Abstract
Background and purpose
Tick‐borne encephalitis (TBE) is a common viral disease in central Europe and Asia. Severe or even lethal neurological symptoms may ensue. With limited therapeutic options, active vaccination against the TBE virus (TBEV) is strongly recommended in endemic areas. A systematic analysis of the clinical picture and cerebral imaging findings associated with TBE was conducted with particular focus on patients who acquired TBE despite previous vaccination.
Methods
A cohort of 52 patients with serologically proven TBE treated at our centre in a 10‐year period who received at least one cerebral magnetic resonance imaging (MRI) was retrospectively described. Extension of MRI changes was systematically assessed by an experienced neuroradiologist. Standard statistical procedures were performed.
Results
Fifty‐two patients with a definite serological diagnosis of TBE were included. The most common presentation was encephalitis (67%). MRI showed TBE‐associated parenchymal lesions in 33% of all patients. Sites of predilection included the periaqueductal grey, the thalamus and the brainstem. Ten patients had received at least one prior active or passive TBEV immunization. All of these had a maximal Rankin Scale score of at least 4. The median number of affected anatomical regions on MRI was significantly higher than in the non‐vaccinated cohort.
Conclusions
To our knowledge, this is the first study systematically describing the peculiarities of MRI in patients vaccinated against TBE. In addition to a severe clinical course, they exhibit more extensive MRI lesions than a non‐vaccinated cohort. Possible reasons for these findings include incomplete seroconversion, more virulent TBEV strains or antibody‐dependent enhancement.
Keywords: encephalitis, magnetic resonance imaging, tick‐borne encephalitis virus, vaccination
Introduction
Tick‐borne encephalitis (TBE) is caused by an RNA virus of the Flaviviridae family. Transmission occurs via infected ticks in most cases, Ixodes ricinus being the vector for the European subtype. Rarely, the disease may be acquired by consumption of contaminated dairy products [1, 2, 3, 4]. In Austria, the introduction and widespread coverage (one or more vaccination doses in >80% of the population) of a vaccine specific for TBE virus (TBEV) has resulted in an 84% reduction of TBE incidence, with a constant incidence of 6 per 100 000 unvaccinated inhabitants [5]. Primary immunization consists of three doses within 12 months, with the first booster after 3 years and every subsequent booster after 5 years [6]. Two preparations – Encepur® and FSME‐IMMUN® – are available in Europe. Cases of TBE after incomplete or complete immunization have been described [2, 7]. Therapeutic options in TBE are limited to supportive care.
The first stage of TBE is characterized by unspecific symptoms such as fever, headache and malaise. Approximately 10% of infected patients suffer from neurological symptoms, which are usually attributed to the second stage: meningitis (approximately 49%–58%), encephalitis (28%–41%) and myelitis and/or polyradiculitis (10%–14%). Patients with an encephalitic manifestation run a high risk of incomplete recovery (up to 46%). The mortality of TBE is approximately 1% [1, 3, 4, 8, 9]. TBE is diagnosed serologically via testing for antibodies in the serum and the cerebrospinal fluid (CSF). False‐positive results may occur post‐vaccination for TBEV or other Flaviviridae. Alternatively, reverse transcription polymerase chain reaction for the detection of TBEV RNA is available. Its sensitivity seems to depend strongly on the timing of this investigation relative to symptom onset [10].
Postmortem and animal studies have identified the thalamus, the basal ganglia, the brainstem and the cerebellar cortex as predilection sites for TBEV. In cases with a positive magnetic resonance imaging, lesions have also been described predominantly in these regions [3, 4, 11, 12]. However, MRI is negative in up to 90% of TBE patients [3, 13].
The primary aim of this study is to describe the radiological and clinical findings in a cohort with serologically proven TBE. The secondary aim is to report the particular presentation in a subgroup of patients who acquired TBE despite previous vaccination. These patients suffer a clinically and radiographically more severe course. Possible reasons include incomplete seroconversion, more virulent TBEV strains or antibody‐dependent enhancement.
