Nucleic acid amplification tests (NAATs) are the primary means of identifying acute infections caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Accurate and fast test results may permit more efficient use of protective and isolation resources and allow rapid therapeutic interventions. We evaluated the analytical and clinical performance characteristics of the Xpert Xpress SARS-CoV-2 (Xpert) test, a rapid, automated molecular test for SARS-CoV-2. Analytical sensitivity and specificity/interference were assessed with infectious SARS-CoV-2; other infectious coronavirus species, including SARS-CoV; and 85 nasopharyngeal swab specimens positive for other respiratory viruses, including endemic human coronaviruses (hCoVs).
KEYWORDS: SARS-CoV-2, COVID-19, Xpert, RT-PCR
ABSTRACT
Nucleic acid amplification tests (NAATs) are the primary means of identifying acute infections caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Accurate and fast test results may permit more efficient use of protective and isolation resources and allow rapid therapeutic interventions. We evaluated the analytical and clinical performance characteristics of the Xpert Xpress SARS-CoV-2 (Xpert) test, a rapid, automated molecular test for SARS-CoV-2. Analytical sensitivity and specificity/interference were assessed with infectious SARS-CoV-2; other infectious coronavirus species, including SARS-CoV; and 85 nasopharyngeal swab specimens positive for other respiratory viruses, including endemic human coronaviruses (hCoVs). Clinical performance was assessed using 483 remnant upper- and lower-respiratory-tract specimens previously analyzed by standard-of-care (SOC) NAATs. The limit of detection of the Xpert test was 0.01 PFU/ml. Other hCoVs, including Middle East respiratory syndrome coronavirus, were not detected by the Xpert test. SARS-CoV, a closely related species in the subgenus Sarbecovirus, was detected by a broad-range target (E) but was distinguished from SARS-CoV-2 (SARS-CoV-2-specific N2 target). Compared to SOC NAATs, the positive agreement of the Xpert test was 219/220 (99.5%), and the negative agreement was 250/261 (95.8%). A third tie-breaker NAAT resolved all but three of the discordant results in favor the Xpert test. The Xpert test provided sensitive and accurate detection of SARS-CoV-2 in a variety of upper- and lower-respiratory-tract specimens. The high sensitivity and short time to results of approximately 45 min may impact patient management.
INTRODUCTION
Laboratory diagnosis of infections caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is usually accomplished by performing nucleic acid amplification tests (NAATs) on respiratory tract specimens. An antibody response is often not detected in the first week to 10 days of symptoms, and antibody testing is therefore generally unhelpful for acute diagnosis (1–3), with virus isolation in culture presenting significant biosafety risks. Upper-respiratory-tract (URT) specimens, such as nasopharyngeal swabs (NPS) and oropharyngeal swabs (OPS), generally have high SARS-CoV-2 loads upon symptom onset (2, 4–6). URT specimens may also have detectable RNA during the presymptomatic period (7), and pediatric patients who remain asymptomatic through the entire course of infection can persistently shed RNA in URT specimens for 2 weeks or longer (4, 8). Importantly, NPS may have higher viral loads than OPS (6). Lower-respiratory-tract (LRT) specimens, including sputum (7, 9) and tracheal aspirates (TA) (10), are often positive for RNA early in disease and remain positive longer than URT sources (5).
NAATs are widely used worldwide to diagnose coronavirus infectious disease 2019 (COVID-19) cases and are the gold standard diagnostic method. At the time of writing, 42 NAATs have been granted in vitro diagnostic emergency use authorization (EUA) by the U.S. Food and Drug Administration (https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd). Some of these NAATs require batch testing, whereas others can be run on demand, providing test results in approximately 1 hour or less. High test sensitivity (negative predictive value) and short time to results can reduce the risk of SARS-CoV-2 transmission (11), may facilitate appropriate use of personal protective equipment and patient isolation, and may be used to guide therapy.
The Xpert Xpress SARS-CoV-2 (Xpert) test (Cepheid, Sunnyvale, CA, USA) received EUA status on 20 March 2020. The Xpert test platform integrates specimen processing, nucleic acid extraction, reverse transcriptase PCR (RT-PCR) amplification of SARS-CoV-2 RNA, and amplicon detection in a single cartridge. Specimens can be tested as soon as they are received, as the testing instrument provides random access to individual cartridges. The test detects the nucleocapsid (N2) gene and the envelope (E) gene, with results generated in approximately 45 min.
