Abstract
Soluble proteins destined for the secretory pathway contain an N-terminal signal peptide that induces their translocation into the endoplasmic reticulum (ER). The importance of N-terminal signal peptides for ER translocation has been extensively examined over the past few decades. However, in the budding yeast Saccharomyces cerevisiae, a few proteins devoid of a signal peptide are still translocated into the ER and then N-glycosyl-ated. Using signal peptide-truncated reporter proteins, here we report the detection of significant translocation of N-terminal signal peptide-truncated proteins in a yeast mutant strain (ste24Δ) that lacks the endopeptidase Ste24 at the ER membrane. Furthermore, several ER/cytosolic proteins, including Sec61, Sec66, and Sec72, were identified as being involved in the translocation process. On the basis of screening for 20 soluble proteins that may be N-glycosylated in the ER in the ste24Δ strain, we identified the transcription factor Rme1 as a protein that is partially N-glycosylated despite the lack of a signal peptide. These results clearly indicate that some proteins lacking a signal peptide can be translocated into the ER and that Ste24 typically suppresses this process.
Keywords: signal peptide, endoplasmic reticulum, protein translocation, Ste24, Rme1, metalloendopeptidase, N-glycosylation, secretory pathway, Spc2, ER, glycosylation, yeast, cell biology
The necessity of a signal peptide for protein transport into the endoplasmic reticulum (ER) was originally demonstrated by Blobel and Dobberstein (1). Various signal peptides are now known to be required for protein transport into each organelle, such as the ER, mitochondria, or peroxisomes, and serve as important targeting signals (2). The N-terminal signal peptide targeting the ER is typically 20∼30 amino acids long and consists of three regions (3). The N, middle, and C regions contain positively charged amino acids, hydrophobic residues, and alanine units, respectively, with the most important region being the middle one, which has a hydrophobic nature (4). The term “N-terminal” is omitted from use here to avoid its frequent usage, except when it is defined. A signal peptide with hydrophobic characteristics is recognized by a signal recognition particle (SRP), and this SRP-mediated protein targeting the ER is referred to as the SRP-dependent pathway (5). In contrast, a signal peptide that has weak hydrophobic characteristics is not recognized efficiently by the SRP, and proteins with these signal peptides are targeted to the ER through the SRP-independent pathway (6, 7). The SRP-dependent pathway is a cotranslational system mediated by SRP and SRP receptor (SR) (5). In the case of the SRP-dependent pathway, a highly hydrophobic signal peptide of newly synthesized proteins on ribosomes is recognized by SRP, which results in protein synthesis being inhibited. The interaction between SRP and SR on the ER membrane facilitates interactions between ribosomes and the translocon Sec61, and protein synthesis is then restarted as proteins are transported into the ER lumen. On the other hand, the SRP-independent pathway is a posttranslational system and is mediated by cytosolic heat shock protein 70 (Hsp70) family proteins, an ER luminal chaperone called BiP, and the Sec62-Sec63 complex (5).
Regardless of SRP dependence, proteins that are destined to be processed through the secretory pathway pass through the translocon Sec61 (8). Schekman and coworkers reported that not only Sec61 but also many other proteins, including Sec62 and Sec63, are involved in the translocation of proteins into the ER (8–11). The Sec61 complex and signal peptide-related components are conserved from bacteria to humans (12). Moreover, recent structural analyses indicated that the Sec61 channel is opened by a signal peptide (13). Thus, a growing body of evidence suggests that signal peptides and Sec proteins are essential components for the translocation of soluble proteins into the ER.
The N-terminal signal peptide-independent transport of carboxypeptidase Y (CPY) into the lumen of the ER was previously reported in the budding yeast Saccharomyces cerevisiae (14). In that study, a deletion mutation causing the removal of the entire CPY signal peptide still resulted in the N-glycosylation of CPY at low efficiency, indicating that there is a signal peptide-independent translocation mechanism for this protein. Furthermore, an N-terminal signal peptide-truncated version of Mid1 (Mid1ΔN23), a subunit of a Ca2+ channel, was still N-glycosylated and functional as a component of the channel (15). This finding indicates that Mid1 is also translocated into the ER in an N-terminal signal peptide-independent manner and suggests that the N-terminal signal peptide-independent translocation of proteins occasionally occurs in budding yeast.
To elucidate the N-terminal signal peptide-independent translocation mechanism, we investigated the factors required using signal peptide-truncated reporter proteins expressed in mutants in which ER function is affected. We found that the N-glycosylation of the signal peptide-removed proteins, CPY*ΔN28 and Pep4ΔN23, was markedly enhanced in the deletion mutant of the STE24 gene encoding a metalloendoprotease located in the ER membrane. In further analyses using mutants known to genetically interact with the STE24 gene and/or those involved in the unfolded protein response (UPR), we identified several ER proteins that affect the efficiency of the detection of signal peptide-independent translocation into the ER lumen. We also found that a portion of transcription factor Rme1 protein carrying no signal peptide per se was translocated into the ER to undergo N-glycosylation in the ste24Δ mutant, which is suggestive of the existence of intrinsic mechanisms controlling the translocation of signal peptideless proteins into the ER. Here, we discuss the importance of a checking system that avoids accidental protein translocation into the ER.
Results
The translocation of N-terminal signal peptide-deprived proteins into the ER was enhanced in ste24Δ cells
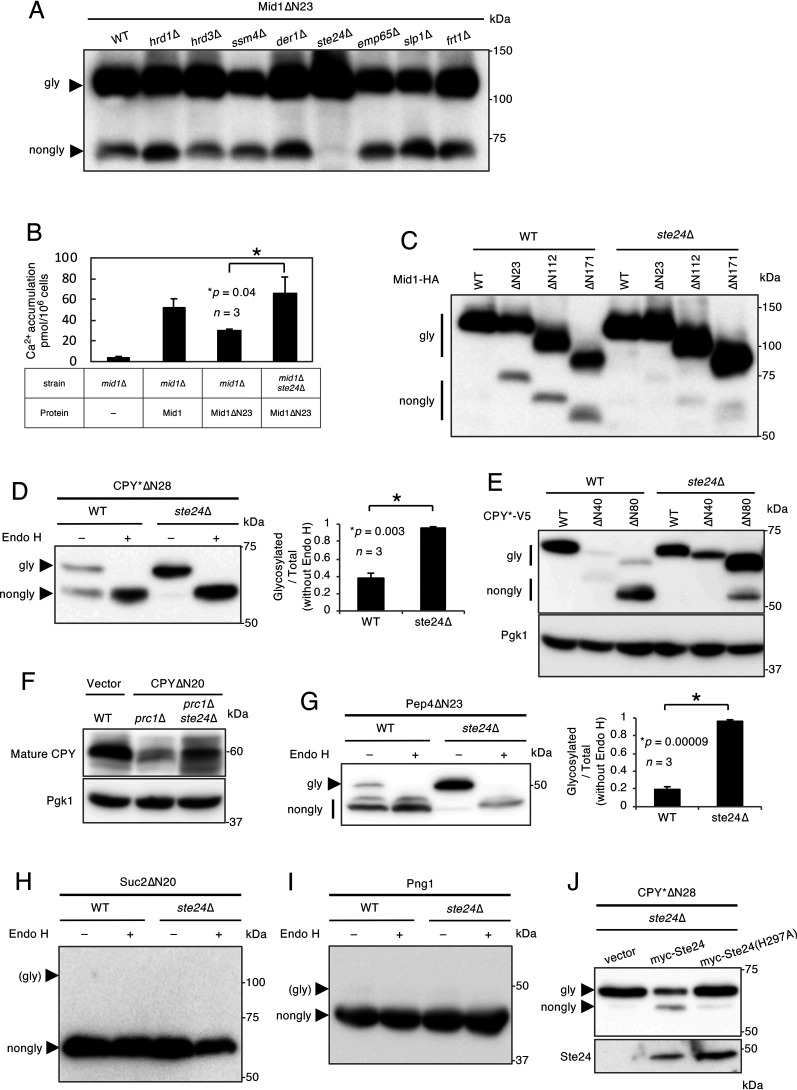
Mid1, a protein that is localized in the ER and plasma membranes and has no transmembrane region, was recently reported to be N-glycosylated and become functional even though its N-terminal signal peptide was removed (15). To identify the components of the N-terminal signal peptide-independent protein translocation pathway, we focused on eight nonessential ER membrane proteins (Hrd1, Hrd3, Ssm4, Der1, Ste24, Emp65, Slp1, and Frt1) involved in several events, including protein recognition and degradation, on the ER membrane (16–22). We constructed gene deletion mutants lacking each of the eight genes with the genetic background of strain H207 (15) and examined the N-glycosylation status of signal peptide-deprived Mid1ΔN23 in these eight mutants. The results obtained indicated that the amount of nonglycosylated Mid1ΔN23 was markedly reduced in the ste24Δ mutant (Fig. 1A), implying that signal peptide-independent translocation is enhanced in ste24Δ cells. In the course of the present study, we noted that although the ratio of N-glycosylated versus nonglycosylated Mid1ΔN23 differed depending on yeast strains with different genetic backgrounds, the effects of the ste24Δ mutation were clearly observed in both strain backgrounds that were tested (Fig. S1).