Methods
Data of all patients with the International Classification of Diseases 10 discharge diagnosis of encephalitis meeting the European Academy of Neurology consensus review criteria of ‘probable TBE’ who were treated between 2007 and 2017 at one of the two neurological departments of the Kepler University Hospital, Linz, Austria, were reviewed [6]. Those patients with a diagnosis of confirmed TBE who received at least one cerebral MRI were included. Clinical data were retrieved from the electronic patient data file. The individuals and/or their general practitioners were contacted for missing details concerning the vaccination scheme.
The following clinical entities were defined:
Meningitis (M): headache, nuchal rigidity, photophobia, nausea, vomiting
Encephalitis (E): according to the criteria of the International Encephalitis Consortium [14]
Myelitis (ME): clinical signs of myelitis and/or suggestive MRI changes on T2‐weighted or contrast‐enhanced spinal MRI
Polyradiculitis (PR): clinical, neuroimaging and/or electrophysiological features compatible with polyradiculitis
MRIs were performed on a 3 T or a 1.5 T scanner using standard protocols consisting of T1, T2, fluid‐attenuated inversion recovery (FLAIR) and diffusion weighted imaging. Contrast medium was injected if the patient had no contraindications. Images were re‐examined by an experienced neuroradiologist (MS) who was blinded to the clinical data of the patients. MRI positivity was defined as hyperintensity on FLAIR/T2 imaging. Extension of MRI changes was assessed by defining 14 anatomical regions and counting the number of regions affected. Additionally, contrast enhancement of radices and/or cranial nerves and meninges was recorded. If the patient had received more than one MRI, the MRI with the most extensive lesion load was considered.
Tick‐borne encephalitis virus antibody titres in serum and CSF were measured via routine protocol enzyme‐linked immunosorbent assay. The diagnosis of TBE was made according to the recommendations of the European Academy of Neurology [6].
Descriptive statistical methods were used. Groups were compared using the Mann–Whitney U test and the chi‐squared test. Statistical significance was assumed for P < 0.05. Statistics were performed by JW. The study was approved by the ethics committee of Upper Austria. The need for consent was waived as this is a retrospective study.
Results
Study population
In all, 245 patients with a diagnosis of encephalitis or meningitis were screened for eligibility. Fifty‐two patients (30 males) with a definite serological diagnosis of TBE were included. All of these patients had neurological symptoms, CSF pleocytosis and at least one of the following (number of vaccinated/non‐vaccinated patients fulfilling each criterion; numbers add up to more than 10/42 as most patients fulfilled more than one criterion):
seroconversion of specific serum and/or CSF immunoglobulin M (IgM) (7/3)
a significant increase of specific serum and/or CSF IgG titres (5/5)
positive CSF/serum indices (IgG and/or IgM) (4/21)
high specific serum and/or CSF IgM titres (i.e. >500 units) and no TBEV vaccination in the previous year (9/42)
immunohistochemical positivity for TBEV in a brain tissue specimen (1/0)
The median age was 58 years (range 19–80). 67% had encephalitis or cerebellitis, 46% meningitis, 25% polyradiculitis or cranial polyneuritis and 10% myelitis. Eighteen patients reported a tick bite within the 4 weeks before hospitalization. Data on previous TBEV vaccination were available for all patients. For individual patient data, see Table 1.
Table 1.