Here, we describe the first multicenter evaluation of the analytical and clinical performance characteristics of the Xpert Xpress SARS-CoV-2 test.
MATERIALS AND METHODS
Cepheid Xpert Xpress SARS-CoV-2 test.
Research use only (RUO)-labeled Xpert kits were provided to study sites. The GeneXpert Dx system (Cepheid, Sunnyvale, CA) is an integrated diagnostic device that performs automated specimen processing and real-time RT-PCR analysis. The Xpert test consists of two main components: (i) the Xpert plastic cartridge, which contains liquid sample-processing and PCR buffers and lyophilized real-time RT-PCR reagents, and (ii) the GeneXpert instrument, which controls intracartridge fluidics and performs real-time RT-PCR analysis. The RUO version of the Xpert test was designed to amplify sequences of the envelope (E), nucleocapsid (N2), and RNA-dependent RNA polymerase (RdRp) genes. Only results from the E and N2 targets are used to generate test results. If both targets are detected, or if only N2 is detected, the test reports a positive result. If only the E target is detected the test reports a presumptive positive result because the target is shared among some members of the Sarbecovirus subgenus of coronaviruses. The RUO version of the Xpert test allows the user to see amplification curves and PCR cycle threshold (CT) values for all three targets. The EUA test version cartridge contains the same reagents as the RUO cartridge. The only difference between the tests is the software, which in the EUA version allows the user to see amplification curves and results for the N2 and E targets only.
Analytical performance. (i) Limit of detection.
The analytical sensitivity of the Xpert test (EUA version) was assessed with one lot of reagent and limiting dilutions of the quantitated SARS-CoV-2 (USA_WA1/2020) according to standard guidelines (12). The virus stock (9.75 × 105 PFU/ml) was obtained from the University of Texas Medical Branch Arbovirus Reference Collection, Galveston, TX. The limit of detection (LOD) was determined by diluting the SARS-CoV-2 in a negative NPS clinical matrix to 7 different levels near the estimated LOD ranging from 0.0200 to 0.0001 PFU/ml. A minimum of 22 replicates were tested at each level, including negatives. Probit regression analysis was utilized to estimate the LOD. The LOD was verified by spiking the SARS-CoV-2 into the negative NPS clinical matrix to the estimated LOD value previously determined by the probit regression analysis. A minimum of 22 replicates were tested for LOD verification.
(ii) Analytical specificity/interference.
In addition to an in silico analysis, a panel of seven microorganisms consisting of four species of the family Coronaviridae (two strains of human coronavirus [hCoV] NL63 [NR44105, four replicates at 8.3E7 copies/ml; NR-470, three replicates at 4.16 × 104 50% tissue culture infective doses {TCID50}/ml]), Middle East respiratory syndrome coronavirus [MERS-CoV] [EMC2012, 4.3 NR-45843, four replicates at 5.36 × 106 copies/ml], SARS-CoV [NR-9547, four replicates at 1.00 × 106 TCID50/ml], and canine coronavirus UCD1 [NR-868, three replicates at 4.16 × 104 TCID50/ml]), Mycobacterium tuberculosis H37Rv, and Mycobacterium bovis BCG (five replicates each at 1.00 × 106 CFU/ml) were tested using the Xpert Xpress SARS-CoV-2 test. Additionally, 85 NPS specimens previously positive for various respiratory viruses (endemic hCoV [NL63, n = 4; 229E, n = 2; HKU1, n = 4; OC43, n = 3], influenza A virus [n = 17], influenza B virus [n = 18], respiratory syncytial virus [RSV] [n = 8], human metapneumovirus [n = 12], rhinovirus [n = 2], influenza A virus-RSV coinfections [n = 6], influenza B virus-hCoV coinfections [n = 2], RSV-rhinovirus coinfections [n = 4], and RSV-hCoV coinfections [n = 3]) were analyzed using the Xpert test. To evaluate for interference in coinfections, 19 specimens positive for influenza virus and/or RSV (7 positive for influenza A virus, 6 positive for influenza B virus, and 6 positive for both influenza A virus and RSV) were spiked with AccuPlex SARS-CoV-2 recombinant virus at 4× LOD (SeraCare, Milford, MA, USA) and analyzed using the Xpert test.
