Abstract
Background: Phosphate stones can be divided into struvite (7%), apatite (20%), and brushite stones (2%). They often present as large staghorn calculi and, therefore, can be challenging to treat. Moreover, it is crucial to obtain a stone-free patient to prevent recurrence. Therefore, local chemolysis can be an interesting tool when complete surgical removal of the stone is impossible or as an adjuvant treatment for residual stone fragments after surgery.
Case Presentation: We present a case of an 84-year old Caucasian man in whom local chemolysis therapy with a citric acid solution resulted in a rapid reduction of the stone load, making less invasive therapy possible.
Conclusion: We describe the procedure, (dis)advantages, and possible indications for local chemolysis.
Keywords: kidney stone, dissolution therapy, chemolysis, stone therapy, Suby G, Renacidin
Introduction and Background
Dissolution therapy through chemolysis has been used in both primary and adjuvant settings in the management of urinary tract stones with varying results since 1924.1 Different types of chemolysis can be used according to stone type. Cystine stones for instance are reported to dissolve with d-penicillamine, tromethamine-E or tiopronin, and N-acetylcysteine. Contrastingly, sodium bicarbonate irrigation can be used to dissolve uric acid stones.1 It is well known that phosphate stones can resolve in acid substances. Two of the most used substances are Renacidin and Suby G. These citric acid solutions were both quite popular treatments until the Food and Drug Administration (FDA) banned the use of Renacidin temporarily because of the report of six possibly related deaths in the 1960s. This ban was withdrawn later on, cautioning to avoid infection and high pressures while irrigating. Nevertheless the concern about possible complications together with the introduction of minimal invasive stone surgery has made it less popular in the past decades. Despite the fact the FDA reapproved the use of Renacidin, there is little available data about chemolysis.
In this case, we used the Suby G solution by Braun, containing isotonic citrate, magnesium oxide, and sodium carbonate with a pH of 4.2 to resolve large phosphate containing stones.
About 20% of renal stones are phosphate stones and consist of struvite, apatite, or brushite.2 These stones are often associated with chronic urinary infection with Proteus, Klebsiella, Morganella, Pseudomonas, and Staphylococcus. The key in the effective treatment of the infectious stones is to render the patients stone free. This can be challenging in complex stones or in patients unfit for surgery. Therefore, chemolysis can be a useful tool in obtaining this goal and has to be considered in carefully selected cases.
Presentation of Case
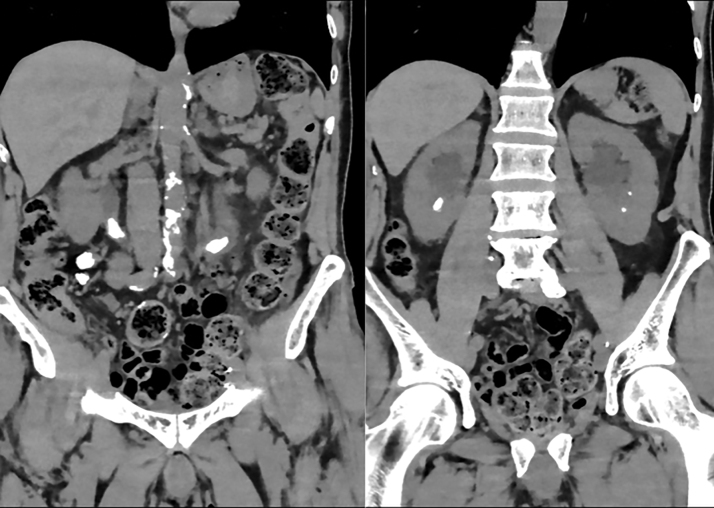
An 84-year old Caucasian patient presents in the outpatient clinic because of repeated asymptomatic positive urine cultures. Medical history reports a cystoprostatectomy with urinary diversion in 2015. Blood sample reveals a normal renal function with a normal leukocyte count and without CRP. Urine culture confirms the growth of Morganella morganii whereas microscopy of the urine shows a pH of 8 with 1016 leukocytes and 181 erythrocytes. A noncontrast CT of the abdomen was done, which shows bilateral proximal ureteral stones with diameters of 23 × 9 × 8 mm (left) and 20 × 11 × 7 mm (right) and densities between 700 and 800 Hounsfield units. In addition, a right lower pole stone and two small left lower pole stones measuring 13 × 8 × 7 mm, 6 × 6 × 4 mm, and 2 × 2 × 2 mm, respectively, were seen (Fig. 1).
FIG. 1.