Epidemiological and clinical details of all TBE patients included in this study
| Sex | Age | Manifestation | Vaccination | Maximal mRS | 1st MRI – time from onset (days) | Last MRI – time from onset (days) | Spinal MRI | First available CSF count (leucocytes/µl) | Mechanical ventilation |
|---|---|---|---|---|---|---|---|---|---|
| f | 66 | M, E, PR | + | 5 | 7 | 28 | 395 | − | |
| f | 60 | E, PR | + | 5 | 3 | 159 | + | 74 | + |
| f | 57 | E | − | 3 | 4 | 13 | 72 | − | |
| m | 48 | M, ME, PR | − | 5 | 6 | 1055 | + | 563 | + |
| m | 64 | E | − | 2 | 18 | 79 | − | ||
| m | 73 | E, PR | − | 4 | 5 | 140 | 52 | − | |
| m | 53 | E, ME, PR | + | 6 | 3 | 24 | + | 224 | + |
| f | 70 | E, PR | − | 2 | 6 | 8 | + | 66 | − |
| f | 44 | E | + | 4 | 5 | 16 | 705 | − | |
| m | 33 | E | − | 2 | 11 | 115 | − | ||
| f | 75 | E | + | 5 | 16 | 53 | − | ||
| f | 77 | E | − | 5 | 25 | 117 | − | ||
| m | 52 | E, PR | + | 5 | 5 | 353 | + | 104 | − |
| f | 68 | E | − | 3 | 15 | 21 | 34 | − | |
| f | 47 | E | − | 3 | 3 | 4 | 159 | − | |
| f | 29 | M | − | 2 | 9 | 143 | − | ||
| f | 74 | E | − | 6 | 4 | 7 | − | ||
| m | 53 | M | − | 1 | 7 | 14 | − | ||
| m | 47 | M | − | 1 | 10 | 129 | − | ||
| m | 48 | E | − | 3 | 11 | 67 | − | ||
| m | 55 | PR | − | 2 | 15 | 86 | − | ||
| m | 58 | M | − | 1 | 6 | 130 | − | ||
| m | 75 | E | − | 3 | 20 | 29 | 82 | − | |
| m | 55 | M | − | 2 | 13 | 72 | − | ||
| m | 54 | E | − | 2 | 7 | 22 | 273 | − | |
| m | 62 | E | − | 2 | 7 | 76 | − | ||
| f | 19 | E | − | 3 | 2 | 194 | − | ||
| m | 54 | E | − | 3 | 3 | 72 | − | ||
| f | 58 | E | − | 3 | 4 | 743 | 39 | − | |
| m | 57 | E | − | 3 | 21 | 102 | − | ||
| m | 56 | M, ME, PR | − | 5 | 1 | 47 | + | 359 | + |
| m | 69 | E | + | 6 | 1 | 11 | 620 | + | |
| m | 73 | E | − | 3 | 4 | 90 | − | ||
| f | 77 | E, PR | − | 5 | 1 | 11 | 64 | + | |
| m | 74 | E | + | 5 | 1 | 84 | − | ||
| m | 66 | M, E | − | 2 | 18 | 54 | − | ||
| m | 80 | M, E | + | 4 | 4 | 16 | 155 | − | |
| f | 29 | M | − | 1 | 3 | 19 | 9 | − | |
| m | 33 | M | − | 1 | 4 | 26 | − | ||
| m | 61 | M | − | 1 | 12 | 733 | 37 | − | |
| m | 55 | M | − | 1 | 4 | 37 | 374 | − | |
| f | 70 | M | − | 4 | 13 | 147 | − | ||
| f | 41 | M | − | 1 | 9 | 205 | − | ||
| f | 60 | M, E | − | 3 | 6 | 220 | − | ||
| m | 78 | M, E | − | 3 | 7 | 116 | − | ||
| f | 37 | M | − | 1 | 8 | 212 | − | ||
| m | 78 | E | − | 5 | 3 | 72 | − | ||
| m | 29 | M, PR | − | 4 | 3 | 16 | + | 665 | − |
| f | 71 | M, E, ME, PR | − | 5 | 6 | + | 258 | − | |
| m | 73 | M, E, ME, PR | + | 5 | 1 | + | 0 | − | |
| f | 49 | M | − | 2 | 17 | 45 | − | ||
| f | 72 | M | − | 1 | 18 | 116 | − |
Presenting neurological symptoms
The most frequent presenting neurological symptoms were disorders of higher order cortical function (consciousness, orientation, cognition, speech etc.; 58% of patients). Other symptoms included pareses of the extremities (27%), cerebellar symptoms (21%), bulbar deficits (13%), extrapyramidal signs (12%) and psychiatric symptoms (4%). The median maximal score on the modified Rankin Scale (mRS) throughout the course of the acute disease [range 6 (death) to 0 (no symptoms)] was 1 in those patients who presented with meningitis only (range 1–2), 5 in those with myelitis and/or polyradiculitis (range 2–6) and 3 in patients with encephalitis without myelitis or polyradiculitis (range 2–6). At least one clinical follow‐up >30 days after symptom onset was available in 16 patients (median time to last follow‐up 236 days; range 42–2554). At the last follow‐up, 44% of these patients were symptom‐free. The mortality rate in the total cohort was 6%.