Figure 1.
Mid1ΔN23, CPY*ΔN28, and Pep4ΔN23 are translocated into the ER. A, Western blot analysis of Mid1ΔN23. Mid1ΔN23 tagged C terminally with the HA antigen was expressed in cells of the WT (strain H207) and indicated isogenic mutants. Extracts from these cells were resolved by SDS-PAGE, and Mid1ΔN23 was visualized by immunoblotting using an anti-HA antibody. B, measurement of Ca2+ accumulation. Ca2+ accumulation in mid1Δ (strain H311) cells bearing an empty vector or the Mid1 or Mid1ΔN23 plasmid or mid1Δ ste24Δ cells bearing the Mid1ΔN23 plasmid was measured using 45CaCl2. n represents the number of biological replicates. C, Western blot analysis of N-terminally truncated mutants of Mid1. Mid1, Mid1ΔN23, Mid1ΔN112, and Mid1ΔN171 were expressed in WT and ste24Δ cells (H207 background). Extracts from these cells were resolved by SDS-PAGE, and proteins were visualized by immunoblotting using an anti-HA antibody. D, Endo H treatment of CPY*ΔN28. CPY*ΔN28 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. CPY*ΔN28 was visualized by immunoblotting using an anti-V5 antibody. The bar graph shows the relative ratio of the glycosylated versus total CPY*ΔN28 proteins. n represents the number of biological replicates. E, Western blot analysis of N-terminally truncated mutants of CPY*. CPY*, CPY*ΔN40, and CPY*ΔN80 were expressed in WT and ste24Δ cells. Extracts from these cells were resolved by SDS-PAGE, and the proteins were visualized by immunoblotting using an anti-V5 antibody. The immunoblot was also probed with an anti-Pgk1 antibody as a loading control. F, Western blot analysis of endogenous CPY and CPYΔN20. CPYΔN20 tagged C terminally with the V5 antigen was expressed in prc1Δ and prc1Δ ste24Δ cells. Cell extracts were resolved by SDS-PAGE. Endogenous CPY and CPYΔN20 were visualized by immunoblotting using an anti-CPY antibody. The immunoblot was also probed with an anti-Pgk1 antibody as a loading control. G, Endo H treatment of Pep4ΔN23. Pep4ΔN23 tagged C terminally with the DYKDDDDK epitope was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Pep4ΔN23 was visualized by immunoblotting using anti-DYKDDDDK antibody. The bar graph shows the relative ratio of the glycosylated versus total Pep4ΔN23 proteins. n represents the number of biological replicates. H, Endo H treatment of Suc2ΔN20. Suc2ΔN20 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Suc2ΔN20 was visualized by immunoblotting using an anti-V5 antibody. I, Endo H treatment of Png1. Png1 tagged C terminally with the V5 antigen was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Png1 was visualized by immunoblotting using an anti-V5 antibody. J, Complementation assay for Ste24. Ste24 or its protease activity-less mutant, Ste24(H297A), N-terminally tagged with the myc epitope, was coexpressed with CPY*ΔN28-V5 in ste24Δ cells. Extracts from these cells were resolved by SDS-PAGE, and proteins were visualized by immunoblotting using anti-myc and -V5 antibodies.
To examine whether Mid1ΔN23 is functional in ste24Δ cells, its functionality was assessed using a Ca2+ accumulation assay. As shown in Fig. 1B, when Mid1ΔN23 was expressed in mid1Δ cells, Ca2+ accumulation was recovered to approximately half that of WT Mid1, but not fully. On the other hand, Ca2+ accumulation was fully recovered in mid1Δ ste24Δ cells, suggesting that Mid1ΔN23 more efficiently localizes to the plasma membrane and becomes functional in mid1Δ ste24Δ cells. To examine whether an N-terminal region outside the signal peptide is required for signal peptide-independent translocation, two additional N-terminal truncation mutants of Mid1, i.e. Mid1ΔN112 and Mid1ΔN171, were examined for N-glycosylation in WT and ste24Δ cells. Figure 1C shows that the two truncation mutants were also more efficiently N-glycosylated in ste24Δ cells, indicating that at least the N-terminal region spanning from 1 to 171 amino acid residues was not required for the N-terminal signal peptide-independent translocation of Mid1 in the ste24Δ background.
The above result showing that the Mid1 protein deprived of the N-terminal signal peptide may be more efficiently translocated into the ER in ste24Δ cells prompted us to examine whether other signal peptide-deprived proteins are translocated in the same manner. We initially selected a variant of CPY, CPY* (23). The rationale behind using CPY* is that this protein, unlike the WT CPY that undergoes multiple proteolytic processes, hardly reached the vacuole; therefore, the N-glycosylated form can be easily distinguished, without proteolytic processing, from the nonglycosylated form by SDS-PAGE. When CPY* containing no signal peptide (CPY*ΔN28) was tested, only 38% of the molecules were N-glycosylated in WT cells (Fig. 1D), suggesting that the CPY*ΔN28 mutant was still translocated into the ER to some extent, as was the case with CPY (14). In contrast, a markedly larger fraction (96%) of N-glycosylated CPY*ΔN28 was detected in ste24Δ cells (Fig. 1D), suggesting that signal peptide-independent translocation looks generally enhanced in ste24Δ cells. To estimate which N-terminal region is necessary for the signal peptide-independent translocation of CPY*, we deleted the N-terminal region up to 40 and 80 amino acid residues to produce CPY*ΔN40 and CPY*ΔN80, respectively, and examined their N-glycosylation in WT and ste24Δ cells. As shown in Fig. 1E, CPY*ΔN40 was undetectable and CPY*ΔN80 was detectable but inefficiently N-glycosylated in WT cells, whereas both proteins were efficiently N-glycosylated in ste24Δ cells. This result suggests that the N-terminal region (up to 80 amino acid residues) of CPY* is unnecessary for signal peptide-independent translocation. In addition to CPY*ΔN28, CPYΔN20 (non-ER-associated degradation [ERAD] substrate), which was expressed in the prc1Δ mutant deficient in vacuolar CPY, was also examined by Western blotting. Strikingly, the intensity of a band corresponding to the vacuolar form of CPY was reduced in prc1Δ cells but significantly recovered in prc1Δ ste24Δ cells (Fig. 1F). This result suggests that once signal peptide-less CPY are translocated into the ER, they can efficiently reach the vacuole.
We also tested the signal peptide-deprived Pep4 (Pep4ΔN23), another well-studied vacuolar protease (24). The results obtained showed that 23% of Pep4ΔN23 molecules were N-glycosylated in WT cells, whereas they were almost completely N-glycosylated in ste24Δ cells (Fig. 1G), further supporting the hypothesis that signal peptide-independent translocation into the ER is generally enhanced in ste24Δ cells.
To test the specificity of signal peptide-independent translocation, we examined Suc2ΔN20, an N-terminal signal peptide-deprived derivative of Suc2 (extracellular invertase) and Png1 (cytosolic protein) containing no intrinsic signal peptide (25, 26). Both proteins have potential N-glycosylation sites. Figure 1H and I shows that N-glycosylation was not observed in either protein in WT or ste24Δ cells. This result suggests that whereas the detection of signal peptide-independent translocation of proteins into the ER is enhanced in ste24Δ cells, strict specificity exists for substrates to be translocated into the ER in ste24Δ cells.
The metalloendoprotease activity of Ste24 is required for the maturation of a-factor and the processing of clogged proteins in the translocon (16, 27). Therefore, we examined whether the protease activity of Ste24 is required to suppress the translocation of signal peptide-deprived proteins into the ER by using the Ste24 mutant without protease activity, Ste24(H297A). As shown in Fig. 1J, nonglycosylated CPY*ΔN28 was increased in cells expressing myc-tagged WT Ste24 but not in those expressing myc-tagged, protease-deficient Ste24(H297A), suggesting that the protease activity of Ste24 is required to suppress the signal peptide-independent protein translocation into the ER.