Clinical performance. (i) Study population.
Patients were referred for COVID-19 testing at seven sites (described in Table S1 in the supplemental material) according to the local criteria at each testing site. Specimens were collected from 1 March through 2 April 2020.
(ii) Specimen collection.
Study sites collected a convenience sample set to enrich for positive specimens. In addition, one site (Los Angeles County/University of Southern California Medical Center) tested specimens from a 4-day point prevalence survey of patients presenting with COVID-19 symptoms during mid-March 2020. Specimen types differed among some sites and included swabs (NPS, OPS, and combined NPS-OPS in the same transport vial) and TA. Swabs were eluted in viral transport medium, while TA were diluted in saline (1 part TA plus 5 parts 0.9% normal saline) for analysis by the Xpert test. Neat TA were analyzed by the SOC NAAT. Specimens were tested in real time by standard-of-care (SOC) NAATs and then stored at −80°C prior to analysis with the Xpert test, except at University Hospital, Newark, NJ, where specimens were tested in real time, within 2 h, by the Xpert test. One site (Manchester, United Kingdom) pretreated specimens with an equal volume of a guanidine hydrochloride buffer and heated them at 80°C. Site-specific specimen conditions and preanalytical procedures are provided in Table S1.
Ethical concerns.
The study protocol was reviewed and approved by local institutional/ethical review boards or was considered to represent quality improvement activities and therefore to be exempt from review.
Study design and oversight.
The study was designed and supervised by the sponsor, Cepheid. Data were collected by investigators at each study site, and statistical analyses were performed by a Cepheid author. Cepheid authors M.J.L. and D.H.P. wrote the first draft of the manuscript. We all vouch for the accuracy and completeness of the data reported.
Standard-of-care laboratory methods.
SARS-CoV-2 testing was performed in real time using a site-specific SOC method described in Table S2 in the supplemental material.
SARS-CoV-2 test comparison.
Clinical performance was assessed by comparing the Xpert test to the sites’ SOC methods. Specimens with inconclusive results from a test, and those with discrepant results between the Xpert and SOC tests, were analyzed by a third method. The tie-breaker methods used at different sites were the Panther Fusion SARS-CoV-2 assay (Hologic, San Diego, CA) (https://www.fda.gov/media/136156/download), Tib-Molbiol LightMix Modular Wuhan Coronavirus E-Gene RT-PCR (Roche, Basel, Switzerland), and the CDC assay (IDT primers and probes) (https://www.fda.gov/media/134922/download). Tib-Molbiol assay conditions were described previously (13).
Statistical analysis.
Positive and negative percent agreement levels for the Xpert test were calculated using two-by-two tables. Wilson’s binomial exact method was used to calculate 95% confidence intervals (CI) of proportions. For the calculation of agreement, inconclusive results by SOC methods were considered positive. Box-and-whisker plots were used to demonstrate different CT values between Xpert test targets and specimen types. The Wilcoxon signed-rank test, 2-tailed, was used to compare median CT values of PCR targets. The Mann-Whitney U test was used to compare CT values of different specimen types.
RESULTS
Analytical performance. (i) Limit of detection.
The LOD was determined using infectious SARS-CoV-2 diluted in negative NPS clinical matrix. The positivity rate observed was ≥95% at a SARS-CoV-2 concentration of 0.005 PFU/ml (Table 1). Of the 22 positive replicates tested at an LOD of 0.01 PFU/ml, 22/22 (100%) reported a positive result. The RdRp target was not assessed, as the EUA-labeled test was used for this study, and the software does not show amplification curves or CT values.
TABLE 1.
