Abstract
The observational CERTITUDE study follows liver transplant patients who completed the SIMCER trial. SIMCER randomized patients at month 1 after transplant to everolimus (EVR) with stepwise tacrolimus (TAC) withdrawal or to standard TAC, both with basiliximab induction and mycophenolic acid ± steroids. After completing SIMCER at 6 months after transplant, 65 EVR‐treated patients and 78 TAC‐treated patients entered CERTITUDE. At month 24 after transplant, 34/65 (52.3%) EVR‐treated patients remained calcineurin inhibitor (CNI) free. Mean estimated glomerular filtration rate (eGFR) was significantly higher with EVR versus TAC during months 3‐12. At month 24, eGFR values were 83.6 versus 75.3 mL/minute/1.73 m2, respectively (P = 0.90) and adjusted mean change in eGFR from randomization was −8.0 versus −13.5 mL/minute/1.73 m2 (P = 0.15). At month 24, 45.9%, 31.1%, and 23.0% of EVR‐treated patients had chronic kidney disease stages 1, 2, and 3, respectively, versus 25.7%, 45.7%, and 28.6% of TAC‐treated patients (P = 0.05). Treated biopsy‐proven acute rejection affected 4 EVR‐treated patients and 2 TAC patients during months 6‐24. Adverse events led to study discontinuation in 15.4% and 7.7% of EVR‐treated and TAC‐treated patients, respectively. Grade 3 or 4 hematological events were rare in both groups. A CNI‐free EVR‐based maintenance regimen appears feasible in approximately half of liver transplant patients. It preserves renal function effectively with good efficacy without compromising safety or hematological tolerance.
Abbreviations
- aMDRD
abbreviated Modification of Diet in Renal Disease
- ANCOVA
analysis of covariance
- BPAR
biopsy‐proven acute rejection
- CI
confidence interval
- CKD
chronic kidney disease
- CNI
calcineurin inhibitor
- CsA
cyclosporine A
- EC‐MPS
enteric‐coated mycophenolate sodium
- eGFR
estimated glomerular filtration rate
- EPO
erythropoietin‐stimulating protein
- EVR
everolimus
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- KDOQI
Kidney Disease Outcomes Quality Initiative
- MACE
major cardiovascular event
- MPA
mycophenolic acid
- mTOR
mammalian target of rapamycin
- RAI
rejection activity index
- SD
standard deviation
- SE
standard error
- TAC
tacrolimus
Graft survival rates after liver transplantation have improved steadily over the last 2 decades, reaching 90% at 1 year and 75% by year 5.1 Patients receiving a liver transplant, however, have become older and more severely ill. Also, they are more frequently transplanted due to malignant diseases.1 It is therefore perhaps unsurprising that improvements in patient survival have plateaued.2
Against this background, the immunosuppressive regimen should take into account the risk of complications that can contribute to longterm morbidity and mortality. The calcineurin inhibitor (CNI) tacrolimus (TAC) is used almost universally after liver transplantation1 based on evidence for reduced rejection and improved outcomes versus cyclosporine A (CsA).3, 4 The nephrotoxic effects of CNI therapy, however, contribute to progressive renal dysfunction after liver transplantation.5, 6 TAC also appears to increase the risk for malignancy in a dose‐dependent manner7, 8 and to be associated with cardiovascular risk factors, such as diabetes mellitus.9
The mammalian target of rapamycin (mTOR) inhibitor everolimus (EVR) offers an alternative immunosuppressant to CNIs, with the potential to reduce CNI‐related nephrotoxicity without loss of efficacy10 and possibly to lower the risk of posttransplant malignancies.11 The randomized H2304 study demonstrated that EVR with reduced TAC was associated with significantly better renal function and comparable efficacy over the first 3 years after liver transplantation compared with standard TAC therapy.12, 13, 14
The recent SIMCER trial examined the use of EVR in combination with mycophenolic acid (MPA) to support a CNI‐free regimen early after liver transplantation.15 At month 1, patients were randomized to start EVR with TAC withdrawal by month 4. All patients received basiliximab induction. At the end of the 6‐month study, the mean gain in estimated glomerular filtration rate (eGFR) from randomization was 14 mL/minute/1.73 m2 higher with EVR compared with the control arm (P < 0.001), with a trend to more frequent treated biopsy‐proven acute rejection (BPAR; 8.9% versus 2.2%; P = 0.06), which was considered as being due to early EVR underexposure.
Patients who completed the SIMCER study were given the option to participate in the observational CERTITUDE study, which followed patients to 5 years after transplant. The aim of CERTITUDE is to provide longterm follow‐up data on renal function (the primary endpoint of SIMCER) as well as on efficacy, safety, and patient care, with a focus on patients receiving a CNI‐free regimen. Results to 2 years after transplant are presented here.
Patients and Methods
Study Design and Conduct
CERTITUDE is a multicenter, observational, prospective study conducted at 13 transplant centers in France (NCT CRAD001HFR03, Eudract 2012‐000137‐39). The enrolled patients were those who completed the 6‐month, multicenter, open‐label SIMCER trial, in which de novo liver transplant patients were randomized at 1 month after transplant to either start EVR with TAC withdrawn by month 4 or to continue standard TAC‐based immunosuppression.15 For patients who agreed to participate in CERTITUDE and who provided the relevant informed consent, study data were collected on entry to CERTITUDE, then at 6‐month intervals until 2 years after transplant, and annually thereafter at routine patient follow‐up visits until 5 years after transplant.
The CERTITUDE study protocol was approved by the French Advisory Committee for Data Processing in Health Research (Comité Consultatif sur le Traitement de l’Information en Matière de Recherche Dans le Domaine de la Santé) and the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés). Written informed consent was obtained from all participants.
