Abstract
Endolysosomes are key players in cell physiology, including molecular exchange, immunity, and environmental adaptation. They are the molecular targets of some pore-forming aerolysin-like proteins (ALPs) that are widely distributed in animals and plants and are functionally related to bacterial toxin aerolysins. βγ-CAT is a complex of an ALP (BmALP1) and a trefoil factor (BmTFF3) in the firebelly toad (Bombina maxima). It is the first example of a secreted endogenous pore-forming protein that modulates the biochemical properties of endolysosomes by inducing pore formation in these intracellular vesicles. Here, using a large array of biochemical and cell biology methods, we report the identification of BmALP3, a paralog of BmALP1 that lacks membrane pore-forming capacity. We noted that both BmALP3 and BmALP1 contain a conserved cysteine in their C-terminal regions. BmALP3 was readily oxidized to a disulfide bond-linked homodimer, and this homodimer then oxidized BmALP1 via disulfide bond exchange, resulting in the dissociation of βγ-CAT subunits and the elimination of biological activity. Consistent with its behavior in vitro, BmALP3 sensed environmental oxygen tension in vivo, leading to modulation of βγ-CAT activity. Interestingly, we found that this C-terminal cysteine site is well conserved in numerous vertebrate ALPs. These findings uncover the existence of a regulatory ALP (BmALP3) that modulates the activity of an active ALP (BmALP1) in a redox-dependent manner, a property that differs from those of bacterial toxin aerolysins.
Keywords: pore-forming protein, aerolysin-like protein (ALP), redox regulation, endolysosomes, bacterial toxin, disulfide, dimerization, βγ-CAT, BmALP3, vesicles
Cellular membranes are essential for defining the border and ensuring the function of all living cells (1, 2). Endolysosomes are functional membrane organelles that are involved in cellular endocytosis and exocytosis and are highly dynamic, playing key roles in diverse cellular processes such as material exchange, signal transduction, infection and immunity, development, and cell death (3–6). Classic membrane receptors, ion channels, and transporters are directly transported to defined cellular membranes after synthesis in the cytoplasm. Pore-forming proteins (PFPs) are secreted as water-soluble proteins; once they reach their target cell membrane, these nonclassic membrane proteins can change their conformation extensively and convert to a transmembrane pore structure (7). In addition to their well-known function as effectors that kill cells (8, 9), emerging evidence suggests that PFPs may play pivotal pathophysiological roles in living organisms, such as neurodegeneration, development, and cell differentiation (10–12), but the related mechanisms remain unclear.
Aerolysin is a β-barrel pore-forming toxin secreted by the Gram-negative bacterium Aeromonas hydrophila (13). Genome sequence and bioinformatic analyses indicated that a variety of proteins from bacteria to vertebrates adopt structures similar to that of aerolysin, and these proteins are termed aerolysin-like proteins (ALPs) (14, 15). Studies on the functions and molecular mechanisms of these ALPs remain in their infancy. Toad skin is naked and is constantly confronted by a complex mixture of potentially injurious factors as it interacts with the environment to ensure sufficient uptake of water, electrolytes, and oxygen (16, 17). Recently, an ALP complex named βγ-CAT was purified and isolated from the skin secretions of the firebelly toad (Bombina maxima). It consists of two subunits, of which BmALP1 (α-subunit) is a βγ-crystallin domain fused with an aerolysin domain and BmTFF3 (β-subunit) is a three-domain trefoil factor (TFF) (18, 19).
The cellular acting pathway of βγ-CAT is characterized by targeting of acidic glycosphingolipids in lipid rafts via a double-receptor binding model to initiate the endocytosis of its BmALP1 subunit and the subsequent oligomerization and pore formation of BmALP1 along the cellular endolysosomal pathways (20). This action results in changes in the biochemical properties of these intracellular vesicles, including increased acidification, which leads to diverse cellular responses and outcomes depending on various cell contexts (21–23). βγ-CAT has been found to be able to trigger inflammasome activation and to stimulate unconventional secretion, as well as to accelerate tissue repair (21, 23). Furthermore, this protein complex has been found to stimulate and to participate in the formation and release of extracellular vesicles to eliminate infecting intracellular pathogens (22). These biological functions suggested that βγ-CAT could play important roles in maintaining mucosal barrier homeostasis, facilitating material exchange via vesicle formation and trafficking, and mediating immune defenses in the toad (18, 21–23).
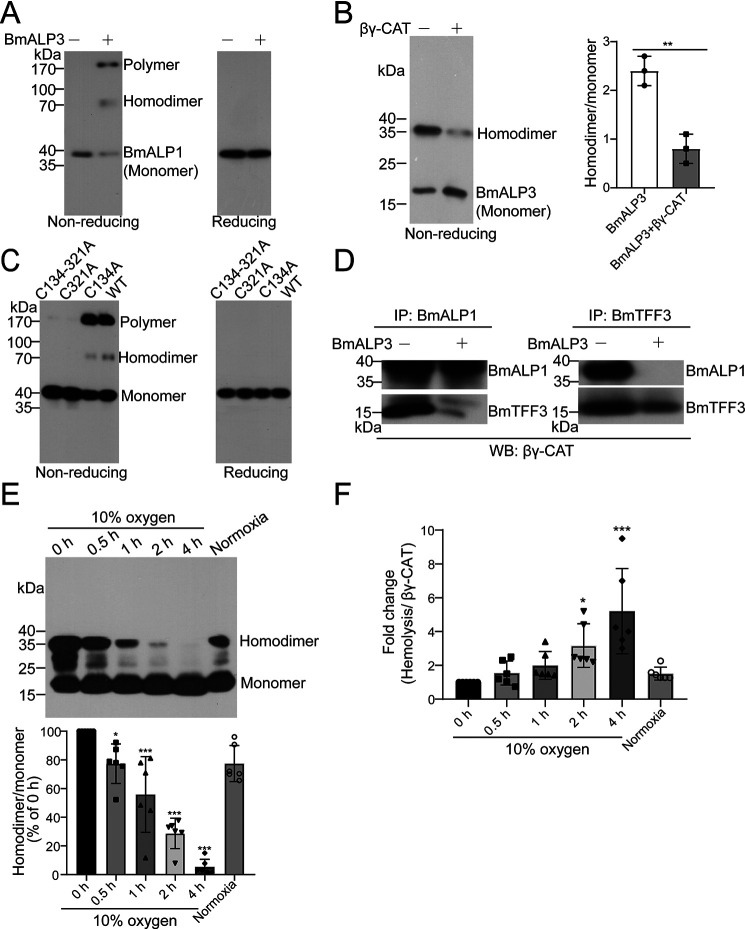
Distinct from classic membrane-integrated ion channels and transporters, βγ-CAT provides the first example of an endogenous secreted β-barrel PFP that extracellularly targets cellular endocytotic pathways to modulate the biochemical contents and properties of endolysosomes. This secreted PFP-mediated action represents a hitherto unknown regulatory mechanism of cell endocytosis and exocytosis via endolysosome modulation. This cellular acting pathway should be tightly regulated, and regulatory proteins of βγ-CAT may exist in the toad. In this study, a paralog of the βγ-CAT BmALP1 subunit (named BmALP3) was identified in B. maxima. BmALP3 homodimer linked by a disulfide bond specifically oxidized BmALP1 into its own homodimer via disulfide bond formation, as well as a water-soluble higher molecular weight polymer, which negatively regulated the assembly and biological functions of the βγ-CAT complex.
Results
BmALP3, a newly identified ALP paralog, interacts with βγ-CAT
To identify βγ-CAT-associated proteins, skin secretions of B. maxima were prepared and subjected to a pulldown assay using anti-βγ-CAT-Sepharose 4B affinity chromatography. A specific band, in addition to the two subunits of βγ-CAT, was found in the eluted proteins of anti-βγ-CAT columns by SDS-PAGE (Fig. 1A). The unknown protein band was further digested by trypsin and analyzed by MS; three MS/MS spectra representing the main components of digest products were selected for de novo sequencing, and the sequences of these peptide fragments matched well with the theoretical sequence deduced from the skin transcriptome of B. maxima (Fig. S1A). Subsequent molecular cloning of the unknown protein showed that its full-length cDNA was 601 bp, and the open reading frame was 471 bp, which encoded 156 amino acids (GenBank accession number MN787048). Sequence alignment revealed that the protein shared 27% sequence identity with the βγ-CAT BmALP1 subunit, suggesting that it was homologous to BmALP1, and we termed it BmALP3. Further sequence analysis revealed that BmALP3 contained only the aerolysin domain; this is different from BmALP1, which contained the βγ-crystallin domain and aerolysin domain (Fig. 1B and Fig. S1B). In addition, a specific conserved cysteine in the C-terminal region was found in both BmALP1 and BmALP3 (Fig. 1B and Fig. S1B).
Figure 1.
