While the coronavirus disease 2019 (COVID-19) pandemic has peaked in many countries already, the current challenge is to assess population immunity on a large scale. Many serological tests are available and require urgent independent validation. Here, we report performance characteristics of Orient Gene Biotech COVID-19 IgG/IgM Rapid Test Cassette (OG) and compare it to Abbott SARS-CoV-2 IgG immunoassay (ASIA). Patients (n = 102) with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase PCR (RT-PCR) were tested.
KEYWORDS: COVID-19, lateral flow assay, rapid test
ABSTRACT
While the coronavirus disease 2019 (COVID-19) pandemic has peaked in many countries already, the current challenge is to assess population immunity on a large scale. Many serological tests are available and require urgent independent validation. Here, we report performance characteristics of Orient Gene Biotech COVID-19 IgG/IgM Rapid Test Cassette (OG) and compare it to Abbott SARS-CoV-2 IgG immunoassay (ASIA). Patients (n = 102) with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase PCR (RT-PCR) were tested. The patients were asymptomatic (n = 2) or had mild (n = 37) or severe symptoms requiring hospitalization in a medical unit (n = 35) or intensive care unit (n = 28). Specificity was evaluated for 42 patients with previous viral and parasitic diseases as well as a high level of rheumatic factor. The sensitivity of OG was 95.8% (95% confidence interval [CI95%], 89.6 to 98.8) for samples collected ≥10 days after the onset of symptoms, which was equivalent to the sensitivity of ASIA of 90.5% (CI95%, 82.8 to 95.6). OG uncovered six false-negative results of ASIA, of which two had only IgM with OG. Specificity was 100% (CI95%, 93.4 to 100) with both tests on samples, including patients infected with endemic coronavirus. Overall, OG performance characteristics indicate that the test is suitable for routine use in clinical laboratories, and its performance is equivalent to that of immunoassay. Testing OG on a larger asymptomatic population may be needed to confirm these results.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has had a major impact on clinical microbiology laboratories. The initial challenge was to increase the capacity of acute COVID-19 diagnosis by reverse transcriptase PCR (RT-PCR) (1). The current challenge is to assess immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), urged by governments to get people back to work and limit economic damage (2). However, our task remains to balance urgency against proper assessment of hundreds of tests simultaneously becoming available on the market. Seroconversion has been shown to occur as early as 7 days after the onset of symptoms (3). Here, we evaluate the Orient Gene Biotech COVID-19 IgG/IgM Rapid Test Cassette (Orient Gene Biotech, Zhejiang, China) (OG) and compare it to the Abbott SARS-CoV-2 IgG immunoassay (ASIA).
MATERIALS AND METHODS
The Orient Gene Biotech COVID-19 IgG/IgM Rapid Test Cassette (OG) is a lateral flow assay (LFA) approved by the European Union (CE mark) and the Chinese National Medical Products Administration, and the FDA recently issued an emergency use authorization (29 May 2020). This test can be performed with 5 μl of serum/plasma or 10 μl of whole blood. The sample is deposited in the specimen well (S) of the test device, and two drops of sample buffer are immediately added to the buffer well (B). The result is read at 10 min. The cassette displays a blue control band that turns red when the test has been performed correctly. IgG and IgM are represented by two separate bands and are read visually. Whole-blood samples used in this study were centrifuged at 1,200 rpm for 20 min, and sera were aliquoted and stored at –20°C upon use. Serum samples (n = 106) were tested from 102 patients with positive SARS-COV-2 RT-PCR (Cobas SARS-CoV-2 test; Roche, Meylan, France) and at least 4 days (4 to 40; median, 18) after the onset of symptoms or positive PCR for asymptomatic patients. Negative samples tested (n = 42) were acquired during the prepandemic period from routine occupational health patients with no known disease (n = 14) or hospitalized patients (n = 28) with a previous pulmonary infection with endemic coronavirus (n = 16), rhinovirus (n = 1), metapneumovirus (n = 1), influenza A virus (n = 1), or syncytial respiratory virus (n = 1). Three patients had recent infection with malaria. Two patients had IgM antibodies (Ab) against cytomegalovirus, and 2 had IgM Ab against Epstein-Barr Virus. A few patients had IgG against HIV (n = 1), hepatitis B virus (n = 1), and toxoplasmosis (n = 1), and two patients had high levels of rheumatic factor. All OG test results were performed and read after 10 min by two clinical microbiologists unblinded regarding the sample group. Indeterminate readings were to be read by a third microbiologist. Concomitantly, all serum samples were tested for IgG using ASIA on Architect Abbott Instrument i2000SR (Abbott, IL, USA) using the manufacturer’s recommended cutoff of 1.4. The study was conducted in accordance with the Declaration of Helsinki. This study was a noninterventional study with no additional sampling from the usual procedures. Biological material and clinical data were obtained only for standard viral diagnostic following physicians’ prescriptions (no specific sampling, no modification of the sampling protocol). Data analyses were conducted using an anonymized database. According to the French Health Public Law (CSP Art L 1121-1.1), such protocol was exempted from informed consent application. Statistical analyses were performed with Prism GraphPad v6.0.