Cerebrospinal fluid
The first lumbar puncture was performed at a median of 5 days after onset of the first symptoms (range 1–30). The median cell count in the first spinal tap was 96 cells (range 0–705). There was no significant difference in cell counts between meningitis, encephalitis, myelitis and polyradiculitis.
Localization and characteristics of MRI lesions
All patients included in this study had at least one cerebral MRI which was performed at a median of 6 days after symptom onset (range 1–25). Parenchymal lesions assumed to be caused by TBEV were found in 33% of all patients. Of those patients with encephalitis or cerebellitis, 16 (46%) had lesions on their cerebral MRI.
Sites of predilection included the periaqueductal grey (17% of all patients), the thalamus (12%) and the brainstem (12%). Supratentorial lobar lesions were seen in 15% of patients. All of these were FLAIR/T2‐positive lesions. The median number of affected anatomical regions per patient was 0 (range 0–8). In those with at least one MRI lesion, the median was 2. Contrast‐enhancing parenchymal lesions were found in only three patients. Meningeal enhancement was seen in 17, radicular/cranial nerve enhancement in seven patients. Diffusion weighted imaging was positive in one patient.
Twenty‐two patients had at least one follow‐up MRI during or after the encephalitis‐related hospitalization with a total of 37 short‐term (i.e. within 3 months after symptom onset) follow‐up MRIs. The last of these MRIs was performed at a median of 14 days (range 2–57) after hospitalization. New lesions relative to the previous MRI were detected in seven patients up to 21 days after hospital admission. On the last short‐term MRI available, six patients had receding, four patients stable and three patients progressive findings relative to the penultimate MRI.
Vaccinated (vTBE) patients
Nine patients of our cohort (17%) had received at least one prior active TBEV immunization; one had been passively immunized 19 years before encephalitis onset. The median age of this group was 68 years (range 44–80) and included six males. At least three of these patients had completed the entire primary immunization scheme. Seven patients had received a booster vaccination within the last 10 years before TBE onset. For details as to the vaccination dates and the formulations used, see Table 2.
Table 2.
Clinical details of vTBE patients
| Patient | Sex | Age | Active versus passive immunization | Known dates of vaccination (month/year) | TBE onset (month/year) | Formulation used for last booster before TBE onset | Serum IgG a | Serum IgM | CSF IgG a | CSF IgM | CSF/serum indices | Further diagnostic markers | Comorbidities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 74 | Active | 4/05 | 6/13 | Encepur® |
27.6: >1000 2.7: 129 000 |
27.6: bl 2.7: pos |
2.7: >1000 | 2.7: pos | Previous chronic alcohol abuse | ||
| 2 | Female | 75 | Active | Approximately 1986 | 7/16 | Unknown |
27.7: pos 2.8: 5100 5.8: 45 600 |
27.7: neg 2.8: neg 5.8: pos |
27.7: pos 2.8: >1000 5.8: >1000 |
27.7: neg 2.8: neg 5.8: pos |
27.7: IgG > 3.67 IgM neg |
||
| 3 | Male | 53 | Passive | 1994 | 7/13 | Unknown | 24.7: neg | 24.7: pos | 24.7: pos | 24.7: pos |
24.7: IgG 1.6 IgM 13.85 |
||
| 4 | Male | 73 | Active | 3/16 | 7/16 | Unknown |
5.7: 769 27.7: 269 000 |
5.7: bl 27.7: pos |
27.7: >1000 | 27.7: pos |
NT titres 27.7: >1280 |
Monoclonal gammopathy of undetermined significance | |