CT scan of the abdomen at initial presentation showing bilateral large little dens proximal ureteral stones and bilateral nephrolithiasis. The image shows us in the left ureter a stone of ∼23 × 9 × 8 mm with a value of 840 HU, in the right ureter a stone of ∼20 × 11 × 7 mm with a value of 727 HU, a large stone in the right lower pole of the kidney of ∼13 × 8 × 7 mm with a value of 691 HU, and two small stones in the left lower pole of the kidney.
Urgent relief of obstruction was obtained through the placement of bilateral nephrostomies and simultaneous introduction of bilateral retrograde ureteral stents under general anesthesia. A rigid 22F rigid cystoscope was inserted through the urinary diversion until both ostia were seen. A Nitinol guidewire was inserted into both ureters, and fluoroscopy showed a correct position of the guidewire before the introduction of a 6 F × 28 cm ureteral stent.
A small and hard stone fragment was collected from the Bricker diversion and was sent for analysis, which showed a 60% struvite and 40% apatite stone. Intravenous temocillin was started and 2 days later, infusion with Suby G through the right nephrostomy was initiated, initially at a rate of 50 mL/h. The infusion rate was doubled to 100 mL/h after 1 day without local or metabolic complications.
CT scan was repeated after 5 days of irrigation that showed disappearance of the right proximal ureteral stone. The left proximal stone had become smaller with a size of ∼17 × 9 × 10 mm, the stone in the lower pole of the right kidney measured 11 × 8 × 4 mm and left renal stones remained unchanged (Fig. 2).
FIG. 2.
CT scan after 5 days of dissolution therapy: the right proximal ureteral stone is not visible anymore, the one at the left side is smaller with a size of ∼17 × 9 × 10 mm, and the stone in the lower pole of the right kidney measures 11 × 8 × 4 mm.
Irrigation was discontinued after 1 week because of an erysipelas at the right nephrostomy insertion side. A few days later, irrigation was restarted through the left nephrostomy and CT scan 4 days later revealed a further dissolution of the left proximal calculus with dimensions of 8 × 8 × 6 mm, as well as the right lower pole stone measuring 8 × 8 × 5 mm (Fig. 3).
FIG. 3.
CT scan 11 days after the start of dissolution: the left proximal stone with dimensions of 8 × 8 × 6 mm and the stone in the lower pole of the right kidney with dimensions of 8 × 8 × 5 mm.
Five days later, we performed a left percutaneous nephrolithotomy (PNL) in an attempt to make the patient stone free. Antegrade flexible ureteroscopy showed no residual stone fragments in the ureter nor in the calices. Follow-up CT scan after 3 months revealed a stone-free left kidney with a residual lower pole fragment on the right side. Further treatment was discussed with the patient who preferred active surveillance for the small residual fragment (Fig. 4).
FIG. 4.
CT scan after 3 months revealed a stone-free left kidney with a residual lower pole fragment on the right side of 5 × 6 × 5 mm.
Discussion and Literature Review
The most important phosphate-containing stone types are apatite (calcium phosphate), brushite (calcium hydrogen phosphate dihydrate), and struvite (magnesium ammonium phosphate hexahydrate). These stones precipitate in alkaline urine (pH >7.2). Infectious stones are primarily composed of magnesium ammonium phosphate hexahydrate but may in addition contain calcium phosphate in the form of carbonate apatite.2
Struvite stones are formed as a result of urinary tract infection with urease-producing bacteria. The most common urease-producing pathogens are Proteus, Providencia, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus species. Proteus mirabilis is the most common organism associated with infectious stones.2 Struvite stones are potentiated by bacterial infection that hydrolyzes urea to ammonium and raises urine pH to neutral or alkaline values. In contrast, apatite stone formation is not caused by infection, although often enhanced in infectious conditions.2 Treatment of struvite and apatite stones includes multiple options: shockwave lithotripsy (SWL), PNL, open surgery, antibiotic therapy, urine dilution, and oral (l-methionine and ammonium chloride) or local (citric acid solutions such as Suby G or Renacidin) acidification with chemolysis.3
Chemolysis can be used as a primary treatment or as an adjuvant procedure in combination with SWL, PNL, or open stone removal.3 Mulvaney reported a stone-free rate of 52% with primary chemolysis in patients with ureteral stones, stone-free rates of 77%–80% were reported in an adjuvant setting (hemiacidrin or Suby G) with a hospital stay up to 34 days.1 In our case, we used chemolysis as a neoadjuvant treatment in an attempt to reduce the stone load. Doing so both ureteral stones resolved, leaving only a few smaller renal stones to treat.