Limits of detection of the Xpert Xpress SARS-CoV-2 test using SARS-CoV-2 in nasopharyngeal-swab clinical matrix
| Concn (PFU/ml) | No. positive/no. of replicates | Mean CT |
Hit rate (%) | |
|---|---|---|---|---|
| E | N2 | |||
| 0.0200 | 22/22 | 35.2 | 38.9 | 100 |
| 0.0100 | 22/22 | 37.1 | 40.1 | 100 |
| 0.0050 | 21/22 | 38.2 | 41.0 | 95.5 |
| 0.0020 | 10/22 | 41.0 | 41.7 | 45.4 |
| 0.0010 | 7/22 | 39.1 | 41.8 | 31.8 |
| 0.0005 | 2/22 | 39.5 | 41.3 | 9.1 |
| 0.0001 | 1/22 | 43.5 | 42.5 | 4.5 |
(ii) Analytical specificity/interference.
All replicates of the non-SARS Coronaviridae species and both M. tuberculosis strains were correctly reported as SARS-CoV-2 negative by the Xpert test. SARS-CoV-1 produced a “SARS-CoV-2 presumptive positive” test result because, as expected, it was detected by the E target (shared among members of the subgenus Sarbecovirus) but not by the N2 target. All 85 NPS clinical specimens containing varied respiratory viruses produced the expected SARS-CoV-2-negative result with the Xpert test. All 19 clinical specimens containing influenza virus or both RSV and influenza virus and spiked with recombinant SARS-CoV-2 at 4× LOD gave the expected SARS-CoV-2-positive result (https://www.fda.gov/media/136314/download).
Clinical performance.
The RUO-labeled Xpert test was used in clinical performance studies and therefore provided CT values for the RdRp target, in addition to the N2 and E targets. The Xpert test was performed on 486 patient specimens with previous SOC comparator method results for SARS-CoV-2. Four Xpert test results were lost permanently due to a single-instrument computer malfunction, and one Xpert test was invalid due to a cartridge error (inadequate sample volume), leaving 481 specimens with reported results by the Xpert test. Specimen sources included NPS (339), combined NPS-OPS (97), TA (30), and OPS (15) (see Table S1).
Compared to all the SOC methods combined, the positive agreement of the Xpert test was 219/220 (99.5%; 95% CI, 97.5 to 99.9%), and the negative agreement was 250/261 (95.8%; 95% CI, 92.6 to 97.6%) (Table 2). Twelve specimens (8 NPS and 4 NPS-OPS) were inconclusive by the New York SARS-CoV-2 real-time RT-PCR diagnostic panel (https://www.fda.gov/media/135847/download) (considered positive for data analysis purposes here). Of these, 11 were positive by the Xpert test and one was presumptive positive (EUA version of the Xpert test). In 4 of these, only the N1 target was detected, and in 8, only the N2 target was detected by the New York EUA method, all with CT values of >36 (data not shown). A single concordant positive specimen was presumptive positive by the Xpert test, with an E target CT value of 38. One NPS specimen was inconclusive by the Quest SARS-CoV-2 rRT-PCR test (https://www.fda.gov/media/136231/download) and negative by the Xpert test. The Quest test reports inconclusive if only a single target (N1 or N3) is detected. We were unable to determine which target was detected by the Quest test. This specimen was negative by a tie-breaker NAAT. Details of discordant specimens and test results are provided in Table 3. Eleven specimens were either positive (n = 10) or presumptive positive (n = 1) (only the E target was detected) by the Xpert test and negative by SOC methods. For the purpose of data analysis here, a presumptive positive result was considered to represent the presence of SARS-CoV-2. Eight of these specimens were positive for SARS-CoV-2 by an alternate test, and three were negative (Table 3). We considered the confirmed positives to be true positives. Notably, pretreatment of specimens with a guanidine hydrochloride buffer followed by heating at 80°C for 25 min did not appear to interfere with the Xpert test. Positive agreement with the reference SOC method at this site was 100%, and the CT values for all three RUO Xpert test targets were not significantly different than those of specimens from other sites that tested swab specimens with no pretreatment (data not shown). However, a head-to-head evaluation of these specimen pretreatments using paired specimens is needed.
TABLE 2.