Eligibility Criteria
Patients who were randomized in the SIMCER trial and attended either the 6‐month or end‐of‐study visit were eligible to enter the CERTITUDE study after providing new written consent unless they had experienced graft loss or been lost to follow‐up. Key exclusion criteria for the SIMCER trial were transplantation with a graft from a living donor or deceased non–heart‐beating donor, transplantation following autoimmune liver hepatitis, primary sclerosing cholangitis or primary biliary cholangitis, and eGFR ≤30 mL/minute/1.73 m2.
Immunosuppression
The immunosuppression protocol for SIMCER has been described previously.15 In brief, all patients received basiliximab induction followed by initial maintenance therapy with TAC (target 6‐10 ng/mL) and enteric‐coated mycophenolate sodium (EC‐MPS; 720 mg twice daily). Intravenous and oral steroids could be given according to local practice. Patients were randomized at week 4 (stratified according to hepatitis C virus [HCV] positivity or negativity and by eGFR at transplant). In the EVR group, the target EVR trough concentration was 6‐10 ng/mL, with the TAC dose reduced stepwise and then discontinued during week 12. In the control arm, TAC was continued (6‐10 ng/mL). All patients continued EC‐MPS to the end of the study with or without steroids.
During the CERTITUDE study, physicians were free to modify the immunosuppressive treatments at any time during follow‐up. The patients were, however, identified according to the therapeutic strategy (with or without CNI) received according to randomization in the SIMCER study.
Study Endpoints
The primary endpoint for the CERTITUDE study was the change in eGFR from randomization in the SIMCER trial (at month 1 after transplant) to months 12, 18, 24, 36, 48, and 60 after transplant. Secondary endpoints included the urinary protein:creatinine ratio; stage of chronic kidney disease (CKD; Kidney Disease Outcomes Quality Initiative [KDOQI] classification); requirement for dialysis or kidney transplantation; incidence and severity of treated BPAR (rejection activity index [RAI] score >3, Banff 97)16; treatment failure (treated BPAR RAI score >3, graft loss, or death); patient and graft survival; major cardiovascular events (MACEs); de novo malignancy; histological HCV recurrence (metavir ≥ F2); overall and de novo diabetes; changes in fasting blood glucose, HbA1c, and lipid levels; immunosuppressive treatments received and the reasons for discontinuation; and adverse events and serious adverse events.
The eGFR was found according to the abbreviated Modification of Diet in Renal Disease (aMDRD).17 BPAR was graded according to the RAI score. Treatment failure was defined as treated BPAR (RAI score >3), graft loss, or death.
Data Analysis
For the primary endpoint (change in eGFR [aMDRD] from randomization), 95% confidence interval (CI) values were estimated, and values were compared between groups using the Wilcoxon rank sum test. Analysis of covariance (ANCOVA) was also used to compare the change in eGFR between groups, with treatment group and planned assessment time as fixed factors and eGFR at baseline as the covariate. Afterward, the change in eGFR was analyzed according to the immunosuppressive regimen received by patients in the following groups:
Remained on EVR as randomized without TAC.
Switched from EVR to TAC.
Remained on TAC as randomized without EVR.
Switched from TAC to EVR.
Characteristics of the 2 initial treatment arms and secondary endpoints were compared using the chi‐square text, Fisher’s exact test, Student t test, or Wilcoxon test, depending on the variable, the distribution of the data, and the number of subgroups. Changes from randomization were assessed by the Wilcoxon signed rank test.
It was estimated that approximately 140 patients would enter the CERTITUDE study based on a 25% dropout rate from the 184 patients randomized in the SIMCER trial.
Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Study Population
Of the 188 patients randomized in the SIMCER study (EVR group, n = 93; TAC group, n = 95), 143 entered CERTITUDE (EVR group, n = 65; TAC group, n = 78). Among the 45 patients who did not enter CERTITUDE, 8 were ineligible (deceased, graft loss, or no study visit at month 6 in the SIMCER trial), 11 declined to participate, 9 were not included due to administrative reasons, and the reason was unknown in the remaining 17 patients. Patient characteristics were similar between groups other than a lower proportion of patients transplanted due to hepatocellular carcinoma (HCC) in the EVR group (18.5% versus 29.5%; Table 1). The follow‐up visit at month 24 was attended by 137 patients (EVR group, n = 63; TAC group, n = 74; Fig. 1A).
Table 1.
Patient Characteristics and Immunosuppression According to Randomized Treatment Groups
| EVR Group (n = 65) | TAC Group (n = 78) | |
|---|---|---|
| Sex, male | 56 (86.2) | 68 (87.2) |
| Age, years* | 57 (9) | 56 (8) |
| Race, white | 61 (93.8) | 74 (94.9) |
| Body mass index, kg/m2 | 23.6 (3.5) | 23.4 (4.2) |
| Reason for liver transplantation | ||
| Alcoholic cirrhosis | 35 (53.8) | 40 (51.3) |
| HCC | 12 (18.5) | 23 (29.5) |
| HCV | 6 (9.2) | 6 (7.7) |
| HBV | 2 (3.1) | 2 (2.6) |
| Other | 10 (15.4) | 7 (9.0) |
| Diabetes† | 20 (30.8) | 23 (29.5) |
| Cold ischemia time ≥12 hours | 5 (7.7) | 3 (3.8) |
| Immunosuppression at month 6 after transplant | ||
| EVR | 60 (92.3) | 3 (3.8) |
| CNI therapy | 15 (23.1) | 77 (98.7) |
| TAC | 15 (23.1) | 76 (97.4) |
| CsA | 0 (0.0) | 1 (1.3) |
| MPA | 53 (81.5) | 69 (88.5) |
| Steroids | 37 (56.9) | 41 (52.6) |
| Immunosuppression at month 24 after transplant‡ | ||
| EVR | 44 (69.8)§ | 18 (24.3) |
| CNI therapy | 29 (46.0) | 68 (91.9) |
| TAC | 28 (44.4) | 67 (90.5)|| |
| CsA | 1 (1.6) | 1 (1.4) |
| MPA | 50 (79.4) | 60 (81.1) |
| Steroids | 19 (30.2) | 19 (25.7) |
Data are given as mean (SD) or n (%).