A new ALP paralog (BmALP3) that interacted with βγ-CAT was identified in B. maxima. A, an unknown protein band (marked with black arrows) was found in the proteins eluted from anti-βγ-CAT antibody columns by SDS-PAGE, with Coomassie Brilliant Blue staining. B, schematic graph of the βγ-CAT BmALP1 subunit and BmALP3. The C-terminal conserved cysteines of BmALP1 and BmALP3 are marked. C, expression profiles of BmALP3 and βγ-CAT in different tissues of B. maxima by Western blotting. D, colocalization of βγ-CAT (green) and BmALP3 (red) in different tissues of toad was observed by laser confocal microscopy. DAPI, 4′,6-diamidino-2-phenylindole. Scale bar, 25 μm. E, the interaction of BmALP3 with βγ-CAT in toad skin secretions was detected by a coimmunoprecipitation assay. WB, Western blotting. All of the data are representative of at least two independent experiments.
Our previous study verified that βγ-CAT was constitutively expressed in different tissues of B. maxima (21). Here, tissue localization analysis of BmALP3 at the mRNA and protein levels revealed that its tissue distribution pattern was consistent with that of βγ-CAT (Fig. 1C and Fig. S1C). Immunofluorescence staining also displayed strong colocalization of BmALP3 with βγ-CAT in different tissues of toad (Fig. 1D). In addition, a coimmunoprecipitation assay was performed and showed a significant interaction between BmALP3 and βγ-CAT in toad skin secretions (Fig. 1E). All of these findings showed that BmALP3 and βγ-CAT existed together, suggesting that the newly identified ALP paralog was involved in the functions of βγ-CAT.
BmALP3 could be oxidized to a homodimer but lacked pore-forming ability
As mentioned above, BmALP3 existed mainly in the skin secretions of B. maxima. Thus, in the subsequent study, natural BmALP3 was purified to better understand its molecular characteristics and functions. B. maxima skin secretions were separated by a DEAE Sephadex A-50 column, and seven protein peaks were obtained; βγ-CAT was distributed in peaks I–VII and BmALP3 was distributed in peaks IV–VII (Fig. 2A). The hemolytic activity of skin secretions is mainly induced by βγ-CAT, as confirmed by an anti-βγ-CAT antibody blocking assay (Fig. S2A). Interestingly, the hemolytic activity of βγ-CAT in each fraction was decreased suddenly in association with the appearance of BmALP3 (Fig. 2A), suggesting that the function of βγ-CAT might be negatively regulated by BmALP3. Since free cysteine was easier to oxidize and BmALP3 contained its only cysteine in its C-terminal region, the reducing agent DTT was added in the following purification. DEAE Sephadex A-50 column peak VII was used in subsequent gel filtration and resulted in the separation of two protein peaks. Peak II was highly purified BmALP3, with an apparent weight of 18 kDa in SDS-PAGE (Fig. 2B).
Figure 2.
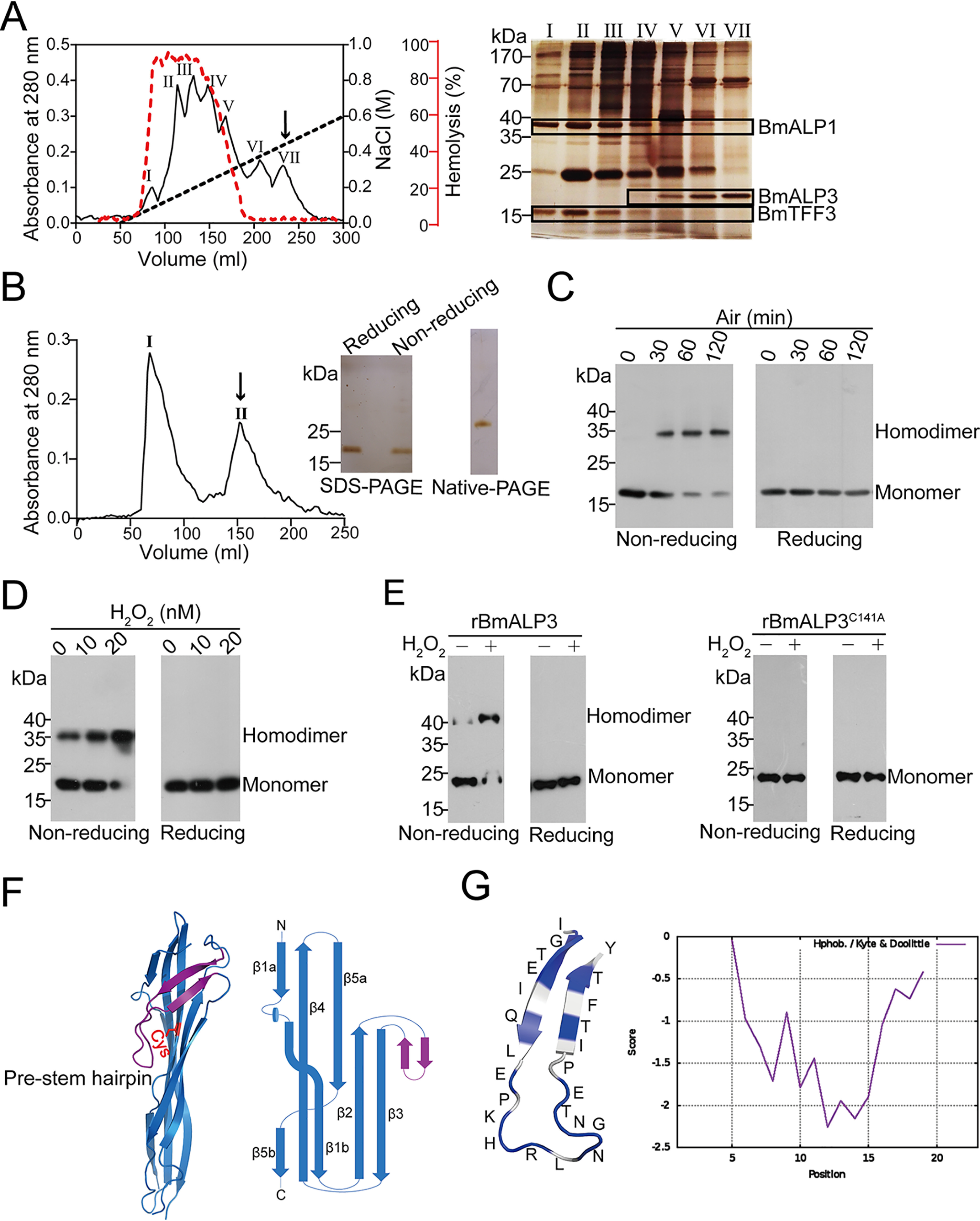
BmALP3 purification, oxidation, and structure determination. A, the isolation of BmALP3 from toad skin secretions was first performed by using a DEAE Sephadex A-50 column. The hemolytic activity of βγ-CAT in each fraction was measured and plotted in the red curve (left). Each peak was then subjected to SDS-PAGE with silver staining, and BmALP3 and βγ-CAT were framed with black boxes (right). B, peak VII from the DEAE Sephadex A-50 column was concentrated (collected from four separations) and then applied to a Sephadex G-50 column. Peak II from the Sephadex G-50 column was known as the highly purified BmALP3. Purified BmALP3 was subjected to SDS-PAGE under reducing and nonreducing conditions and native PAGE with silver staining. C and D, purified BmALP3 was exposed to air for different times (C) or treated with various concentrations of H2O2 (D) and then detected by Western blotting with reducing and nonreducing SDS-PAGE. E, recombinant rBmALP3 and mutant rBmALP3C141A were treated with or without H2O2 and then detected by Western blotting with reducing and nonreducing SDS-PAGE. F, cartoon representation of the BmALP3 structure (left) and topological view of the structure (right) (PDB code 6LH8). The pre-stem hairpin is colored purple. The conserved cysteine at the C-terminal is colored red. G, the pre-stem hairpin of BmALP3 harbored more hydrophilic amino acids. Hydrophilic amino acids are colored blue, and hydrophobic acids are colored gray (left). The hydrophobicity of the pre-stem hairpin was calculated according to the Kyte-Doolittle scale (right). All of the data are representative of at least two independent experiments.
In the following study, Western blotting revealed that a new 35-kDa band was found in nonreducing SDS-PAGE when purified BmALP3 was exposed to air for different times (Fig. 2C) or treated with H2O2 at different concentrations (Fig. 2D), whereas the 35-kDa band disappeared under reducing conditions (Fig. 2, C and D). A reasonable explanation was that the 35-kDa band was the dimeric form of BmALP3 under oxidative conditions. A point mutation assay also verified that the conserved C-terminal cysteine (Cys141) was responsible for BmALP3 homodimer formation under oxidative conditions (Fig. 2E). To further study whether BmALP3 dimerization was mediated by a disulfide bond, a MS assay was performed and showed that a peptide at m/z 6108.4 in the homodimer was observed after enzyme digestion, whereas this ion disappeared along with the appearance of a new ion with m/z 3055.1, which is close to the theoretical m/z value of the peptide that contains Cys141, when DTT was added (Fig. S2B). This implied that a disulfide bond may exist in the homodimer. Unfortunately, we failed to obtain the MALDI-postsource decay spectra of both ions because the ion intensity was too low for MS/MS analysis. To increase the signal intensity of the enzyme-digested peptide that contained Cys141, Glu150 was mutated to Arg. Similar to BmALP3, rBmALP3E150R could also be oxidized to a homodimer. A disulfide bond-linked peptide was found in the rBmALP3E150R homodimer by MS/MS (Fig. S2C). These results demonstrated that BmALP3 could be oxidized to a disulfide bond-linked homodimer via the conserved C-terminal cysteine under oxidative conditions.
