Abstract
The gastrointestinal tract is a highly proliferative and regenerative tissue. The intestine also harbors a large and diverse microbial population collectively called the gut microbiome (microbiota). The microbiome–intestine cross-talk includes a dynamic exchange of gaseous signaling mediators generated by bacterial and intestinal metabolisms. Moreover, the microbiome initiates and maintains the hypoxic environment of the intestine that is critical for nutrient absorption, intestinal barrier function, and innate and adaptive immune responses in the mucosal cells of the intestine. The response to hypoxia is mediated by hypoxia-inducible factors (HIFs). In hypoxic conditions, the HIF activation regulates the expression of a cohort of genes that promote adaptation to hypoxia. Physiologically, HIF-dependent genes contribute to the aforementioned maintenance of epithelial barrier function, nutrient absorption, and immune regulation. However, chronic HIF activation exacerbates disease conditions, leading to intestinal injury, inflammation, and colorectal cancer. In this review, we aim to outline the major roles of physiological and pathological hypoxic conditions in the maintenance of intestinal homeostasis and in the onset and progression of disease with a major focus on understanding the complex pathophysiology of the intestine
Keywords: hypoxia, hypoxia-inducible factor 1α (HIF-1α), HIF-2α, mucosal barrier, hypoxia-inducible factor (HIF), intestinal epithelium, intestinal metabolism, inflammatory bowel disease (IBD), inflammation, iron, iron metabolism, colitis, colon cancer
The primary functions of the intestine are absorption and processing of nutrients, fluid homeostasis, removal of waste, and the maintenance of oral tolerance toward luminal antigens (1). The intestine is a highly unique tissue that is adjacent to a diverse and dense microbial population, called the microbiota. The microbiota is essential in the breakdown of dietary nutrients and regulation of intestinal and systemic immune responses. Microbiota also produce small molecules critical for intestinal metabolism. In addition, bacterial metabolism generates several gasses that can modulate cellular function. The microbiota mostly consists of anaerobes that decrease environmental O2, and therefore the intestine is highly hypoxic compared with most issues. Due to the central role of O2 in our biology, the intestine is highly dependent on the adaptive pathways activated by hypoxia. This is a rapidly evolving and changing field at the intersection between physiology and biological chemistry. Recent studies demonstrate a central role of oxygen dynamics in regulating intestinal homeostasis and disruption of the oxygen gradient is responsible for many intestinal diseases, including inflammatory bowel disease (IBD) and colorectal cancer (CRC) (2). Currently, several new drugs that target oxygen-sensing pathways have been approved or are in clinical trials. In this review, we summarize the unique gas exchange and metabolic cross-talk of the microbiota and intestine that are critical in maintaining a hypoxic response in intestinal health and disease.
Regulation of oxygen tension in the intestine by the microbiota
In an adult humans, the intestinal epithelium forms a monolayer of cells that covers an area of ∼250–300 m2 (3). The small intestine is organized into crypt-villus units. Villi are finger-like projections that are essential in the absorption of nutrients. At the base of each villus, invaginations form the crypt where intestinal stem cells reside. The colon is distally localized along the gastrointestinal tract to the small intestine and is comprised only of crypts. The small intestinal and colonic epithelium consist of highly specialized post-mitotic cells, including enterocytes that help in absorption and goblet, Paneth, and enteroendocrine cells that aid in secretion of intestinal hormones, mucus, and anti-bacterial peptides. The small intestinal and colonic epithelium renew after every 5–6 days from mitotic stem cells and early progenitor transit-amplifying cells. Underneath the epithelium, there resides a vascular and lymphatic network critical for nutrient transport and tissue oxygenation. Moreover, intestinal tissue oxygenation is regulated by luminal oxygen sources, local metabolism due to the high cell turnover, and intestinal vascularization.
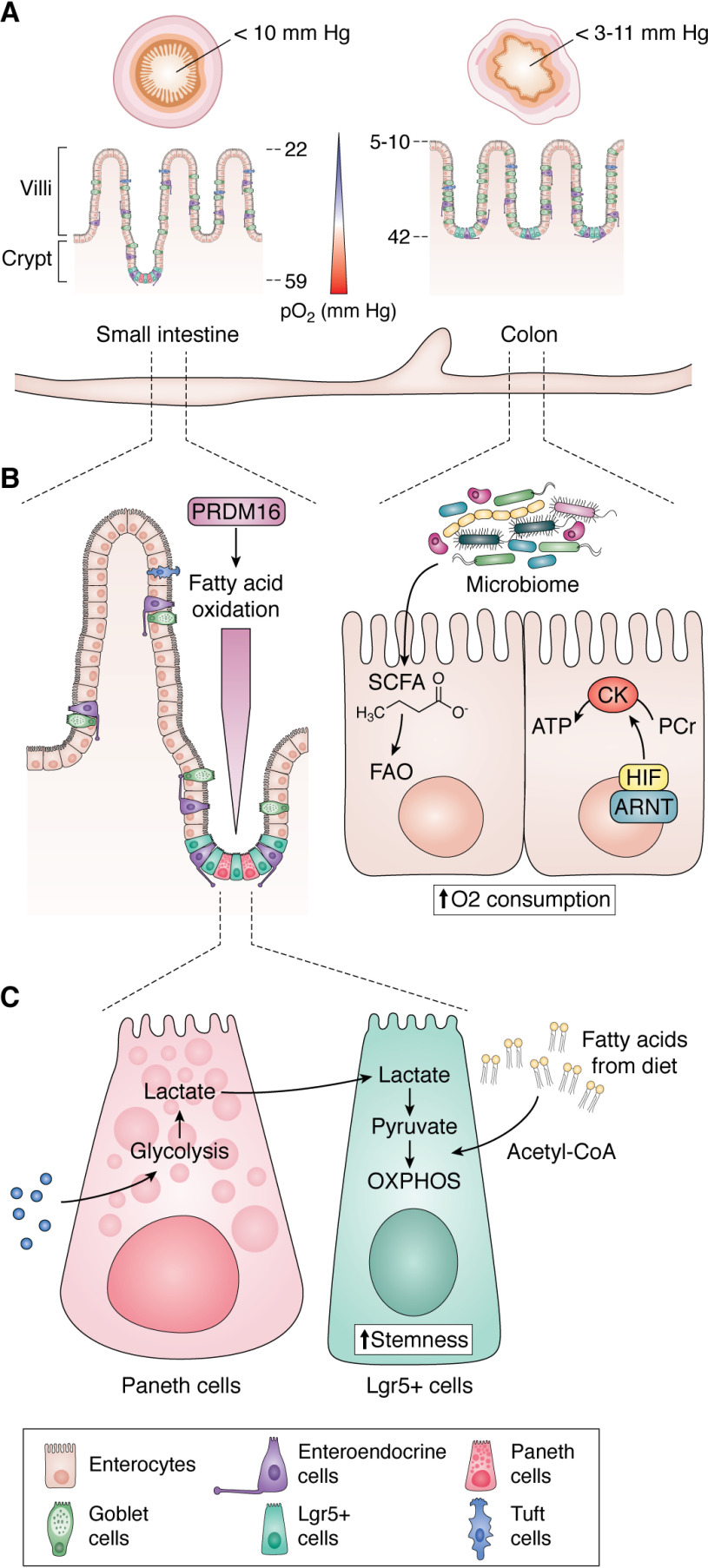
A unique oxygen gradient exists within the human intestinal tract. A vertical oxygen gradient has been documented in more distal, colonic regions of the gastrointestinal (GI) tract, from the anaerobic lumen across the epithelium to the richly vascularized subepithelial mucosa (4). The partial pressure (pO2) of air at sea level is ∼145 mm Hg (∼21% O2). In contrast, at baseline, epithelial cells lining the intestinal mucosa exist in a relatively low-pO2 environment. Noninvasive techniques such as EPR oximetry that measures pO2 of tissues estimate 42–71 mm Hg (7–10%) across the colonic muscle wall to around ∼42 mm Hg (∼6%) in the vascularized submucosa, 5-10 mm Hg near the crypt-lumen interface, and 11 (∼2%) and 3 mm Hg (∼0.4%) in the lumen of ascending and sigmoid colon, respectively (5). EPR oximetry estimates pO2 of 59 mm Hg (8%) in the small intestinal wall to around 22 mm Hg (3%) at the villus tip and <10 mm Hg (2%) in the small intestinal lumen (Fig. 1A) (6). In comparison, the healthy lung alveoli have a pO2 of 100–110 mm Hg, and 60–65 mm Hg is observed in the periportal area of the liver (7). Lymphoid tissues such as bone marrow and spleen have a lower oxygen concentration of 50 mm Hg (8) and 25–35 mm Hg (9), respectively. The pO2 drops precipitously along the radial axis from the intestinal submucosa to the lumen, which is home to trillions of anaerobic microbes.
Figure 1.
Intestinal O2 and metabolic regulation. A, countercurrent blood flow reduces local pO2 along the crypt-villus axis and the microbiome alters pO2 from small intestine to the colon. B, transit-amplifying cells of the proximal small intestine rely on fatty acid oxidation (FAO) transcriptionally regulated by PRMD16 to support the differentiation of intestinal epithelial cells. Colonocytes rely on SCFAs for fuel. Increased utilization of SCFAs increases oxygen consumption and activates HIF activity, contributing to the basal hypoxic tone of the intestine. During high ATP demand in the intestine, HIF-1α can replenish the ATP pool via the PCr/CK system. C, Lgr5+ stem cells are dependent on lactate exchange from neighboring Paneth cells. Paneth cells fuel oxidative phosphorylation to maintain stemness and diets high in fat fuel fatty acid oxidation to increase the stemness of Lgr5 stem cell cells.
The microbiota is critical in establishing the hypoxic intestinal microenvironment. Newborn intestines are relatively better oxygenated compared with those of adults. Recent data demonstrate that maternal microbiota seeds the offspring microbiota at birth. Diversity and density of the microbiota increases and stabilizes to adult-like by the first 3 years of life (10, 11). Studies demonstrate that the initial bacterial community is composed of aerobic and facultative anaerobic bacteria that consume oxygen in the intestine and thus provide an optimal niche for anaerobic bacteria (12). The number and diversity of commensals increase down the intestinal longitudinal axis from the small intestine to the colon (12). Moreover, the utilization of oxygen by aerobic bacteria near the epithelium and rise in tissue oxygenation lead to an increase in the population of aerotolerant microbes at the epithelia-lumen interface and renders the central portion of the lumen deficient in oxygen (13). The variation in luminal oxygen concentration in intestinal disease and the consequent effects on hosts are not currently known. However, many GI diseases are thought to have high oxidative stress, which can influence luminal oxygen levels and lead to dysbiosis (2). In addition, some studies show that the use of antibiotics can also alter the intestinal oxygen gradient and the microbiota (14).
