Abstract
Background: Primary malignant melanoma (PMM) of the urinary tract is a rare entity, with only 28 cases reported in the literature. We present an interesting case of a 27-year-old Caucasian woman, with family history of melanoma, who initially presented with gross hematuria, and was subsequently found to have PMM of the bladder.
Case Presentation: Initially diagnosis was made through transurethral resection of the bladder tumor with clinical suspicion of residual disease in the patient. Subsequently, she underwent robotic partial cystectomy with pelvic lymph node dissection followed by 1 year of pembrolizumab, a PD-1 checkpoint inhibitor. Subsequent imaging demonstrated no evidence of metastatic disease or local recurrence.
Conclusion: This case report presents a unique management of a rare pathological diagnosis with the use of robotic partial cystectomy, and a PD-1 checkpoint inhibitor therapy that ultimately has led to a 2-year recurrence-free survival period for this young patient.
Keywords: melanoma, primary malignant melanoma, bladder tumor, robotic partial cystectomy, pembrolizumab, immunotherapy
Introduction and Background
Primary malignant melanoma (PMM) of the urinary tract is rare, with the urethra being the most common site; the urinary bladder is involved less frequently.1 A comprehensive review published in 2018 identified 28 acceptable cases.2 The pathogenesis of PMM of the bladder is not known but theorized to be related to rare examples of melanosis involving the bladder. Historically, the prognosis for patients with PMM of the urinary tract has been poor.1 We present a unique case of a 27-year-old Caucasian woman with PMM of the bladder that was treated with robotic partial cystectomy, and subsequently pembrolizumab, a PD-1 checkpoint inhibitor therapy.
Presentation of Case
A 27-year-old Caucasian woman with history of bipolar disorder, atopic dermatitis, and significant family history of melanoma presented to an urgent care for evaluation of painless gross hematuria. She was treated with antibiotics for urinary tract infection without any relief. Several months later, she presented to the emergency room for evaluation of persistent painless gross hematuria. An ultrasonography was performed, which was notable for a bladder mass. She was evaluated by an outside urologist, and a CT abdomen/pelvis was ordered, which confirmed a 1.9 × 1.7 × 2.6 cm (anterior-posterior × axial × craniocaudal) mass at the right anterior bladder wall (Fig. 1). Transurethral resection of the bladder tumor (TURBT) was performed. Interestingly, the pathology showed the tumor as positive for SOX, S-100, Melan-A/MART1, and HMB-45, all consistent with melanoma (Fig. 2). Lamina propria was involved; however, there was minimal muscle tissue present without any tumor involvement. She was then referred to our tertiary academic center for further management.
FIG. 1.
A preoperative abdominal CT scan shows a 1.9 × 1.7 × 2.6 cm (anterior-posterior × axial × craniocaudal) right anterior bladder wall enhancing soft tissue mass (black arrow). (A) Axial without contrast, (B) coronal without contrast, and (C) coronal with contrast.
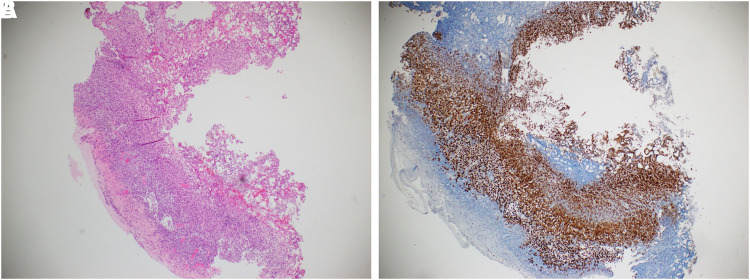
FIG. 2.
Patient's pre-partial cystectomy bladder repeat biopsy shows expected obliteration of normal urothelial architecture by spreading tumor and positive SOX staining. (A) H&E stain of right anterior bladder resection site showing suspicious morphological features. (B) SOX positive immunohistochemistry stain confirming melanoma. H&E, hematoxylin and eosin.
On physical examination, patient had no significant findings. Her Eastern Cooperative Oncology Group (ECOG) score was 0. A comprehensive skin examination revealed multiple tan-brown macules and papules with reassuring clinical and dermatoscopic features consistent with benign nevi. She was discussed at the multidisciplinary tumor board, and the decision was made to proceed with repeat TURBT and random bladder biopsy to confirm solitary focus of disease in bladder, and to determine muscle invasion. A positron emission tomography-CT was obtained, and no other evidence of cancer was noted. MRI brain was negative for any evidence of malignancy. She then underwent repeat TURBT with random bladder biopsies, which demonstrated localized malignant melanoma with extensive lamina propria invasion to the right anterior bladder wall; random bladder biopsies were negative for malignancy. CARIS profiling was performed and was positive for BRAF V600E mutation, PTEN Exon 9 mutation, PDL1—2+, and with tumor mutational load high. The patient was then offered robotic partial cystectomy with immunotherapy, and she elected to proceed with the surgical intervention.
Patient then underwent robotic partial cystectomy, and bilateral lymph node dissection. After transperitoneal access was obtained, 0-degree lens camera was placed with four total 8-mm robotic trocars, similar to robotic prostatectomy configuration. A 12-mm assistant trocar was placed in the right anterior axillary line. The da Vinci Xi robotic system was then docked to the trocars. The anterior peritoneum and space of Retzius was taken down lateral to the medial umbilical ligaments to drop the bladder posteriorly.