Sec61, Sec66, and Sec72 are required for signal peptide-independent protein translocation
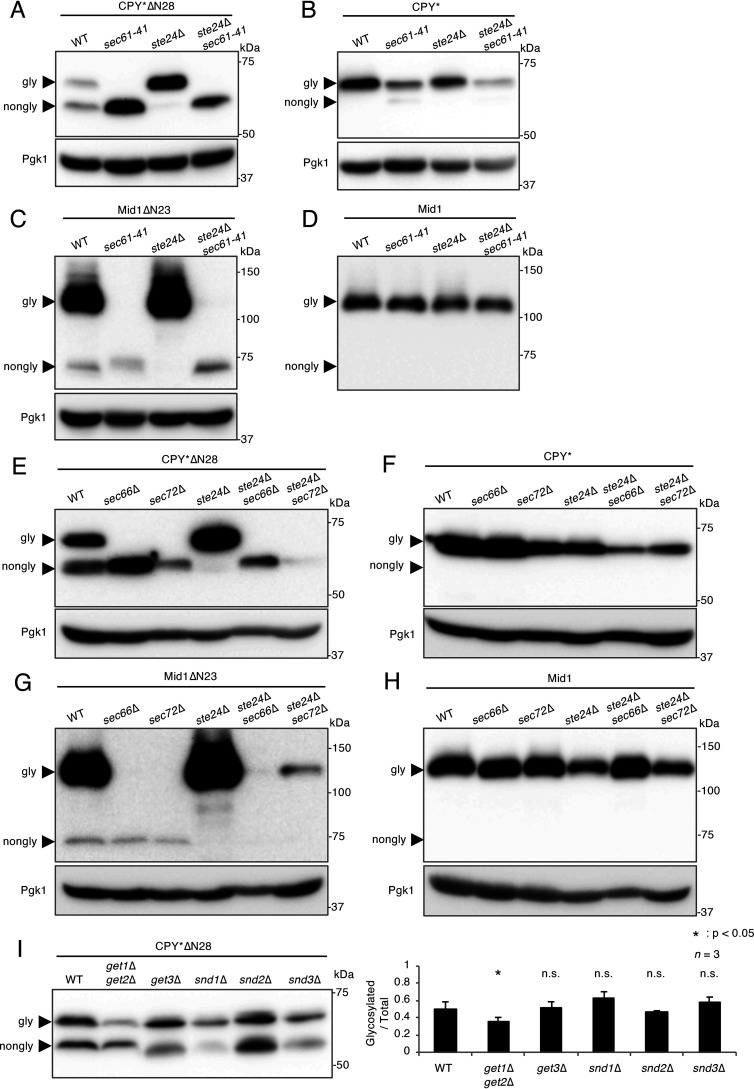
Because Ste24 was found to be involved in the suppression of the signal peptide-independent translocation of various substrates, we investigated the translocation mechanism contributing to this pathway. To confirm whether Sec61, a well-conserved protein translocation channel on the ER membrane, is required for this process, the N-glycosylation status of CPY*ΔN28 and Mid1ΔN23 was examined in the cold-sensitive sec61 mutant sec61-41. All experiments were performed at a permissive temperature of 30 °C. Figure 2A and C shows that the N-glycosylation of CPY*ΔN28 and Mid1ΔN23 was suppressed in sec61-41 cells, suggesting that these two proteins are translocated into the ER through the Sec61 translocon. To confirm that the glycosylation deficiency for these proteins was not because of a general N-glycosylation defect, the N-glycosylation status of signal peptide-bearing CPY* and WT Mid1 were examined in the same sec61-41 mutant at the permissive temperature. Figure 2B and D shows that CPY* and Mid1 were both efficiently N-glycosylated in ste24Δ sec61-41 cells, implying that N-glycosylation activity was normal in cells used in the present study. Therefore, we concluded that the marked decreases observed in glycosylated CPY*ΔN28 and Mid1ΔN23 proteins in sec61-41 cells were not because of a general N-glycosylation defect. Comparisons between Fig. 2A and B and between Fig. 2C and D indicated that the N-glycosylation efficiencies of CPY*ΔN28 and Mid1ΔN23 were markedly lower than those of CPY* and Mid1 in sec61-41 cells, even at the permissive temperature, which is suggestive of the importance of the N-terminal signal peptide in the sec61-41 mutant.
Figure 2.
Sec61, Sec66, and Sec72 are required for the translocation of N-terminal signal peptide-deprived proteins into the ER. A, Western blot analysis of CPY*ΔN28 expressed in the cold-sensitive sec61-41 mutant. CPY*ΔN28 was expressed in the indicated cells at the permissive temperature (30 °C). Extracts from these cells were resolved by SDS-PAGE, and CPY*ΔN28 was visualized by immunoblotting using an anti-V5 antibody. B, Western blot analysis of CPY* expressed in the sec61-41 mutant. CPY* was expressed and analyzed as described above. C, Western blot analysis of Mid1ΔN23 expressed in the sec61-41 mutant. Mid1ΔN23-HA was expressed and analyzed as described above. D, Western blot analysis of WT Mid1 expressed in the sec61-41 mutant. Mid1-HA was expressed and analyzed as described above. E, Western blot analysis of CPY*ΔN28 expressed in the sec66 and sec72 mutants. CPY*ΔN28-V5 was expressed and analyzed as described above. F, Western blot analysis of CPY* expressed in the sec66 and sec72 mutants. CPY*-V5 was expressed and analyzed as described above. G, Western blot analysis of Mid1ΔN23 expressed in the sec66 and sec72 mutants. Mid1ΔN23-HA was expressed and analyzed as described above. H, Western blot analysis of WT Mid1 expressed in the sec66 and sec72 mutants. Mid1-HA was expressed and analyzed as described above. I, Western blot analysis of CPY*ΔN28 in the get and snd mutants. CPY*ΔN28-V5 was expressed in the get and snd mutants. Extracts from the mutants were analyzed as described above. The ratio of glycosylated versus total CPY*ΔN28 proteins was represented as a bar graph. n represents the number of biological replicates.
In addition to Sec61, the Sec62-Sec63 complex (composed of Sec62, Sec63, Sec66, and Sec72) is essential for posttranslational protein translocation into the ER (6). Therefore, we examined the role of the Sec66 and Sec72 subunits (nonessential components of the Sec62-Sec63 complex) in this pathway. To examine whether Sec66 and Sec72 are required for this pathway, the N-glycosylation statuses of CPY*ΔN28 and Mid1ΔN23 were examined in the sec66Δ and sec72Δ backgrounds. As shown in Fig. 2E and G, the relative amounts of N-glycosylated CPYΔN28 and Mid1ΔN23 were markedly lower in ste24Δ sec66Δ and ste24Δ sec72Δ cells than in ste24Δ cells. This result suggests that the Sec66 and Sec72 subunits are essential for the translocation of signal peptide-deprived substrates. An analysis of signal peptide-bearing CPY* and Mid1 in these mutants showed normal N-glycosylation, suggesting that the decreases observed in glycosylated CPY*ΔN28 and Mid1ΔN23 proteins were not because of general underglycosylation (Fig. 2F and H). These results indicate that the Sec66 and Sec72 subunits are required for signal peptide-independent protein translocation into the ER and that the pathway occurs in a posttranslational event.
The GET (guided entry of tail-anchor) pathway is a protein transport mechanism for tail-anchored proteins (28). C-terminally anchored proteins, such as the Sbh1, Sbh2, and SNARE proteins, are targeted to the ER membrane by the GET pathway. In addition to the GET pathway, a novel backup protein targeting system to the ER that may compensate for a defect in the GET pathway was recently reported (29). In that study, Snd (SRP-independent targeting) proteins were identified through genome-wide screening; Snd1 is a cytosolic protein that interacts with ribosomal proteins, whereas Snd2 (Env10) and Snd3 (Pho88) are localized in the ER membrane (30). Thus, Snd components are localized in the cytosol and ER. To elucidate the relationship between the signal peptide-independent and GET/SND pathways, Western blot analyses of CPY*ΔN28 were performed using get and snd mutant cells. The results obtained indicated that the N-glycosylation of CPY*ΔN28 was slightly suppressed in the get1Δ get2Δ mutant only but not in the other mutants shown in Fig. 2I, suggesting that the signal peptide-independent protein translocation pathway is partially dependent on the GET pathway and independent of the SND pathway.
Effects of other ER proteins that genetically interact with Ste24 on the signal peptide-independent translocation of proteins
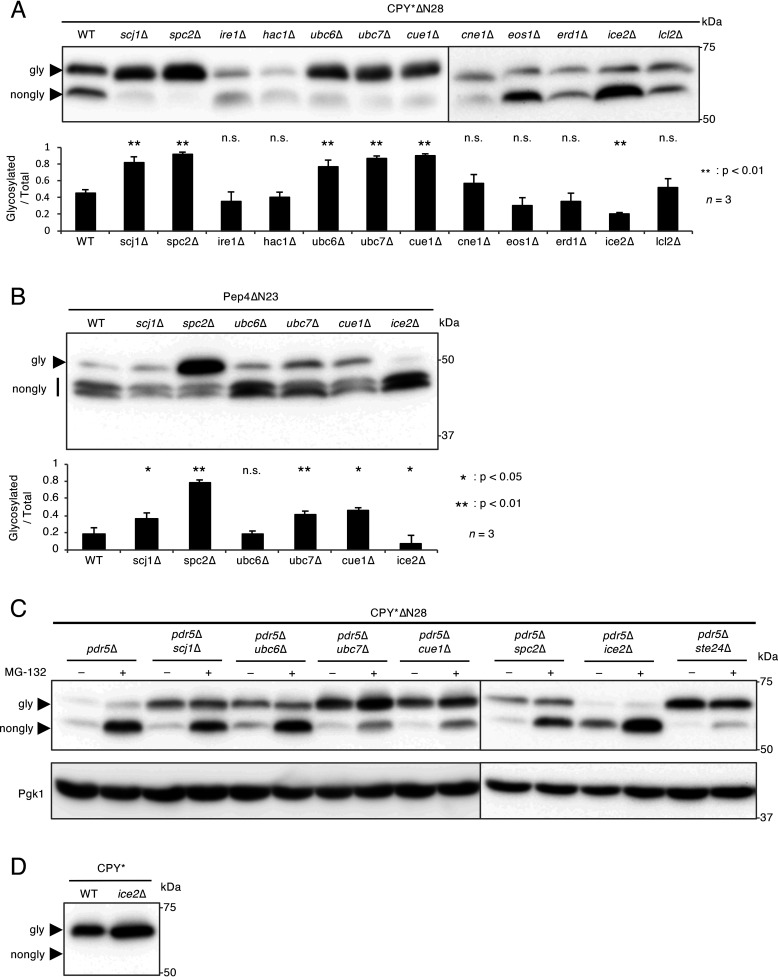
To obtain deeper insights into the molecular mechanisms underlying N-terminal signal peptide-independent protein translocation into the ER, we focused on genes that genetically interact with STE24, as reported in TheCellMap database (profile similarities, 21 genes; negative interactions, 283 genes) (RRID:SCR_018728) (31). In consideration of the upregulation of the UPR (unfolded protein response) in ste24Δ cells (32), we selected 12 genes from a list of TheCellMap: the SCJ1, SPC2, IRE1, HAC1, UBC6, UBC7, CUE1, CNE1, EOS1, ERD1, ICE2, and LCL2 genes. All of these genes, except for UBC6, were shown to elicit the UPR when they were deleted (32), whereas UBC6 was required for the ER-associated degradation of a subset of proteins (33). We examined the N-glycosylation status of CPY*ΔN28 in the mutants lacking each one of these genes and found that N-glycosylated CPY*ΔN28 levels were markedly higher in spc2Δ cells (93%) than in WT cells (45%) (Fig. 3A). The N-glycosylation of Pep4ΔN23 was also significantly increased in spc2Δ cells (Fig. 3B). These results indicate that not only Ste24 but also Spc2 are somehow required for the efficient suppression of signal peptide-independent protein translocation into the ER.