At randomization in the SIMCER study.
At entry to the SIMCER study.
EVR group, n = 63; TAC group, n = 74.
31 patients also received TAC at month 24.
19 patients also received EVR at month 24.
Figure 1.
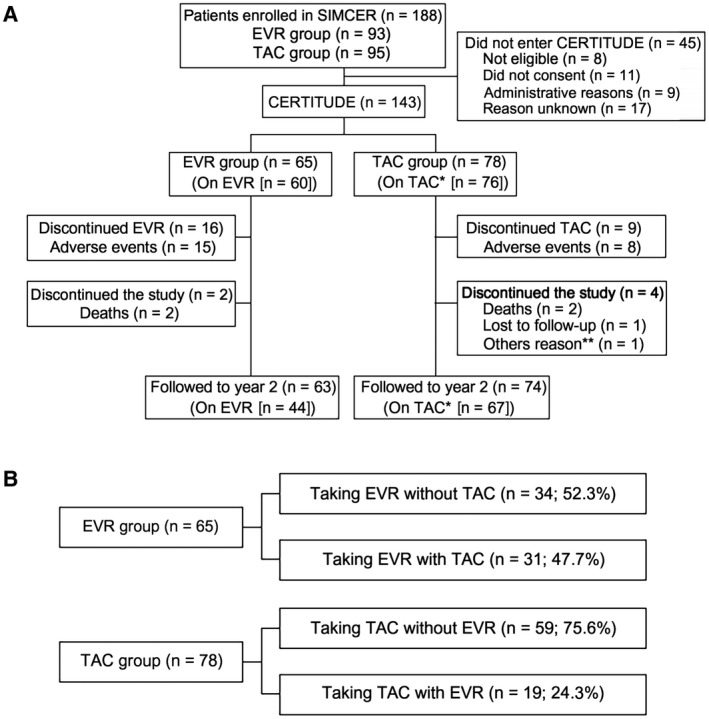
(A) Patient allocation and (B) patient subgroups according to immunosuppression at month 24 after transplant. *1 patient was receiving CsA. **Patient relocated.
Immunosuppression
At entry to CERTITUDE, 60/65 (92.3%) patients in the EVR group were still receiving EVR, and 15 were receiving TAC. Thus, 45/65 (69.2%) patients were being given EVR in a CNI‐free regimen. In the TAC group, 76/78 (97.4%) patients were still receiving TAC, 1 was receiving CsA, and 3 patients had started EVR, 1 of whom was on a CNI‐free regimen. Use of steroids and MPA was comparable between groups (Table 1).
Immunosuppression at month 24 after transplant is summarized in Table 1. Because CERTITUDE is an observational study, physicians are free to modify the immunosuppressive regimen at any time during follow‐up. Of the 65 patients in the EVR group, 31 (47.7%) patients received TAC at some point during months 6‐24, whereas 34 (52.3%) patients remained on a CNI‐free regimen (Fig. 1B). By month 24, 44/63 (69.8%) patients in the EVR group were still receiving EVR. In the TAC group, 19 (24.3%) patients were given EVR at some point during months 6‐24 (Fig. 1B). At month 24, 67/74 (90.5%) patients were still receiving TAC at month 24.
In the EVR group, the mean (standard deviation [SD]) EVR trough concentration at month 6 and at month 24 was 7.7 (3.3) and 7.1 (2.7) ng/mL, respectively, among patients receiving CNI‐free therapy and 8.3 (3.6) and 8.3 (4.1) ng/mL, respectively, among patients who had restarted TAC. In the TAC group, the mean trough concentration at month 6 and month 24 was 7.9 (2.5) and 6.3 (2.0) ng/mL, respectively, for patients receiving TAC without EVR and 8.4 (3.4) and 2.6 (0.7) ng/mL, respectively, for TAC‐treated patients in whom EVR was introduced. There were no marked differences between the groups in MPA or steroid use at month 24 (Table 1). The mean (SD) dose of EC‐MPS was 987 (415) and 911 (453) mg/day in patients receiving EVR alone or with TAC, respectively, and 1028 (439) and 1008 (372) mg/day in patients receiving TAC alone or with EVR, respectively.
Renal Function
At the end of the SIMCER study, the mean ± SD eGFR in the patients included in CERTITUDE (n = 143) was 83.42 ± 27.93 mL/minute/1.73 m2, and in the patients who were not included, it was 80.99 ± 31.48 mL/minute/1.73 m2. Observed mean (SD) eGFR was significantly higher in the EVR group during months 3‐12, with a nonsignificant trend to higher eGFR thereafter (Fig. 2A). At month 24, mean (SD) eGFR in the EVR group and the TAC group was 83.6 (24.8) and 75.3 (29.6) mL/minute/1.73 m2, respectively (P = 0.09).
Figure 2.