Hypoxia and HIF signaling
Oxygen and metabolic regulation of HIFs
The adaptation to hypoxia at the cellular level in the intestine is regulated primarily by hypoxia-inducible factors (HIFs) (2). In hypoxic conditions, HIF stabilization drives the expression of genes that aid in the adaptation to hypoxia, which primarily includes regulators of erythropoiesis (erythropoietin; EPO), angiogenesis (vascular endothelial growth factor; VEGF), and metabolism (glycolytic enzymes) (15–17). HIFs are a heterodimer of an α- and β-subunit. The HIF-α subunit belongs to the basic helix-hoop-helix Per-Arnt-Sim (bHLH-PAS) family of transcription factors (18). Vertebrates have three α-subunits: HIF-1α, HIF-2α, and HIF-3α. This review primarily focuses on HIF-1α and HIF-2α; very little has been done to assess the role of HIF-3α in the intestine.
The N-terminal region of HIF-α subunit contains a domain that is required for DNA binding and heterodimerization (19). HIF-α subunit contains a highly conserved oxygen-dependent degradation domain (ODD). The ODD domain contains two prolines that are hydroxylated, 402 and 562 on HIF-1α and prolines 405 and 531 on HIF-2α (20). Hydroxylation of HIF-α leads to the proteasomal degradation. The enzymes that hydroxylate HIF-α, the prolyl hydroxylase domain enzymes (PHDs), consisting of PHD1 (EGLN2), PHD2 (EGLN1), and PHD3 (EGLN3), are the major oxygen sensors in a cell. PHDs are α-ketoglutarate–dependent dioxygenases. PHDs use O2 to hydroxylate the HIF-α subunit on the prolines in the ODD. Hydroxylation enables the binding of the Von Hippel–Lindau (VHL) tumor suppressor protein, which is an E3 ubiquitin ligase (21–23). In low-oxygen conditions, the PHD enzymes can no longer utilize O2 for HIF-α hydroxylation (Fig. 2). HIF-α is stabilized and heterodimerizes with a constitutively expressed HIF-β subunit also known as aryl hydrocarbon receptor nuclear translocator (ARNT) (24). This heterodimerization enables binding to HIF response elements (HREs) in the promoters of target genes (25, 26).
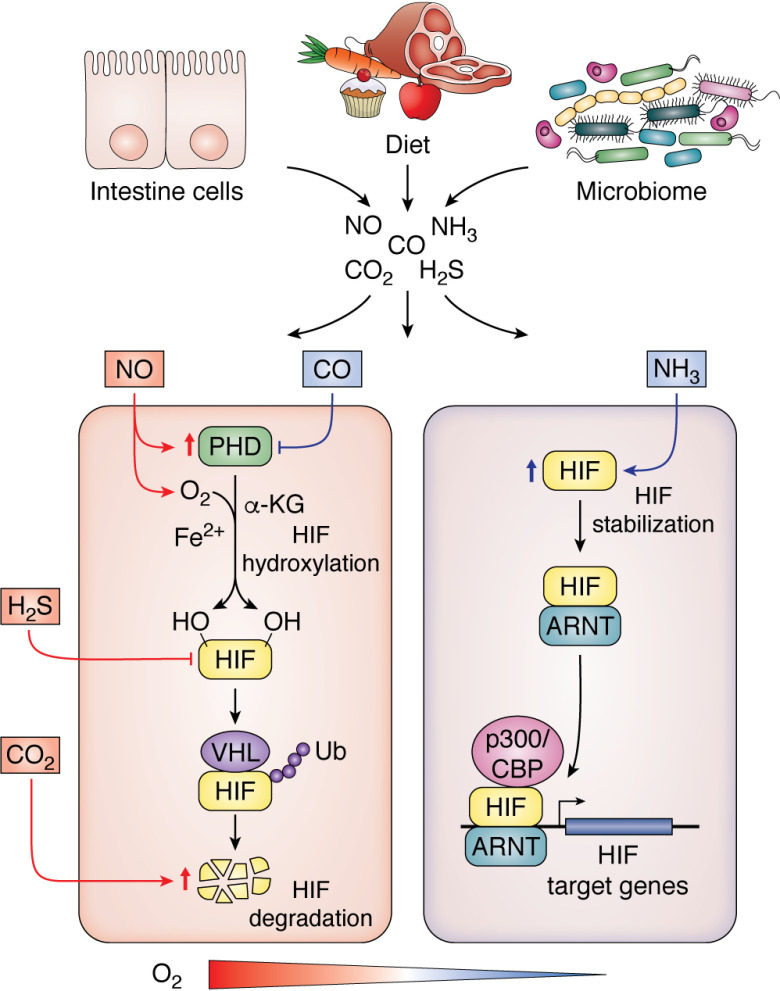
Figure 2.
Intestinal gas exchange and hypoxic signaling. In the presence of O2, α-ketoglutarate (α-KG), and Fe2+, PHD enzymes hydroxylate the two proline residues on HIF, enabling the binding of VHL, a tumor suppressor protein, to the HIF-α subunit. VHL binding is coupled with E3 ubiquitin ligase activity, which degrades HIF-α subunits under normoxic conditions. As oxygen, iron, or α-ketoglutarate levels decrease in a cell, PHDs are no longer active. In addition, if succinate, fumarate, or ROS levels are elevated, PHD activity is decreased. HIF-α is stabilized and binds to ARNT to activate transcription of target genes. The intestinal cells, diet, and microbiome generate numerous gaseous signaling mediators that cross-talk with HIF signaling. NO, H2S, and CO2 inhibit HIF activity, whereas carbon monoxide and NH3 activate HIF (red lines indicate inhibitory mechanism, and blue lines denote activation).
Hypoxia is the best-studied mechanism leading to the activation of HIFs. However, PHDs use both O2 and α-ketoglutarate as substrates, leading to protein hydroxylation, succinate, and CO2 as products of the reaction. α-Ketoglutarate is an intermediate metabolite of the TCA cycle. In intestinal epithelial cells, α-ketoglutarate is not limiting and is required for succinyl-CoA production. The requirement of α-ketoglutarate for PHD activity thus links HIF stability to cellular metabolism. Isocitrate dehydrogenase 1 (IDH1) and IDH2 are enzymes that generate α-ketoglutarate, and mutations in these enzymes show a robust HIF gene signature (27). IDH mutations lead to decreased IDH activity and lower conversion of isocitrate to α-ketoglutarate and a gain of neomorphic enzyme activity, resulting in high levels of 2-hydroxyglutarate (28). 2-Hydroxyglutarate is a competitive inhibitor of α-ketoglutarate that decreases PHD activity (28). In addition, other metabolites important in cell metabolism can also alter HIF-α stability. Succinate is generated following HIF-α hydroxylation. Tumors with elevated succinate levels due to mutations in succinate dehydrogenase increase HIF-1α stability (29). Succinate levels are also elevated in LPS-treated macrophages, resulting in activation of HIF-1α and a glycolytic phenotype (30). Because succinate is generated following HIF-α hydroxylation, succinate can directly inhibit PHD activity by product inhibition. PHDs require Fe2+ as an essential co-factor for enzymatic activity (31). Therefore, limiting intracellular Fe2+ levels potently activate HIFs. Last, mitochondrial reactive oxygen species (ROS) are critical for the activation of HIFs (32, 33). The exact mechanism of ROS-mediated HIF-1α stabilization is unknown. But it is shown that hypoxia increases mitochondrial ROS generation at Complex III, which causes accumulation of HIF-1α protein. This response is lost in cells depleted of mitochondrial DNA (ρ° cells) (32). Several mitochondrial intermediates, such as succinate and fumarate, result in ROS production and can indirectly activate HIF-1α (34, 35). Succinate can drive reverse electron transport through complex II, which is a major source of ROS (36). Fumarate directly binds the antioxidant GSH in vitro and in vivo to produce a novel metabolite that can decrease NADPH levels and increase mitochondrial ROS (34). The mechanisms by which redox balance alters HIF-1α stability have not been clearly defined, but ROS can induce nonenzymatic decarboxylation of α-ketoglutarate and/or oxidize Fe2+ to Fe3+.
Studies assessing the role of mitochondrial intermediates and/or ROS cross-talk to HIF pathways have not assessed the role of these pathways in vivo in the intestine. Moreover, the majority of studies have focused on HIF-1α. Although HIF-1α and HIF-2α share 48% overall amino acid identity and can bind to the same response elements, they exhibit considerable differences in their expression pattern and function. They can regulate both distinct and overlapping genes. HIF-1α is ubiquitously expressed in all cell types (37). HIF-2α expression is selective (38–42), but it is highly expressed in intestinal epithelial cells (43, 44). HIF-1α regulates 80% of the glycolytic-related genes, including phosphofructokinase, pyruvate dehydrogenase kinase, and lactate dehydrogenase A. HIF-2α selectively regulates EPO, and iron-regulatory genes, such as DMT1 and CYBRD1 (which encodes DCYTB) (45). In addition, both HIF-1α and HIF-2α induce a large battery of genes that are up-regulated during injury, inflammation, and cancer. Thus, studies are needed to demonstrate the interplay of redox balance and mitochondrial metabolism on HIF-2α expression and activity. It will be critical to define how mitochondrial metabolism and redox balance integrate and contribute to regulate HIF-1α and HIF-2α expression in the hypoxic intestinal environment.
Hypoxia and intestinal cellular metabolism
During low-oxygen conditions, cells adapt to hypoxic stress by inducing the expression of several genes involved in energy metabolism. Many of the metabolic responses to hypoxia are orchestrated by HIFs. The roles of HIF-1α and/or HIF-2α in the induction of genes encoding for enzymes involved in glycolytic, carbohydrate, fatty acid, mitochondrial, and peroxisomal metabolism have been reviewed extensively elsewhere (46, 47). HIFs fulfill the high cellular metabolic demands for glucose, protein, and lipids in oxygen starved cells, and this function is conserved in many cell types and tissues. Moreover, in tissues the cellular metabolic pathways utilized for growth and function largely depend on the proliferative nature of the cell type. As mentioned above, the colon and small intestine contain both highly proliferative stem cells and transit-amplifying progenitor population and post-mitotic differentiated cells (48). The post-mitotic differentiated cells have a high rate of energy expenditure due to energy-consuming digestive, secretory, and absorptive processes (49). Recent work identified PRDM16 as a central transcription factor that regulates intestinal fatty acid oxidation. The deletion of PRDM16 in mice triggered apoptosis in the transit-amplifying progenitor cells (50). This led to a decrease in epithelial cell differentiation (50). The critical role of PRMD16-induced fatty acid oxidation was localized to the proximal small intestine and was dispensable for the distal small intestine or colonic metabolism (Fig. 1B).