Figure 3.
Spc2 is involved in the suppression of N-terminal signal peptide-independent protein translocation. A, Western blot analysis of CPY*ΔN28 expressed in mutants defective in ER functions. CPY*ΔN28-V5 was expressed and analyzed as described in the legend to Fig. 1. The bar graph shows the relative ratio of glycosylated versus total CPY*ΔN28 proteins. n represents the number of biological replicates. B, Western blot analysis of Pep4ΔN23 expressed in the scj1Δ, spc2Δ, ubc6Δ, ubc7Δ, cue1Δ, and ice2Δ mutants. Pep4ΔN23 tagged N terminally with the DYKDDDDK epitope was expressed and analyzed as described above. The ratio of glycosylated versus total Pep4ΔN23 proteins was represented as a bar graph. n represents the number of biological replicates. C, accumulation of nonglycosylated CPY*ΔN28 expressed in the pdr5Δ scj1Δ, pdr5Δ ubc6Δ, pdr5Δ ubc7Δ, pdr5Δ cue1Δ, pdr5Δ spc2Δ, pdr5Δ ice2Δ, and pdr5Δ ste24Δ mutants treated with MG-132. CPY*ΔN28-V5 was expressed in the indicated cells. Cells were treated with 50 μm MG-132 for 1 h before protein extraction. Samples were subjected to SDS-PAGE followed by immunoblotting using an anti-V5 antibody. The immunoblot was also probed with an anti-Pgk1 antibody as a loading control. D, Western blot analysis of CPY* expressed in the ice2Δ mutant. CPY*-V5 was expressed and analyzed as described above.
In addition to spc2Δ cells, the levels of N-glycosylated CPY*ΔN28 were increased in scj1Δ, ubc6Δ, ubc7Δ, and cue1Δ cells (Fig. 3A). These mutant cells exhibit a defect in ERAD, and CPY* is an ERAD substrate (33–36). ERAD substrates are retrotranslocated into the cytosol and undergo proteasomal degradation (33, 37, 38). Therefore, it remained unclear whether the increased amount of N-glycosylated CPY*ΔN28 in these cells is caused by accelerated signal peptide-independent protein translocation or, alternatively, because of a defect in ERAD (i.e. a defect in the degradation of N-glycosylated proteins). To clarify this issue, the N-glycosylation of CPY*ΔN28 was examined in the presence of the proteasome inhibitor, MG-132. If an ERAD deficiency is the cause of the increasing amount of N-glycosylated CPY*ΔN28, the amount of N-glycosylated CPY*ΔN28 may be elevated in MG132-treated cells. To examine this possibility, we introduced the pdr5Δ mutation into the test strains, and the resulting strains became susceptible to MG-132 because PDR5 encodes a multidrug transporter that facilitates the export of small chemicals (35). Figure 3C shows that the amount of N-glycosylated CPY*ΔN28 was only slightly increased by MG-132 in pdr5Δ cells, whereas nonglycosylated CPY*ΔN28 was highly increased by this reagent in the same cells. This result suggests that the translocation of CPY*ΔN28 into the ER is hardly promoted by suppressing the degradation of CPY*ΔN28. However, under MG-132 treatment, the amount of N-glycosylated CPY*ΔN28 was increased in the pdr5Δ ubc7Δ and pdr5Δ cue1Δ cells but not in the pdr5Δ scj1Δ, pdr5Δ ubc6Δ, pdr5Δ spc2Δ, pdr5Δ ice2Δ, and pdr5Δ ste24Δ cells (Fig. 3C). Therefore, the increasing amounts of N-glycosylated CPY*ΔN28 in ubc7Δ and cue1Δ cells appear to be attributed to both an enhancement of signal peptide-independent protein translocation and a defect in ERAD. Consistent with the above results for CPY*ΔN28, when Pep4ΔN23 was used as a substrate, the amount of the N-glycosylated form was only slightly increased in scj1Δ, ubc7Δ, and cue1Δ cells but not in ubc6Δ cells (Fig. 3B), suggesting that Scj1, Ubc7, and Cue1 somehow affect signal peptide-independent protein translocation into the ER; however, their contribution is markedly smaller than that of Ste24 or Spc2.
It is also notable that, unlike pdr5Δ ste24Δ, nonglycosylated CPY*ΔN28 was significantly accumulated in pdr5Δ spc2Δ upon inhibition of proteasome activity (Fig. 3C). The result implies that the effect of Spc2 is weaker than that of Ste24 for the suppression of signal peptide-independent translocation of proteins, and the effect may have something to do with the differential stabilization of glycosylated and nonglycosylated proteins.
It is important to note that the N-glycosylation of CPY*ΔN28 and Pep4ΔN23 was suppressed in ice2Δ cells defective in the distribution of the cortical ER network (Fig. 3A and B). This strain did not exhibit a defect in the N-glycosylation of N-terminal signal peptide-containing CPY* (Fig. 3D), suggesting that the reduced levels of N-glycosylated CPY*ΔN28 and Pep4ΔN23 observed in ice2Δ cells were not because of general hypoglycosylation. Collectively, these results indicate that Ste24, Spc2, Scj1, Ubc7, and Cue1 more or less suppress, whereas Ice2 positively regulates, signal peptide-independent protein translocation into the ER.
The soluble nuclear protein Rme1 is translocated into the ER in ste24Δ mutant
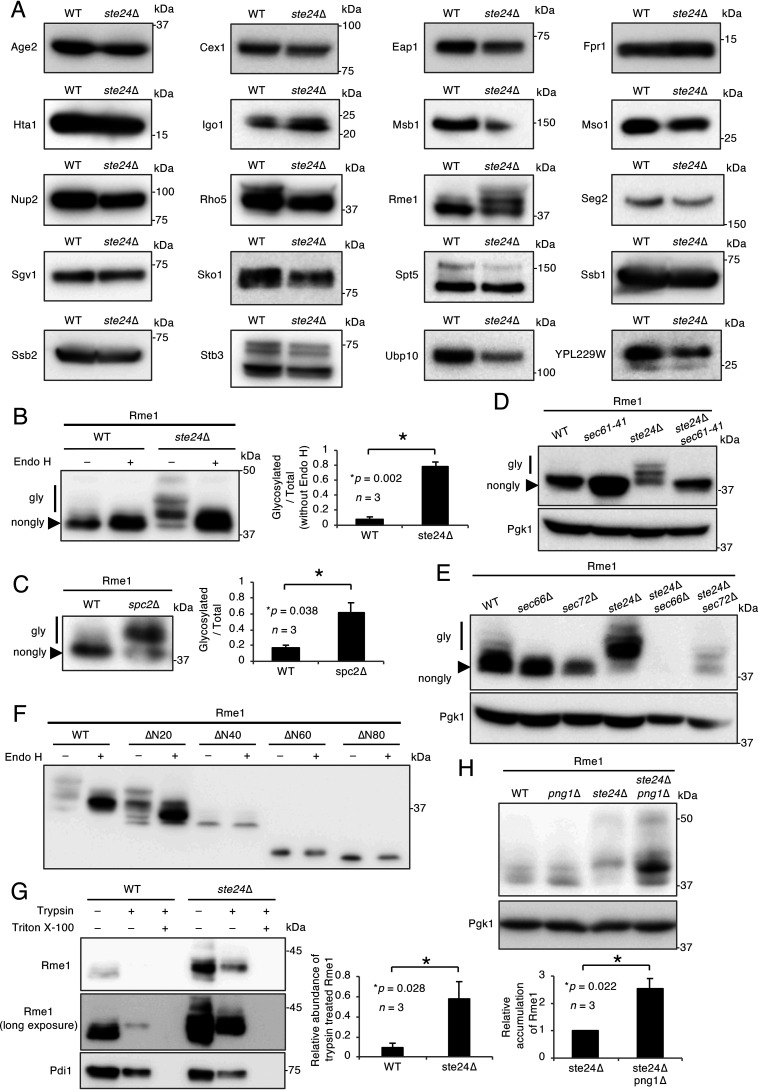
Because CPY*ΔN28, Pep4ΔN23, and Mid1ΔN23 are transported into the ER in an N-terminal signal peptide-independent manner, proteins without an intrinsic N-terminal signal peptide may also be translocated by a signal peptide-independent mechanism. We previously identified cytosolic N-glycosylated proteins as potential endogenous substrates for cytosolic peptide:N-glycanase (PNGase) in S. cerevisiae (39). Among them, according to the SGD (Saccharomyces Genome Database), we identified 34 proteins that contained neither an N-terminal signal peptide nor a transmembrane region (Table S1). In the present study, we successfully cloned 20 of the 34 candidates and compared the sizes of these proteins in WT and ste24Δ cells by Western blotting (Fig. 4A). Among the 20 proteins tested, an electrophoretic band shift was observed only with Rme1. The Rme1 sample was treated with Endo H, and its N-glycosylation status was assessed. Figure 4B shows that 9 and 78% of the Rme1 molecules were N-glycosylated in WT and ste24Δ cells, respectively. In addition, 62% of Rme1 was also N-glycosylated in spc2Δ cells (Fig. 4C). These results suggest that some Rme1 proteins are translocated into the ER in the ste24Δ and spc2Δ mutants by a mechanism similar to that for N-terminal signal peptide-deprived proteins.