| 5 | Male | 52 | Active | 1/01, 5/05, 5/11 | 4/17 | FSME‐IMMUN® | 5.5: >1000 | 5.5: pos | 5.5: 420 | 5.5: pos |
5.5: IgG > 2.1 IgM > 5.98 |
||
| 6 | Male | 80 | Active | Approximately 1993 | 9/8 | Unknown | 24.9: >1000 | 24.9: >1000 | 24.9: 833 | 24.9: 334 | Type 2 diabetes | ||
| 7 | Male | 69 | Active | 3/98, 4/98, 4/99, 4/02 | 7/11 | Unknown |
22.7: 2800 27.7: 7500 2.8: 15 500 |
22.7: neg 27.7: pos 2.8: pos |
22.7: 495 | 22.7: neg |
30.7: IgG 5.99 IgM 18.23 |
NT titres 22.7: 60 27.7: 240 2.8: 320 Brain tissue pos for TBEV |
CREST syndrome |
| 8 | Female | 44 | Active | 5/90, 6/90, 6/91, 6/94, 2001, 8/10, 9/10, 8/11 | 8/14 | FSME‐IMMUN® |
20.8: 189 770 RU/ml 26.8: 185 880 RU/ml |
20.8: 2.610 RU/ml 26.8: 2.950 RU/ml |
20.8: neg 26.8: pos |
20.8: neg 26.8: bl |
Ulcerative colitis, previous therapies with azathioprine, adalimumab, methotrexate, infliximab | ||
| 9 | Female | 60 | Active | 5/93, 6/93, 2/94, ?/97, 2/00, 3/03, 5/08 | 9/09 | FSME‐IMMUN® |
14.10: 67 800 17.11: 384 400 |
14.10: pos 17.11: pos |
30.9: 478 14.10: >1000 11.11: >1000 17.11: >1000 |
30.9: neg 14.10: pos 11.11: pos 17.11: pos |
NT titres 14.10: >1280 17.11: >1280 |
||
| 10 | Female | 66 | Active | 4/98, 4/01, 5/05 | 7/07 | FSME‐IMMUN® |
12.7: >1000 17.7: >1000 |
12.7: bl 17.7: >1000 |
12.7: >1000 17.7: >1000 |
12.7: >1000 17.7: >1000 |
Polyarthritis |
bl, borderline; CSF, cerebrospinal fluid; Ig, immunoglobulin; neg, negative; nt, tick‐borne encephalitis virus neutralization test; pos, positive; TBE, tick‐borne encephalitis; vTBE, vaccinated against the TBE virus. Only comorbidities potentially causing immunosuppression are listed.
Date (day.month): titre (VIE [vienna] units unless stated otherwise).
All of the vTBE patients suffered from encephalitis, two from myelitis and five from polyradiculitis, and were severely affected with a median maximal mRS score of 5 (range 4–6). Cerebral lesions were found in eight patients, three of them with contrast‐enhancing parenchymal lesions. The median number of affected anatomical regions in vTBE patients was significantly higher at a median of 2 (range 0–8) than in non‐vaccinated patients (median 0; range 0–4). For a direct comparison of MRI findings in the vaccinated versus non‐vaccinated group, see Table 3. Imaging examples are shown in Figs 1 and 2.
Table 3.
Affected anatomical regions (MRI) in all TBE patients included in this study
| Patient | Sex, age (years) | Thalamus | Lentiform ncl. | Caudate ncl. | Internal capsule | Corpus callosum | Frontal lobe | Parietal lobe | Occipital lobe | Temporal lobe | Brainstem | Cerebellum | Periaqu. grey | Cerebell. peduncle |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m, 74 | |||||||||||||
| 2 | f, 75 | |||||||||||||
| 3 | m, 53 | |||||||||||||
| 4 | m, 73 | |||||||||||||
| 5 | m, 52 | |||||||||||||
| 6 | m, 80 | |||||||||||||
| 7 | m, 69 | |||||||||||||
| 8 | f, 44 | |||||||||||||
| 9 | f, 60 | |||||||||||||
| 10 | f, 66 | |||||||||||||
| 11 | m, 73 | |||||||||||||
| 12 | f, 70 | |||||||||||||
| 13 | f, 57 | |||||||||||||
| 14 | m, 73 | |||||||||||||
| 15 | f, 77 | |||||||||||||
| 16 | f, 58 | |||||||||||||
| 17 | f, 74 | |||||||||||||
| 18 | f, 70 | |||||||||||||
| 19 | m, 56 | |||||||||||||
| 20–52 |
cerebell., cerebellar; f, female; m, male; MRI, magnetic resonance imaging; ncl., nucleus; periaqu., periaqueductal; TBE, tick‐borne encephalitis.
Patients marked light grey are patients vaccinated against TBE virus. The numbering corresponds to table 2. Patients 20–52 had no lesions on MRI.
Figure 1.