A critical regulator of tissue homeostasis is crypt base columnar (CBC) stem cells (51). CBC stem cells represent a population of rapidly dividing cells at the base of a crypt in the small intestine (52). CBC stem cells are responsible for all terminally differentiated intestinal epithelial cells (enterocytes, Paneth, goblet, enteroendocrine, tuft, and M cells) (53). There is an increasing demand for mitochondrial oxidative phosphorylation in CBCs to sustain the immense amount of cell turnover in the small intestine (54). To fuel the oxidative phosphorylation of CBCs, Paneth cells that intercalate between CBCs are involved in continuous metabolic exchange with CBCs in which lactate from Paneth cells fuels oxidative phosphorylation in CBCs (Fig. 1B). Inhibition of glycolysis led to a decrease in lactate production in Paneth cells and subsequently altered Lgr5+ CBC function and a decrease in crypt maturation (55). Consistent with these data, the promotion of fatty acid oxidation to fuel oxidative phosphorylation enhances small intestinal stem cell function (56, 57). The colon is relatively more hypoxic than the small intestine and largely relies on commensally derived fuel sources in the form of short-chain fatty acids (SCFAs) (58). The SCFA butyrate can reach up to 30 mm in the colon, and it serves as an ideal metabolic substrate for colonic epithelial cells as acetyl-CoA derived from butyrate is readily made accessible for oxidative phosphorylation (59). A healthy colon derives about 30% of energy from butyrate (Fig. 1B) (60). Butyrate via fueling oxidative phosphorylation (O2-consuming process) is critical for initiating and sustaining a hypoxic/HIF gradient in the intestine (Fig. 1B) (61). A recent report by Kelly et al. (61) highlights the importance of butyrate in influencing colonic epithelial cell growth through the regulation of physiologic hypoxia. Butyrate increases colon epithelial O2 consumption via driving oxidative phosphorylation and stabilizes HIF (61, 62). Depleting microbiota by antibiotics reduced butyrate levels in the colon and decreased HIF expression, which could be restored by supplementing butyrate. Regulation of cellular metabolism is a well-conserved HIF transcriptional program. However, the disruption of HIF-1α or HIF-2α in the intestinal epithelial results in no major basal phenotype in mice. Therefore, extensive work in this area is needed to understand whether HIF-1α and HIF-2α play overlapping roles in regulating intestinal epithelial cellular metabolism or if additional HIF-independent pathways regulate intestinal metabolism. During stress conditions, such as in intestinal injury, the injured foci is highly hypoxic, attributable to several factors that disrupt the oxygen gradient that is established in the normal intestine. Inflammatory tissue injury leads to vascular injury, which diminishes the countercurrent circulatory exchange in the intestine. Moreover, the influx of highly active immune cells that are dependent on continuous glycolysis to fuel rapid ATP production can consume local oxygen and drive local intestinal hypoxia (63). Moreover, wound repair is a highly energy-demanding process that requires increased ATP to maintain hemostasis, inflammation, proliferation, and remodeling that occurs for the tissue to fully heal (64). HIFs are critical in the intestinal wound repair response by sustaining ATP pools through regulation of the creatine/creatine kinase (Cr/CK) axis. HIF-2α regulates and coordinates the expression of CKs, an essential metabolic enzyme for ATP generation. Cr can be phosphorylated by CK, resulting in phosphocreatine (PCr) and ADP. Thus, PCr is a stable readily accessible substrate of high-energy phosphate for the replenishment of the ATP pool during high energy demand (65) (Fig. 1B).
Hypoxic cross-talk with gaseous signaling mediators
The intestinal microenvironment due to both microbiome and host metabolism leads to high levels of biological gases and provides a unique microenvironment, where many gaseous molecules interact with HIFs. This complex interaction plays a central role in health and disease. The small, endogenous, and diffusible gaseous mediators nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and ammonia (NH4) play numerous physiological roles. We summarize what is known about these gasotransmitters and cross-talk to hypoxic signaling.
Nitric oxide
In the gastrointestinal tract, NO production is regulated via nitric oxide synthase or by bacterially mediated mechanisms (66). The constitutively expressed and inducible isoforms of nitric oxide synthase are responsible for the enzymatic production of NO. Nitric oxide can also be formed from dietary nitrate, which in the oral cavity is reduced by bacterial reductases to nitrite, yielding NO gas after acidification in the gastric lumen (67). Nitric oxide production from the reaction of hydrogen peroxide with arginine is another example of nonenzymatic NO production (68). NO can readily interact with cytochrome c oxidase (CoX), the terminal enzyme in the mitochondrial electron transport chain and the primary site of cellular O2 consumption in mammals (69). Many factors, including local NO and O2 concentrations and also the redox state of CoX, regulate NO binding to CoX (70, 71). Under certain instances, which are not completely understood, NO can bind to CoX in the presence of O2, leading to increased O2 availability for prolyl hydroxylation of HIF-1α in HEK293T cells (72, 73). Moreover, multiple studies have shown that NO attenuates HIF-1α accumulation and DNA binding during hypoxia via promoting free cellular iron and/or restoring PHD activity (72, 74–78). During hypoxic conditions, when O2 concentrations are low and CoX is reduced, competitive binding of NO inhibits CoX activity, resulting in decreased O2 consumption and redistribution of cellular O2 (79). Under normoxic conditions, NO is also found to antagonize iron chelator–induced HIF-1α accumulation, which was ascribed to increases in intracellular free iron (80). These data demonstrate that NO directly alters hypoxic sensing.
A study in HEK293 cells has established the mechanism through which NO activates PHD enzymes and destabilizes HIF-1α. Damage of cellular mitochondria increases 3-nitrotyrosine and formation of peroxynitrite in cells. These byproducts increase cellular concentration of α-ketoglutarate and iron, which are essential to activate prolyl hydroxylases (22, 81).
Hydrogen sulfide
Like NO, H2S is produced in abundance in the intestine from microbiota as well as host epithelial cells (82, 83). In the colon lumen, several bacterial species, including Clostridium, Fusobacterium, Escherichia, Salmonella, Klebsiella, Streptococcus, Enterobacter, and Desulfovibrio, can convert cysteine into H2S (84). In fact, numerous bacterial groups convert cysteine to H2S, pyruvate, and ammonia by cysteine desulfhydrase activity (84). H2S measured in the colonic lumen varies from high micromolar to low millimolar concentrations (85, 86). The production of H2S is also influenced by diet; for example, increased protein consumption leads to higher production of H2S, as shown in rat studies (87). In eukaryotic cells, cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase regulate H2S production (88–90). The first evidence of a functional cross-talk of H2S and hypoxia was demonstrated in Caenorhabditis elegans, as the knockdown of HIF alters organismal survival under 50-ppm H2S exposure (91). In mammalian vascular smooth muscle cells, a 300 μm concentration of the H2S donor sodium hydrosulfide (NaHS) induced the up-regulation of HIF-1α mRNA and protein (Fig. 2) (92). However, lower concentrations of about 10 μm NaHS significantly lowered HIF-1α protein levels under both hypoxia and iron chelation (93). In a study utilizing HCT116, HeLa, and HEK293T cells, both HIF-1α ubiquitination and HIF-1α degradation were not changed by H2S treatment. To further elucidate the mechanism, cycloheximide, a translation inhibitor, was assessed. Cycloheximide abrogated the effect of H2S on HIF-1α expression, demonstrating that H2S inhibits HIF-1α translation (94).
Ammonia
Intestinal ammonia is derived via two major routes. Ammonia is liberated from urea in the intestinal lumen by enzymes known as ureases (95). Ureases are products of bacteria that convert urea to ammonia and carbon dioxide. The breakdown of amino acids also generates free ammonia. Ammonia is considered a toxic byproduct of metabolism that leads to cellular stresses. Ammonia is known to inhibit cell growth and cellular metabolism (96). Several studies have reported that increased concentrations of ammonia in cell culture medium may induce cellular apoptosis by increasing levels of ROS (97–99). There exists a negative feedback loop via HIF-1α to mitigate the cellular toxicities of ammonia. In a study on ovarian cancer cells, ammonia blunted PHD activity by unidentified mechanisms and thus stabilized HIF. Several studies demonstrate that ammonium donors such as ammonium chloride lead to increased levels of NO, which may be responsible for inhibition of PHD enzymes and subsequent activation of HIF-1α (100, 101). HIF-1α activation is critical for decreasing ammonia-induced toxicity by modifying cellular energy metabolism and cellular stress response through up-regulation of glycolytic target genes such as PFKFB3 and GLUT-1 (glucose transporter) via HIF (102).
Carbon monoxide
The major cellular mechanism by which carbon monoxide is produced is through heme breakdown by heme oxygenases. Heme degradation results in hydrogen peroxide, Fe2+, and CO (103). Roughly 40–60% of dietary iron is in the form of heme iron, and the intestine expresses several heme oxygenases to liberate iron from heme and produce CO. CO is critical in smooth muscle membrane potential, allowing mechanical contractile responses in the gut (104). CO activates HIF-1α via an indirect mechanism of altering redox balance. In a study in primary cells of human bronchial smooth muscle, CO via mitochondrial oxidases leads to an increase in the production of superoxide and hydrogen peroxide (105). As discussed above, mitochondrial ROS activates HIF-1α, by inhibiting O2 sensors such as PHD enzymes, most likely by oxidation of the Fe(II) that is necessary for PHD function (Fig. 2) (106).
Carbon dioxide (CO2)
CO2 is a major intestinal gas (107). CO2 is a waste metabolite produced by cellular metabolism in glycolysis and the TCA cycle. Moreover, CO2 can be produced following food intake and is the major gas generated in distal small intestine and colon due to bacterial fermentation (107). Although the physiological levels of CO2 produced in the intestine are not thought to have a major role in HIF signaling, hypercapnia (increased CO2 levels) is associated with several diseased states, such as respiratory disorders, which can lead to altered HIF signaling (108). Several studies have established increased localization and subsequent degradation of HIF-1α in lysosomes, which are central hubs for protein degradation via high acidity and proteolytic enzyme activity (109, 110). Although the mechanisms are still not clear, a study utilizing HEK293, A549, HeLa, and HCT116 cells demonstrates that CO2 increases HIF-1α degradation in lysosomes (108) (Fig. 2).
Physiological hypoxia in regulation of barrier function and nutrient absorption
Barrier function
The intestine serves as a crucial barrier between the external (luminal) and internal (vascular) compartments. This barrier is dynamic in nature and maintained mostly by the presence of a mucus layer, intercellular tight junctions, and adherens junctions. Numerous studies have revealed that HIF provides a barrier-protective program in the intestine (111–114). Mouse models disrupted for HIF-1α specifically in intestinal epithelial cells or cell models with a knockdown of HIF-1α demonstrate major defects in the mucosal barrier integrity. A major line of defense to the commensal population is the production of mucus. Goblet cells are specialized intestinal epithelial cells that secrete mucus. HIF-1α directly regulates the transcription of several mucins, which are the major glycoproteins in mucus (115, 116). Moreover, the mucus layer contains a battery of anti-microbial peptides. β-Defensin-1 is secreted by the epithelium into the mucin layer to protect against commensal overgrowth and pathogen infiltration. β-Defensin-1 expression requires HIF-1α activation (117). In addition to the mucus layer, the tight junctions form the core mechanism regulating intestinal barrier integrity. A major tight junctional protein claudin-1 is directly regulated by HIF-1α. Cell models in which HIF-1α is knocked down demonstrate a defect in forming a tight barrier, which can be completely rescued by claudin-1 expression (Fig. 3) (118).