Agreement of Xpert Xpress SARS-CoV-2 test and comparator RT-PCR tests
| Comparator (targets) | No. of results (Xpert/comparator)a
|
PPAb (95% CI) | NPAc (95% CI) | |||
|---|---|---|---|---|---|---|
| Pos/Pos | Pos/Neg | Neg/Pos | Neg/Neg | |||
| All methods | 219 | 11 | 1 | 250 | 99.5 (97.5–99.9) | 95.8 (92.6–97.6) |
| Quest SARS-CoV-2 rRT-PCR (N1, N3) | 12 | 0 | 1 | 75 | 92.3 (66.7–98.6) | 100 (95.1–100) |
| RealStar SARS-CoV-2 RT-PCR kit 1.0 (S, E) | 60 | 0 | 0 | 69 | 100 (94.0–100) | 100 (94.7–100) |
| New York SARS-CoV-2 real-time RT- PCR diagnostic panel (N1, N2) | 74 | 2 | 0 | 23 | 100 (94.2–100) | 92.0 (75.0–97.8) |
| Inhouse (RdRp) | 30 | 9 | 0 | 26 | 100 (88.7–100) | 74.3(57.9–85.8) |
| Allplex 2019-nCoV assay, GeneFinder COVID-19 Plus RealAmp kit (E, N, RdRp) | 35 | 0 | 0 | 44 | 100 (90.1–100) | 100 (92.0–100) |
| Abbott RealTime SARS-CoV-2 assay (N, RdRp) | 8 | 0 | 0 | 10 | 100 (67.6–100) | 100 (77.2–100) |
| Simplexa COVID-19 direct (ORF1ab, S) | 0 | 0 | 0 | 3 | 100 (43.9–100) | |
Pos, positive; Neg, negative.
PPA, positive percent agreement {[Pos/Pos ÷ (Pos/Pos + Neg/Pos)] × 100}.
NPA, negative percent agreement {[Neg/Neg ÷ (Neg/Neg + Pos/Neg)] × 100}.
TABLE 3.
Specimens with discrepant test results
| Specimen ID | Specimen source | Xpert result | Comparator result; method | Result of additional testing |
|---|---|---|---|---|
| 2 | NPS | Negative | Inconclusive (N1 or N3 detected; unknown CT value); Quest SARS-CoV-2 | Negative (CDC 2019-nCoV real-time RT-PCR diagnostic panel) |
| RUO3695 | TA | Positive (E CT, 40.6; N2 CT, 40.1) | Negative; New York SARS-CoV-2 | Negative (SARS-CoV-2 assay [Panther Fusion system]; Hologic) |
| RUO4152 | NPS/OPS | Presumptive positive (E CT, 40.6) | Negative; New York SARS-CoV-2 | Negative (SARS-CoV-2 assay [Panther Fusion system]; Hologic) |
| M207300204 | NPS/OPS | Positive (N2 CT, 40.6) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 36.6) (Roche) |
| M207300207 | NPS/OPS | Positive (E CT, 33.7; N2 CT, 37.6) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 34.3) (Roche) |
| M207300219 | NPS/OPS | Positive (N2 CT, 40.1) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 36.9) (Roche) |
| M207300223 | NPS/OPS | Positive (E CT, 35.7; N2 CT, 38.3) | Negative; Charité Virology RdRp | Negative (E gene RT-PCR) (Roche) |
| M207300259 | NPS | Positive (RdRp CT, 31.7; E CT, 33.2; N2 CT, 28.9) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 37.1) (Roche) |
| M207300260 | NPS/OPS | Positive (RdRp CT, 33.2; E CT, 35.6; N2 CT, 28.6) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 33.1) (Roche) |
| M207300261 | NPS | Positive (RdRp CT, 36.8; E CT, 34.6; N2 CT, 28.6) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 33.6) (Roche) |
| M207300262 | NPS/OPS | Positive (RdRp CT, 35.0; E CT, 35.3; N2 CT, 28.5) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 29.5) (Roche) |
| M207300263 | NPS/OPS | Positive (N2 CT, 28.5) | Negative; Charité Virology RdRp | Positive (E gene RT-PCR; CT, 36.5) (Roche) |
Specimens with concordant positive results were more likely to have all three targets detected by the RUO version of the Xpert test than specimens positive only by the Xpert test (Table 4). Specimens positive by the Xpert test and negative by SOC methods were more likely to have only the E target or the N2 target detected.