vTBE patient (passive immunization; no. 3 in Table 2) with FLAIR‐positive lesions in the mesial temporal and frontal lobe, the brainstem including the periaqueductal grey and the cerebellum. The patient’s lesions in the thalamus, lentiform and caudate nucleus do not show on the selected slice.
Figure 2.
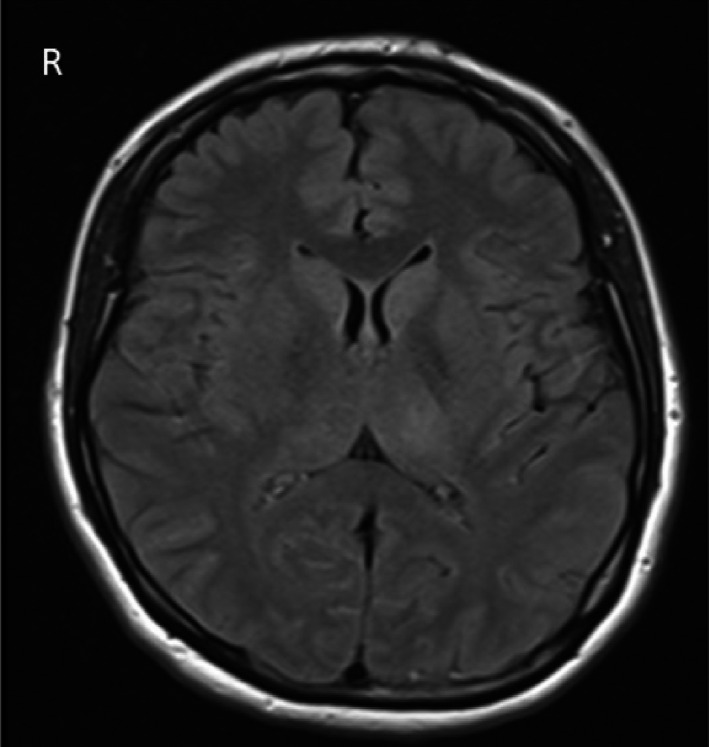
vTBE patient (active immunization; no. 8 in Table 2) with FLAIR‐positive lesions in the thalamus and caudate nucleus (the patient’s brainstem lesions do not show on the selected slice).
There was no significant difference in the CSF cell count between the TBE (median 88; range 7–665) and vTBE (median 130; range 0–705) groups. Five out of 10 vTBE patients were seropositive for anti‐TBEV IgM in the first serum tested at a median of 5 days after onset of the first symptoms; three patients were negative, two had borderline titres. In all of the latter patients, the titres turned clearly positive on the second testing. The initial CSF was IgM positive in six, negative in three and borderline in one vTBE patient(s). In contrast, initial IgM testing was positive in 93% (serum) and 87% (CSF) of unvaccinated patients.
Discussion
The most frequent TBEV‐associated neurological symptoms were disorders of cognitive and motor function. The latter was particularly common in patients who suffered from myelitis and/or polyradiculitis, often causing significant long‐term invalidity. Persisting disability was also seen after encephalitis and may result from residual focal cerebral lesions or a post‐encephalitic syndrome, which comprises cognitive and psychiatric symptoms [3].
The sensitivity of cerebral imaging was low: 33% of all patients and 46% of patients with clinical encephalitis had a positive MRI. This figure is higher than that found in previous reports (17%–20% [3, 11]), possibly due to more advanced imaging techniques (i.e. more patients with 3 T MRI). Furthermore, all of our MRIs were seen by a specialized neuroradiologist. A possible alternative confounder may be the timing of the investigation as a time‐dependent course of MRI alterations has been described [13]. 42% of our patients received multiple images, reducing the likelihood of missed temporary lesions. Two of these patients displayed new lesions on day 21 after hospital admission compared to their respective MRIs on day 5 and day 8. This suggests that MRI changes may appear late, providing the rationale for repeat MRI in case of diagnostic doubt.
A novel finding of our study is the increased extension of cerebral MRI lesions in our cohort of vTBE patients, going along with a more severe clinical course. Contrast‐enhancing lesions were only found in this group, probably reflecting a propensity for a blood–brain barrier breakdown. More severe clinical courses of TBE have been seen in patients after complete and incomplete active vaccination compared to those without previous immunization [1, 2, 7, 15, 16, 17, 18]. Sporadically, exceptionally pronounced MRI alterations have been documented in these patients as well [8, 19].