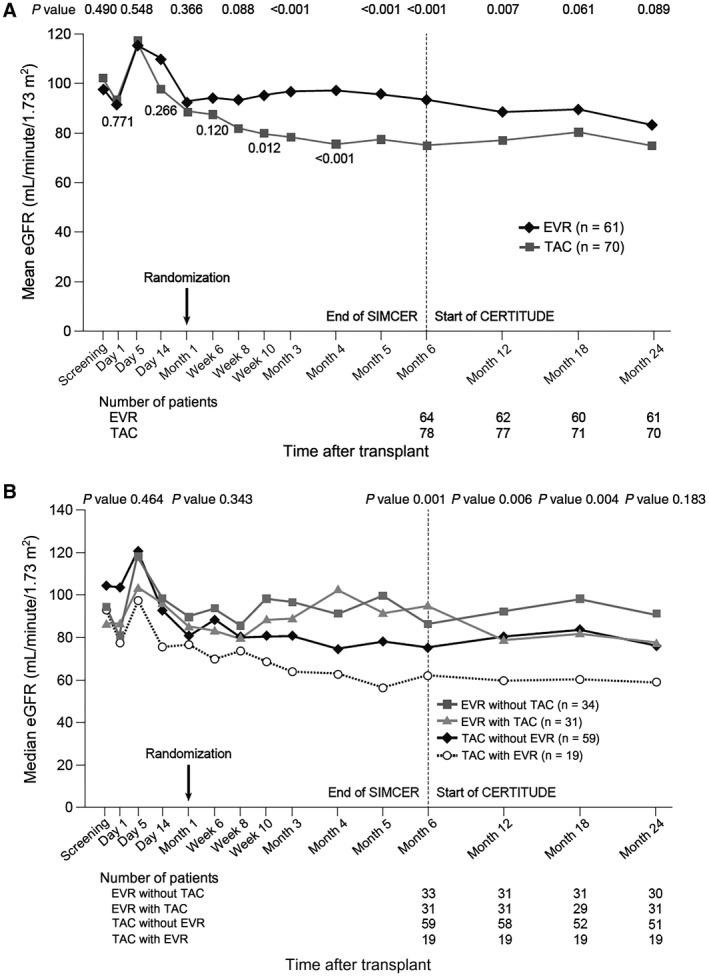
(A) Mean eGFR (aMDRD) from randomization to month 24 after transplant according to randomized treatment group. (B) Median eGFR (aMDRD) from randomization to month 24 after transplant according to treatment received.
Mean (SD) eGFR decreased by 10.2 (34.2) mL/minute/1.73 m2 in the EVR group and by 12.6 mL/minute/1.73 m2 in the TAC group from randomization to month 24 (median, 11.4 and 8.6 mL/minute/1.73 m2, respectively; P = 0.77). When analyzed by ANCOVA, the adjusted mean (standard error [SE]) values for change from randomization to month 24 were −8.0 (2.8) mL/minute/1.73 m2 and −13.5 (2.6) mL/minute/1.73 m2, respectively, a difference of 5.5 mL/minute/1.73 m2 (95% CI, −2.0 to 13.1 mL/minute/1.73 m2; P = 0.15).
When renal function was assessed according to the actual immunosuppressive regimen administered, from month 6 until month 18, there were significant differences in eGFR between the subpopulations of patients receiving EVR only, EVR with TAC, TAC only, or TAC with EVR, with patients given EVR only consistently having the highest eGFR (Fig. 2B). The highest median eGFR at month 24 was seen in the subpopulation of 34 patients who received EVR without TAC (90.8 mL/minute/1.73 m2 compared with 58.8 mL/minute/1.73 m2 for patients given TAC with EVR). The smallest median decrease in eGFR from randomization to month 24 was seen in the subpopulation given EVR without TAC (−5.3 mL/minute/1.73 m2 compared with −13.0 mL/minute/1.73 m2 for patients given TAC with EVR).
The incidences of CKD stages 1, 2, and 3 were significantly in favor of the EVR group versus the TAC group at month 6 (P < 0.001 overall), and they showed a trend in favor of the EVR group at month 24: 45.9%, 31.1%, and 23.0% of EVR‐treated patients had CKD stages 1, 2, and 3, respectively, versus 25.7%, 45.7%, and 28.6% of TAC‐treated patients (P = 0.05; Fig. 3).No patient had CKD stage 4 or stage 5, or required dialysis or kidney transplantation, at either month 6 or month 24. When assessed by the type of immunosuppression actually given, the incidence of CKD stage 3 at month 24 was 6.7% (2/30) for EVR only, 38.7% (12/31) for EVR with TAC, 16.9% (10/59) for TAC only, and 52.6% (10/19) for TAC with EVR.
Figure 3.
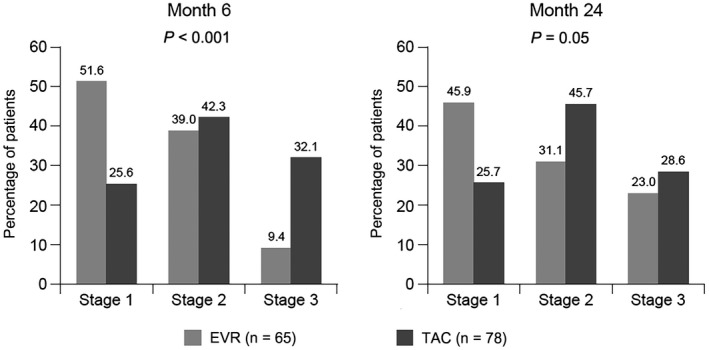
KDOQI stage at months 6 and 24 according to randomized treatment group.