TABLE 4.
Distribution of Xpert Xpress SARS-CoV-2 positive results by assay target reactivity
| Category | Xpert targets detected [no. specimens with target detected/no. tested (%)]a
|
|||
|---|---|---|---|---|
| E, N2, RdRp | E, N2 | N2 | E | |
| Xpert positive/SOC positive | 168/199 (84.4) | 29/199 (14.6) | 1/199 (0.5) | 1/199 (0.5) |
| Xpert positive/SOC negative | 5/11 (45.5) | 2/11 (18.2) | 1/11 (9.1) | 3/11 (27.3) |
Twelve specimens were inconclusive by the New York EUA method, and 21 specimens tested at Rutgers University Hospital are not included because the EUA version of the Xpert test was used (no RdRp target). Both E and N2 targets were detected by the Xpert EUA test in 10 of these specimens, and the N2 target alone and E target alone were each detected in 1 specimen.
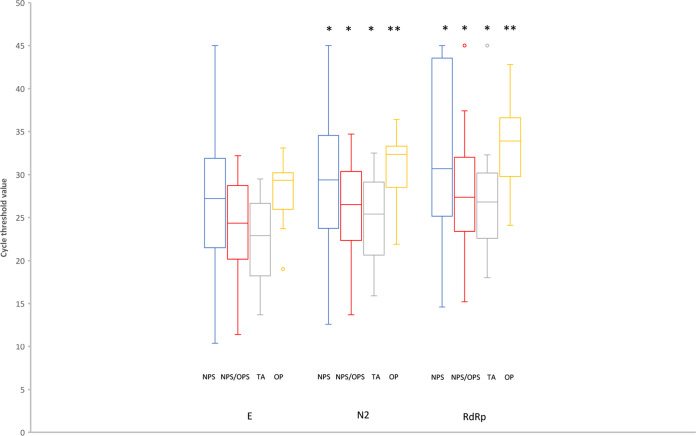
The distribution of CT values for the E, N2, and RdRp targets in the RUO version Xpert test in concordant positive specimens is shown in Fig. 1. The E target had lower CT values than the N2 and RdRp targets, while the RdRp target was consistently the least sensitive. Tracheal aspirates had lower CT values than upper respiratory tract swab specimens, but this difference was significant only for NPS and OPS specimens (tracheal aspirate E target mean CT versus NPS, P = 0.003, and versus OPS, P = 0.002; N2 target versus NPS, P = 0.005, and versus OPS, P = 0.002; RdRp target versus NPS, P = 0.018, and versus OPS, P = 0.009).
FIG 1.
Distribution of Xpert Xpress SARS-CoV-2 CT values in concordant positive specimens. Twelve specimens inconclusive by the New York EUA method and 21 specimens tested at Rutgers University Hospital are not included because the EUA version of the Xpert test was used (no RdRp target). The box center lines are medians. The boxes represent upper and lower quartiles. The bars represent minimum and maximum values, except for RdRp NPS/OPS and RdRp TA, where the maximum values are outliers. *, P < 0.0001 for comparison with the E target; **, P = 0.005 for comparison with the E target.
DISCUSSION
The data presented here indicate that the analytical and clinical performance of the Xpert Xpress SARS-CoV-2 test meets or exceeds that of reference laboratory comparator assays in an easy-to-use, on-demand format that generates results in about 45 min. A recently published study showed excellent agreement between the Xpert test and the Cobas SARS-CoV-2 test (Roche Molecular Systems, Branchburg, NJ) (14). Another recent study showed excellent agreement between the Xpert test and the Panther Fusion SARS-CoV-2 assay (Hologic) (15). The ability of the Xpert test to rapidly deliver a definitive diagnosis has the potential to reduce disease transmission (11).