Since BmALP3 belongs to the ALP family, its oligomerization and pore-forming capacity were detected in a subsequent study. Given that BmALP3 existed in both the monomeric and dimeric states, we first detected the proportion of prepared BmALP3 monomer and homodimer before the following assay (Fig. S2D). A hemolytic assay on human red blood cells (RBCs) was performed to reflect pore-forming ability and revealed that there was no significant hemolysis when the concentration of BmALP3 was up to 1.28 mg/ml, while high hemolytic activity was observed after treatment with βγ-CAT (Fig. S2E). Oligomerization was the key step for ALP pore formation (14, 24). Western blotting showed that there was no oligomer observed after cells (RBCs and toad peritoneal cells) or liposomes were treated with BmALP3, whereas the SDS-stable oligomer was observed in βγ-CAT-treated samples (Fig. S2, F–H). These findings indicated that BmALP3 lacks the typical pore-forming ability, which is different from previously reported ALPs.
As a novel ALP that lacks the pore-forming ability, the three-dimensional structure of the BmALP3 monomer at 1.73 Å was next determined by the single-wavelength anomalous diffraction method (Table S1). The whole structure of BmALP3 could be divided into two distinct β-sandwiches; the N-terminal β-sandwich was formed by a twisted antiparallel five-stranded β-sheet together with a long hairpin, whereas the C-terminal β-sandwich comprised a three‐stranded antiparallel β‐sheet packing against a two-stranded β-sheet (Fig. 2F).The structural comparison revealed that it harbored the conserved aerolysin fold, which was similar to typical ALP family members, such as Dln1 (25) and aerolysin (26) (Fig. S2I). However, the long hairpin of BmALP3 between strand β2 and strand β3 was enriched in hydrophilic amino acids (Fig. 2G), which was significantly different from the amphipathic pre-stem loop of previously reported ALPs (14, 24). Notably, the conserved amphipathic pre-stem loop is pivotal for the pore-forming capacity of ALPs (14). The hydrophilic hairpin may explain the poor pore-forming ability of BmALP3.
All of the above findings revealed that, unlike typical ALP members, BmALP3 could be oxidized to a disulfide-linked homodimer but lacked the pore-forming ability, suggesting that it performs some novel functions that are different from those of classic ALPs, such as negative regulation.
Oxidized BmALP3 negatively regulates the function of βγ-CAT in vitro and in vivo
To probe the direct effects of BmALP3 on βγ-CAT, natural purified BmALP3, recombinant rBmALP3, and mutant rBmALP3C141A were used, and the hemolytic activity and oligomerization of βγ-CAT on RBCs and toad peritoneal cells were detected in vitro. Interestingly, both the hemolytic activity and oligomerization of βγ-CAT were largely attenuated by BmALP3 and rBmALP3 in a dose-dependent manner (Fig. 3, A and B, and Fig. S3, A–C). However, these behaviors were not affected by rBmALP3C141A (Fig. 3A and Fig. S3, D and E). In contrast to natural BmALP3 or recombinant rBmALP3, rBmALP3C141A could not be oxidized to form a homodimer. The above findings suggested that the functions of βγ-CAT could be negatively regulated by oxidized BmALP3 homodimer rather than reduced monomer. To validate this hypothesis, fully reduced BmALP3 was prepared through pretreatment with DTT and applied in the in vitro activity inhibition assay. As expected, similar to rBmALP3C141A, the hemolytic activity and oligomerization of βγ-CAT were not affected by reduced BmALP3 under DTT treatment (Fig. 3, C and D, and Fig. S3F). The above findings indicated that BmALP3 negatively regulated the functions of βγ-CAT under oxidative conditions rather than under reducing conditions.
Figure 3.
BmALP3 negatively regulated the function of βγ-CAT under oxidative conditions. A, βγ-CAT was first incubated with various concentrations of natural BmALP3, rBmALP3, or rBmALP3C141A and then added to RBCs. The hemolysis of βγ-CAT was tested. B, βγ-CAT was first incubated with various concentrations of BmALP3 and then added to toad peritoneal cells. The oligomerization of βγ-CAT was detected by Western blotting. C, BmALP3 was pretreated with DTT, incubated with βγ-CAT, and added to RBCs. The hemolysis of βγ-CAT was tested. The data represent the mean ± S.D. from 3 technical replicates. ***, p < 0.001 by ANOVA with post hoc contrasts by Dunnett's multiple-comparison test. D, BmALP3 was pretreated with DTT, incubated with βγ-CAT, and added to toad peritoneal cells. The oligomerization of βγ-CAT was detected by Western blotting. E and F, toads were injected intraperitoneally with bacteria. The number of peritoneal bacteria was then counted 24 h after infection. To assess the influence of BmALP3 on the bacterial clearance ability of βγ-CAT, βγ-CAT pretreated with BmALP3 was intraperitoneally injected 4 h before bacterial infection (E). To assess the influence of BmALP3 on toad bacterial clearance, BmALP3 alone was intraperitoneally injected 4 h before bacterial infection (F). The data represent the mean ± S.D. from 8 toads. *, p < 0.05; ***, p < 0.001 by unpaired t test. All of the data are representative of at least two independent experiments.
A previous study demonstrated that βγ-CAT could reduce the infectious bacterial load in the toad peritoneum and protect toads against bacterial infection (21). To further examine whether BmALP3 could inhibit βγ-CAT in vivo and affect the microbial clearance of toads, a toad peritoneal bacterial infection model was used. The results showed that the ability of βγ-CAT to induce rapid bacterial clearance was inhibited by intraperitoneal injection of BmALP3 and βγ-CAT together (Fig. 3E), and BmALP3 alone also attenuated the bacterial clearance ability of toads (Fig. 3F). All of these findings suggested that oxidized BmALP3 negatively regulates the function of βγ-CAT in vivo and in vitro, while reduced BmALP3 has no impact on the function of βγ-CAT.
BmALP3 inhibits βγ-CAT by triggering oxidation of BmALP1 and dissociation of βγ-CAT
To further study the mechanism by which oxidized BmALP3 negatively regulates βγ-CAT, we first detected the states of two subunits of βγ-CAT under nonreducing conditions after treatment with BmALP3. Western blotting of BmALP1 showed that two new bands appeared in nonreducing SDS-PAGE, one band at ∼70 kDa and another at ∼170 kDa, whereas the two new bands were not found in reducing SDS-PAGE (Fig. 4A). A reasonable explanation was that the two new bands were the dimeric and polymeric forms of BmALP1 that were oxidized by BmALP3. It is worth noting that the BmALP1 high-molecular-weight polymer could be reduced to a monomeric form (Fig. 4A), indicating that the reversible polymer was obviously different from the SDS-resistant oligomer that formed in cells or liposomes, as described above; thus, we termed this form the polymer to distinguish it from the SDS-resistant oligomer. In addition to BmALP1 being oxidized to a homodimer and a polymer, we next observed that the BmALP3 homodimer was reduced to its monomeric form in nonreducing SDS-PAGE after incubation with βγ-CAT (Fig. 4B). Previous studies demonstrated that the BmTFF3 subunit has no free cysteine (18, 20). Accordingly, Western blotting of BmTFF3 revealed that there was no obvious band position change in BmTFF3 in reducing or nonreducing SDS-PAGE after treatment with BmALP3 (Fig. S4A). A point mutation assay was used to probe which cysteine was responsible for BmALP1 dimerization and polymerization, and it showed that the formation of the BmALP1 homodimer and polymer were both dependent on the conserved C-terminal cysteine Cys321 (Fig. 4C). Further MS detection also verified that both the BmALP1 dimeric band and the polymeric band were linked by a disulfide bond via Cys321 (Fig. S4B). These results suggested that BmALP1 could be oxidized by the BmALP3 homodimer and form its own homodimer, as well as a polymer, via disulfide bond exchange.
Figure 4.