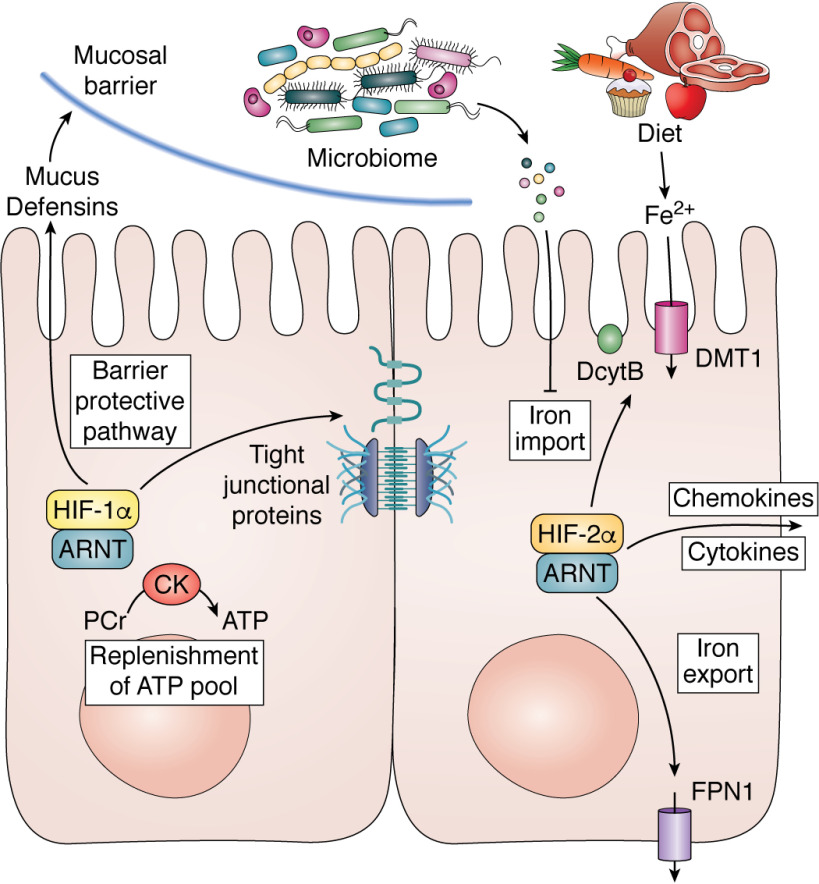
Figure 3.
The role of HIF-1α and HIF-2α in intestinal homeostasis. Activation of HIF-1α in the intestine induces a barrier-protective pathway by increasing mucus, defensin, and tight junctional proteins and replenishing the ATP pool during injury via the PCr/CK system. HIF-2α activation increases the expression of iron-absorptive genes and pro-inflammatory cytokines and chemokines.
In addition to direct regulation of the barrier, HIF-1α mediates several indirect mechanisms to maintain barrier integrity. Trefoil factor-3 is a direct target gene of HIF-1α and plays a critical role in the repair of epithelial surfaces. HIF-1α also regulates the expression of two important membrane-bound proteins, CD39 (ecto-apyrase) and CD73 (ecto-5′-nucleotidase). CD39 is required for enzymatic conversion of ATP/ADP to AMP, and CD73 degrades AMP to adenosine (119). The degradation of ATP by CD39 and CD73 is critical for restoring the barrier (113). Adenosine binding to adenosine 2B receptor activates a signaling cascade initiated by cAMP, resulting in reorganization and increase expression of tight junctions to repair barrier function following injury (120). Last, as mentioned above, injury repair is a highly energy-demanding process, and HIFs are critical in regulating intestinal energy metabolism via the creatine kinase/PCr axis (Fig. 3) (65, 111).
Nutrient absorption
Apart from being a critical barrier, the intestinal epithelium is required for nutrient absorption. Approximately 9 liters of fluid from consumed liquids and secreted digestive fluids per day are absorbed by the epithelium. The function of transporting fluid is carried out through highly coordinated events of ion transport following the regulation of salt and water transport between the lumen of the intestine and the bloodstream. One of the major roles of the intestinal epithelium is in iron absorption, which supports the production of red blood cells or erythropoiesis. HIF-2α is a key regulator in iron absorption (Fig. 3). Iron absorption is locally regulated via the apical expression of a ferric reductase (Dcytb) that reduces iron to Fe2+. Fe2+ is transported into an intestinal epithelial cell by divalent metal transporter-1 (DMT-1; also known as Slc11a2). Iron is either stored in ferritin or exported out via a basolateral transporter ferroportin (FPN; also known as Slc40a1) (45). HIF-2α can directly regulate the expression DcytB, DMT-1, and FPN in conditions of increased iron demand (pregnancy, blood loss, and iron-deficient diet) (121–123). Moreover, there is a liver-derived peptide hormone, hepcidin, that regulates intestinal iron absorption. Hepcidin binds to FPN, resulting in its internalization and degradation (124). Therefore, high hepcidin levels result in degradation of FPN and reduced iron mobilization into the plasma. In times when increased iron absorption is needed (iron deficiency, bleeding, or pregnancy), hepcidin is potently down-regulated, leading to FPN stabilization in the intestine and increased iron export from the intestine to circulation. Recent findings establish a molecular association between liver hepcidin and intestinal HIF-2α (125). A decrease in hepcidin expression results in high iron efflux from the intestinal epithelium. This leads to an iron-deficient state in the epithelium. As mentioned above, PHD enzymes require iron; thus, low hepcidin can increase HIF-2α by decreasing PHD activity. This results in a feed-forward cycle, increasing the expression of apical and basolateral iron transporters. Therefore, in diseases such as hereditary hemochromatosis (iron overload), β-thalassemia, and sickle cell disease, where hepcidin expression is decreased, HIF-2α is essential in the hyperabsorption of dietary iron (126, 127). Last, microbiota cross-talk is crucial for regulating iron homeostasis (128). A recent study identified two iron-responsive gut bacterial metabolites, 1,3-diamino propane and reuterin, as potent inhibitors of HIF-2α via inhibition of heterodimerization. HIF-1α and HIF-2α intestinal epithelial disruption is not associated with major nutrition deficiencies outside of anemia due to the lack of iron absorption. However whole-genome transcriptional responses following HIF-1α and HIF-2α activation have shown several nutrient transporters and/or associated proteins to be highly altered (129, 130). This suggests that the hypoxic/HIF response is essential for iron absorption but could play a modulatory role with respect to other nutrients, such as amino acids, glucose, and fatty acids. Indeed, during nutrient excess, such as a high-fat diet, intestinal HIF-2α is central in the regulation of obesity and secondary pathological events such as liver steatosis and glucose tolerance (131).
Pathological hypoxia in IBD and cancer
Chronic intestinal injury, such as in IBD, is associated with chronic pathological hypoxia (132, 133). Chronic hypoxia can contribute to disease development and progression through the modulation of epithelial and immune responses. This section will focus on intestinal epithelial roles of hypoxia/HIF. However, it should be noted that as immune cells move from the oxygen-rich vasculature to hypoxic inflamed foci, both function and metabolism of immune cells are highly altered and have been recently reviewed (134). IBD is categorized into two major groups: ulcerative colitis (UC) and Crohn's disease (CD) (135). In UC patients, inflammation is localized in the colonic region. In CD patients, inflammatory lesions are seen throughout the GI tract, with ulcerated mucosa and granulomas (136). Both HIF-1α and HIF-2α are expressed in the intestinal epithelial cells of UC and CD patients and in mouse models of colitis (44, 65, 137). As mentioned above, HIF-1α plays a critical role in the mucosal barrier, and conditional deletion of HIF-1α in many acute models of colitis leads to a profound exacerbation of the disease (43). HIF-2α directly regulates a chemokine/cytokine network critical for the recruitment of neutrophils and activation of several pro-inflammatory mediators (2, 44, 129). Moreover, in radiation-induced intestinal toxicity models, HIF-2α is essential in restoring epithelial integrity and decreasing apoptosis by inducing angiogenic gene expression (138). Together, HIF-1α and HIF-2α are critical in the intestinal response following injury, which requires both a neutrophil response to remove the injurious stimuli and restoration of the mucosal barrier. However, in IBD or other chronic models, constitutive HIF-2α but not HIF-1α activation can lead to spontaneous colitis or increase the susceptibility of intestinal injury in acute models of colitis by activating pro-inflammatory genes and decreasing barrier function (44, 65, 139, 140). In chronic hypoxia, HIF-1α expression can be decreased via a miRNA-155, resulting in inhibition of HIF-1α translation (141). Therefore, in chronic colitis that is associated with chronic hypoxia, unrestricted activation of HIF-2α with reduced expression of HIF-1α may potentiate inflammation and injury.
Colon cancer
Due to the continuous growth of cancer cells and high metabolic demand, hypoxia is a hallmark feature of all solid tumors (142, 143). Hypoxia is associated with increased growth, survival, proliferation, invasion, and metastasis and confers resistance to chemo- and radiotherapies (144, 145). CRC remains the second-leading cause of cancer-related deaths in the United States (146). CRC can be either sporadic in nature or inflammation induced. Both sporadic CRC and colitis-associated colon cancer (CAC) share similar genetic alterations; however, there are heterochronic differences in key genes or pathways that distinguish the two types. More than 80% of sporadic CRCs are linked with a loss-of-function mutation of a tumor suppressor gene, adenomatous polyposis coli (APC) (147). Inflammation is the major factor and important driver of tumorigenesis in CAC (148). Compared with sporadic CRC, TP53 mutations occur at an early stage in CAC and usually precede APC mutations in the progression of CAC (149, 150). Studies have reported increased protein expression of HIF-2α in both sporadic CRC and CAC (2). According to the Cancer Genome Atlas, <4% of colon cancers harbor mutations in Vhl, Hif-2α, or PHD enzymes (PHD1–PHD3) (151, 152). Decreased oxygen concentration or altered mitochondrial metabolism in colon cancer are probable mechanisms for HIF activation. However, studies have also reported the role of epigenetic mechanisms in HIF activation (153). Alteration in the methylation pattern of the Hif-2α gene is observed in CRC patients (154). A key role of HIF-2α in colon cancer has been established by HIF-2α knockdown studies on colon cancer–derived cell lines and mouse models (155). These studies demonstrate a decrease in cell growth following HIF-2α knockdown in colorectal cancer cell lines. However, some studies point toward a contrasting anti-tumor role of HIF-2α. Loss of HIF-2α in human SW480 cells caused increased cell growth and proliferation and decreased apoptotic activity (156). These differences may arise due to heterogeneity in different colon cancer–derived cell lines. In mouse models of both sporadic CRC and CAC, activation of HIF-2α increases tumor multiplicity and progression in intestinal epithelial cells (129, 155, 157, 158). Activation of HIF-1α in intestinal epithelial cells did not account for an increase in tumorigenesis in sporadic CRC or CAC mouse models (130). However, it is still unclear whether the activation of HIF-1α in CRC may play a role in response to therapy. Adenosine is a highly immunosuppressive metabolite regulated by HIF-1α and is currently being targeted for cancer immunotherapies (159). Furthermore, the administration of acriflavine, which inhibits both HIF-1α and HIF-2α decreased tumor growth in CAC mouse models significantly (160).