Vaccine failure may result from incomplete seroconversion or the specific absence of antibodies to neutralizing epitopes despite the presence of antiviral antibodies detected with immunoassays. These patients may be predisposed to a more severe disease course [1, 16, 17]. However, seroconversion rates are high after active TBE vaccination (up to 99.5% after three shots, 91%–92% with an irregular schedule [16, 20]). Serological follow‐up studies in adults have revealed long‐term persistence of protective immunity following at least one booster immunization [21].
Another patient‐specific immunological factor may be the velocity of the antibody defence. Low early CSF IgM response is an immunological peculiarity associated with the development of encephalitic symptoms and vaccination failure [18, 22, 23]. It was possible to verify this delayed induction of specific IgM in our vTBE cohort. Alternatively, vTBE may be due to a selection effect of particularly virulent strains of TBEV in a vaccinated population [4]. RNA sequencing of the virions found in the relevant geographical areas should be performed to test this hypothesis.
For another flavivirus – dengue virus – antibody‐dependent enhancement of the disease has been suggested [24, 25]. This theory claims that within a specific concentration range antibodies bind but do not neutralize virions, thereby facilitating virus entry and replication in immune cells. In a recent trial, children and adolescents showed evidence of a higher risk of severe disease and hospitalization in vaccinated persons who had not previously been exposed to dengue compared to those who had [24]. Further exploration of this hypothesis would require prospective studies.
In summary, a cohort of TBE patients was systematically described with particular focus on imaging results. The subgroup of vTBE patients is of particular interest. To our knowledge, this is the first study systematically describing the peculiarities of MRI in this entity. Limitations of this study include its retrospective character and the lack of complete vaccination data in some patients. Furthermore, the anti‐TBEV Ig CSF serum index was not available for 60% of vTBE patients. Also, no children, who may have a more favourable course of disease, were included.
Disclosure of conflicts of interest
Dr Wagner reports personal fees from UCB, outside the submitted work; Dr von Oertzen reports personal fees and non‐financial support from Eisai Pharma GmbH Vienna, grants, personal fees and non‐financial support from UCB Pharma GmbH Vienna, non‐financial support from Medtronic Austria GmbH, grants, personal fees and non‐financial support from Novartis Pharma, personal fees from Roche Pharma, personal fees from Biogen Idec Austria, personal fees from Liva Nova, personal fees from Sanofi‐Aventis GmbH, grants from Grossegger & Drbal GmbH, outside the submitted work; Dr Krehan reports grants from Bayer, grants from Boehringer Ingelheim, outside the submitted work. The other authors report no conflicts of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Lindquist L, Vapalahti O. Tick‐borne encephalitis. Lancet 2008; 371: 1861–1871. [DOI] [PubMed] [Google Scholar]
- 2. Zavadska D, Odzelevica Z, Karelis G, et al Tick‐borne encephalitis: a 43‐year summary of epidemiological and clinical data from Latvia (1973 to 2016). PLoS One 2018; 13: e0204844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaiser R. The clinical and epidemiological profile of tick‐borne encephalitis in southern Germany 1994–98: a prospective study of 656 patients. Brain J Neurol 1999; 122(Pt 11): 2067–2078. [DOI] [PubMed] [Google Scholar]
- 4. Lenhard T, Ott D, Jakob NJ, et al Predictors, neuroimaging characteristics and long‐term outcome of severe European tick‐borne encephalitis: a prospective cohort study. PLoS One 2016; 11: e0154143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinz FX, Stiasny K, Holzmann H, et al Emergence of tick‐borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Euro Surveill 2015; 20: 9–16. [DOI] [PubMed] [Google Scholar]