Crowell was the first to describe stone dissolution by direct irrigation in 1924. In 1943, Suby and Albright introduced urologic solution G or Suby solution for dissolution of renal calculi. Solution G contains isotonic citrate, magnesium oxide, and sodium carbonate. In 1957, Mulvaney presented an alternative for solution G: Renacidin. Renacidin has a similar pH and buffering capacity as Suby G solution but contains malonic and gluconic acids.4 Renacidin has had a complex history with mixed results. In the early 1960s, the FDA banned the use of Renacidin after the report of six deaths during percutanous chemolysis. Further investigations revealed that they all had the same cause: urosepsis because of increased intrarenal pressure by obstructed ureteral catheters. Afterward the FDA reapproved the use of Renacidin as an adjunct to dissolve catheter and bladder calculi and in 1990 extended the indication to renal and ureteral calculi.4
Possible complications of chemolysis are urosepsis and hypermagnesemia by systemic absorption of the product caused by an increased intrarenal pressure (>15–25 cm H2O) or leakage. It is of uttermost importance to only proceed when there are no signs of acute infection and when low intrarenal pressure is guaranteed. During the treatment, constant monitoring of flank pain, fever, or elevated intrarenal pressure is necessary. It is crucial to secure renal outflow, as mentioned in the literature.1 Symptoms of hypermagnesemia after systemic absorption of the irrigant are mental confusion, nausea, and decreased deep tendon reflexes with serum concentrations of 5–6 mg/dL and a decreased ventilatory rate, hypotension, and dysrhythmias with serum concentrations from 7 to 9 mg/dL.
Dissolving fragmentscan cause obstruction, which can lead to an increase of intrarenal pressure. It is advisable to secure continuous drainage of the solution through the introduction of a Double-J stent stent and an indwelling bladder catheter to preserve a low intrarenal pressure. Furthermore, it is recommended to wait 24 hours after an invasive procedure before starting the irrigation to prevent extravasation. Practically, irrigation with saline is initiated and chemolysis is only started if no leakage, fever, or flank discomfort occurs. The maximum velocity of irrigation is 120 mL/h. The chemolysis can be stopped 24–48 hours after absence of visible parts on imaging.1
Some studies show a higher rate of toxicity with Renacidin than with Suby G.3 With the introduction of percutaneous lithotomy (1976) and SWL (1980), the stone dissolution therapy became less popular, not only because these treatments were considered as minimal invasive but probably also because of the previous FDA warnings, the time-consuming treatment protocols (labor-intensive for the nurses), patient noncompliance, the need for additional ureteral and nephrostomy tubes, the need for additional imaging, prolonged hospitalization, and metabolic complications such as hypermagnesemia. During the treatment, prolonged antibiotic therapy is required and deep venous thrombosis prophylaxis has to be considered because of the immobility of the patient.1
However, this case demonstrates that it can be an effective treatment option in carefully selected patients. Chemolysis can be an option in high-risk patients wherein standard treatments (such as percutaneous lithotomy) are contraindicated, that is, patients with bleeding disorders or on anticoagulants, patients unfit to undergo general anesthesia, or in patients with multiple residual fragments and/or inaccessible fragments after endoscopic procedures.1
Conclusion
Percutaneous chemolysis can be a safe and effective method for dissolution of phosphate containing urinary calculi if it is correctly used in a low-pressure system with continuous drainage, in the absence of infection, and with close monitoring. It can be used in both primary or (neo)adjuvant setting. However, available data are scarce, stressing the importance of further prospective trials.
Abbreviations Used
- CT
computed tomography
- HU
Hounsfield units
- PNL
percutaneous nephrolithotomy
- SWL
shockwave lithotripsy
Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Cite this article as: Schillebeeckx C, Vander Eeckt K, Ost D, Van den Branden M, Deconinck S (2020) Kidney stone dissolution therapy in phosphate stones: A case report, Journal of Endourology Case Reports 6:1, 45–48, DOI: 10.1089/cren.2019.0076.
References
- 1. Kachrilas S, Papatsoris A, Bach C, Bourdoumis A, Zaman F, Masood J, Buchholz N. The current role of percutaneous chemolysis in the management of urolithiasis: Review and results. Urolithiasis 2013;41:323–326 [DOI] [PubMed] [Google Scholar]
- 2. Hesse A, Heimbach D. Causes of phosphate stone formation and the importance of metaphylaxis by urinary acidification. World J Urol 1999;17:308–315 [DOI] [PubMed] [Google Scholar]
- 3. Heimbach D, Jacobs D, Müller SC, Hesse A. Chemolitholysis and lithotripsy of infectious urinary stones—An in vitro study. Urol Int 2002;69:212–218 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez RD, Whiting BM, Canales BK. The history of kidney stone dissolution therapy: 50 years of optimism and frustration with Renacidin. J Endourol 2012;26:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]