Conclusion and perspectives
The remarkable differences in baseline O2 tension between mucosal tissues in the gastrointestinal tract play a distinctive role in intestinal homeostasis and inflammation. The roles of HIFs in regulating tissue barrier function, metabolism, and inflammatory and immune response in the intestine are areas of active research. Many studies have highlighted the therapeutic potential of targeting hypoxia signaling pathways in intestinal disease. A battery of PHD inhibitors to stabilize HIF-1α has proven to be protective in several models of IBD (132, 161, 162). Of the different inhibitors that are under investigation in clinical trials, the majority are competitive inhibitors of PHD co-substrate 2-oxoglutarate (163). There are three known PHD enzymes in the intestine as discussed above. Currently, the substrate specificity and selectivity are not known for each PHD. A detailed understanding of PHD selectivity in different intestinal cells may help to envisage the effect of pan-hydroxylase inhibition. Recently, direct inhibitors of HIF-2α have been characterized and could be useful in IBD and CRC treatments (164–166). Structural studies demonstrated a unique hydrophobic pocket on HIF-2α, which subsequently has been used to target small molecules to inhibit HIF-2α heterodimerization (165). These are the first on-target inhibitors of HIF-2α and are in clinical trials for studies of clear cell renal cell carcinomas (167, 168). Additionally, inhibition of HIF-2α signaling is effective in decreasing iron accumulation in iron overload models (169). New pharmacological agents to target oxygen-sensing pathways underscore the importance of characterizing how HIF-1α and HIF-2α alter disease progression. The definition of precise mechanisms by which HIF-1α and HIF-2α lead to overlapping and distinct cellular responses is needed. This will be critical for combinatorial treatment. In IBD, a HIF-1α activator in conjunction with HIF-2α inhibitors may provide the best treatment response; however, anemia will be exacerbated. As we define the specific roles of HIF-1α and HIF-2α, we will better be able to monitor the beneficial versus deleterious effects of pharmacological targeting of the oxygen-sensing pathways. Last, an understanding of how HIF-1α and HIF-2α are temporally regulated and how this alters downstream response is needed. This will be critical to better target HIF-1α and HIF-2α in chronic diseases and cancer.
Acknowledgments
This work was supported by National Institutes of Health Grants CA148828 and DK095201, DK034933 (University of Michigan Center for Gastrointestinal Research), and CA046592 (University of Michigan Rogel Cancer Center).
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- IBD
- inflammatory bowel disease
- CRC
- colorectal cancer
- EPO
- erythropoietin
- VEGF
- vascular endothelial growth factor
- ODD
- oxygen-dependent degradation domain
- PHD
- prolyl hydroxylase domain enzyme
- VHL
- Von Hippel–Lindau
- ARNT
- aryl hydrocarbon receptor nuclear translocator
- HIF
- hypoxia-inducible factor
- HRE
- HIF response element
- IDH
- isocitrate dehydrogenase
- ROS
- reactive oxygen species
- CBC
- crypt base columnar
- SCFA
- short-chain fatty acid
- Cr
- creatine
- CK
- creatine kinase
- PCr
- phosphocreatine
- CoX
- cytochrome c oxidase
- FPN
- ferroportin
- UC
- ulcerative colitis
- CD
- Crohn's disease
- GI
- gastrointestinal
- CAC
- colitis-associated colon cancer.
References
- 1. Taylor C. T., and Colgan S. P. (2007) Hypoxia and gastrointestinal disease. J. Mol. Med. (Berl.) 85, 1295–1300 10.1007/s00109-007-0277-z [DOI] [PubMed] [Google Scholar]
- 2. Ramakrishnan S. K., and Shah Y. M. (2016) Role of intestinal HIF-2α in health and disease. Annu. Rev. Physiol. 78, 301–325 10.1146/annurev-physiol-021115-105202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lundquist P., and Artursson P. (2016) Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 106, 256–276 10.1016/j.addr.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 4. Colgan S. P., Campbell E. L., and Kominsky D. J. (2016) Hypoxia and mucosal inflammation. Annu. Rev. Pathol. 11, 77–100 10.1146/annurev-pathol-012615-044231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He G., Shankar R. A., Chzhan M., Samouilov A., Kuppusamy P., and Zweier J. L. (1999) Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. U. S. A. 96, 4586–4591 10.1073/pnas.96.8.4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher E. M., Khan M., Salisbury R., and Kuppusamy P. (2013) Noninvasive monitoring of small intestinal oxygen in a rat model of chronic mesenteric ischemia. Cell Biochem. Biophys. 67, 451–459 10.1007/s12013-013-9611-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson G. K., Tennant D. A., and McKeating J. A. (2014) Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J. Hepatol. 61, 1397–1406 10.1016/j.jhep.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 8. Carreau A., El Hafny-Rahbi B., Matejuk A., Grillon C., and Kieda C. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 15, 1239–1253 10.1111/j.1582-4934.2011.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caldwell C. C., Kojima H., Lukashev D., Armstrong J., Farber M., Apasov S. G., and Sitkovsky M. V. (2001) Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 167, 6140–6149 10.4049/jimmunol.167.11.6140 [DOI] [PubMed] [Google Scholar]
- 10. Reyman M., van Houten M. A., van Baarle D., Bosch A. A. T. M., Man W. H., Chu M. L. J. N., Arp K., Watson R. L., Sanders E. A. M., Fuentes S., and Bogaert D. (2019) Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 10, 4997 10.1038/s41467-019-13014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sommer F., Anderson J. M., Bharti R., Raes J., and Rosenstiel P. (2017) The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 15, 630–638 10.1038/nrmicro.2017.58 [DOI] [PubMed] [Google Scholar]
- 12. Donaldson G. P., Lee S. M., and Mazmanian S. K. (2016) Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 10.1038/nrmicro3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albenberg L., Esipova T. V., Judge C. P., Bittinger K., Chen J., Laughlin A., Grunberg S., Baldassano R. N., Lewis J. D., Li H., Thom S. R., Bushman F. D., Vinogradov S. A., and Wu G. D. (2014) Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147, 1055–1063.e1058 10.1053/j.gastro.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rivera-Chávez F., Zhang L. F., Faber F., Lopez C. A., Byndloss M. X., Olsan E. E., Xu G., Velazquez E. M., Lebrilla C. B., Winter S. E., and Bäumler A. J. (2016) Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19, 443–454 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carmeliet P., Dor Y., Herbert J. M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P., Koch C. J., Ratcliffe P., Moons L., Jain R. K., Collen D., et al. (1998) Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 10.1038/28867 [DOI] [PubMed] [Google Scholar]
- 16. Eckardt K. U., and Kurtz A. (2005) Regulation of erythropoietin production. Eur. J. Clin. Invest. 35, Suppl. 3, 13–19 10.1111/j.1365-2362.2005.01525.x [DOI] [PubMed] [Google Scholar]
- 17. Semenza G. L., Roth P. H., Fang H. M., and Wang G. L. (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 [PubMed] [Google Scholar]
- 18. Schito L., and Semenza G. L. (2016) Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer 2, 758–770 10.1016/j.trecan.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 19. Wu D., Potluri N., Lu J., Kim Y., and Rastinejad F. (2015) Structural integration in hypoxia-inducible factors. Nature 524, 303–308 10.1038/nature14883 [DOI] [PubMed] [Google Scholar]
- 20. Chan D. A., Sutphin P. D., Yen S. E., and Giaccia A. J. (2005) Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1α. Mol. Cell Biol. 25, 6415–6426 10.1128/MCB.25.15.6415-6426.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., and Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 22. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., and Kaelin W. G. Jr. (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 10.1126/science.1059817 [DOI] [PubMed] [Google Scholar]
- 23. Ohh M., Park C. W., Ivan M., Hoffman M. A., Kim T. Y., Huang L. E., Pavletich N., Chau V., and Kaelin W. G. (2000) Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the Von Hippel-Lindau protein. Nat. Cell Biol. 2, 423–427 10.1038/35017054 [DOI] [PubMed] [Google Scholar]
- 24. Wang G. L., Jiang B. H., Rue E. A., and Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 92, 5510–5514 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Rourke J. F., Dachs G. U., Gleadle J. M., Maxwell P. H., Pugh C. W., Stratford I. J., Wood S. M., and Ratcliffe P. J. (1997) Hypoxia response elements. Oncol. Res. 9, 327–332 [PubMed] [Google Scholar]
- 26. Wiener C. M., Booth G., and Semenza G. L. (1996) In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 225, 485–488 10.1006/bbrc.1996.1199 [DOI] [PubMed] [Google Scholar]
- 27. Zhao S., Lin Y., Xu W., Jiang W., Zha Z., Wang P., Yu W., Li Z., Gong L., Peng Y., Ding J., Lei Q., Guan K. L., and Xiong Y. (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science 324, 261–265 10.1126/science.1170944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koivunen P., Lee S., Duncan C. G., Lopez G., Lu G., Ramkissoon S., Losman J. A., Joensuu P., Bergmann U., Gross S., Travins J., Weiss S., Looper R., Ligon K. L., Verhaak R. G., et al. (2012) Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 10.1038/nature10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selak M. A., Armour S. M., MacKenzie E. D., Boulahbel H., Watson D. G., Mansfield K. D., Pan Y., Simon M. C., Thompson C. B., and Gottlieb E. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 10.1016/j.ccr.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 30. Tannahill G. M., Curtis A. M., Adamik J., Palsson-McDermott E. M., McGettrick A. F., Goel G., Frezza C., Bernard N. J., Kelly B., Foley N. H., Zheng L., Gardet A., Tong Z., Jany S. S., Corr S. C., et al. (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 10.1038/nature11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hewitson K. S., McNeill L. A., Elkins J. M., and Schofield C. J. (2003) The role of iron and 2-oxoglutarate oxygenases in signalling. Biochem. Soc. Trans. 31, 510–515 10.1042/bst0310510 [DOI] [PubMed] [Google Scholar]
- 32. Chandel N. S., McClintock D. S., Feliciano C. E., Wood T. M., Melendez J. A., Rodriguez A. M., and Schumacker P. T. (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 10.1074/jbc.M001914200 [DOI] [PubMed] [Google Scholar]