Only one‐third of patients had a protein:creatinine ratio assessment at month 24, and the median (range) protein:creatinine ratio was similar in both groups (EVR group, 10 [10‐50] mg/mmol; TAC group, 10 [0‐670] mg/mmol). In the subgroups of patients receiving EVR only, EVR with TAC, TAC only, and TAC with EVR, the median (range) protein:creatinine ratio at month 24 was 0.01 (0.01‐0.05) g/mmol, 0.01 (0.01‐0.03) g/mmol, 0.01 (0.00‐0.67) g/mmol, and 0.06 (0.01‐0.07) g/mmol, respectively.
Efficacy
After entering the CERTITUDE study at month 6 after transplant, 6 (9.2%) patients in the EVR group and 4 (5.1%) patients in the TAC group experienced treatment failure (treated BPAR [RAI >3 or indeterminate], graft loss, or death) by month 24 (Table 2). The most common contributing event was treated BPAR (EVR group, n = 4; TAC group, n = 2), with only 1 patient in each group experiencing treated BPAR after month 12.
Table 2.
Efficacy Endpoints According to Time Period
| SIMCER | CERTITUDE | |||||||
|---|---|---|---|---|---|---|---|---|
| Randomization to Month 6 | Months > 6‐12 | Months > 12‐18 | Months > 18‐24 | |||||
| EVR (n = 65) | TAC (n = 78) | EVR (n = 65) | TAC (n = 78) | EVR (n = 65) | TAC (n = 78) | EVR (n = 65) | TAC (n = 78) | |
| Treatment failure* | 6 (9.2) | 2 (2.6) | 4 (6.2) | 1 (1.3) | 2 (3.1) | 1 (1.3) | 0 | 2 (2.6) |
| ≥1 treated BPAR† | 6 (9.2) | 2 (2.6) | 3 (4.6) | 1 (1.3) | 1 (1.5) | 1 (1.3) | 0 | 0 |
| Severity of treated BPAR† | ||||||||
| RAI 4/5, Banff grade 1 | 5 (83.3) | 1 (50.0) | 2 (66.7) | 1 (100.0) | 1 (100.0) | 0 | — | — |
| RAI 6/7, Banff grade 2 | 1 (16.7) | 1 (50.0) | 0 | 0 | 0 | 1 (100.0) | — | — |
| Indeterminate | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | — | — |
| Death, n (%) | 0 | 0 | 1 (1.5) | 0 | 1 (1.5) | 0 | 0 | 2 (2.6) |
| Graft loss, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Treated BPAR (RAI score >3), graft loss, or death.
RAI score >3 or indeterminate.
Between month 6 and month 24, 2 patients died in the EVR group (sepsis, n = 1; malignant lung neoplasm, n = 1), and 2 patients died in the TAC group (metastatic renal cancer/respiratory distress, n = 1; cardiopulmonary arrest/multiple organ failure, n = 1). None of the deaths had a suspected relation to the study drug. There were no graft losses.
When efficacy events were analyzed from randomization in SIMCER to month 24, treatment failure occurred in 18.5% of patients in the EVR group versus 7.7% of those in the TAC group (P = 0.05). The odds ratio was 2.93 (95% CI, 0.99‐8.65; P = 0.05).
Safety
Between months 6 and 24 after transplant, 100% of patients in the EVR group and 92.3% of patients in the TAC group reported 1 or more adverse event (Table 3); adverse events with a suspected relation to EVR were reported by 47.7% of patients in the EVR group (Supporting Table 1). Serious adverse events were reported in 66.2% and 43.6% of patients in the EVR and TAC groups, respectively, during months 6 to 24. During this period, adverse events led to study discontinuation in 15.4% and 7.7% of patients randomized to EVR or to TAC, respectively. Adverse events reported between randomization and month 24 are summarized in Supporting Table 2.
Table 3.
Adverse Events Reported Between Month 6 and Month 24 According to Randomized Treatment Groups
| EVR Group (n = 65) | TAC Group (n = 78) | |
|---|---|---|
| Adverse events | ||
| At least 1 adverse event | 65 (100.0) | 72 (92.3) |
| At least 1 adverse event with suspected relation to EVR | 31 (47.7) | 9 (11.5) |
| At least 1 adverse event leading to study drug discontinuation | 10 (15.4) | 6 (7.7) |
| Adverse events occurring in >10% of patients in either group | ||
| Cholestasis | 9 (13.8) | 18 (23.1) |
| Hepatocellular injury | 7 (10.8) | 5 (6.4) |
| Peripheral edema | 9 (13.8) | 5 (6.4) |
| Anemia | 6 (9.2) | 8 (10.3) |
| Hypertension | 9 (13.8) | 7 (9.0) |
| Dyslipidemia | 9 (13.8) | 3 (3.8) |
| Weight increased | 4 (6.2) | 10 (12.8) |
| Hypertriglyceridemia | 8 (12.3) | 6 (7.7) |
| Diabetes mellitus | 4 (6.2) | 9 (11.5) |
| Hypercholesterolemia | 8 (12.3) | 4 (5.1) |
| Serious adverse events | ||
| At least 1 serious adverse event | 43 (66.2) | 34 (43.6) |
| Serious adverse events occurring in >5% of patients in either group | ||
| Abdominal pain | 4 (6.2) | 1 (1.3) |
| Liver transplant rejection | 4 (6.2) | 1 (1.3) |
| Acute kidney injury | 1 (1.5) | 4 (5.1) |
Adverse Events
During months 6 to 24, peripheral edema, dyslipidemia, hypertriglyceridemia, and hypercholesterolemia were more common in the EVR group, whereas cholestasis, increased weight, and diabetes mellitus were more common in the TAC group (Table 3). Cytomegalovirus infection was reported in 1.5% and 6.4% of patients in the EVR and TAC groups, respectively, between months 6 and 24. Proteinuria was reported as an adverse event in 1 (1.5%) patient in the EVR group and 3 (3.8%) patients in the TAC group.