BmALP3 inhibits βγ-CAT by triggering the oxidation of BmALP1 and the dissociation of βγ-CAT. A, βγ-CAT was first incubated with BmALP3. Then, the state of BmALP1 was detected by Western blotting using an anti-BmALP1 antibody in nonreducing and reducing SDS-PAGE. B, βγ-CAT was first incubated with BmALP3. Then, the BmALP3 homodimer and monomer were detected by Western blotting using an anti-BmALP3 antibody in nonreducing SDS-PAGE (left), and the bands were semiquantified with ImageJ (right). The data represent the mean ± S.D. of triplicate samples. **, p < 0.01 by unpaired t test. C, recombinant rBmALP1 (WT) and rBmALP1 mutants were first incubated with BmALP3. Then, the state was detected by Western blotting using an anti-BmALP1 antibody in nonreducing SDS-PAGE. D, βγ-CAT was first incubated with BmALP3. Then, immunoprecipitation (IP) was performed with anti-BmALP1 and anti-BmTFF3 antibodies, and detection was performed by Western blotting (WB) using an anti-βγ-CAT antibody. E and F, toads were exposed to hypoxic conditions (10% oxygen) for different times and recovered under normal oxygen conditions (20.95%). The toad skin secretions were collected and diluted to 1 mg/ml. The BmALP3 homodimer and monomer were detected by Western blotting (top) and semiquantified with ImageJ (bottom) (E). The specific hemolytic activity of βγ-CAT in skin secretions was also detected (F). The data represent the mean ± S.D. from 6 toads. *, p < 0.05; ***, p < 0.001 by ANOVA with post hoc contrasts by Dunnett's multiple-comparison test. All of the data are representative of at least two independent experiments.
A previous study revealed that both the BmALP1 and BmTFF3 subunits were required for the functions of βγ-CAT and the dissociation of the BmALP1 and BmTFF3 subunits would lead to functional loss of βγ-CAT (18, 20). The above results showed that BmALP1, rather than BmTFF3, could be oxidized by BmALP3, which prompted us to determine whether BmALP1 oxidation could lead to the disassociation of βγ-CAT and the elimination of its biological activity. To validate this hypothesis, immunoprecipitation assays of BmALP1 and BmTFF3 were performed and showed that the two subunits of βγ-CAT were dissociated after treatment with BmALP3 (Fig. 4D). All of these findings indicated that BmALP3 inhibits the function of βγ-CAT by triggering oxidation of BmALP1 and disassociation of βγ-CAT.
We also detected the direct effects of other oxidative conditions on βγ-CAT, including air, H2O2, and recombinant B. maxima oxidoreductase peroxiredoxin 6 (rBmPrx6). Western blotting of BmALP1 showed that all three oxidative conditions had no obvious effect on the βγ-CAT BmALP1 subunit (Fig. S4, C–E). In addition, the same negative results were observed when βγ-CAT was treated with toad skin secretions that lacked BmALP3 and βγ-CAT (Fig. S4F). These findings further indicated that the central role of BmALP3 is to act as a paralog to negatively regulate βγ-CAT functions.
BmALP3 senses environmental oxygen tension in vivo
Toad skin is a specific organ that can take up oxygen and help respiration (27). To investigate the physiological relevance of the specific redox-dependent regulatory pattern of these two ALPs, acute hypoxia experiments were performed. The results showed that the percentage of BmALP3 in toad skin secretions showed no obvious change when toads were exposed to low-oxygen (10%) conditions (Fig. S4G). However, the BmALP3 homodimer was rapidly transformed to its reduced monomer under hypoxic conditions (Fig. 4E). As described above, the hemolytic activity of toad skin secretions was mainly induced by βγ-CAT (Fig. S2A). Here, a hemolysis assay showed that the hemolytic activity of βγ-CAT in skin secretions was elevated along with a decrease in the BmALP3 homodimer (Fig. 4F). Interestingly, the specific hemolytic activity of βγ-CAT decreased with increasing amounts of the BmALP3 homodimer when the low-oxygen toads were exposed to normal oxygen conditions (20.95%). Our results suggested that BmALP3 could be secreted as a sensor of oxygen in the toad skin and change its redox form depending on the oxygen tension, thus participating in the regulation of βγ-CAT functions.
Discussion
Secreted PFPs have been identified in organisms from all kingdoms of life (28). The majority of studies on PFPs focused on their role in cell death, including roles as virulence factors in pathogens or effectors in the host immune system (7, 29, 30). βγ-CAT is an endogenous secreted PFP (BmALP1) and TFF (BmTFF3) complex. By acting on and modulating cellular endocytotic and exocytotic pathways, this protein complex has been proposed to play functional roles in maintaining mucosal barrier homeostasis while facilitating material exchange, as well as providing immune defense via cellular membranal vesicle formation and modulation of endolysosomes (18, 21–23). Regulatory elements must exist for the effective formation and dissociation of this protein complex. Indeed, the present study uncovered an endogenous paralog of BmALP1 (named BmALP3) as a negative regulator of this βγ-CAT protein complex.
Unlike different domain-fused full-length ALPs from vertebrates (14, 31), BmALP3 is composed of an aerolysin-like domain only (Fig. 1B and Fig. S1B). Its pre-stem hairpin was not amphipathic but was enriched in hydrophilic amino acids, which may explain its lack of membrane pore-forming capacity (Fig. 2G). Both BmALP1 and BmALP3 contain a conserved cysteine in their C-terminal regions (Fig. 1B and Fig. S1B). The BmALP3 homodimer linked by a disulfide bond could oxidize BmALP1 to generate its own homodimer, as well as a high-molecular-weight polymer, via BmALP1 disulfide bond formation, which rendered BmALP3 back to its reduced monomer at the same time. The specific disulfide bridge exchange process between these two ALP paralogs led to the dissociation of βγ-CAT and the elimination of its biological activity (Fig. 5).
Figure 5.
Proposed model of the action of BmALP3 in negatively regulating the function of βγ-CAT. As an endogenous negative regulator of βγ-CAT, BmALP3 was homologous to the βγ-CAT BmALP1 subunit. Both BmALP3 and BmALP1 contained the conserved cysteine in their C-terminal regions. In toad skin secretions, BmALP3 was sensitive to environmental oxidative conditions (O2 tension and ROS levels), which induced the protein to convert to its homodimer linked by a disulfide bond. The BmALP3 homodimer oxidized BmALP1 via disulfide bond exchange. This action resulted in the formation of BmALP1 homodimers and water-soluble polymers, leading to dissociation of βγ-CAT subunits and loss of biological activity. Additionally, given the important physiological roles of βγ-CAT, we proposed that putative unknown positive regulators may exist and promote the activation of βγ-CAT to exert its biological functions, such as immunity, maintenance of the mucosal barrier, and vesicular trafficking of materials (21–23).
It is generally thought that ALPs are secreted in a stable water-soluble form. Once they reach their target cell membrane, they can undergo a large conformation change and be converted to a transmembrane SDS-stable oligomerized state (14, 28). To prevent premature conversion to their membrane-inserting forms, bacterial ALPs are produced as proproteins, and an activation process exists (32). The present study provided the first evidence that vertebrate ALPs could reversibly convert to homodimers, as well as water-soluble polymers, as exemplified by BmALP1, which might be a protective mechanism by which vertebrate ALPs avoid conversion to their membrane-inserting form before reaching the target cell membranes. In addition, although the BmALP1 homodimer and polymer could be transformed into the monomer by treatment with DTT, the βγ-CAT activity could not be recovered (Fig. S5). Given the important physiological roles of βγ-CAT in B. maxima, several possibilities for positive regulation may exist in toads to promote the activation and assembly of βγ-CAT. First, because oxidized BmALP1 was inactivated, physiological reducing elements in toads, such as reductase, might act as potential positive regulators to reduce and to activate βγ-CAT. Second, the oligomerization and activity of bacterial aerolysins and zebrafish Dln1 could be promoted in a microenvironment of relatively lower pH via protonation of histidine (25, 33), Histidine existed in the βγ-CAT BmALP1 subunit, and lower pH may also promote the action of βγ-CAT. The effects of pH values and the potential positive regulatory elements on regulation of βγ-CAT in vitro and in vivo are the focus of ongoing studies (Fig. 5). Bacterial aerolysin from A. hydrophila must be activated by proteolytic cleavage of a C-terminal fragment (13). However, the possible activation mechanisms for vertebrate ALPs remain unknown. Depending on the redox state, membrane-active BmALP1 of βγ-CAT reversibly changed between the monomer and the homodimer, as well as the water-soluble polymer (Figs. 4 and 5). This work provided a novel possible activation mechanism for vertebrate ALPs, which is completely different from that of bacterial aerolysin (Fig. S6A).
BmALP1, BmALP3, and BmTFF3 are relatively abundant protein components in toad B. maxima skin secretions (Fig. 1). Lacking a signal peptide, both BmALP1 and BmALP3 possess the same N-acetylation posttranslational modification (Fig. S7). It is reasonable to speculate that BmALP1 and BmALP3 may be secreted by the same unconventional secretory pathway, making it easier for BmALP3 to interact with and to regulate BmALP1 in vivo. In contrast, possessing a typical signal peptide in its precursor (18), the TFF subunit of βγ-CAT BmTFF3 should be secreted by a distinctive classic pathway. Large amounts of free BmTFF3 exist in B. maxima skin secretions (20). The BmALP1 monomer could interact with BmTFF3 to form the biologically active βγ-CAT protein complex in the toad skin. The function of BmTFF3 in this protein complex should be as a chaperon that can stabilize the BmALP1 monomer and deliver BmALP1 to proper membrane targets via the double-receptor binding model of βγ-CAT (20). Peroxiredoxin, air, H2O2, and toad skin secretions lacking BmALP3 were not able to directly target βγ-CAT to oxidize BmALP1 under our assay conditions (Fig. S4), emphasizing the specific regulatory effect of BmALP3 on BmALP1. Taken together, these findings highlight the necessity of βγ-CAT complex formation as well as the strict and specific regulatory mechanisms of the βγ-CAT pathway. It is worth noting that, apart from the newly characterized oxidized BmALP3, other molecules that might interact (with or even without redox dependence) with the ALP complex βγ-CAT to modulate the activity of endolysosomes, such as free acidic glycosphingolipids, which could interact with βγ-CAT and affect endolysosome regulation (20), could not be excluded.