Flexible cystoscopy was then performed to observe the previous resection site of the tumor. The illumination of the cystoscope was observed through the bladder and 1 cm margins were then scored. A 2 × 2 cm segment of the entire bladder wall was then excised. The bladder was then reconstructed in two layers using 2-0 Vicryl suture. The bladder was then tested, with no extravasation identified. Bilateral pelvic lymph node dissection was then performed. The limits of node dissection were as follows: cephalad—the bifurcation of the common iliac artery, caudad—the node of Cloquet, medial—the obturator vessels, and lateral—the pelvic sidewall. Lymph node packets were removed together in the Endocatch bag with the bladder specimen. A Jackson-Pratt drain drain was placed as well as a 22F Foley catheter in the bladder.
No residual malignant melanoma was identified, and all lymph nodes were negative for malignancy. A cystogram was then performed in the clinic 1 week after the operation, which did not reveal any extravasation. The Foley catheter was then removed. Patient was started on pembrolizumab, an anti-PD-1 immunotherapy, for 1 year (18 treatments) and tolerated well overall. Patient also had continued surveillance with cystoscopy, bladder urine cytologies, and abdominal cross-sectional imaging with CT and MRI. At 2-year follow-up, she remains disease free with no evidence of recurrence.
Discussion and Literature Review
PMM of the urinary bladder has been known to occur over a wide age range (7–82), with a slightly higher prevalence in men.2 Gross hematuria is the most common presentation, which is regarded as a late symptom of a locally advanced melanoma.1 In the literature, there are a wide range of treatment options. Surgical treatments such as TURBT, partial cystectomy, and radical cystectomy are usually the primary treatments. Chemotherapy, radiation therapy, intravesical Bacillus Calmette-Guérin, and immunotherapy have also been used in a few patients.2
Historically, patient with metastatic PMM had a poor survival.2 Oncology recommended adjuvant pembrolizumab immunotherapy to give her the best possible survival advantage.3 Thus, taking into consideration the overall prognosis of the patient, her ECOG score, and the extensive lamina propria invasion, a partial cystectomy with lymph node dissection followed by immunotherapy was her most favorable option. This option would also avoid the morbidity associated with radical cystectomy. The technique for the robotic partial cystectomy was like that of a partial cystectomy for bladder cancer, as this was confirmed to be a solitary lesion by negative random bladder biopsies.
The pathogenesis of PMM of the bladder is unknown but theorized to be related to rare examples of melanosis involving the bladder. Melanosis of the bladder is characterized by multifocal and diffuse pigmentation of the bladder mucosa, which was not present on initial cystoscopy of our patient.1 Specific to melanoma of the bladder, there are no characteristic gross pathologic features. Microscopically the tumors have typical features of melanoma: nests of large pleomorphic cells with macronuclei and prominent nucleoli.1 Melanin pigment can be present in varying quantities. Immunohistochemistry shows typical features of melanoma with positive HMB45, melan-A, and S-100 protein without expression of epithelial markers. Primary melanoma of bladder has the following diagnostic criteria: (1) absence of any previous skin lesion, (2) absence of cutaneous malignant melanoma, (3) absence of primary visceral malignant melanoma, (4) recurrence pattern showing consistency with the primary tumor diagnosis, and (5) atypical melanocytes at the tumor margin on microscopic examination. However, metastatic melanoma is much more common than primary tumors.2
Before immunotherapy, despite the variety of therapies available for primary melanomas of the bladder, the prognosis of patients with this melanoma of the bladder was quite poor.2 Previously, most patients died because of widespread metastases within 3 years of the initial diagnosis.2 Pembrolizumab is a humanized monoclonal IgG4 PD1 antibody blocking the signaling of both PDL1 and PDL2 ligands.4 It was first approved by the FDA in September 2014 for the treatment of advanced melanoma, and has since then expanded its indications for use, including in patients with urothelial carcinoma. It was primarily studied as a first-line treatment of patients who were ineligible for cisplatin-containing therapy and as second-line treatment for patients whose disease progressed on or after platinum-containing chemotherapy.4 Patients treated with pembrolizumab have been reported to have improved progression-free survival and overall survival in both melanoma and bladder cancer patients.4 Also, pembrolizumab used as an adjuvant therapy for high-risk stage III melanoma, 200 mg of pembrolizumab administered every 3 weeks for up to 1 year, resulted in significantly longer recurrence-free survival than placebo, with no new toxic effects identified.3 Pembrolizumab is well tolerated with most common adverse reactions being fatigue, pruritus, diarrhea, decreased appetite, and rash.4 Our young patient was treated with pembrolizumab based on the immunohistochemical analysis of the bladder tumor, and has had a 2-year recurrence-free survival without any significant toxicities since then.
Conclusion
PMM of the bladder is a rare entity, and the optimal management algorithm for this disease is not well delineated in the literature. In this case, we have demonstrated an effective treatment paradigm that led to a 2-year recurrence-free survival. In particular, the usage of immunotherapy proved to be vital in preventing recurrence. Further research is required to determine its success in other patients with PMM.
Abbreviations Used
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- MRI
magnetic resonance imaging
- PMM
primary malignant melanoma
- TURBT
transurethral resection of the bladder tumor
Disclosure Statement
No competing financial interests exist.
Cite this article as: Chaus FM, Craig M, Bracamonte E, Sundararajan S, Lee BR (2019) Primary malignant melanoma of the bladder treated by robotic partial cystectomy and immunotherapy, Journal of Endourology Case Reports 5:4, 151–153, DOI: 10.1089/cren.2019.0031.
References
- 1. Venyo AK. Melanoma of the urinary bladder: A review of the literature. Surg Res Pract 2014;2014:605802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barillaro F, Camilli M, Dessanti P, et al. Primary melanoma of the bladder: Case report and review of the literature. Arch Ital Urol Androl 2018;90:224–226 [DOI] [PubMed] [Google Scholar]
- 3. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–1801 [DOI] [PubMed] [Google Scholar]
- 4. Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat Rev 2017;54:58–67 [DOI] [PubMed] [Google Scholar]