- 33. Martínez-Reyes I., Diebold L. P., Kong H., Schieber M., Huang H., Hensley C. T., Mehta M. M., Wang T., Santos J. H., Woychik R., Dufour E., Spelbrink J. N., Weinberg S. E., Zhao Y., DeBerardinis R. J., et al. (2016) TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61, 199–209 10.1016/j.molcel.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sullivan L. B., Martinez-Garcia E., Nguyen H., Mullen A. R., Dufour E., Sudarshan S., Licht J. D., Deberardinis R. J., and Chandel N. S. (2013) The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 10.1016/j.molcel.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mills E. L., Kelly B., Logan A., Costa A. S. H., Varma M., Bryant C. E., Tourlomousis P., Dabritz J. H. M., Gottlieb E., Latorre I., Corr S. C., McManus G., Ryan D., Jacobs H. T., Szibor M., Xavier R. J., Braun T., Frezza C., Murphy M. P., and O'Neill L. A. (2016) Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e413 10.1016/j.cell.2016.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chouchani E. T., Pell V. R., Gaude E., Aksentijević D., Sundier S. Y., Robb E. L., Logan A., Nadtochiy S. M., Ord E. N. J., Smith A. C., Eyassu F., Shirley R., Hu C. H., Dare A. J., James A. M., et al. (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu C. J., Wang L. Y., Chodosh L. A., Keith B., and Simon M. C. (2003) Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol. Cell Biol. 23, 9361–9374 10.1128/mcb.23.24.9361-9374.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keith B., Johnson R. S., and Simon M. C. (2011) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 10.1038/nrc3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talks K. L., Turley H., Gatter K. C., Maxwell P. H., Pugh C. W., Ratcliffe P. J., and Harris A. L. (2000) The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411–421 10.1016/S0002-9440(10)64554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenberger C., Mandriota S., Jürgensen J. S., Wiesener M. S., Hörstrup J. H., Frei U., Ratcliffe P. J., Maxwell P. H., Bachmann S., and Eckardt K. U. (2002) Expression of hypoxia-inducible factor-1α and -2α in hypoxic and ischemic rat kidneys. J. Am. Soc. Nephrol. 13, 1721–1732 10.1097/01.asn.0000017223.49823.2a [DOI] [PubMed] [Google Scholar]
- 41. Wiesener M. S., Turley H., Allen W. E., Willam C., Eckardt K. U., Talks K. L., Wood S. M., Gatter K. C., Harris A. L., Pugh C. W., Ratcliffe P. J., and Maxwell P. H. (1998) Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92, 2260–2268 10.1182/blood.V92.7.2260.2260_2260_2268 [DOI] [PubMed] [Google Scholar]
- 42. Wiesener M. S., Jürgensen J. S., Rosenberger C., Scholze C. K., Hörstrup J. H., Warnecke C., Mandriota S., Bechmann I., Frei U. A., Pugh C. W., Ratcliffe P. J., Bachmann S., Maxwell P. H., and Eckardt K. U. (2003) Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 17, 271–273 10.1096/fj.02-0445fje [DOI] [PubMed] [Google Scholar]
- 43. Karhausen J., Furuta G. T., Tomaszewski J. E., Johnson R. S., Colgan S. P., and Haase V. H. (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 10.1172/JCI200421086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xue X., Ramakrishnan S., Anderson E., Taylor M., Zimmermann E. M., Spence J. R., Huang S., Greenson J. K., and Shah Y. M. (2013) Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145, 831–841 10.1053/j.gastro.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shah Y. M., and Xie L. (2014) Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 146, 630–642 10.1053/j.gastro.2013.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samanta D., and Semenza G. L. (2018) Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim. Biophys. Acta Rev. Cancer 1870, 15–22 10.1016/j.bbcan.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 47. Xie H., and Simon M. C. (2017) Oxygen availability and metabolic reprogramming in cancer. J. Biol. Chem. 292, 16825–16832 10.1074/jbc.R117.799973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rangel-Huerta E., and Maldonado E. (2017) Transit-amplifying cells in the fast lane from stem cells towards differentiation. Stem Cells Int. 2017, 7602951 10.1155/2017/7602951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Der Schoor S. R., Reeds P. J., Stoll B., Henry J. F., Rosenberger J. R., Burrin D. G., and Van Goudoever J. B. (2002) The high metabolic cost of a functional gut. Gastroenterology 123, 1931–1940 10.1053/gast.2002.37062 [DOI] [PubMed] [Google Scholar]
- 50. Stine R. R., Sakers A. P., TeSlaa T., Kissig M., Stine Z. E., Kwon C. W., Cheng L., Lim H. W., Kaestner K. H., Rabinowitz J. D., and Seale P. (2019) PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell 25, 830–845.e8 10.1016/j.stem.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Umar S. (2010) Intestinal stem cells. Curr. Gastroenterol. Rep. 12, 340–348 10.1007/s11894-010-0130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barker N., van de Wetering M., and Clevers H. (2008) The intestinal stem cell. Genes Dev. 22, 1856–1864 10.1101/gad.1674008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schuijers J., and Clevers H. (2012) Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 31, 2685–2696 10.1038/emboj.2012.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jackson D. N., and Theiss A. L. (2019) Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 1–20 10.1080/19490976.2019.1592421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodríguez-Colman M. J., Schewe M., Meerlo M., Stigter E., Gerrits J., Pras-Raves M., Sacchetti A., Hornsveld M., Oost K. C., Snippert H. J., Verhoeven-Duif N., Fodde R., and Burgering B. M. (2017) Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427 10.1038/nature21673 [DOI] [PubMed] [Google Scholar]
- 56. Mihaylova M. M., Cheng C. W., Cao A. Q., Tripathi S., Mana M. D., Bauer-Rowe K. E., Abu-Remaileh M., Clavain L., Erdemir A., Lewis C. A., Freinkman E., Dickey A. S., La Spada A. R., Huang Y., Bell G. W., Deshpande V., Carmeliet P., Katajisto P., Sabatini D. M., and Yilmaz Ö. (2018) Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778.e4 10.1016/j.stem.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beyaz S., Mana M. D., Roper J., Kedrin D., Saadatpour A., Hong S. J., Bauer-Rowe K. E., Xifaras M. E., Akkad A., Arias E., Pinello L., Katz Y., Shinagare S., Abu-Remaileh M., Mihaylova M. M., et al. (2016) High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 10.1038/nature17173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D. J., and Bakker B. M. (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamer H. M., Jonkers D., Venema K., Vanhoutvin S., Troost F. J., and Brummer R. J. (2008) Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 27, 104–119 10.1111/j.1365-2036.2007.03562.x [DOI] [PubMed] [Google Scholar]
- 60. Donohoe D. R., Garge N., Zhang X., Sun W., O'Connell T. M., Bunger M. K., and Bultman S. J. (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kelly C. J., Zheng L., Campbell E. L., Saeedi B., Scholz C. C., Bayless A. J., Wilson K. E., Glover L. E., Kominsky D. J., Magnuson A., Weir T. L., Ehrentraut S. F., Pickel C., Kuhn K. A., Lanis J. M., et al. (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Inai T., Kobayashi J., and Shibata Y. (1999) Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 78, 849–855 10.1016/S0171-9335(99)80086-7 [DOI] [PubMed] [Google Scholar]
- 63. Campbell E. L., Bruyninckx W. J., Kelly C. J., Glover L. E., McNamee E. N., Bowers B. E., Bayless A. J., Scully M., Saeedi B. J., Golden-Mason L., Ehrentraut S. F., Curtis V. F., Burgess A., Garvey J. F., Sorensen A., et al. (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 10.1016/j.immuni.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iizuka M., and Konno S. (2011) Wound healing of intestinal epithelial cells. World J. Gastroenterol. 17, 2161–2171 10.3748/wjg.v17.i17.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glover L. E., Bowers B. E., Saeedi B., Ehrentraut S. F., Campbell E. L., Bayless A. J., Dobrinskikh E., Kendrick A. A., Kelly C. J., Burgess A., Miller L., Kominsky D. J., Jedlicka P., and Colgan S. P. (2013) Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl. Acad. Sci. U. S. A. 110, 19820–19825 10.1073/pnas.1302840110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah V., Lyford G., Gores G., and Farrugia G. (2004) Nitric oxide in gastrointestinal health and disease. Gastroenterology 126, 903–913 10.1053/j.gastro.2003.11.046 [DOI] [PubMed] [Google Scholar]
- 67. Tiso M., and Schechter A. N. (2015) Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 10, e0119712 10.1371/journal.pone.0119712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thomas S. R., Chen K., and Keaney J. F. Jr. (2002) Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 277, 6017–6024 10.1074/jbc.M109107200 [DOI] [PubMed] [Google Scholar]
- 69. Srinivasan S., and Avadhani N. G. (2012) Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 53, 1252–1263 10.1016/j.freeradbiomed.2012.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tengan C. H., and Moraes C. T. (2017) NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta Bioenerg. 1858, 573–581 10.1016/j.bbabio.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Olson N., and van der Vliet A. (2011) Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 25, 125–137 10.1016/j.niox.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kozhukhar A. V., Yasinska I. M., and Sumbayev V. V. (2006) Nitric oxide inhibits HIF-1α protein accumulation under hypoxic conditions: implication of 2-oxoglutarate and iron. Biochimie 88, 411–418 10.1016/j.biochi.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 73. Sarti P., Forte E., Giuffrè A., Mastronicola D., Magnifico M. C., and Arese M. (2012) The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: reactions, effectors and pathophysiology. Int. J. Cell Biol. 2012, 571067 10.1155/2012/571067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hagen T., Taylor C. T., Lam F., and Moncada S. (2003) Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302, 1975–1978 10.1126/science.1088805 [DOI] [PubMed] [Google Scholar]
- 75. Huang L. E., Willmore W. G., Gu J., Goldberg M. A., and Bunn H. F. (1999) Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J. Biol. Chem. 274, 9038–9044 10.1074/jbc.274.13.9038 [DOI] [PubMed] [Google Scholar]
- 76. Berchner-Pfannschmidt U., Yamac H., Trinidad B., and Fandrey J. (2007) Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J. Biol. Chem. 282, 1788–1796 10.1074/jbc.M607065200 [DOI] [PubMed] [Google Scholar]
- 77. Wang G. L., and Semenza G. L. (1993) Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82, 3610–3615 10.1182/blood.V82.12.3610.bloodjournal82123610 [DOI] [PubMed] [Google Scholar]
- 78. Zhou J., Köhl R., Herr B., Frank R., and Brüne B. (2006) Calpain mediates a von Hippel-Lindau protein-independent destruction of hypoxia-inducible factor-1α. Mol Biol Cell 17, 1549–1558 10.1091/mbc.e05-08-0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taylor C. T., and Moncada S. (2010) Nitric oxide, cytochrome c oxidase, and the cellular response to hypoxia. Arterioscler. Thromb. Vasc. Biol. 30, 643–647 10.1161/ATVBAHA.108.181628 [DOI] [PubMed] [Google Scholar]