As a negative regulator of βγ-CAT, BmALP3 inhibited the function of the protein complex under oxidative conditions (Fig. 3 and Fig. S3). This regulatory manner should have physiological relevance in light of the biological role of βγ-CAT. First, toad skin is a respiratory organ that absorbs oxygen (27). Oxygen in the air could oxidize BmALP3 and inhibit the activity of βγ-CAT (Figs. 1 and 3). The oxidized BmALP3 homodimer was transformed to the reduced monomer when toads were exposed to hypoxic conditions, and the activity of βγ-CAT was up-regulated at the same time (Fig. 4). These results suggested that BmALP3 was sensitive to environmental oxidative conditions and that βγ-CAT might not function when oxygen is abundant. Under conditions of hypoxia, how βγ-CAT and BmALP3 act as stress factors to help toad skin respiration and mechanisms inside are interesting future challenges. Second, as an effective protein machine that protects hosts from microbial infection, βγ-CAT could form pores in endolysosomes, leading to inflammasome activation and interleukin-1β release (21). Continuous and excessive inflammasome activation is harmful to the host, and such a process should be controlled strictly (34, 35). Inflammasome activation generates reactive oxygen species (ROS), and ROS such as H2O2 could oxidize BmALP3 and then inhibit the function of βγ-CAT (Fig. 1 and 3), generating negative feedback to prevent uncontrollable inflammation.
To our knowledge, BmALP3, which acts as a regulatory ALP, is the first example of paralog regulation via redox modulation on an executive ALP with pore-forming capacity (BmALP1). This paralog regulatory mode also occurs in other kind of PFPs, although the detailed mechanisms vary depending on the different types of PFPs. BCL-2 family members are positively or negatively regulated by distinct family members via paralog binding. Complement factors are activated by their paralogs via proteolysis (36, 37). With advances in genome sequencing, it has been revealed that many ALP isoforms are present in the genome of vertebrate species, such as Danio rerio and Xenopus laevis (14). The present study revealed that BmALP3 acts as a negative regulator of membrane insertion BmALP1. However, the possibility that an ALP paralog might act as a positive regulator of a membrane insertion ALP could not be excluded. The investigation of possible interactions and regulatory relationships among these ALP paralogs in the same species will be a fascinating subject in the future.
The regulatory mode of BmALP3 on BmALP1 relied on the redox reaction of a conserved C-terminal cysteine (Figs. 1 and 4), indicating that this conserved residue was a key regulatory site in B. maxima ALPs. Interestingly, the conserved C-terminal cysteine does not appear only in B. maxima ALPs. The sequence alignment of ALPs from vertebrates revealed that this cysteine site is highly conserved from fishes to reptiles. Particularly, although the site was mutated to serine in birds, it is also highly conserved in various bird species (Fig. S6B). This phenomenon highlights the key role of this site in the regulation of vertebrate ALPs. Our work may provide clues for understanding the possible regulatory patterns and mechanisms of vertebrate ALPs. Accordingly, redox regulation may serve as a regulatory mode for ALPs from fishes to reptiles. For ALPs from birds, phosphorylation of the serine residues might be an alternative regulatory mechanism. It is worthwhile to point out that our work has revealed the possible existence of regulatory ALPs and executive ALPs, with the former lacking membrane insertion activity, which should serve as an important indication in the future study of ALPs.
In conclusion, the present study identified a novel ALP, BmALP3, which could act as an endogenous regulator of the BmALP1 and BmTFF3 complex βγ-CAT. Distinct from BmALP1, BmALP3 lacks membrane pore-forming capacity. Particularly, BmALP3 is sensitive to environmental oxidative conditions (O2 tension and ROS levels). The BmALP3 homodimer oxidized BmALP1 via disulfide bond exchange. This action resulted in the formation of BmALP1 homodimer and a water-soluble polymer, leading to dissociation of βγ-CAT subunits and loss of biological activity (Fig. 5). These findings revealed a hitherto unknown redox-dependent paralog regulatory pattern of vertebrate ALPs.
Experimental procedures
Animals
The collection and feeding of toads (B. maxima) were performed as described previously (21). All procedures and the care and handing of animals were approved by the Ethics Committee of the Kunming Institute of Zoology, Chinese Academy of Sciences.
Antibodies, reagents, and cell lines
Rabbit and mouse polyclonal antibodies against βγ-CAT, BmALP3, BmALP1, and BmTFF3 were produced as described previously (38). Mouse mAb against β-actin (sc-47778) was purchased from Santa Cruz Biotechnology. HRP-conjugated AffiniPure goat anti-rabbit IgG (H+L) (SA00001-2), HRP-conjugated AffiniPure goat anti-mouse IgG (H+L) (SA00001-1), Cy3-conjugated AffiniPure goat anti-mouse IgG (H+L) (SA00009-1), and FITC-conjugated AffiniPure goat anti-rabbit IgG (H+L) (SA00003-2) were purchased from Proteintech Group.
Human RBCs were obtained from Yunnan Kunming Blood Service. Toad (B. maxima) peritoneal cells were isolated as described previously (21). For short-term culture, toad cells were cultured in DMEM/F12 (BI) supplemented with 10% heat-inactivated FBS (BI), and these toad cells were maintained under an atmosphere of 5% CO2 at 26 °C.
Sepharose 4B affinity chromatography
Anti-βγ-CAT antibody-Sepharose 4B and rabbit IgG-Sepharose 4B were used for the method described previously (39). Briefly, anti-βγ-CAT antibody and rabbit IgG were coupled to CNBr-activated Sepharose 4B beads (GE Biosciences), and B. maxima skin secretions were loaded on a column packed with anti-βγ-CAT antibody and rabbit IgG-Sepharose 4B that had been previously equilibrated with equilibration buffer (20 mM Tris-HCl, pH 7.4). The column was washed extensively with equilibration buffer containing 0.15 M NaCl. Proteins specifically binding to the column were eluted with equilibration buffer containing 1 M NaCl. The eluted fractions were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250 (Sigma).
MS analysis
The MS analysis was performed as described previously (40). Briefly, target proteins were separated by SDS-PAGE with Coomassie Brilliant Blue staining, and the proteins bands were cut into small pieces for subsequent destaining and enzymatic digestion. For BmALP3 identification and posttranslational modification analysis, the gel pieces were destained with 25 mM NH4HCO3 and 50% acetonitrile at room temperature, reduced with 10 mM DTT at 37 °C for 60 min, and blocked with 30 mM iodoacetamide at room temperature for 45 min. BmALP1 and BmALP3 were digested by Glu-C and trypsin, respectively, overnight at 37 °C. For disulfide bond determination, target protein bands were separated by SDS-PAGE under nonreducing conditions and processed in the same way as mentioned above, except for the DTT/iodoacetamide steps. BmALP1 homodimer and polymer bands were digested with trypsin, the BmALP3 homodimer band was digested with trypsin and thermolysin, and the BmALP3E150R homodimer band were digested with trypsin and chymotrypsin. Digested products (1 μl) dissolved in 25 mM NH4HCO3 were mixed with an equal volume of α-cyano-4-hydroxycinnimic acid (5 mg/ml, dissolved in 50% acetonitrile with 0.1% TFA) and then spotted on a sample plate for crystallization at room temperature. Positive MS and MS/MS data were acquired with an AutoFlex Speed MALDI TOF/TOF mass spectrometer (Bruker Daltonik GmbH, Leipzig, Germany). FlexAnalysis v.3.3 (for peak list generation) and BioTools v.3.2 software provided by the manufacturer, combined with manual annotation, were employed for MS/MS spectrum interpretation. The mass tolerance for MS/MS ion spectra was ±0.5 Da.
Molecular cloning of BmALP3
The molecular cloning assay was performed as described previously (18). For cloning of BmALP3, RNA was extracted from the toad skin using an RNA Easy kit (Qiagen) according to the manufacturer's instructions. The total RNA was used to synthesize cDNA (TaKaRa) according to the protocol. To obtain the complete cDNA sequences, 3′- and 5′-rapid amplification of cDNA ends was performed using the GeneRacer kit (Invitrogen). The amplified fragments were cloned into a pGEM-T Easy vector (Promega) and sequenced.