The overall incidence of diabetes (defined as treatment with antidiabetic medication; EVR group, 47.7%, n = 31/65; TAC group, 41.0%, n = 32/78) and the incidence of de novo diabetes among patients who were nondiabetic at randomization (EVR group, 4.4%, n = 2/45; TAC group, 0.0%, n = 0/55) showed no marked differences in the EVR and TAC groups at month 24.
At baseline, arterial hypertension was found in 28 (48.3%) patients in the TAC group and 13 (28.3%) in the EVR group. At baseline, 43.6% patients in the TAC group were taking antihypertensive drugs, and 8.82% of hypertensive patients in this group were on angiotensin inhibitors. In the EVR group, 47.7% were taking antihypertensive drugs, and 9.47% of hypertensive patients in this group were on angiotensin inhibitors. There were no MACEs in the EVR group. Three patients experienced a MACE in the TAC group (hospitalization for heart failure, hospitalization for acute coronary syndrome, and death from cardiovascular causes; P = 0.25).
No patients in the EVR group and 3 patients in the TAC group developed HCC recurrence (0.0% versus 3.8%; P = 0.25). De novo malignancies occurred between randomization and month 24 in 2 patients in the EVR group (fatal bronchopulmonary carcinoma, n = 1; prostate adenocarcinoma, n = 1), and 1 patient in the TAC group (renal/prostate cancer).
Hematology
Between randomization and month 24, no patient in either group experienced grade 4 thrombocytopenia (platelet count 25,000‐50,000/mm3) or leukopenia (white blood cell count <1000/mm3). In the EVR group, 1 patient had grade 4 anemia (hemoglobin level <6.5 g/dL). Regarding grade 3 events, 1 patient in the EVR group had thrombocytopenia (platelet count 25,000‐50,000/mm3)or leukopenia (white blood cell count 1000‐2000/mm3); 5 patients in the EVR group and 6 patients in the TAC group had leukopenia (white blood cell count <2000/mm3); and 3 patients in the EVR group and 1 TAC‐treated patient had anemia (hemoglobin level <8.0 g/dL; Supporting Table 3). The administration of erythropoietin‐stimulating protein (EPO) was left to the physician’s discretion. Overall, only 1 patient in the EVR group was taking EPO, and none in the TAC group did.
Biochemistry
The mean levels in the EVR versus TAC groups of fasting blood glucose (6.7 versus 6.5 mmol/L), total cholesterol (5.4 versus 5.1 mmol/L), low‐density lipoprotein cholesterol (3.2 mmol/L in both groups), high‐density lipoprotein cholesterol (1.2 mmol/L in both groups), and triglycerides (2.1 versus 2.0 mmol/L) were comparable between treatment groups at month 24. However, full lipid data were provided for only half the patients (EVR group, n = 33; TAC group, n = 36). Use of lipid‐lowering drugs (38.1% versus 14.9%) and hypoglycemic drugs (44.3% versus 31.1%) at month 24 was higher in the EVR group versus the TAC group.
Discussion
In this observational study following patients who completed the randomized 6‐month SIMCER study, EVR with MPA and early TAC withdrawal preserved renal function to month 24 after transplant. Observed mean eGFR was significantly higher in the EVR group after completing SIMCER up to month 12 after transplant with a nonsignificant trend to superiority thereafter, although the primary endpoint of adjusted eGFR lost significance by month 24. Almost half the EVR‐treated patients did not progress beyond CKD grade 1 compared with only a quarter of the TAC‐treated patients. Strikingly, the subgroup of patients who continued to receive CNI‐free EVR therapy had a median eGFR of ~90 mL/minute/1.73 m2 at month 12. Treated rejection was rare in both groups after month 6 after transplant, and hematological tolerability was good.
The loss of significance for the primary endpoint by month 24 likely reflected the extensive changes to immunosuppression regimens. After completion of the SIMCER study, immunosuppression was at the discretion of the investigator. By month 24, only ~70% of patients were randomized to EVR in a CNI‐free regimen. The subgroup of patients who remained on EVR without restarting TAC showed the highest eGFR at 2 years, compatible with 1‐year results from the PROTECT study which showed that the renal benefit was higher in patients who remain on EVR versus those who discontinued.14 In the TAC group of our study, conversely, EVR was initiated in 24% of patients. Consistent with these changes to immunosuppression, mean eGFR decreased in the EVR arm after completion of SIMCER but plateaued in the TAC group such that the between‐group difference lessened. Nevertheless, despite the high proportion of switches in immunosuppression, mean eGFR was still ~8 mL/minute/1.73 m2 higher in the EVR group versus the TAC arm at month 24, and fewer patients progressed beyond CKD stage 1. It is unlikely that many patients will switch immunosuppression after month 24, and further follow‐up will determine if this numerical difference is maintained long term. The prevalence of CDK stage 3 was higher in the EVR‐TAC (38.7%) and TAC‐EVR (52.6%) groups compared with the group with TAC alone (19.6%). TAC was introduced in 46% of patients in the EVR arm, and EVR was introduced in 24% of patients in the TAC arm; therefore we assume that the low proportion of CKD stage 3 in the TAC arm was due, in part, to the selected TAC group as those patients with CKD stage 3 in this arm have mostly an introduction of EVR and figure in the TAC + EVR group.