- 80. Callapina M., Zhou J., Schnitzer S., Metzen E., Lohr C., Deitmer J. W., and Brüne B. (2005) Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1α accumulation–implications for prolyl hydroxylase activity and iron. Exp. Cell Res. 306, 274–284 10.1016/j.yexcr.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 81. Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., et al. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- 82. Blachier F., Beaumont M., and Kim E. (2019) Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 22, 68–75 10.1097/MCO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 83. Linden D. R. (2014) Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 20, 818–830 10.1089/ars.2013.5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tomasova L., Konopelski P., and Ufnal M. (2016) Gut bacteria and hydrogen sulfide: the new old players in circulatory system homeostasis. Molecules 21, 1558 10.3390/molecules21111558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Szabo C., Ransy C., Módis K., Andriamihaja M., Murghes B., Coletta C., Olah G., Yanagi K., and Bouillaud F. (2014) Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 171, 2099–2122 10.1111/bph.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kolluru G. K., Shen X., Bir S. C., and Kevil C. G. (2013) Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35, 5–20 10.1016/j.niox.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kabil O., Vitvitsky V., and Banerjee R. (2014) Sulfur as a signaling nutrient through hydrogen sulfide. Annu. Rev. Nutr. 34, 171–205 10.1146/annurev-nutr-071813-105654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Banerjee R. (2017) Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 37, 115–121 10.1016/j.cbpa.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kabil O., Vitvitsky V., Xie P., and Banerjee R. (2011) The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 15, 363–372 10.1089/ars.2010.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yadav P. K., Yamada K., Chiku T., Koutmos M., and Banerjee R. (2013) Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 288, 20002–20013 10.1074/jbc.M113.466177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Budde M. W., and Roth M. B. (2011) The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics 189, 521–532 10.1534/genetics.111.129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu X., Pan L., Zhuo Y., Gong Q., Rose P., and Zhu Y. (2010) Hypoxia-inducible factor-1α is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol. Pharm. Bull. 33, 1550–1554 10.1248/bpb.33.1550 [DOI] [PubMed] [Google Scholar]
- 93. Kai S., Tanaka T., Daijo H., Harada H., Kishimoto S., Suzuki K., Takabuchi S., Takenaga K., Fukuda K., and Hirota K. (2012) Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von Hippel-Lindau- and mitochondria-dependent manner. Antioxid. Redox Signal. 16, 203–216 10.1089/ars.2011.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang J., Cao J., Weng Q., Wu R., Yan Y., Jing H., Zhu H., He Q., and Yang B. (2010) Suppression of hypoxia-inducible factor 1α (HIF-1α) by tirapazamine is dependent on eIF2α phosphorylation rather than the mTORC1/4E-BP1 pathway. PLoS ONE 5, e13910 10.1371/journal.pone.0013910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Konieczna I., Zarnowiec P., Kwinkowski M., Kolesinska B., Fraczyk J., Kaminski Z., and Kaca W. (2012) Bacterial urease and its role in long-lasting human diseases. Curr. Protein Pept. Sci. 13, 789–806 10.2174/138920312804871094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gottlieb E., Armour S. M., Harris M. H., and Thompson C. B. (2003) Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 10, 709–717 10.1038/sj.cdd.4401231 [DOI] [PubMed] [Google Scholar]
- 97. Bobermin L. D., Wartchow K. M., Flores M. P., Leite M. C., Quincozes-Santos A., and Gonçalves C. A. (2015) Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase 1. Neurotoxicology 49, 28–35 10.1016/j.neuro.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 98. Cruz H. J., Freitas C. M., Alves P. M., Moreira J. L., and Carrondo M. J. (2000) Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb. Technol. 27, 43–52 10.1016/S0141-0229(00)00151-4 [DOI] [PubMed] [Google Scholar]
- 99. Martinelle K., and Häggström L. (1993) Mechanisms of ammonia and ammonium ion toxicity in animal cells: transport across cell membranes. J. Biotechnol. 30, 339–350 10.1016/0168-1656(93)90148-G [DOI] [PubMed] [Google Scholar]
- 100. Swamy M., Zakaria A. Z., Govindasamy C., Sirajudeen K. N., and Nadiger H. A. (2005) Effects of acute ammonia toxicity on nitric oxide (NO), citrulline-NO cycle enzymes, arginase and related metabolites in different regions of rat brain. Neurosci. Res. 53, 116–122 10.1016/j.neures.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 101. Kruczek C., Görg B., Keitel V., Bidmon H. J., Schliess F., and Häussinger D. (2011) Ammonia increases nitric oxide, free Zn2+, and metallothionein mRNA expression in cultured rat astrocytes. Biol. Chem. 392, 1155–1165 10.1515/BC.2011.199 [DOI] [PubMed] [Google Scholar]
- 102. Kitajima S., Lee K. L., Hikasa H., Sun W., Huang R. Y., Yang H., Matsunaga S., Yamaguchi T., Araki M., Kato H., and Poellinger L. (2017) Hypoxia-inducible factor-1α promotes cell survival during ammonia stress response in ovarian cancer stem-like cells. Oncotarget 8, 114481–114494 10.18632/oncotarget.23010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ryter S. W. (2019) Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch. Biochem. Biophys. 678, 108186 10.1016/j.abb.2019.108186 [DOI] [PubMed] [Google Scholar]
- 104. Szurszewski J. H., and Farrugia G. (2004) Carbon monoxide is an endogenous hyperpolarizing factor in the gastrointestinal tract. Neurogastroenterol. Motil. 16, Suppl 1, 81–85 10.1111/j.1743-3150.2004.00480.x [DOI] [PubMed] [Google Scholar]
- 105. Taillé C., El-Benna J., Lanone S., Boczkowski J., and Motterlini R. (2005) Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 280, 25350–25360 10.1074/jbc.M503512200 [DOI] [PubMed] [Google Scholar]
- 106. Hagen T. (2012) Oxygen versus reactive oxygen in the regulation of HIF-1α: the balance tips. Biochem. Res. Int. 2012, 436981 10.1155/2012/436981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kalantar-Zadeh K., Berean K. J., Burgell R. E., Muir J. G., and Gibson P. R. (2019) Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 16, 733–747 10.1038/s41575-019-0193-z [DOI] [PubMed] [Google Scholar]
- 108. Selfridge A. C., Cavadas M. A., Scholz C. C., Campbell E. L., Welch L. C., Lecuona E., Colgan S. P., Barrett K. E., Sporn P. H., Sznajder J. I., Cummins E. P., and Taylor C. T. (2016) Hypercapnia suppresses the HIF-dependent adaptive response to hypoxia. J. Biol. Chem. 291, 11800–11808 10.1074/jbc.M116.713941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ferreira J. V., Fôfo H., Bejarano E., Bento C. F., Ramalho J. S., Girão H., and Pereira P. (2013) STUB1/CHIP is required for HIF1A degradation by chaperone-mediated autophagy. Autophagy 9, 1349–1366 10.4161/auto.25190 [DOI] [PubMed] [Google Scholar]
- 110. Ferreira J. V., Soares A. R., Ramalho J. S., Pereira P., and Girao H. (2015) K63 linked ubiquitin chain formation is a signal for HIF1A degradation by chaperone-mediated autophagy. Sci. Rep. 5, 10210 10.1038/srep10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Furuta G. T., Turner J. R., Taylor C. T., Hershberg R. M., Comerford K., Narravula S., Podolsky D. K., and Colgan S. P. (2001) Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 10.1084/jem.193.9.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Comerford K. M., Wallace T. J., Karhausen J., Louis N. A., Montalto M. C., and Colgan S. P. (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62, 3387–3394 [PubMed] [Google Scholar]
- 113. Synnestvedt K., Furuta G. T., Comerford K. M., Louis N., Karhausen J., Eltzschig H. K., Hansen K. R., Thompson L. F., and Colgan S. P. (2002) Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 10.1172/JCI0215337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eltzschig H. K., Ibla J. C., Furuta G. T., Leonard M. O., Jacobson K. A., Enjyoji K., Robson S. C., and Colgan S. P. (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 10.1084/jem.20030891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Louis N. A., Hamilton K. E., Canny G., Shekels L. L., Ho S. B., and Colgan S. P. (2006) Selective induction of mucin-3 by hypoxia in intestinal epithelia. J. Cell. Biochem. 99, 1616–1627 10.1002/jcb.20947 [DOI] [PubMed] [Google Scholar]
- 116. Young H. W., Williams O. W., Chandra D., Bellinghausen L. K., Perez G., Suarez A., Tuvim M. J., Roy M. G., Alexander S. N., Moghaddam S. J., Adachi R., Blackburn M. R., Dickey B. F., and Evans C. M. (2007) Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am. J. Respir. Cell Mol. Biol. 37, 273–290 10.1165/rcmb.2005-0460OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kelly C. J., Glover L. E., Campbell E. L., Kominsky D. J., Ehrentraut S. F., Bowers B. E., Bayless A. J., Saeedi B. J., and Colgan S. P. (2013) Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 6, 1110–1118 10.1038/mi.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Saeedi B. J., Kao D. J., Kitzenberg D. A., Dobrinskikh E., Schwisow K. D., Masterson J. C., Kendrick A. A., Kelly C. J., Bayless A. J., Kominsky D. J., Campbell E. L., Kuhn K. A., Furuta G. T., Colgan S. P., and Glover L. E. (2015) HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 26, 2252–2262 10.1091/mbc.E14-07-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Allard B., Longhi M. S., Robson S. C., and Stagg J. (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Aherne C. M., Saeedi B., Collins C. B., Masterson J. C., McNamee E. N., Perrenoud L., Rapp C. R., Curtis V. F., Bayless A., Fletcher A., Glover L. E., Evans C. M., Jedlicka P., Furuta G. T., de Zoeten E. F., et al. (2015) Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol. 8, 1324–1338 10.1038/mi.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mastrogiannaki M., Matak P., Keith B., Simon M. C., Vaulont S., and Peyssonnaux C. (2009) HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Invest. 119, 1159–1166 10.1172/JCI38499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Taylor M., Qu A., Anderson E. R., Matsubara T., Martin A., Gonzalez F. J., and Shah Y. M. (2011) Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140, 2044–2055 10.1053/j.gastro.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shah Y. M., Matsubara T., Ito S., Yim S. H., and Gonzalez F. J. (2009) Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metabol. 9, 152–164 10.1016/j.cmet.2008.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., and Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 125. Schwartz A. J., Das N. K., Ramakrishnan S. K., Jain C., Jurkovic M. T., Wu J., Nemeth E., Lakhal-Littleton S., Colacino J. A., and Shah Y. M. (2019) Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Invest. 129, 336–348 10.1172/JCI122359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Das N., Xie L., Ramakrishnan S. K., Campbell A., Rivella S., and Shah Y. M. (2015) Intestine-specific disruption of hypoxia-inducible factor (HIF)-2α improves anemia in sickle cell disease. J. Biol. Chem. 290, 23523–23527 10.1074/jbc.C115.681643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mastrogiannaki M., Matak P., Delga S., Deschemin J. C., Vaulont S., and Peyssonnaux C. (2012) Deletion of HIF-2α in the enterocytes decreases the severity of tissue iron loading in hepcidin knockout mice. Blood 119, 587–590 10.1182/blood-2011-09-380337 [DOI] [PubMed] [Google Scholar]