We found that the test generated fewer inconclusive or presumptive results than the reference methods, which improves the actionability of the results overall. The combination of E and N2 targets provided the highest sensitivity across the range of specimen types tested, and therefore, we excluded the RdRp target in the EUA version of the test. The test design for the E target allows detection of a broad range of SARS/bat coronaviruses, including SARS-CoV-2, so the potential effects of genetic drift can be avoided. We observed a wide variation in viral load, as inferred from CT values, among the upper-respiratory-tract specimens collected in this study. The high sensitivity of the test, with a limit of detection of 0.01 PFU/ml, allows the test to be used on a variety of specimen types. We observed the greatest range of values, estimated at 0.01 to 106 PFU/ml, within NPS specimens using the CT values we obtained during our studies to determine the limit-of-detection range as a reference. Low viral loads can be explained by a variety of mechanisms, including inefficient specimen collection, sampling too early or too late in the course of infection, and low levels of viral shedding overall. High viral-load values, on the other hand, are obtainable only by efficient specimen collection in the setting of high levels of viral shedding. The relationship between SARS-CoV-2 load values and disease outcome has been controversial; a recent study in Hong Kong showed that respiratory tract specimen viral loads did not correlate with disease severity (16), whereas in a study of hospitalized patients in Nanchang, China, severely ill patients had viral-load values 60-fold higher than those with milder disease courses and took longer to achieve undetectable levels (17). Additional studies combining measures of viral load and host response patterns will likely be necessary to better understand the best predictors of disease outcome.
During the recovery phase of the epidemic and in upcoming respiratory virus seasons, there will likely be a role for NAAT prior to elective medical or dental procedures and for determining the carrier status of persons at risk of infection, such as first responders, household contacts of COVID-19 patients, and exposed health care workers. An ongoing debate is the safety of performing molecular testing for SARS-CoV-2 in settings such as outpatient clinics, where biosafety cabinets are not available. The U.S. CDC recommends that sample processing be performed inside a biosafety cabinet, but when one is not available, sample processing can be performed behind a barrier (face or bench shield) when procedures are not likely to generate aerosols (https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html). The Xpert test has been authorized by the FDA for use in point-of-care settings by trained nonlaboratorians. Sample processing involves a single addition of 300 μl of sample to a GeneXpert cartridge. The cartridge is then closed permanently. Within several minutes after starting the RT-PCR run, the sample is mixed with guanidine thiocyanate inside the cartridge. An alternative approach would be to deploy mobile laboratories with high-capacity systems capable of performing many tests simultaneously and equipped with appropriate biocontainment equipment. Such mobile laboratory capacity has already been deployed to find active cases of tuberculosis among at-risk populations in South Africa (18). Similar mobile laboratory capacity could potentially be made available in the current outbreak and potential future seasonal outbreaks and could be used to find active cases in nursing homes and for testing other less ambulatory populations, with results available on site and in an actionable time frame. Anonymous test results can also be uploaded immediately from the testing systems into a cloud-based system for public health reporting, a feature that has been incorporated into nearly all of the hundreds of systems used for tuberculosis testing in South Africa (19).
In summary, we have described a highly sensitive and specific test that can enable decentralized testing for SARS-CoV-2 and that is suitable for testing multiple specimen types and delivers results in real time. Many hospitals in the United States and over 23,000 locations worldwide already have GeneXpert systems in place that can perform from 1 to 80 tests at a time, so implementation of this technology has the potential for significant global impact. Since testing can be performed in local hospital laboratories in an on-demand manner, the extended turnaround times associated with reference laboratory testing can be avoided. Currently, the best use of this technology is likely to be in acute-care hospitals in high-prevalence settings, where rapid triage decisions need to be made regarding patient disposition and isolation and the targeted use of personal protective equipment for health care workers and potentially lifesaving treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rachel Hicken, Johns Hopkins University School of Medicine, for assistance with testing. We thank Jennifer Dien Bard (LAC and USC) for assistance with discrepant resolution testing and Brad Spellberg (LAC and USC) for assistance with IRB preparation. We thank NYC Public Health Laboratory, NYC Department of Health and Mental Hygiene, staff for technical support and Gregory Berry for assistance with discrepant resolution testing. We thank Beryl Oppenheim and Pierre Le Roux (Cepheid) for managing European study sites.
Cepheid provided Xpert Xpress test kits.