After month 6, treated BPAR was rare in both groups, and all 3 of the episodes in the EVR group for which results were available were graded as mild. The overall rate of treated BPAR from randomization to 2 years (15.4%) in the EVR group was similar to the 1‐year incidence in the EVR/CNI‐free group in the PROTECT study (17.7%)14 and lower than in the H2304 study (26.4%)12 where basiliximab was not given. Inclusion of MPA in the CNI‐free EVR‐based regimen is likely to have helped reduce graft rejection.
The adverse events reported in the EVR group are consistent with the known safety profile of mTOR inhibitors.18 Equally, the higher rate of diarrhea in the TAC arm is as expected,19 as was the lower rate of cytomegalovirus infection in the EVR‐treated cohort, based on evidence from kidney20 and heart21 transplantation. Immunosuppressive regimen modification due to adverse events was more frequent in the EVR group from randomization to month 24. Here, MPA was included in the EVR‐based regimen with the aim of maintaining immunosuppressive efficacy while permitting lower EVR exposure. The mean EVR trough concentration at the end of the SIMCER study (ie, month 6 after transplant) was 7.9 ng/mL15 compared with 9.3 ng/mL at month 7 in PROTECT.14
There has been interest in using mTOR inhibitors for patients undergoing liver transplantation for HCC22 because of studies suggesting a lower risk for HCC recurrence23, 24 and improved short‐term outcomes in patients where HCC does recur.25 In our study, at 2 years after transplant, HCC recurrence was seen only in the TAC arm, but numbers were low, thus not allowing a thorough between‐group comparison. The mTOR inhibitors could also potentially lower the risk for cardiovascular events by reducing CNI‐related hypertensive and diabetogenic effects26 and possibly via direct cardioprotective effects,27, 28, 29, 30 as suggested in a recent analysis of 1‐year data from kidney transplantation.31 In our smaller cohort, MACEs occurred only in the TAC group (3.8%).
The major weakness of the study is its observational design. Therefore, a high proportion of patients were switched to different immunosuppressive regimens. Although this is representative of real‐world practice, any effects of using EVR in a CNI‐free regimen would be diminished. Additionally, although follow‐up was good in the CERTITUDE trial, with 137/143 patients followed to month 24, 45/188 patients in the SIMCER study did not enter CERTITUDE, notably due to patients’ refusal and for administrative reasons. There were also missing data at the 2‐year visit; notably, the protein:creatinine ratio was provided in few patients, indicating that this is not a standard follow‐up assessment in many transplant centers.
In conclusion, evidence from this observational study indicates that an EVR‐based maintenance regimen appears feasible in approximately half of liver transplant patients during routine practice. Such a regimen seems to preserve renal function effectively with favorable antirejection efficacy and without compromising safety or hematological tolerance. However, no one‐size‐fits‐all approach could be applied, and it remains important to tailor therapy to suit patient characteristics through the assessment of individual risk/benefit balance. In CERTITUDE, patients are being followed to 5 years after transplant to provide longterm information.
Supporting information
See Editorial on Page 1745
The study was funded by Novartis Pharma SAS, Rueil‐Malmaison, France. Support from a medical writer was also funded by Novartis Pharma SAS.
Faouzi Saliba has received speaker's honoraria and/or research grants from Novartis, Astellas, Chiesi, Gilead Sciences, AbbVie, Merck Sharp & Dohme, Pfizer, Gambro, Baxter, and Vital Therapies. Christophe Duvoux has received speaker’s honoraria and/or research grants from Novartis, Astellas, Chiesi, and Sandoz and advises for Novartis, Astellas, and Sandoz. Sébastien Dharancy has received speaker’s honoraria and/or research grants from Novartis, Otsuka Pharmaceutical, Chiesi, Gilead Sciences, Astellas, and Nanobiotix and consults for Novartis, Nanobiotix, and Astellas. Jérôme Dumortier has been a clinical investigator, speaker, and/or consultant for Astellas, Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme, Novartis, and Chiesi. Yvon Calmus has been a member of advisory boards and/or received research grants from Novartis, Astellas, and Intercept Pharmaceuticals. Jean Gugenheim has received research grants from Astellas, Novartis, Roche, and Merck Sharp & Dohme. Nassim Kamar has received speaker’s honoraria and/or research grants and has been a member of advisory boards from Novartis, Astellas, Gilead Sciences, Merck Sharp & Dohme, Neovii, Sanofi, and Amgen and consults for and has travel grants from Novartis, Astellas, and Chiesi. Ephrem Salamé has been a member of an advisory board for Novartis and has received research grants from Novartis, Astellas, and Chiesi. François Durand has received research grants, speaker’s fees, and consultant fees from Novartis, Astellas, Gilead Sciences, and Bristol‐Myers Squibb; consults for Astellas and Gilead Sciences; advises for Gilead Sciences; is on the speaker’s bureau for Sequana Therapeutics; and has travel grants from Sequana Therapeutics, Cheisi, and Astellas. Georges Pageaux has received speaker’s honoraria and/or is a member of advisory boards for Novartis, Astellas, Gilead Sciences, and Bristol‐Myers Squibb and has grants from Novartis and Astellas. Vincent Leroy has received speaker's honoraria and/or is a member of advisory boards for Chiesi, AbbVie, Gilead Sciences, Merck Sharp & Dohme, and Intercept. Hakam Gharbi, Cécile Masson, and Malka Tindel are employees of Novartis. Filomena Conti has been a member of an advisory board for Chiesi.
All authors except Hakam Gharbi, Cécile Masson, and Malka Tindel enrolled patients and collected study data. Hakam Gharbi provided medical input. All authors critically reviewed the manuscript and approved the final version for publication. The scientific committee had input on the study design.