Immunofluorescence assay
The immunofluorescence assay was performed as described previously (41). To determine the localization of BmALP3 and βγ-CAT in B. maxima, paraffin-embedded sections were routinely dewaxed, hydrated, and rinsed and underwent antigen repair. Then, the samples were incubated with 3% BSA in PBS to block nonspecific binding by primary antibodies. Sections mounted on slides were incubated with rabbit anti-βγ-CAT primary antibody and mouse anti-BmALP3 primary antibody at 4 °C overnight; rabbit IgG and mouse IgG served as isotype controls. After being washed three times with Phosphate buffered saline with Tween-20 (PBST), slides were incubated with FITC-conjugated goat anti-rabbit IgG and Cy3-conjugated goat anti-mouse IgG for 1 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific). Finally, the slides were observed using a confocal microscope (Olympus FV1000).
Purification of BmALP3
Toad (B. maxima) skin secretions were dissolved in 10 ml of 50 mM Tris-HCl buffer, pH 7.45, containing 5 mM EDTA, dialyzed against the same buffer at 4 °C overnight, and centrifuged. The supernatant was loaded on a DEAE Sephadex A-50 column. Elution was performed at 12 ml/h with a linear NaCl gradient, collecting fractions of 2 ml/tube. Peak VII from the Sephadex A-50 column was concentrated (collected from four separations). Peak VII from the Sephadex A-50 column with added 10 mM DTT was then applied to a Sephadex G-50 column equilibrated with 50 mM Tris-HCl buffer, pH 7.8, containing 150 mM NaCl and 5 mM EDTA. Elution was achieved with the same buffer at a flow rate of 9 ml/h, collecting fractions of 1.5 ml/tube. All of these procedures were performed at 4 °C, and the protein concentration was estimated from the absorbance at 280 nm. Peak II from the Sephadex G-50 column was purified BmALP3, and the purity of BmALP3 was analyzed by SDS-PAGE under reducing and nonreducing conditions and by native PAGE with silver staining. The purified BmALP3 was exposed to air or treated with H2O2 for the oxidation assay immediately. The remaining BmALP3 was dialyzed against PBS at 4 °C for 12 h and stored at −20 °C for further studies.
Hemolysis assay
Hemolytic activity was determined for BmALP3, βγ-CAT, and each separated fraction from the DEAE Sephadex A-50 column. Human RBCs (6 × 107 cells/ml) were incubated with various concentrations of BmALP3 (0–1280 μg/ml) and βγ-CAT (0–1280 μg/ml) and a 50 μg/ml concentration of the DEAE Sephadex A-50 column-separated fraction at 37 °C for 30 min. Hemolysis was detected as described previously (18). To determine whether the hemolysis of toad skin secretions was mainly induced by βγ-CAT, various concentrations of toad skin secretions (25, 50, and 100 μg/ml) were incubated with 200 μg/ml anti-βγ-CAT antibody or rabbit IgG at 37 °C for 30 min and then the hemolytic activity of the mixed sample was detected as mentioned above.
Western blotting
Prepared protein samples were subjected to SDS-PAGE and transferred to a PVDF membrane. The membrane was subsequently blocked with 3% BSA and incubated with the appropriate primary and secondary antibodies. Protein bands were visualized with SuperSignal chemiluminescence reagents (Pierce), as described previously (42). The protein bands were semiquantified with ImageJ software. For data presented as bar graphs, control samples are set as 100%, and the percentage change in intensity is reported.
Tissue distribution of BmALP3 and βγ-CAT
Toad tissues were sampled from intact adult toads. Total RNAs of these tissues were purified using an RNA Easy kit and converted to the corresponding cDNAs as described above for molecular cloning of BmALP3 and then were assessed by semiquantitative PCR, using the primers listed in Table S2. Total proteins of these tissues were obtained as described previously (42) and then assayed by Western blotting.
Oxidation of BmALP3
For the oxidation of BmALP3, 0.18 μg/ml purified BmALP3 was exposed to air at room temperature for different times (0, 30, 60, and 120 min) or treated with different concentrations of H2O2 (0, 10, and 20 nM) at room temperature for 10 min. To determine the oxidation of recombinant rBmALP3 and rBmALP3C141A, 0.2 μg/ml rBmALP3 and rBmALP3C141A were treated with 20 nM H2O2 at room temperature for 10 min. All protein samples were analyzed by Western blotting using an anti-BmALP3 antibody under reducing and nonreducing conditions.
Oligomerization assay
For the oligomerization of BmALP3 and βγ-CAT, RBCs (6 × 107 cells/ml) and liposomes (dioleoylphosphatidylcholine, dioleoylphosphatidylethanolamine, and dioleoylphosphatidylserine, 9:9:2, 0.2 mg/ml) were incubated with 1 μg/ml βγ-CAT or 18 μg/ml BmALP3 in 1 ml of PBS at 37 °C for 30 min. Toad peritoneal cells (4 × 105 cells/ml) were incubated with 7.2 μg/ml βγ-CAT or 18 μg/ml BmALP3 in 1 ml of PBS at room temperature for 30 min. The cells and liposomes were collected by centrifugation at 12,000 × g for 30 min at 4 °C and washed five times with PBS. The cell supernatants were concentrated to 1/20th of the original volume. Finally, the cell precipitate and concentrated supernatant were subjected to SDS-PAGE and analyzed by Western blotting using anti-BmALP3 and anti-βγ-CAT antibodies, respectively.
Coimmunoprecipitation
To detect the interactions of BmALP3 with βγ-CAT in the toad skin secretions, a coimmunoprecipitation assay was performed as reported previously (42). Briefly, after preclearance with protein A-agarose beads, the toad skin secretions were incubated with primary antibody overnight at 4 °C. The protein A-agarose beads were added to the toad skin secretions and incubated for 2 h at 4 °C. The beads were pulled down and washed three times; the immune complexes were removed from the beads by boiling for 15 min in SDS-PAGE sample buffer and were detected by Western blotting.
To detect whether BmALP3 triggered the dissociation of βγ-CAT, βγ-CAT (7.2 μg/ml) was incubated with BmALP3 (18 μg/ml) at room temperature for 30 min. Coimmunoprecipitation was performed as described above, using anti-BmALP1 and anti-BmTFF3 antibodies.
Recombinant expression
The genes encoding rBmPrx6, rBmALP3, rBmALP3 mutants, rBmALP1, and rBmALP1 mutants were synthesized in GeneCreate Biotech, using an Escherichia coli codon preference table, and then cloned into the expression vector pET-28a with an N-terminal His6 tag. All expression vectors were transformed into Escherichia coli strain BL21 (DE3) cells (Novagen). Transformed cells were grown at 37 °C to an A600 of 0.6 and then induced with 0.1 mM isopropyl 1-thio-β-d-galactopyranoside for 12 h at 28 °C. After centrifugation, the harvested cells were lysed by sonication. All of the recombinant proteins were purified with a nickel-nitrilotriacetic acid affinity chromatography column in buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 10 mM imidazole, 10 mM DTT) and eluted with 300 mM imidazole according to the manufacturer's instructions (Sigma). The purified recombinant proteins were dialyzed against PBS at 4 °C for 12 h.
Crystallization, data collection, and structure determination
To obtain the crystal structure of BmALP3 monomer, native rBmALP3 was overexpressed as described above, and the selenium-selenomethionine (SeMet)-substituted rBmALP3 was cultured in a different medium M9 broth by a methionine-biosynthesis inhibition method (43). All crystals were grown at 289 K using the hanging-drop vapor diffusion method. Crystals of the native rBmALP3 were grown for ∼30 days, to a decent size, in buffer containing 0.2 M MgCl2·6H2O, 25% (w/v) PEG 3350, and 0.1 M bis-Tris, pH 5.7. Crystals of the SeMet-rBmALP3 protein were obtained from 0.2 M MgCl2·6H2O, 25% (w/v) PEG 3350, 0.1 M bis-Tris, pH 6.4. All crystals, with the protector 20% glycerol, were flash-frozen at 100 K in a liquid nitrogen stream. The data sets were collected on beamline BL17U of synchrotron radiation at the Shanghai Synchrotron Radiation Facility (44) and were processed and scaled with HKL-2000 and Scalepack (43). The phase problem was solved by calculating the position of Se atoms by the single-wavelength anomalous dispersion method (45), obtaining the initial model using AutoSol of Phenix to find the position of Se, and running the AutoBuild module to calculate and to build other atoms of SeMet-BmALP3 (46, 47). The initial structure of native rBmALP3 was determined by the molecular replacement method using Molrep of the CCP4i package (48, 49). Subsequently, these structures were rebuilt and checked using Coot (50) and then using Refmac5 (51) of CCP4i, and Refine (52) of the Phenix suite refined these models interactively and accurately. The final structures were evaluated with MolProbity (53). The crystallographic parameters are listed in Table S1. All figures of structures were prepared with PyMOL.
In vitro inhibition of βγ-CAT
For the inhibition assay of βγ-CAT hemolytic activity, 1 μg/ml βγ-CAT was first mixed with various concentrations of natural BmALP3, rBmALP3, or rBmALP3C141A (0–23.04 μg/ml) at room temperature for 30 min, and then the hemolysis in the mixed samples was detected as described for the hemolysis assay. To determine the inhibitory effects of reduced BmALP3, 23.04 μg/ml BmALP3 was pretreated with 10 mM DTT at room temperature for 30 min and then incubated with 1 μg/ml βγ-CAT at room temperature for 30 min. Finally, the hemolysis in the mixed samples was detected as described for the hemolysis assay.