- 6. Taba P, Schmutzhard E, Forsberg P, et al EAN consensus review on prevention, diagnosis and management of tick‐borne encephalitis. Eur J Neurol 2017; 24: 1214–e61. [DOI] [PubMed] [Google Scholar]
- 7. Hansson K, Rosdahl A, Insulander M, et al Tick‐borne encephalitis (TBE) vaccine failures: a ten‐year retrospective study supporting the rationale for adding an extra priming dose in individuals from the age of 50 years. Clin Infect Dis 2020; 70: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valdueza JM, Weber JR, Harms L, Bock A. Severe tick borne encephalomyelitis after tick bite and passive immunisation. J Neurol Neurosurg Psychiatry 1996; 60: 593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Czupryna P, Moniuszko A, Pancewicz SA, Grygorczuk S, Kondrusik M, Zajkowska J. Tick‐borne encephalitis in Poland in years 1993–2008 – epidemiology and clinical presentation. A retrospective study of 687 patients. Eur J Neurol 2011; 18: 673–679. [DOI] [PubMed] [Google Scholar]
- 10. Kaiser R. Tick‐borne encephalitis. Nervenarzt 2016; 87: 667–680. [DOI] [PubMed] [Google Scholar]
- 11. Zawadzki R, Garkowski A, Kubas B, et al Evaluation of imaging methods in tick‐borne encephalitis. Pol J Radiol 2017; 82: 742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Stülpnagel C, Winkler P, Koch J, et al MRI‐imaging and clinical findings of eleven children with tick‐borne encephalitis and review of the literature. Eur J Paediatr Neurol 2016; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 13. Pichler A, Sellner J, Harutyunyan G, et al Magnetic resonance imaging and clinical findings in adults with tick‐borne encephalitis. J Neurol Sci 2017; 375: 266–269. [DOI] [PubMed] [Google Scholar]
- 14. Venkatesan A, Tunkel AR, Bloch KC, et al Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin Infect Dis 2013; 57: 1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lotrič‐Furlan S, Bogovič P, Avšič‐Županc T, Jelovšek M, Lusa L, Strle F. Tick‐borne encephalitis in patients vaccinated against this disease. J Intern Med 2017; 282: 142–155. [DOI] [PubMed] [Google Scholar]
- 16. Bender A, Jäger G, Scheuerer W, Feddersen B, Kaiser R, Pfister H‐W. Two severe cases of tick‐borne encephalitis despite complete active vaccination – the significance of neutralizing antibodies. J Neurol 2004; 251: 353–354. [DOI] [PubMed] [Google Scholar]
- 17. Kaiser R, Holzmann H. Laboratory findings in tick‐borne encephalitis – correlation with clinical outcome. Infection 2000; 28: 78–84. [DOI] [PubMed] [Google Scholar]
- 18. Günther G, Haglund M, Lindquist L, Sköldenberg B, Forsgren M. Intrathecal IgM, IgA and IgG antibody response in tick‐borne encephalitis. Long‐term follow‐up related to clinical course and outcome. Clin Diagn Virol 1997; 8: 17–29. [DOI] [PubMed] [Google Scholar]
- 19. Lorenzl S, Pfister HW, Padovan C, Yousry T. MRI abnormalities in tick‐borne encephalitis. Lancet 1996; 347: 698–699. [DOI] [PubMed] [Google Scholar]
- 20. Heinz FX, Stiasny K, Holzmann H, et al Vaccination and tick‐borne encephalitis, central Europe. Emerg Infect Dis 2013; 19: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rendi‐Wagner P, Zent O, Jilg W, Plentz A, Beran J, Kollaritsch H. Persistence of antibodies after vaccination against tick‐borne encephalitis. Int J Med Microbiol 2006; 296: 202–207. [DOI] [PubMed] [Google Scholar]
- 22. Andersson CR, Vene S, Insulander M, Lindquist L, Lundkvist A, Günther G. Vaccine failures after active immunisation against tick‐borne encephalitis. Vaccine 2010; 28: 2827–2831. [DOI] [PubMed] [Google Scholar]
- 23. Kleiter I, Jilg W, Bogdahn U, Steinbrecher A. Delayed humoral immunity in a patient with severe tick‐borne encephalitis after complete active vaccination. Infection 2007; 35: 26–29. [DOI] [PubMed] [Google Scholar]
- 24. Sridhar S, Luedtke A, Langevin E, et al Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379: 327–340. [DOI] [PubMed] [Google Scholar]
- 25. Katzelnick LC, Gresh L, Halloran ME, et al Antibody‐dependent enhancement of severe dengue disease in humans. Science 2017; 358: 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