Figure 4.
The transcription factor Rme1 is N-glycosylated in a signal peptide-independent manner. A, Western blot analysis for the screening of signal peptide- and transmembrane domain-less proteins that undergo N-glycosylation in the ste24Δ mutant. V5-tagged candidate proteins were expressed and analyzed as described above. B, Endo H treatment of Rme1. Rme1-V5 was expressed in WT and ste24Δ cells. Cell extracts were mock treated (−) or digested (+) with Endo H and resolved by SDS-PAGE. Rme1 was detected as described above. The bar graph shows the relative ratio of glycosylated versus total Rme1 proteins. n represents the number of biological replicates. C, Western blot analysis of Rme1 expressed in the spc2Δ mutant. Rme1-V5 was expressed in the indicated cells. Extracts from these cells were analyzed as described above. The bar graph shows the relative ratio of glycosylated versus total Rme1 proteins. n represents the number of biological replicates. D, Western blot analysis of Rme1 expressed in the sec61-41 mutant. Rme1-V5 was expressed in the indicated cells at the permissive temperature (30 °C). Extracts from these cells were analyzed as described above. E, Western blot analysis of Rme1 expressed in the sec66 and sec72 mutants. Rme1-V5 was expressed in the sec66 and sec72 mutants. Extracts from these cells were analyzed as described above. F, Western blot analysis of the N-terminally truncated mutant of Rme1. V5-tagged Rme1, Rme1ΔN20, Rme1ΔN40, Rme1ΔN60, and Rme1ΔN80 were expressed in ste24Δ cells. Extracts from these cells were analyzed as described above. G, Protease protection assay of Rme1. Rme1-V5 and Pdi1-HA were coexpressed in WT and ste24Δ cells. The protease protection assay was performed using these strains. Samples were resolved by SDS-PAGE, and Rme1-V5 or Pdi1-HA was visualized by immunoblotting using an anti-V5 or an anti-HA antibody, respectively. The bar graph shows the relative abundance of trypsin-treated Rme1 normalized by Pdi1-HA. H, Western blot analysis of Rme1 expressed in the ste24Δ png1Δ mutant. Rme1-V5 was expressed in WT, png1Δ, ste24Δ, and ste24Δ png1Δ cells. Extracts from these cells were analyzed as described above. The bar graph shows the amount of Rme1 relative to that of Pgk1 expressed in ste24Δ and ste24Δ png1Δ cells. n represents the number of biological replicates.
To investigate Sec protein dependence in the translocation of Rme1, Western blotting of Rme1 was performed with the sec mutants used above, such as sec61-41, sec66Δ, and sec72Δ. Figure 4D and E shows that the N-glycosylation of Rme1 in the absence of the STE24 gene was restricted in all three sec mutants tested, suggesting that Rme1 is translocated into the ER through the function of the Sec61 complex and the Sec66 and Sec72 subunits. To examine whether the N-terminal region of Rme1 is required for signal peptide-independent translocation, the N-glycosylation of the N-terminally truncated forms of Rme1 was investigated in ste24Δ cells. The results obtained showed that intact Rme1 and Rme1Δ20, but not Rme1Δ40, Rme1Δ60, or Rme1Δ80, were N-glycosylated, indicating that the N-terminal region (at least up to 20 amino acid residues) was not necessary for signal peptide-independent translocation into the ER (Fig. 4F). To confirm the ER luminal localization of Rme1 in WT and ste24Δ cells, protease protection assays were carried out. In both strains, the ER luminal protein Pdi1 was detected in trypsin-treated samples but not in trypsin- and Triton X-100-treated samples (Fig. 4G, lower). In WT cells, weak but significant levels of N-glycosylated Rme1 were detected in the trypsin-treated sample (Fig. 4G, middle). The N-glycosylation of the trypsin-treated Rme1 was confirmed by an Endo H treatment (Fig. S2A). These results indicate that at least a part of N-glycosylated Rme1 is localized in the ER lumen in WT cells. In ste24Δ cells, the luminal localization of N-glycosylated Rme1 was significantly increased (Fig. 4G, upper and middle), clearly indicating that the translocation of Rme1 into the ER is significantly enhanced in ste24Δ cells. Similarly, N-glycosylated CPY*-V5(ΔN80) or Pep4ΔN23 was found to be exclusively localized in the luminal side (Fig. S2). Collectively, these results suggest that there is an intrinsic system that suppresses signal peptide-independent protein translocation into the ER in budding yeast.
A previous study reported that the mammalian ER-associated transcription factor Nfe2L1 (Nrf1) was retrotranslocated to the cytosol and deglycosylated by the peptide:N-glycanase Ngly1, a homolog of yeast Png1 (40). The deglycosylation of Nfe2L1 is required for its full activation as a transcription factor (41). Because Rme1 is also a transcription factor (42), we examined the N-glycosylation status of Rme1 in png1Δ cells. Figure 4H shows that an N-glycosylated form of Rme1 accumulated in ste24Δ png1Δ cells, suggesting that Png1 is involved in the deglycosylation and/or degradation of the N-glycosylated form of Rme1. Therefore, the transcription factor Rme1 is translocated into the ER to undergo N-glycosylation when Ste24 or Spc2 is nonfunctional, and the N-glycosylated form of this transcription factor may eventually undergo deglycosylation by Png1 in the cytosol.
Discussion
A novel role for Ste24: suppressing the intrusion of signal peptideless proteins into the ER
In the present study, we identified several factors that are involved in the suppression of signal peptide-independent protein translocation into the ER in S. cerevisiae, one of which is Ste24, a highly conserved ER membrane metalloendopeptidase with its active site located on the cytosolic side. We confirmed that the N-terminal signal peptide-removed derivatives of vacuolar CPY and Pep4, CPY*ΔN28 and Pep4ΔN23, were translocated into the ER and then underwent N-glycosylation. Although a similar phenomenon has already been reported (14, 15), the present results are novel, because we discovered that the translocation of CPY*ΔN28 and Pep4ΔN23 was enhanced in the ste24Δ mutant. We also found that the cytosolic protein Png1, which does not intrinsically carry a signal peptide, was not translocated into the ER in the same mutant. These results suggest that the Ste24 protein is involved in suppressing the N-terminal signal peptide-independent translocation of specific proteins into the ER.
Ste24 was previously shown to be involved in the processing of the peptide pheromone a-factor in the ER membrane (27), and the deletion of the STE24 gene resulted in the UPR (32). Moreover, Ste24 was reported to take part in the clearance of clogging proteins on the Sec61 complex using its own protease activity (16). Interestingly, the activity of Ste24 to suppress signal peptide-independent protein translocation also depends on its own protease activity. We found that the Ste24(H297A) protein with no protease activity did not exhibit suppressive activity. Therefore, we propose that Ste24, by its protease activity, is involved in the elimination of N-terminal signal peptideless proteins that are about to intrude into the ER at the translocon complex. However, because only nonglycosylated CPY*ΔN28 was accumulated in the WT cells by MG-132 treatment, it should not be a general ERAD defect that causes the accumulation of signal peptideless proteins in ste24Δ cells. Rather, it should be a specific system dedicated to the suppression of signal peptide-independent translocation, just like the clearance of clogged proteins on the Sec61 complex.