Funding Statement
Cepheid provided Xpert Xpress test kits.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. 28 March 2020. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haveri A, Smura T, Kuivanen S, Österlund P, Hepojoki J, Ikonen N, Pitkäpaasi M, Blomqvist S, Rönkkö E, Kantele A, Strandin T, Kallio-Kokko H, Mannonen L, Lappalainen M, Broas M, Jiang M, Siira L, Salminen M, Puumalainen T, Sane J, Melin M, Vapalahti O, Savolainen-Kopra C. March 2020. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill 25. doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, Wang Q, Tan L, Wu W, Tang S, Xiong Z, Zheng S. 30 March 2020. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. J Clin Microbiol doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, Zhang Y, Zhang H, Jia R, Liu P, Wang X, Ge Y, Xia A, Tian H, Chang H, Wang C, Li J, Wang J, Zeng M. 28 February 2020. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 1 April 2020. Virological assessment of hospitalized patients with COVID-2019. Nature doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen H-L, Peiris M, Wu J. 2020. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. 2020. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kam K-Q, Yung CF, Cui L, Lin Tzer Pin R, Mak TM, Maiwald M, Li J, Chong CY, Nadua K, Tan NWH, Thoon KC. 28 February 2020. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, Gao G, Wang S, Ma C, Xie R, Wang F, Tan C, Zhu L, Guo Y, Zhang F. 28 March 2020. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KK-W, Tsang O-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, Yip CC-Y, Cai J-P, Chan J-C, Chik T-H, Lau D-L, Choi C-C, Chen L-L, Chan W-M, Chan K-H, Ip JD, Ng A-K, Poon R-S, Luo C-T, Cheng V-C, Chan J-W, Hung I-N, Chen Z, Chen H, Yuen K-Y. 23 March 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong XM, Yang L, di Chu H, Fan M. 2020. Effect of delay in diagnosis on transmission of COVID-19. Math Biosci Eng 17:2725–2740. doi: 10.3934/mbe.2020149. [DOI] [PubMed] [Google Scholar]
- 12.CLSI. 2012. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline, 2nd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Chan J-W, Yip CC-Y, To KK-W, Tang T-C, Wong S-Y, Leung K-H, Fung A-F, Ng A-K, Zou Z, Tsoi H-W, Choi G-Y, Tam AR, Cheng V-C, Chan K-H, Tsang O-Y, Yuen K-Y. 2020. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol 58:e00310-20. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran A, Beavis KG, Matushek SM, Ciaglia C, Francois N, Tesic V, Love N. 17 April 2020. The detection of SARS-CoV-2 using the Cepheid Xpert Xpress SARS-CoV-2 and Roche Cobas SARS-CoV-2 assays. J Clin Microbiol doi: 10.1128/JCM.00772-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen W, Smith E, Manji R, Schron D, Berry GJ. 24 April 2020. Clinical evaluation of three sample-to-answer platforms for the detection of SARS-CoV-2. J Clin Microbiol doi: 10.1128/JCM.00783-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lui G, Ling L, Lai CK, Tso EY, Fung KS, Chan V, Ho TH, Luk F, Chen Z, Ng JK, Chow K-M, Cheng PK, Chan RC, Tsang DN, Gomersall C, Hui DS, Chan PK. 18 April 2020. Viral dynamics of SARS-CoV-2 across a spectrum of disease severity in COVID-19. J Infect doi: 10.1016/j.jinf.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Yan L-M, Wan L, Xiang T-X, Le A, Liu J-M, Peiris M, Poon LLM, Zhang W. 19 March 2020. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassett IV, Forman LS, Govere S, Thulare H, Frank SC, Mhlongo B, Losina E. 2019. Test and Treat TB: a pilot trial of GeneXpert MTB/RIF screening on a mobile HIV testing unit in South Africa. BMC Infect Dis 19:110. doi: 10.1186/s12879-019-3738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens WS, Cunningham B, Cassim N, Gous N, Scott LE. 2016. Cloud-based surveillance, connectivity, and distribution of the GeneXpert analyzers for diagnosis of tuberculosis (TB) and multiple-drug-resistant TB in South Africa, p 707–718. In Persing DH, Tenover FC, Hayden RT, Leven M, Miller MB, Nolte FS, Tang Y-W, van Belkum A (ed), Molecular microbiology: diagnostic principles and practice, 3rd ed ASM Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.