For the inhibition assay of βγ-CAT oligomerization, 7.2 μg/ml βγ-CAT was first mixed with various concentrations of natural BmALP3, rBmALP3, or rBmALP3C141A (0–18 μg/ml) at room temperature for 30 min, and then the oligomerization of mixed samples in the precipitate of RBCs and toad peritoneal cells was detected by Western blotting using anti-βγ-CAT antibody, as described for the oligomerization assay. To determine the inhibitory effects of reduced BmALP3, 18 μg/ml BmALP3 was pretreated with 10 mM DTT at room temperature for 30 min and then incubated with 7.2 μg/ml βγ-CAT at room temperature for 30 min. Finally, the oligomerization of mixed samples in the precipitate of RBCs and toad peritoneal cells was detected by Western blotting using anti-βγ-CAT antibody, as described for the oligomerization assay.
In vivo toad peritoneal bacterial infection assay
In vivo toad peritoneal experiments were performed as described previously (21). For bacterial clearance, toads weighing 25 ± 5 g were injected intraperitoneally with 1 × 108 colony-forming units of bacteria (A. hydrophila). The number of peritoneal bacteria was then counted 24 h after infection. To assess the influence of βγ-CAT on bacterial clearance, 28.8 μg/kg βγ-CAT was intraperitoneally injected 4 h before bacterial infection. To assess the influence of BmALP3 on the bacterial clearance ability of βγ-CAT, 28.8 μg/kg βγ-CAT and 72 μg/kg BmALP3 were intraperitoneally injected together 4 h before bacterial infection. To assess the influence of BmALP3 on toad bacterial clearance, 72 μg/kg BmALP3 alone was intraperitoneally injected 4 h before bacterial infection.
Oxidation of βγ-CAT, recombinant rBmALP1, and rBmALP1 mutants
For the oxidation of βγ-CAT, 7.2 μg/ml βγ-CAT was exposed to air at room temperature for different times (0, 30, and 60 min) or incubated with 1 μM H2O2, 25 μg/ml BmPrx6, and 18 μg/ml BmALP3 at room temperature for 30 min. Then, the samples were detected by Western blotting using an anti-BmALP1 antibody or anti-BmTFF3 antibody under nonreducing or reducing conditions. The state of BmALP3 after incubation with βγ-CAT at room temperature for 30 min was detected by Western blotting using an anti-BmALP3 antibody under nonreducing conditions.
To determine whether other factors present in toad skin secretions could oxidize βγ-CAT, both BmALP3 and βγ-CAT were removed from toad skin secretions by immunodepletion. Briefly, 200 μg/ml toad skin secretions were incubated with 2 mg/ml mixed antibodies (anti-BmALP3 and anti-βγ-CAT antibodies) and 2 mg/ml rabbit IgG at 4 °C overnight. Then, protein A-agarose beads were added to the processed toad skin secretions and incubated for 2 h at 4 °C. Finally, the mixed samples were centrifuged at 12,000 × g for 30 min at 4 °C, and the supernatants were collected. The effect of immunodepletion was detected by Western blotting using anti-BmALP3 and anti-βγ-CAT antibodies. Various concentrations of prepared toad skin secretions (0, 50, and 100 μg/ml) were incubated with 7.2 μg/ml βγ-CAT at room temperature for 30 min, and the state of the βγ-CAT BmALP1 subunit was detected by Western blotting under nonreducing conditions.
For the oxidation of rBmALP1 and its mutants, 4 μg/ml rBmALP1 and mutants were incubated with 18 μg/ml BmALP3 at room temperature for 30 min. Then, the samples were detected by Western blotting under nonreducing conditions.
Acute hypoxia assay
Toads weighing 25 ± 5 g were exposed to hypoxic conditions (10% oxygen) for different times (0, 0.5, 1, 2, and 4 h) and recovered under normal oxygen conditions (20.95%) for 4 h. The toad skin secretions were collected, diluted to 1 mg/ml, and added to SDS-PAGE loading buffer under the corresponding oxygen conditions. The percentages of BmALP3 and βγ-CAT in toad skin secretions were detected by Western blotting under reducing conditions. BmALP3 homodimers and monomers were detected by Western blotting under nonreducing conditions. The specific hemolytic activity of βγ-CAT in toad skin secretions (2.5 μg/ml) was detected as described for the hemolysis assay.
Statistical analysis
All experimental values are expressed as mean ± S.D. Each individual experiment was repeated at least two times. All data were analyzed using Prism v8.0 software. Two-sample comparisons were performed using Student's t test. Multiple comparisons were performed using one-way analysis of variance (ANOVA), with post hoc contrasts by Dunnett's multiple-comparison test; p values of <0.05 were considered statistically significant.
Data availability
The structures presented in this paper have been deposited in the Protein Data Bank with the following codes: 6LHZ and 6LH8. The raw MS files are available through Zenodo accession number 3813475. GenBank accession number MN787048. All remaining data are contained within the article.
Supplementary Material
This article contains supporting information.
Author contributions—Q. W., X. B., L. Z., W. L., Y. X., S. L., M. T., X. L., X. G., and Y. Z. data curation; Q. W., X. B., and L. Z. methodology; Q. W. writing-original draft; Q. W., X. L., X. G., and Y. Z. writing-review and editing; Y. Z. project administration; X. G. and Y. Z. funding acquisition.
Funding and additional information—This work was supported by grants from the National Natural Science Foundation of China (Grants 31572268, U1602225, and 31872226) and the Yunling Scholar Program to Y.Z., the Light of West China Talents Training Program of the Chinese Academy of Sciences, and the Project of Applied Basic Research of Yunnan Province (Grant 2018FB049) to X.G.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- PFP
- pore-forming protein
- ALP
- aerolysin-like protein
- TFF
- trefoil factor
- RBC
- red blood cell
- ROS
- reactive oxygen species
- SeMet
- selenomethionine
- ANOVA
- analysis of variance
- βγ-CAT
- βγ-crystallin-aerolysin and trefoil factor.
References
- 1. Bischofberger M., Gonzalez M. R., and van der Goot F. G. (2009) Membrane injury by pore-forming proteins. Curr. Opin. Cell Biol. 21, 589–595 10.1016/j.ceb.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 2. Bischofberger M., Iacovache I., and van der Goot F. G. (2012) Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275 10.1016/j.chom.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 3. Afghah Z., Chen X., and Geiger J. D. (2020) Role of endolysosomes and inter-organellar signaling in brain disease. Neurobiol. Dis. 134, 104670 10.1016/j.nbd.2019.104670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang F., Gomez-Sintes R., and Boya P. (2018) Lysosomal membrane permeabilization and cell death. Traffic 19, 918–931 10.1111/tra.12613 [DOI] [PubMed] [Google Scholar]
- 5. Perrin P., Jongsma M. L., Neefjes J., and Berlin I. (2019) The labyrinth unfolds: architectural rearrangements of the endolysosomal system in antigen-presenting cells. Curr. Opin. Immunol. 58, 1–8 10.1016/j.coi.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 6. Scita G., and Di Fiore P. P. (2010) The endocytic matrix. Nature 463, 464–473 10.1038/nature08910 [DOI] [PubMed] [Google Scholar]
- 7. Parker M. W., and Feil S. C. (2005) Pore-forming protein toxins: from structure to function. Prog. Biophys. Mol. Biol. 88, 91–142 10.1016/j.pbiomolbio.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 8. Salvador-Gallego R., Mund M., Cosentino K., Schneider J., Unsay J., Schraermeyer U., Engelhardt J., Ries J., and Garcia-Saez A. J. (2016) Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 35, 389–401 10.15252/embj.201593384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V. G., Wu H., and Lieberman J. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bharadwaj P., Wijesekara N., Liyanapathirana M., Newsholme P., Ittner L., Fraser P., and Verdile G. (2017) The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J. Alzheimers Dis. 59, 421–432 10.3233/JAD-161192 [DOI] [PubMed] [Google Scholar]
- 11. Galvin B. D., Kim S., and Horvitz H. R. (2008) Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33. Genetics 179, 403–417 10.1534/genetics.108.087221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogawa M., Takahashi T. C., Takabatake T., and Takeshima K. (1998) Isolation and characterization of a gene expressed mainly in the gastric epithelium, a novel member of the ep 37 family that belongs to the βγ-crystallin superfamily. Dev. Growth Differ. 40, 465–473 10.1046/j.1440-169x.1998.t01-2-00001.x [DOI] [PubMed] [Google Scholar]
- 13. Fivaz M., Abrami L., Tsitrin Y., and van der Goot F. G. (2001) Aerolysin from Aeromonas hydrophila and related toxins. Curr. Top. Microbiol. Immunol. 257, 35–52 [DOI] [PubMed] [Google Scholar]