Spc2 also affects the suppression of the signal peptide-independent protein translocation into the ER, whereas its precise role remains unclear
We also found that Spc2, a noncatalytic subunit of the signal peptidase complex (43), suppressed N-terminal signal peptide-independent protein translocation. We demonstrated that the N-glycosylation of the CPY*ΔN28 and Pep4ΔN23 proteins was facilitated in the spc2Δ mutant. This suppression may not require signal peptidase activity, because Spc1, another noncatalytic subunit structurally related to Spc2, did not show this suppression effect (Fig. S3). In addition, in genome-wide screening for proteins that are required for protein quality control, Spc2, but not Spc1, was identified together with Ste24 (16). Coimmunoprecipitation experiments with anti-Spc2 antibodies revealed that Spc2 associates with Sbh1 and Sbh2, the β subunits of the Sec61 complex and Ssh1 (a Sec61 homolog) complex, respectively (44). Thus, Spc2 may participate in the suppression process as a subcomponent of the translocon complex. Spc2 may be required for the efficient action of Ste24 in suppressing the entry of N-terminal signal peptideless proteins into the translocon complex. However, as shown in Fig. 3C, nonglycosylated CPY*ΔN28 was significantly accumulated by MG-132 treatment in pdr5Δ spc2Δ cells compared with the results of pdr5Δ ste24Δ cells. The result implies that the effect of Spc2 does not suppress the signal peptide-independent translocation of proteins but has something to do with the differential stabilization of glycosylated and nonglycosylated proteins. Obviously further careful experiments will be required to clarify the precise role of Spc2 in this signal peptide-independent translocation of proteins into the ER lumen.
Scj1, Ubc7, and Cue1 play a translocation-suppressive role, whereas Ice2 has the opposite role
The present results demonstrated that Scj1, Ubc7, and Cue1 are, to some extent, required to suppress the signal peptide-independent translocation of CPY*ΔN28 and Pep4ΔN23, indicating that these proteins are also involved in suppressing signal peptide-independent translocation into the ER. Because Ubc7 is a ubiquitin-conjugating enzyme (45) and Cue1 is a ubiquitin-binding protein that recruits Ubc7 to the ER surface (36), these proteins may take part in the specific degradation process of signal peptideless proteins that differs from the general ERAD. Unlike Ubc7 and Cue1, Scj1 is localized in the ER lumen, so it is unclear how it suppresses the translocation of signal peptideless protein.
In contrast, Ice2 was found to be required for N-terminal signal peptide-independent protein translocation into the ER and, thus, appeared to be a supporting factor for this translocation process. Ice2 is an integral membrane protein that is localized in the ER, and no human orthologue has been identified to date. This protein is required for the distribution of the cortical ER network and is involved in the UPR under low-zinc conditions (46, 47). Ice2 is also required to target the nuclear protein Trm1 to the inner nuclear membrane (48). Thus, Ice2 may act as a targeting factor for signal peptideless proteins at the ER surface.
Signal peptide-independent translocation into the ER is posttranslational
We found that Sec66 and Sec72 (subunits of the Sec62-Sec63 complex), as well as the Sec61 complex, are required for the signal peptide-independent protein translocation of CPY*ΔN28 and Rme1 into the ER, suggesting that the substrates of this pathway are posttranslationally translocated into the ER through the Sec61 channel. This mechanism is different from that of nonclassical protein secretion (or leaderless secretion). Nonclassical protein secretion has long been studied and demonstrated to be independent of an N-terminal signal peptide and the ER-Golgi transport pathway (49–51). Thus, the present study has provided insights into regulation of a protein transport mechanism that can be designated the N-terminal signal peptide-independent translocation mechanism.
We also found that the N-glycosylation of CPY*ΔN28 through the N-terminal signal peptide-independent pathway was slightly suppressed in the get1Δ get2Δ mutant but not in the snd1Δ, snd2Δ, or snd3Δ mutant, suggesting that the signal peptide-independent protein translocation pathway is slightly dependent on the GET pathway and independent of the SND pathway. The ER localization of the secretory pathway tail-anchored proteins Sbh1 and Sbh2 (β subunits of the Sec61 complex) was shown to be impaired in the get1Δ get2Δ mutant (28). Therefore, it is plausible that signal peptide-independent protein translocation in the present study was positively regulated by the GET pathway through Sbh1 and Sbh2; however, its contribution was limited.
The transcription factor Rme1 with no intrinsic signal peptide is translocated into the ER in ste24Δ cells
Our screening for signal peptide-less soluble proteins that may be translocated into the ER in the ste24Δ mutant identified the transcription factor Rme1 from the 20 candidates we selected as soluble proteins. Furthermore, Spc2 was also suggested to be involved in this suppression. Our result showing that N-glycosylated Rme1 was increased in the png1Δ mutant defective in peptide:N-glycanase suggests the physiological importance of the N-glycosylation/deglycosylation status of Rme1. As a transcription factor, Rme1 is involved in physiologically significant events: it prevents meiosis, promotes mitosis, mediates the cell type control of sporulation, and relocalizes from the nucleus to the cytosol upon DNA replication stress (42, 52, 53). An interesting example can be found in mammalian cells: the ER-associated transcription factor Nfe2L1 (nuclear factor erythroid 2-related factor 1) is retrotranslocated to the cytosol, in which it is deglycosylated by Ngly1 (40). Deglycosylation is pivotal for the full activation of the Nfe2L1 transcription factor (41, 54). Although the N-glycosylation of signal peptideless Rme1 is unequivocally demonstrated, the physiological significance and mechanistic insights of this result warrant further investigation.
Truncation assay suggests that the 21–40 amino acid residues of Rme1 are important for its translocation into the ER. It was previously reported that ∼20% of random sequences (relatively hydrophobic) can replace the normal signal sequence of Suc2 (55). The internal signal sequence of ovalbumin was also reported (56, 57). Our results imply that internal sequences of Rme1 can function as an internal signal peptide.
It is also important to note that the translocation of proteins with no signal peptide into the ER to allow N-glycosylation is not yeast specific. Secretome studies identified human-secreted N-glycoproteins that do not contain a signal peptide or transmembrane region (58–61). Some of these glycoprotein members may be translocated into the ER by a signal peptide-independent pathway. It is not clear whether signal peptide-independent protein translocation is “active translocation” or “wrong insertion.” Irrespective of the mechanism, however, the translocation of proteins with no signal peptide into the ER appears to be universal in eukaryotic cells.
The Ste24 homolog is found in humans as ZMPSTE24, which is required for lamin A maturation (27); thus, ZMPSTE24 is a causative gene of laminopathies (62, 63). If ZMPSTE24 has a function similar to that of the yeast Ste24, namely, the elimination of proteins translocable into the ER in a signal peptide-independent fashion, this process may be compromised in patients with laminopathies. Therefore, further studies are needed to clarify whether there is indeed a similar protein translocation system in humans and if ZMPSTE24 is involved in this system.
Experimental procedures
Yeast strains and plasmid construction
The yeast strains and plasmids used in the present study are listed in Tables S2 and S3. If there is no notation, the genetic background is BY4741. Detailed information on primer sequences is available upon request. The DNA sequences of the constructs were confirmed using BigDye ver. 3.1 and an ABI DNA sequencer (3730xl).
Preparation of yeast cell extracts and Western blotting
The preparation of yeast cell extracts and Western blotting were performed as previously described (64). Unless otherwise specified, equal amounts (1 optical density at 600 nm unit) of total protein extracts were loaded in each lane. Antibodies were used at the following dilutions: 1:20,000 for anti-V5 (46-0705, Thermo Fisher Scientific, MA), 1:10,000 for anti-DYKDDDDK (018-22381, Wako Pure Chemical Industries, Japan), 1:20,000 for anti-HA (H9658, Sigma, MO), 1:5000 for anti-myc (017-21871, Wako Pure Chemical Industries, Japan), 1:10,000 for anti-CPY(10A5B5, Thermo Fisher Scientific, MA), and 1:10,000 for anti-Pgk1 (22C5, Thermo Fisher Scientific, MA).
Measurement of Ca2+ accumulation
Measurements of Ca2+ accumulation were performed as previously described (65). The mating pheromone α-factor was ordered from the Center for Analytical Instruments, National Institute for Basic Biology, Okazaki, Japan.
MG-132 treatment
Cells were grown and MG-132 (3175-V; Peptide Institute, Japan) was added to the cultures (final concentration, 50 μm) to inhibit proteasomes. Cultures were collected at the indicated times, and the extracted proteins were subjected to a Western blot analysis.
