
- 64.Alley SC, Okeley NM, Senter PD: Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol 2010, 14:529–537. [DOI] [PubMed] [Google Scholar]
- 65.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, Senter PD, Wahl AF: In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res 2005, 11:843–852. [PubMed] [Google Scholar]
- 66.Dorywalska M, Strop P, Melton-Witt JA, Hasa-Moreno A, Farias SE, Galindo Casas M, Delaria K, Lui V, Poulsen K, Loo C et al. : Effect of attachment site on stability of cleavable antibody drug conjugates. Bioconjug Chem 2015, 26:650–659. [DOI] [PubMed] [Google Scholar]
- 67.Gorovits B, Krinos-Fiorotti C: Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother 2013, 62:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nani RR, Gorka AP, Nagaya T, Kobayashi H, Schnermann MJ: Near-IR light-mediated cleavage of antibody-drug conjugates using cyanine photocages. Angew Chem Int Ed Engl 2015, 54:13635–13638.• Application of the heptamethine cyanine scaffold for near-IR antibody-drug cleavage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderl J, Faulstich H, Hechler T, Kulke M: Antibody-drug conjugate payloads. Methods Mol Biol 2013, 1045:51–70. [DOI] [PubMed] [Google Scholar]
- 70.Patterson DM, Nazarova LA, Prescher JA: Finding the right (bioorthogonal) chemistry. ACS Chem Biol 2014, 9:592–605. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Chen PR: Development and application of bond cleavage reactions in bioorthogonal chemistry. Nature Chem Biol 2016, 12:129–137.• An overview of the development and use of bond cleavage reactions in chemical biology. [DOI] [PubMed] [Google Scholar]