- 14. Szczesny P., Iacovache I., Muszewska A., Ginalski K., van der Goot F. G., and Grynberg M. (2011) Extending the aerolysin family: from bacteria to vertebrates. PLoS ONE 6, e20349 10.1371/journal.pone.0020349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y. (2015) Why do we study animal toxins? Zool. Res. 36, 183–222 10.13918/j.issn.2095-8137.2015.4.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu X., and Lai R. (2015) The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 115, 1760–1846 10.1021/cr4006704 [DOI] [PubMed] [Google Scholar]
- 17. Jared S. R., and Rao J. P. (2017) Transepithelial sodium transport across frog skin. Adv. Physiol. Educ. 41, 444–447 10.1152/advan.00115.2016 [DOI] [PubMed] [Google Scholar]
- 18. Liu S. B., He Y. Y., Zhang Y., Lee W. H., Qian J. Q., Lai R., and Jin Y. (2008) A novel non-lens βγ-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS ONE 3, e1770 10.1371/journal.pone.0001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao Q., Xiang Y., Zeng L., Ma X. T., Lee W. H., and Zhang Y. (2011) Characterization of the βγ-crystallin domains of βγ-CAT, a non-lens βγ-crystallin and trefoil factor complex, from the skin of the toad Bombina maxima. Biochimie 93, 1865–1872 10.1016/j.biochi.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 20. Guo X. L., Liu L. Z., Wang Q. Q., Liang J. Y., Lee W. H., Xiang Y., Li S. A., and Zhang Y. (2019) Endogenous pore-forming protein complex targets acidic glycosphingolipids in lipid rafts to initiate endolysosome regulation. Commun. Biol. 2, 59 10.1038/s42003-019-0304-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang Y., Yan C., Guo X., Zhou K., Li S., Gao Q., Wang X., Zhao F., Liu J., Lee W.-H., and Zhang Y. (2014) Host-derived, pore-forming toxin–like protein and trefoil factor complex protects the host against microbial infection. Proc. Natl. Acad. Sci. U.S.A. 111, 6702–6707 10.1073/pnas.1321317111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S. A., Liu L., Guo X. L., Zhang Y. Y., Xiang Y., Wang Q. Q., Lee W. H., and Zhang Y. (2017) Host pore-forming protein complex neutralizes the acidification of endocytic organelles to counteract intracellular pathogens. J. Infect. Dis. 215, 1753–1763 10.1093/infdis/jix183 [DOI] [PubMed] [Google Scholar]
- 23. Gao Z. H., Deng C. J., Xie Y. Y., Guo X. L., Wang Q. Q., Liu L. Z., Lee W. H., Li S. A., and Zhang Y. (2019) Pore-forming toxin-like protein complex expressed by frog promotes tissue repair. FASEB J. 33, 782–795 10.1096/fj.201800087R [DOI] [PubMed] [Google Scholar]
- 24. Cirauqui N., Abriata L. A., van der Goot F. G., and Dal Peraro M. (2017) Structural, physicochemical and dynamic features conserved within the aerolysin pore-forming toxin family. Sci. Rep. 7, 13932 10.1038/s41598-017-13714-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jia N., Liu N., Cheng W., Jiang Y. L., Sun H., Chen L. L., Peng J., Zhang Y., Ding Y. H., Zhang Z. H., Wang X., Cai G., Wang J., Dong M. Q., Zhang Z., et al. (2016) Structural basis for receptor recognition and pore formation of a zebrafish aerolysin-like protein. EMBO Rep. 17, 235–248 10.15252/embr.201540851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parker M. W., Buckley J. T., Postma J. P. M., Tucker A. D., Leonard K., Pattus F., and Tsernoglou D. (1994) Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 367, 292–295 10.1038/367292a0 [DOI] [PubMed] [Google Scholar]
- 27. Demori I., Rashed Z. E., Corradino V., Catalano A., Rovegno L., Queirolo L., Salvidio S., Biggi E., Zanotti-Russo M., Canesi L., Catenazzi A., and Grasselli E. (2019) Peptides for skin protection and healing in amphibians. Molecules 24, 347 10.3390/molecules24020347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dal Peraro M., and van der Goot F. G. (2016) Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 10.1038/nrmicro.2015.3 [DOI] [PubMed] [Google Scholar]
- 29. Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.-C., and Shao F. (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- 30. Evavold C. L., Ruan J., Tan Y., Xia S., Wu H., and Kagan J. C. (2018) The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 10.1016/j.immuni.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang L., Rouge P., and Van Damme E. J. M. (2017) Amaranthin-like proteins with aerolysin domains in plants. Front. Plant Sci. 8, 1368 10.3389/fpls.2017.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knapp O., Stiles B., and Popoff M. R. (2010) The aerolysin-like toxin family of cytolytic, pore-forming toxins. Open Toxinol. J. 3, 53–68 10.2174/1875414701003010053 [DOI] [Google Scholar]
- 33. Buckley J. T., Wilmsen H. U., Lesieur C., Schulze A., Pattus F., Parker M. W., and van der Goot F. G. (1995) Protonation of histidine-132 promotes oligomerization of the channel-forming toxin aerolysin. Biochemistry 34, 16450–16455 10.1021/bi00050a028 [DOI] [PubMed] [Google Scholar]
- 34. Heneka M. T., McManus R. M., and Latz E. (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 19, 610–621 10.1038/s41583-018-0055-7 [DOI] [PubMed] [Google Scholar]
- 35. Wang S., Yuan Y. H., Chen N. H., and Wang H. B. (2019) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson's disease. Int. Immunopharmacol. 67, 458–464 10.1016/j.intimp.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 36. Siddiqui W. A., Ahad A., and Ahsan H. (2015) The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch. Toxicol. 89, 289–317 10.1007/s00204-014-1448-7 [DOI] [PubMed] [Google Scholar]
- 37. Merle N. S., Church S. E., Fremeaux-Bacchi V., and Roumenina L. T. (2015) Complement system part I: molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao F., Yan C., Wang X., Yang Y., Wang G., Lee W., Xiang Y., and Zhang Y. (2014) Comprehensive transcriptome profiling and functional analysis of the frog (Bombina maxima) immune system. DNA Res. 21, 1–13 10.1093/dnares/dst035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y., Yu G., Wang Y., Xiang Y., Gao Q., Jiang P., Zhang J., Lee W., and Zhang Y. (2011) Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cell. Mol. Life Sci. 68, 3771–3780 10.1007/s00018-011-0678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeng L., Sun Q. Y., Jin Y., Zhang Y., Lee W. H., and Zhang Y. (2012) Molecular cloning and characterization of a complement-depleting factor from king cobra, Ophiophagus hannah. Toxicon 60, 290–301 10.1016/j.toxicon.2012.04.344 [DOI] [PubMed] [Google Scholar]
- 41. Wang Y. J., Guo X. L., Li S. A., Zhao Y. Q., Liu Z. C., Lee W. H., Xiang Y., and Zhang Y. (2014) Prohibitin is involved in the activated internalization and degradation of protease-activated receptor 1. Biochim. Biophys. Acta 1843, 1393–1401 10.1016/j.bbamcr.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y., Wang Y., Xiang Y., Lee W., and Zhang Y. (2012) Prohibitins are involved in protease-activated receptor 1-mediated platelet aggregation. J. Thromb. Haemost. 10, 411–418 10.1111/j.1538-7836.2011.04607.x [DOI] [PubMed] [Google Scholar]
- 43. Shen H., Zhu Y., Wang C., Yan H., Teng M., and Li X. (2016) Structural and histone binding ability characterization of the ARB2 domain of a histone deacetylase Hda1 from Saccharomyces cerevisiae. Sci. Rep. 6, 33905 10.1038/srep33905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Q.-S., Zhang K.-H., Cui Y., Wang Z.-J., Pan Q.-Y., Liu K., Sun B., Zhou H., Li M.-J., Xu Q., Xu C.-Y., Yu F., and He J.-H. (2018) Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl. Sci. Tech. 29, 68 10.1007/s41365-018-0398-9 [DOI] [Google Scholar]
- 45. Brodersen D. E., de La Fortelle E., Vonrhein C., Bricogne G., Nyborg J., and Kjeldgaard M. (2000) Applications of single-wavelength anomalous dispersion at high and atomic resolution. Acta Crystallogr. D Biol. Crystallogr. 56, 431–441 10.1107/s0907444900000834 [DOI] [PubMed] [Google Scholar]
- 46. Adams P. D., Afonine P. V., Bunkoczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Terwilliger T. C., Grosse-Kunstleve R. W., Afonine P. V., Moriarty N. W., Zwart P. H., Hung L. W., Read R. J., and Adams P. D. (2008) Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 10.1107/S090744490705024X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., and Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 10.1107/s0907444902016657 [DOI] [PubMed] [Google Scholar]
- 49. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 51. Murshudov G. N., Skubak P., Lebedev A. A., Pannu N. S., Steiner R. A., Nicholls R. A., Winn M. D., Long F., and Vagin A. A. (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., and Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 10.1107/S0907444912001308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen V. B., Arendall W. B. III, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 10.1107/S0907444909042073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The structures presented in this paper have been deposited in the Protein Data Bank with the following codes: 6LHZ and 6LH8. The raw MS files are available through Zenodo accession number 3813475. GenBank accession number MN787048. All remaining data are contained within the article.