Protease protection assay
Yeast cells (40 optical density at 600 nm units) were cultured and collected by centrifugation at 1,000 × g for 3 min. The collected cells were washed with 1 m sorbitol and resuspended in 10 ml of a spheroplasting solution (50 mm phosphate buffer [pH 6.8], 1 m sorbitol, 10 mm EDTA, 0.1% β-mercaptoethanol, 0.1 mg/ml Zymolyase 20T [07663-91, Nacalai Tesque, Japan]). Samples were incubated at 30 °C with rotation at 100 rpm for 1 h. After spheroplasting, the cells were collected by centrifugation at 1,000 × g for 3 min, washed with 1 M sorbitol, and resuspended in 400 µl of osmotic lysis buffer (20 mm Tris-HCl [pH 8.0]). Samples were incubated at −80 °C for 30 min and thawed at room temperature. The samples were then divided into aliquots of 100 µl and were treated with 1 ml of 20 mm Tris-HCl buffer (control reaction) or a trypsin (20233, Thermo Fisher Scientific, MA) solution in the same buffer (final concentration, 20 µg/ml) with or without Triton X-100 (final concentration 1%). Samples were incubated at 37 °C for 15 min. The proteolysis reaction was stopped by adding 100% (w/v) TCA (final concentration, 10%, w/v). Samples were incubated on ice for 30 min and centrifuged at 15,000 × g at 4 °C for 10 min. Supernatants were discarded, and pellet fractions were washed twice with acetone and once with 70% ethanol. The resulting pellets were resuspended in SDS-PAGE sample buffer (60 mm Tris-HCl [pH 6.8], 5% glycerol, 2% SDS, 4% β-mercaptoethanol, 0.0025% bromphenol blue, 8 M urea), incubated at 95 °C for 5 min, and subjected to Western blot analysis.
Statistical analysis
The significance of differences was evaluated using the Student's t test, with maximum p values of <0.05 being required for significance. All n are biological replicates.
Data availability
Data not shown in this article are available upon request from the corresponding authors.
Supplementary Material
Acknowledgments
We thank the former members of the Glycometabolome Team (RIKEN) for their fruitful discussions. Special thanks go to Ms. Yae Tsuchiya and Ms. Tsugiyo Matsuda (RIKEN) for their technical support. T. S. thank Hyun Kim (School of Biological Sciences, College of Natural Sciences, Seoul National University, South Korea) for valuable suggestions on this study.
This article contains supporting information.
Author contributions—A. H. and T. S. conceptualization; A. H., H. I., and M. K. resources; A. H. data curation; A. H. and T. S. supervision; A. H., H. I., and T. S. funding acquisition; A. H., K. I., T. C., and H. I. validation; A. H., K. I., T. C., and M. K. investigation; A. H., K. I., T. C., and M. K. visualization; A. H., K. I., H. I., and T. S. writing-original draft; A. H., H. I., and T. S. project administration; A. H., K. I., H. I., and T. S. writing-review and editing; K. I. methodology.
Funding and additional information—This work was supported, in part, by Grants-in-Aid for Scientific Research (C) (25440058) (to A. H.), (19K06539) (to H. I.), and (B) (25291030) (to T. S.), by a Grant-in-Aid for Young Scientists (17K15089) (to A. H.) from the Ministry of Education, Science, Sports and Culture of Japan, and by a grant for Incentive Research Projects (RIKEN) (to A. H.).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- SRP
- signal recognition particle
- SR
- SRP receptor
- CPY
- carboxypeptidase Y
- GET
- guided entry of tail-anchor
- Snd
- SRP-independent targeting
- UPR
- unfolded protein response.
References
- 1. Blobel G., and Dobberstein B. (1975) Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851 10.1083/jcb.67.3.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunze M., and Berger J. (2015) The similarity between N-terminal targeting signals for protein import into different organelles and its evolutionary relevance. Front. Physiol. 6, 259 10.3389/fphys.2015.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heijine V. G. (1990) The signal peptide. J. Membr. Biol. 115, 195–201 [DOI] [PubMed] [Google Scholar]
- 4. Nilsson I., Lara P., Hessa T., Johnson A. E., Heijne G. V., and Karamyshev A. L. (2015) The code for directing proteins for translocation across ER membrane: SRP cotranslationally recognizes specific features of a signal sequence. J. Mol. Biol. 427, 1191–1201 10.1016/j.jmb.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmermann R., Eyrisch S., Ahmad M., and Helms V. (2011) Protein translocation across the ER membrane. Biochim. Biophys. Acta 1808, 912–924 10.1016/j.bbamem.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 6. Ng D. T. W., Brown J. D., and Walter P. (1996) Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 134, 269–278 10.1083/jcb.134.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson N., Powis K., and High S. (2013) Post-translational translocation into the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2403–2409 10.1016/j.bbamcr.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 8. Deshaies R. J., and Schekman R. (1987) A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J. Cell Biol. 105, 633–645 10.1083/jcb.105.2.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novick P., Field C., and Schekman R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- 10. Novick P., and Schekman R. (1979) Secretion and cell-surface growth are blocked in a temperature- sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U S A 76, 1858–1862 10.1073/pnas.76.4.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., and Schekman R. (1989) Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J. Cell Biol. 109, 2641–2652 10.1083/jcb.109.6.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dudek J., Pfeffer S., Lee P., Jung M., Cavalié A., Helms V., Förster F., and Zimmermann R. (2015) Protein transport into the human endoplasmic reticulum. J. Mol. Biol. 427, 1159–1175 10.1016/j.jmb.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 13. Voorhees R. M., and Hegde R. S. (2016) Structure of the Sec61 channel opened by a signal sequence. Science 351, 88–91 10.1126/science.aad4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blachly-Dyson E., and Stevens T. H. (1987) Yeast carboxypeptidase Y can be translocated and glycosylated without its amino-terminal signal sequence. J. Cell Biol. 104, 1183–1191 10.1083/jcb.104.5.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iida K., Teng J., Cho T., Yoshikawa-Kimura S., and Iida H. (2017) Posttranslational processing and membrane translocation of the yeast regulatory Mid1 subunit of the Cch1/VGCC/NALCN cation channel family. J. Biol. Chem. 292, 20570–20582 10.1074/jbc.M117.810283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ast T., Michaelis S., and Schuldiner M. (2016) Article the protease Ste24 clears clogged translocons. Cell 164, 103–114 10.1016/j.cell.2015.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S., Xu C., Larrimore K. E., and Ng D. T. W. (2017) Slp1-Emp65: a guardian factor that protects folding polypeptides from promiscuous degradation. Cell 171, 346–350 10.1016/j.cell.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 18. Pin F. J., Donnell A. F. O., Pagant S., Piao H. L., Miller J. P., Fields S., Miller E. A., and Cyert M. S. (2011) Hph1 and Hph2 are novel components of the Sec63/Sec62 posttranslational translocation complex that aid in vacuolar proton ATPase biogenesis. Eukaryot. Cell 10, 63–71 10.1128/EC.00241-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carvalho P., Goder V., and Rapoport T. A. (2006) Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126, 361–373 10.1016/j.cell.2006.05.043 [DOI] [PubMed] [Google Scholar]
- 20. Mehnert M., Sommer T., and Jarosch E. (2014) Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane. Nat. Cell Biol. 16, 77–86 10.1038/ncb2882 [DOI] [PubMed] [Google Scholar]
- 21. Mehnert M., Sommermeyer F., Berger M., Kumar Lakshmipathy S., Gauss R., Aebi M., Jarosch E., and Sommer T. (2015) The interplay of Hrd3 and the molecular chaperone system ensures efficient degradation of malfolded secretory proteins. Mol. Biol. Cell 26, 185–194 10.1091/mbc.E14-07-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carvalho P., Stanley A. M., and Rapoport T. A. (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143, 579–591 10.1016/j.cell.2010.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finger A., Knop M., and Wolf D. H. (1993) Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur. J. Biochem. 218, 565–574 10.1111/j.1432-1033.1993.tb18410.x [DOI] [PubMed] [Google Scholar]
- 24. Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., and Stevens T. H. (1986) PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol. Cell. Biol. 6, 2490–2499 10.1128/mcb.6.7.2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams R. S., Trumbly R. J., MacColl R., Trimble R. B., and Maley F. (1985) Comparative properties of amplified external and internal invertase from the yeast SUC2 gene. J. Biol. Chem. 260, 13334–13341 [PubMed] [Google Scholar]