RESULTS
Patients (n = 102) characteristics with a positive SARS-COV-2 RT-PCR are detailed in Table 1. Patients were asymptomatic (n = 2) or had mild symptoms (n = 37) or severe symptoms requiring hospitalization in a medical unit (n = 35) or intensive care unit (n = 28).
TABLE 1.
Clinical characteristics of 102 patients with a positive SARS-COV-2 RT-PCR resulta
| Characteristics | No. of patients (n = 102) (%) |
|---|---|
| Male | 59 (57.8) |
| Disease severity | |
| Asymptomatic | 2 (1.9) |
| Mild | 37 (36.3) |
| Severe (medical unit) | 35 (34.3) |
| Critical (ICU) | 28 (27.5) |
| Patients with 2 consecutive sera | 4 (3.9) |
The mean age of the patient population was 52 (±16) years. ICU, intensive care unit.
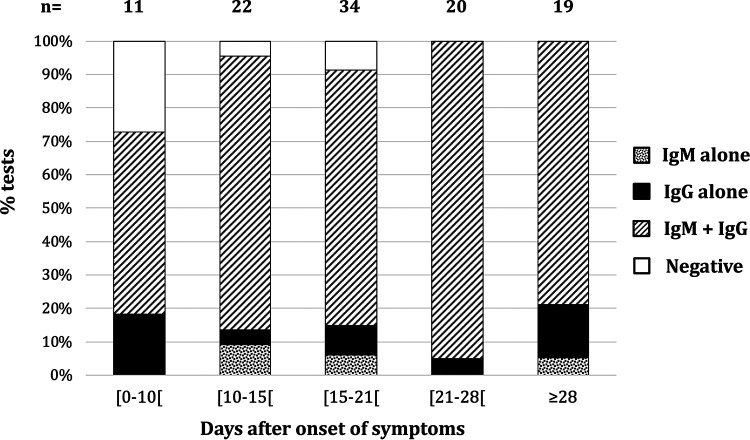
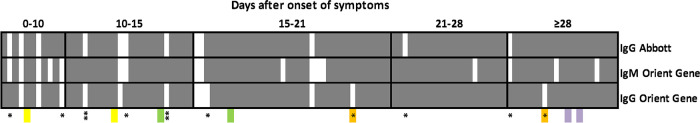
OG global sensitivity, including all samples regardless of sampling time to the onset of symptoms was 93.4% (95% confidence interval [CI95%], 86.9 to 97.3) (99/106) and 95.8% (CI95%, 89.6 to 98.8) (91/95) for samples collected ≥10 days after the onset of symptoms or after positive PCR. Results of the test according to the number of days from the onset of symptoms are presented in Fig. 1. The global sensitivity of ASIA was 88.7% (CI95%, 81.1 to 94.0) (94/106) and 90.5% (CI95%, 82.8 to 95.6) (86/95) for samples collected ≥10 days after the onset of symptoms or a positive PCR result. A total of six patients tested positive with OG and negative with ASIA. For two of these patients, OG showed IgM only. The remaining four patients had IgG according to OG. Discrepancies between ASIA and OG in IgG are labeled in Fig. 2. All patients had mild symptoms, and sera were collected on days 6, 12, 22, and 28, respectively, after the onset of symptoms. ASIA indexes for these patients were 0.02, 0.16, 0.47, and 1.33, respectively (recommended cutoff, 1.4).
FIG 1.
Percentage of Orient Gene tests displaying IgM alone, IgG alone, or both IgM and IgG according to the number of days from the onset of symptoms.
FIG 2.
Global overview of results of 106 serum samples from patients with a positive SARS-COV-2 RT-PCR tested for IgG with Abbott SARS-CoV-2 IgG assay and tested for IgG and IgM with Orient Gene Rapid Test Cassette (OG). Data are shown according to the number of days from the onset of symptoms. Positive tests are represented by gray row, negative tests by white rows. Discrepancies between IgG results by OG and ASIA are indicated by a single asterisk. Serum samples positive for IgM only by OG are indicated by two asterisks. Colored boxes represent samples from the same patients (n = 4).
Seven patients tested negative with OG; samples from three of these patients were collected <10 days after the onset of symptoms. Out of these three patients, one who was sampled on day 9 after the onset of symptom was positive by ASIA with an index of 4.1, while the two others remained negative with respective indexes of 0.05 and 0.46. The remaining four patients negative by OG were negative on days 12, 15, 15, and 18, respectively, and all displayed acquired immunodeficiencies that could explain an inability to synthesize immunoglobulins. Acquired immunodeficiencies resulted from hematological malignancies (n = 2) and metastatic lung carcinoma (n = 1) and glioblastoma (n = 1) both under chemotherapy and/or radiotherapy associated with severe malnutrition (albumin of <25 g/liter). Concomitantly, ASIA was also negative for these four patients with indexes of <0.3 in all cases.