REFERENCES
- 1. Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2015 annual data report: liver. Am J Transplant 2017;17(suppl 1):174‐251. [DOI] [PubMed] [Google Scholar]
- 2. Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, et al.; for European Liver and Intestine Transplant Association (ELITA) . Evolution of indications and results of liver transplantation in Europe. a report from the European Liver Transplant Registry (ELTR). J Hepatol 2012;57:675‐688. [DOI] [PubMed] [Google Scholar]
- 3. Muduma G, Saunders R, Odeyemi I, Pollock RF. Systematic review and meta‐analysis of tacrolimus versus ciclosporin as primary immunosuppression after liver transplant. PLoS One 2016;11:e0160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev 2006;4:CD005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morard I, Mentha G, Spahr L, Majno P, Hadengue A, Huber O, et al. Long‐term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin Transplant 2006;20:96‐101. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez‐Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta‐analysis. Am J Transplant 2012;12:2797‐2814. [DOI] [PubMed] [Google Scholar]
- 7. Wimmer CD, Angele MK, Schwarz B, Pratschke S, Rentsch M, Khandoga A, et al. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int 2013;26:999‐1006. [DOI] [PubMed] [Google Scholar]
- 8. Carenco C, Assenat E, Faure S, Duny Y, Danan G, Bismuth M, et al. Tacrolimus and the risk of solid cancers after liver transplant: a dose effect relationship. Am J Transplant 2015;15:678‐686. [DOI] [PubMed] [Google Scholar]
- 9. Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new‐onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation 2010;89:1134‐1140. [DOI] [PubMed] [Google Scholar]
- 10. Lin M, Mittal S, Sahebjam F, Rana A, Sood GK. Everolimus with early withdrawal or reduced‐dose calcineurin inhibitors improves renal function in liver transplant recipients: a systematic review and meta‐analysis. Clin Transplant 2017;31 (2). [DOI] [PubMed] [Google Scholar]
- 11. Holdaas H, De Simone P, Zuckermann A. Everolimus and malignancy after solid organ transplantation: a clinical update. J Transplant 2016;2016:4369574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al.; for H2304 Study Group . Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:3008‐3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al.; for H2304 Study Group . Renal function at 2 years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant 2013;13:1734‐1745. [DOI] [PubMed] [Google Scholar]
- 14. Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, et al.; for H2304 Study Group . Three‐year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow‐up results from a randomized, multicenter study. Transplantation 2015;99:1455‐1462. [DOI] [PubMed] [Google Scholar]
- 15. Saliba F, Duvoux C, Gugenheim J, Kamar N, Dharancy S, Salamé E, et al. Efficacy and safety of everolimus and mycophenolic acid with early tacrolimus withdrawal after liver transplantation: a multicenter, randomized trial. Am J Transplant 2017;17:1843‐1852. [DOI] [PubMed] [Google Scholar]
- 16. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999;55:713‐723. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 18. Murakami N, Riella LV, Funakoshi T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta‐analysis. Am J Transplant 2014;14:2317‐2327. [DOI] [PubMed] [Google Scholar]
- 19. Ekberg H, Bernasconi C, Nöldeke J, Yussim A, Mjörnstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant 2010;25:2004‐2010. [DOI] [PubMed] [Google Scholar]
- 20. Brennan DC, Legendre C, Patel D, Mange K, Wiland A, McCague K, Shihab FS. Cytomegalovirus incidence between everolimus versus mycophenolate in de novo renal transplants: pooled analysis of three clinical trials. Am J Transplant 2011;11:2453‐2462. [DOI] [PubMed] [Google Scholar]
- 21. Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis 2013;15:150‐162. [DOI] [PubMed] [Google Scholar]
- 22. Duvoux C, Toso C. mTOR inhibitor therapy: does it prevent HCC recurrence after liver transplantation? Transplant Rev (Orlando) 2015;29:168‐174. [DOI] [PubMed] [Google Scholar]
- 23. Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus‐based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010;51:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 24. Menon KV, Hakeem AR, Heaton ND. Meta‐analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37:411‐419. [DOI] [PubMed] [Google Scholar]
- 25. Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open‐label phase 3 trial. Transplantation 2016;100:116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeier M, Van Der Giet M. Calcineurin inhibitor sparing regimens using m‐target of rapamycin inhibitors: an opportunity to improve cardiovascular risk following kidney transplantation? Transpl Int 2011;24:30‐42. [DOI] [PubMed] [Google Scholar]
- 27. Joannidès R, Monteil C, de Ligny BH, Westeel PF, Iacob M, Thervet E, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant 2011;11:2414‐2422. [DOI] [PubMed] [Google Scholar]
- 28. Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, et al. Inhibition of mTOR reduces chronic pressure‐overload cardiac hypertrophy and fibrosis. J Hypertens 2006;24:1663‐1670. [DOI] [PubMed] [Google Scholar]
- 29. Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, et al. Beneficial effects of mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 2009;54:2435‐2446. [DOI] [PubMed] [Google Scholar]
- 30. Yong K, Nguyen HD, Hii L, Chan DT, Boudville N, Messineo A, et al. Association of a change in immunosuppressive regimen with hemodynamic and inflammatory markers of cardiovascular disease after kidney transplantation. Am J Hypertens 2013;26:843‐849. [DOI] [PubMed] [Google Scholar]
- 31. Holdaas H, de Fijter JW, Cruzado JM, Massari P, Nashan B, Kanellis J, et al.; for ELEVATE Study Group . Cardiovascular parameters to 2 years after kidney transplantation following early switch to everolimus without calcineurin inhibitor therapy: an analysis of the randomized ELEVATE study. Transplantation 2017;101:2612‐2620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