- 26. Suzuki T., Park H., Hollingsworth N. M., Sternglanz R., and Lennarz W. J. (2000) PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J. Cell Biol. 149, 1039–1052 10.1083/jcb.149.5.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michaelis S., and Barrowman J. (2012) Biogenesis of the Saccharomyces cerevisiae Pheromone a-Factor, from Yeast Mating to Human Disease. Microbiol. Mol. Biol. Rev. 76, 626–651 10.1128/MMBR.00010-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuldiner M., Metz J., Schmid V., Denic V., Rakwalska M., Schmitt H. D., Schwappach B., and Weissman J. S. (2008) The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell 134, 634–645 10.1016/j.cell.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aviram N., Ast T., Costa E. A., Arakel E. C., Chuartzman S. G., Jan C. H., Haßdenteufel S., Dudek J., Jung M., Schorr S., Zimmermann R., Schwappach B., Weissman J. S., and Schuldiner M. (2016) The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature 540, 134–138 10.1038/nature20169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleischer T. C., Weaver C. M., Mcafee K. J., Jennings J. L., and Link A. J. (2006) Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 20, 1294–1307 10.1101/gad.1422006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Usaj M., Tan Y., Wang W., VanderSluis B., Zou A., Myers C. L., Costanzo M., Andrews B., and Boone C. (2017) TheCellMap.org: a web-accessible database for visualizing and mining the global yeast genetic interaction network. G3 7, 1539–1549 10.1534/g3.117.040220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jonikas M. C., Collins S. R., Denic V., Oh E., Quan E. M., Schmid V., Weibezahn J., Schwappach B., Walter P., Weissman J. S., and Schuldiner M. (2009) Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323, 1693–1697 10.1126/science.1167983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiller M. M., Finger A., Schweiger M., and Wolf D. H. (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273, 1725–1728 10.1126/science.273.5282.1725 [DOI] [PubMed] [Google Scholar]
- 34. Nishikawa S., Fewell S. W., Kato Y., Brodsky J. L., and Endo T. (2001) Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J. Cell Biol. 153, 1061–1069 10.1083/jcb.153.5.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosomi A., and Suzuki T. (2015) Cytoplasmic peptide:N-glycanase cleaves N-glycans on a carboxypeptidase Y mutant during ERAD in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1850, 612–619 10.1016/j.bbagen.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 36. Biederer T., Biederer T., Volkwein C., and Sommer T. (1997) Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278, 1806–1809 10.1126/science.278.5344.1806 [DOI] [PubMed] [Google Scholar]
- 37. Jensen T. J., Loo M. A., Pind S., Williams D., Goldberg A. L., and Riordan J. R. (1995) Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135 10.1016/0092-8674(95)90241-4 [DOI] [PubMed] [Google Scholar]
- 38. Ward C. L., Omura S., and Kopito R. R. (1995) Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 10.1016/0092-8674(95)90240-6 [DOI] [PubMed] [Google Scholar]
- 39. Hosomi A., Fujita M., Tomioka A., Kaji H., and Suzuki T. (2016) Identification of PNGase-dependent ERAD substrates in Saccharomyces cerevisiae. Biochem. J. 473, 3001–3012 10.1042/BCJ20160453 [DOI] [PubMed] [Google Scholar]
- 40. Yang K., Huang R., Fujihira H., Suzuki T., and Yan N. (2018) N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J. Exp. Med. 215, 2600–2616 10.1084/jem.20180783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehrbach N. J., Breen P. C., and Ruvkun G. (2019) Protein sequence editing of SKN-1A/Nrf1 by peptide:N-glycanase controls proteasome gene expression. Cell 177, 737–750 10.1016/j.cell.2019.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Covitz P. A., Herskowitz I., and Mitchell A. P. (1991) The yeast RME1 gene encodes a putative zinc finger protein that is directly repressed by a l-alpha 2. Genes Dev. 5, 1982–1989 10.1101/gad.5.11.1982 [DOI] [PubMed] [Google Scholar]
- 43. Mullins C., Meyer H., Hartmann E., Green N., and Fang H. (1996) Structurally related Spc1p and Spc2p of yeast signal peptidase complex are functionally distinct. J. Biol. Chem. 271, 29094–29099 10.1074/jbc.271.46.29094 [DOI] [PubMed] [Google Scholar]
- 44. Antonin W., Meyer H., and Hartmann E. (2000) Interactions between Spc2p and other components of the endoplasmic reticulum translocation sites of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275, 34068–34072 10.1074/jbc.M006126200 [DOI] [PubMed] [Google Scholar]
- 45. Jungmann J., Reins H.-A., Schobert C., and Jentsch S. (1993) Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature 361, 369–371 10.1038/361369a0 [DOI] [PubMed] [Google Scholar]
- 46. Martin P. E. D., Du Y., Novick P., and Ferro-Novick S. (2005) Ice2p is important for the distribution and structure of the cortical ER network in Saccharomyces cerevisiae. J. Cell Sci. 118, 65–77 10.1242/jcs.01583 [DOI] [PubMed] [Google Scholar]
- 47. North M., Steffen J., Loguinov A. V., Zimmerman G. R., Vulpe C. D., and Eide D. J. (2012) Genome-wide functional profiling identifies genes and processes important for zinc-limited growth of Saccharomyces cerevisiae. PLoS Genet. 8, e1002699 10.1371/journal.pgen.1002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murthi A., and Hopper A. K. (2005) Genome-wide screen for inner nuclear membrane protein targeting in Saccharomyces cerevisiae: roles for N-acetylation and an integral membrane protein. Genetics 170, 1553–1560 10.1534/genetics.105.043620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nombela C., Gil C., and Chaffin W. L. J. (2006) Non-conventional protein secretion in yeast. Trends Microbiol. 14, 15–21 10.1016/j.tim.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 50. Ding Y., Wang J., Wang J., Stierhof Y. D., Robinson D. G., and Jiang L. (2012) Unconventional protein secretion. Trends Plant Sci. 17, 606–615 10.1016/j.tplants.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 51. Ponpuak M., Mandell M. A., Kimura T., Chauhan S., Cleyrat C., and Deretic V. (2015) Secretory autophagy. Curr. Opin. Cell Biol. 35, 106–116 10.1016/j.ceb.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rine J., Sprague G. F., Herskowitz I. R. A., Mata M., Mata M., and Mata M. (1981) rme1 mutation of Saccharomyces cerevisiae: map position and bypass of mating type locus control of sporulation. Mol. Cell. Biol. 1, 958–960 10.1128/mcb.1.10.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tkach J. M., Yimit A., Lee A. Y., Riffle M., Costanzo M., Jaschob D., Hendry J. A., Ou J., Moffat J., Boone C., Davis T. N., Nislow C., and Brown G. W. (2012) Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol. 14, 966–976 10.1038/ncb2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tomlin F. M., Gerling-Driessen U. I. M., Liu Y., Flynn R. A., Vangala J. R., Lentz C. S., Clauder-Muenster S., Jakob P., Mueller W. F., Ordon D., Paulsen M., Matsui N., Foley D., Rafalko A., Suzuki T., et al. (2017) Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent. Sci. 3, 1143–1155 10.1021/acscentsci.7b00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaiser C. A., Preuss D., Grisafi P., and Botstein D. (1987) Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science 235, 312–317 10.1126/science.3541205 [DOI] [PubMed] [Google Scholar]
- 56. Lingappa V. R., Lingappa J. R., and Blobel G. (1979) Chicken ovalbumin contains an internal signal sequence. Nature 281, 117–121 10.1038/281117a0 [DOI] [PubMed] [Google Scholar]
- 57. Robinson A., Meredith C., and Austen B. M. (1986) Isolation and properties of the signal region from ovalbumin. FEBS Lett. 203, 243–246 10.1016/0014-5793(86)80751-7 [DOI] [PubMed] [Google Scholar]
- 58. Lin Q., Lim H. S. R., Lin H. L., Tan H. T., Lim T. K., Cheong W. K., Cheah P. Y., Tang C. L., Chow P. K. H., and Chung M. C. M. (2015) Analysis of colorectal cancer glyco-secretome identifies laminin β-1 (LAMB1) as a potential serological biomarker for colorectal cancer. Proteomics 15, 3905–3920 10.1002/pmic.201500236 [DOI] [PubMed] [Google Scholar]
- 59. Li X., Jiang J., Zhao X., Zhao Y., Cao Q., Zhao Q., Han H., Wang J., Yu Z., Peng B., Ying W., and Qian X. (2016) In-depth analysis of secretome and N-glycosecretome of human hepatocellular carcinoma metastatic cell lines shed light on metastasis correlated proteins. Oncotarget 7, 22031–22049 10.18632/oncotarget.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sugahara D., Tomioka A., Sato T., Narimatsu H., and Kaji H. (2015) Large-scale identification of secretome glycoproteins recognized by wisteria floribunda agglutinin: a glycoproteomic approach to biomarker discovery. Proteomics 15, 2921–2933 10.1002/pmic.201400443 [DOI] [PubMed] [Google Scholar]
- 61. Boersema P. J., Geiger T., Wis J. R., and Mann M. (2013) Quantification of the N-glycosylated secretome by super-SILAC during breast cancer progression and in human blood samples. Mol. Cell. Proteomics 12, 158–171 10.1074/mcp.M112.023614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Agarwal A. K., Fryns J., Auchus R. J., and Garg A. (2003) Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, 1995–2001 10.1093/hmg/ddg213 [DOI] [PubMed] [Google Scholar]
- 63. Barrowman J., Wiley P. A., Hudon-Miller S. E., Hrycyna C. A., and Michaelis S. (2012) Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum. Mol. Genet. 21, 4084–4093 10.1093/hmg/dds233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hosomi A., Tanabe K., Hirayama H., Kim I., Rao H., and Suzuki T. (2010) Identification of an Htm1 (EDEM)-dependent, Mns1-independent endoplasmic reticulum-associated degradation (ERAD) pathway in Saccharomyces cerevisiae: application of a novel assay for glycoprotein ERAD. J. Biol. Chem. 285, 24324–24334 10.1074/jbc.M109.095919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iida H., Nakamura H., Ono T., Okumura M. S., and Anraku Y. (1994) MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14, 8259–8271 10.1128/mcb.14.12.8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not shown in this article are available upon request from the corresponding authors.