- 128. Das N. K., Schwartz A. J., Barthel G., Inohara N., Liu Q., Sankar A., Hill D. R., Ma X., Lamberg O., Schnizlein M. K., Arques J. L., Spence J. R., Nunez G., Patterson A. D., Sun D., Young V. B., and Shah Y. M. (2020) Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 31, 115–130.e6 10.1016/j.cmet.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Triner D., Xue X., Schwartz A. J., Jung I., Colacino J. A., and Shah Y. M. (2017) Epithelial hypoxia-inducible factor 2α facilitates the progression of colon tumors through recruiting neutrophils. Mol. Cell Biol. 37, 10.1128/MCB.00481-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xue X., Ramakrishnan S. K., and Shah Y. M. (2014) Activation of HIF-1α does not increase intestinal tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G187–G195 10.1152/ajpgi.00112.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xie C., Yagai T., Luo Y., Liang X., Chen T., Wang Q., Sun D., Zhao J., Ramakrishnan S. K., Sun L., Jiang C., Xue X., Tian Y., Krausz K. W., Patterson A. D., et al. (2017) Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 23, 1298–1308 10.1038/nm.4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Taylor C. T., Doherty G., Fallon P. G., and Cummins E. P. (2016) Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Invest. 126, 3716–3724 10.1172/JCI84433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Taylor C. T. (2018) Hypoxia in the gut. Cell Mol. Gastroenterol. Hepatol. 5, 61–62 10.1016/j.jcmgh.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Colgan S. P., Furuta G. T., and Taylor C. T. (2020) Hypoxia and innate immunity: keeping up with the HIFsters. Annu. Rev. Immunol. 10.1146/annurev-immunol-100819-121537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Perler B., Ungaro R., Baird G., Mallette M., Bright R., Shah S., Shapiro J., and Sands B. E. (2019) Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 19, 47 10.1186/s12876-019-0963-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tontini G. E., Vecchi M., Pastorelli L., Neurath M. F., and Neumann H. (2015) Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J. Gastroenterol. 21, 21–46 10.3748/wjg.v21.i1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Giatromanolaki A., Sivridis E., Maltezos E., Papazoglou D., Simopoulos C., Gatter K. C., Harris A. L., and Koukourakis M. I. (2003) Hypoxia inducible factor 1α and 2α overexpression in inflammatory bowel disease. J. Clin. Pathol. 56, 209–213 10.1136/jcp.56.3.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Olcina M. M., and Giaccia A. J. (2016) Reducing radiation-induced gastrointestinal toxicity—the role of the PHD/HIF axis. J. Clin. Invest. 126, 3708–3715 10.1172/JCI84432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xie L., Xue X., Taylor M., Ramakrishnan S. K., Nagaoka K., Hao C., Gonzalez F. J., and Shah Y. M. (2014) Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol. Cell Biol. 34, 3013–3023 10.1128/MCB.00324-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Taniguchi C. M., Miao Y. R., Diep A. N., Wu C., Rankin E. B., Atwood T. F., Xing L., and Giaccia A. J. (2014) PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med. 6, 236ra64 10.1126/scitranslmed.3008523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bruning U., Cerone L., Neufeld Z., Fitzpatrick S. F., Cheong A., Scholz C. C., Simpson D. A., Leonard M. O., Tambuwala M. M., Cummins E. P., and Taylor C. T. (2011) MicroRNA-155 promotes resolution of hypoxia-inducible factor 1α activity during prolonged hypoxia. Mol. Cell Biol. 31, 4087–4096 10.1128/MCB.01276-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Muz B., de la Puente P., Azab F., and Azab A. K. (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl.) 3, 83–92 10.2147/HP.S93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Challapalli A., Carroll L., and Aboagye E. O. (2017) Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 5, 225–253 10.1007/s40336-017-0231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tang Y. A., Chen Y. F., Bao Y., Mahara S., Yatim S., Oguz G., Lee P. L., Feng M., Cai Y., Tan E. Y., Fong S. S., Yang Z. H., Lan P., Wu X. J., and Yu Q. (2018) Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 115, E5990–E5999 10.1073/pnas.1801348115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Evensen N. A., Li Y., Kuscu C., Liu J., Cathcart J., Banach A., Zhang Q., Li E., Joshi S., Yang J., Denoya P. I., Pastorekova S., Zucker S., Shroyer K. R., and Cao J. (2015) Hypoxia promotes colon cancer dissemination through up-regulation of cell migration-inducing protein (CEMIP). Oncotarget 6, 20723–20739 10.18632/oncotarget.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bhandari A., Woodhouse M., and Gupta S. (2017) Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the U.S.A.: a SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. 65, 311–315 10.1136/jim-2016-000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang L., and Shay J. W. (2017) Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst 109, djw332 10.1093/jnci/djw332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kameyama H., Nagahashi M., Shimada Y., Tajima Y., Ichikawa H., Nakano M., Sakata J., Kobayashi T., Narayanan S., Takabe K., and Wakai T. (2018) Genomic characterization of colitis-associated colorectal cancer. World J. Surg. Oncol. 16, 121 10.1186/s12957-018-1428-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sakai E., Fukuyo M., Matsusaka K., Ohata K., Doi N., Takane K., Matsuhashi N., Fukushima J., Nakajima A., and Kaneda A. (2016) TP53 mutation at early stage of colorectal cancer progression from two types of laterally spreading tumors. Cancer Sci. 107, 820–827 10.1111/cas.12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Romano M., DE Francesco F., Zarantonello L., Ruffolo C., Ferraro G. A., Zanus G., Giordano A., Bassi N., and Cillo U. (2016) From inflammation to cancer in inflammatory bowel disease: molecular perspectives. Anticancer Res. 36, 1447–1460 [PubMed] [Google Scholar]
- 151. Cancer Genome Atlas Network (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Goto T., Marusawa H., and Chiba T. (2013) Landscape of genetic aberrations detected in human colorectal cancers. Gastroenterology 145, 686–688 10.1053/j.gastro.2013.07.029 [DOI] [PubMed] [Google Scholar]
- 153. Choudhry H., and Harris A. L. (2018) Advances in hypoxia-inducible factor biology. Cell Metab. 27, 281–298 10.1016/j.cmet.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 154. de Cubas A. A., and Rathmell W. K. (2018) Epigenetic modifiers: activities in renal cell carcinoma. Nat. Rev. Urol. 15, 599–614 10.1038/s41585-018-0052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ma X., Zhang H., Xue X., and Shah Y. M. (2017) Hypoxia-inducible factor 2α (HIF-2α) promotes colon cancer growth by potentiating Yes-associated protein 1 (YAP1) activity. J. Biol. Chem. 292, 17046–17056 10.1074/jbc.M117.805655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Imamura T., Kikuchi H., Herraiz M. T., Park D. Y., Mizukami Y., Mino-Kenduson M., Lynch M. P., Rueda B. R., Benita Y., Xavier R. J., and Chung D. C. (2009) HIF-1α and HIF-2α have divergent roles in colon cancer. Int. J. Cancer 124, 763–771 10.1002/ijc.24032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Xue X., Ramakrishnan S. K., Weisz K., Triner D., Xie L., Attili D., Pant A., Győrffy B., Zhan M., Carter-Su C., Hardiman K. M., Wang T. D., Dame M. K., Varani J., Brenner D., et al. (2016) Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 24, 447–461 10.1016/j.cmet.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xue X., Taylor M., Anderson E., Hao C., Qu A., Greenson J. K., Zimmermann E. M., Gonzalez F. J., and Shah Y. M. (2012) Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 72, 2285–2293 10.1158/0008-5472.CAN-11-3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hammami A., Allard D., Allard B., and Stagg J. (2019) Targeting the adenosine pathway for cancer immunotherapy. Semin. Immunol. 42, 101304 10.1016/j.smim.2019.101304 [DOI] [PubMed] [Google Scholar]
- 160. Lee K., Zhang H., Qian D. Z., Rey S., Liu J. O., and Semenza G. L. (2009) Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. U. S. A. 106, 17910–17915 10.1073/pnas.0909353106 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 161. Cummins E. P., Seeballuck F., Keely S. J., Mangan N. E., Callanan J. J., Fallon P. G., and Taylor C. T. (2008) The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165 10.1053/j.gastro.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 162. Robinson A., Keely S., Karhausen J., Gerich M. E., Furuta G. T., and Colgan S. P. (2008) Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 10.1053/j.gastro.2007.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chan M. C., Holt-Martyn J. P., Schofield C. J., and Ratcliffe P. J. (2016) Pharmacological targeting of the HIF hydroxylases—a new field in medicine development. Mol. Aspects Med. 47–48, 54–75 10.1016/j.mam.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 164. Zimmer M., Ebert B. L., Neil C., Brenner K., Papaioannou I., Melas A., Tolliday N., Lamb J., Pantopoulos K., Golub T., and Iliopoulos O. (2008) Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol. Cell 32, 838–848 10.1016/j.molcel.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Scheuermann T. H., Tomchick D. R., Machius M., Guo Y., Bruick R. K., and Gardner K. H. (2009) Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc. Natl. Acad. Sci. U. S. A. 106, 450–455 10.1073/pnas.0808092106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Scheuermann T. H., Li Q., Ma H. W., Key J., Zhang L., Chen R., Garcia J. A., Naidoo J., Longgood J., Frantz D. E., Tambar U. K., Gardner K. H., and Bruick R. K. (2013) Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 9, 271–276 10.1038/nchembio.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Chen W., Hill H., Christie A., Kim M. S., Holloman E., Pavia-Jimenez A., Homayoun F., Ma Y., Patel N., Yell P., Hao G., Yousuf Q., Joyce A., Pedrosa I., Geiger H., et al. (2016) Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 10.1038/nature19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Cho H., Du X., Rizzi J. P., Liberzon E., Chakraborty A. A., Gao W., Carvo I., Signoretti S., Bruick R. K., Josey J. A., Wallace E. M., and Kaelin W. G. (2016) On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature 539, 107–111 10.1038/nature19795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Anderson E. R., Taylor M., Xue X., Ramakrishnan S. K., Martin A., Xie L., Bredell B. X., Gardenghi S., Rivella S., and Shah Y. M. (2013) Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc. Natl. Acad. Sci. U. S. A. 110, E4922–E4930 10.1073/pnas.1314197110 [DOI] [PMC free article] [PubMed] [Google Scholar]