Four patients displayed only IgM with OG, one of whom was negative for IgG on days 19 and 30 (Fig. 2, orange). For this patient, IgG was detected with ASIA with indexes of 3.71 and 4.28 at day 19 and 30, respectively. The three other patients were sampled ≤15 days after onset of symptoms, and two of the three were also negative by ASIA.
The overall agreement between OG and ASIA focusing only on IgG detection was 92.5% (98/106), and the results are displayed in Fig. 2. Both asymptomatic patients tested positive on days 13 and 18, respectively, after positive RT-PCR with both techniques. One patient was tested with OG using both serum and whole blood showing bands of similar intensity. The specificity of the test was 100% (CI95%, 93.4 to 100) with no cross-reactivity observed with endemic coronavirus and other infections tested as well as with high level of rheumatic factors known to be a frequent cause of false-positive results. The specificity of ASIA was tested on the same samples and was also 100% (CI95%, 93.4 to 100). Among the 106 samples screened, no indeterminate reading required a third reading, and all tests were valid according to the control line.
DISCUSSION
In this study, the performance characteristics of OG were evaluated and showed a specificity of 100% and a sensitivity of 95.8% for samples collected ≥10 days after the onset of symptoms or a positive PCR result. This rapid test performance was equivalent to that of the Abbott SARS-CoV-2 IgG assay. This immunoassay sensitivity was 90.5% in our study and 82.4% in a recent publication for samples collected ≥10 days after the onset of symptoms (4). In that same study, the sensitivity was found to be 100% for samples collected ≥17 days after the onset of symptoms. However, using the 17-day cutoff to evaluate our data, we found a sensitivity of 94.9% (CI95%, 85.9 to 98.9). This difference could be explained by a different patient population. Indeed, IgG was not detected with ASIA for samples collected ≥10 days from the onset of symptoms or PCR positivity compared to OG in six patients, all with mild symptoms, including five from occupational health. Bryan et al. report their serum specimens came mostly from hospitalized patients and thus probably from patients with a severe or critical disease (4). Furthermore, our population was younger, with a mean age of 52 years, while 65.6% were older than 60 in the study by Bryan et al. In our study, all patients with IgG detected only with OG were <40 years old. Younger patients may have a different immunoglobulin synthesis kinetics and/or have lower levels of Ab. The ability of this rapid test to detect IgM concomitantly to IgG is a substantial advantage, and global sensitivity was increased compared to that of ASIA to assess seroreactivity to SARS-CoV-2. OG also performs better than other rapid tests evaluated (5, 6). OG performance characteristics were also assessed very recently in the United Kingdom and showed a sensitivity of 100% for samples collected ≥13 days after the onset of symptoms. The specificity was 96%, in contrast to the 100% specificity we found (6).
Overall, it is necessary to remind readers that both tests described here measure seroreactivity, which may or may not reflect immunity. OG characteristics are suitable for routine use in clinical laboratories for patients with onset of symptoms ≥10 days ago, and its performance is equivalent to that of ASIA. Testing this lateral flow assay on a larger asymptomatic population may be needed to confirm our results. OG could certainly help clinical microbiologists and governments at estimating the number of people who have been infected and provide an acceptable product to be used in outpatient and home settings.
ACKNOWLEDGMENTS
We acknowledge specifically François Gnemmi and Thierry Poiraud from Menarini for providing the tests, Charles Bourget for his technical assistance, Florence Morin from the immunology department who provided sera positive for rheumatic factor, and all the laboratory staff of the Saint-Louis hospital virology department.
The members of the Saint-Louis CORE (COvid REsearch) group follow: G. Archer, A. Benattia, A. Bergeron, L. Bondeelle, J. D. Bouaziz, D. Bouda, D. Boutboul, I. Brindel Berthon, E. Bugnet, S. Caillat Zucman, S. Cassonnet, K. Celli Lebras, J. Chabert, M. L. Chaix, S. Chevret, M. Clément, C. Davoine, N. De Castro, E. De Kerviler, C. De Margerie-Mellon, C. Delaugerre, F. Depret, B. Denis, L. Djaghout, C. Dupin, D. Farge-Bancel, C. Fauvaux, E. Feredj, D. Feyeux, J. P. Fontaine, V. Fremeaux-Bacchi, L. Galicier, S. Harel, A. L. Jegu, E. Kozakiewicz, M. Lebel, A. Baye, J. Le Goff, P. Le Guen, E. Lengline, G. Liegeon, G. Lorillon, I. Madelaine Chambrin, G. Martin de Frémont, M. Meunier, J. M. Molina, F. Morin, E. Oksenhendler, R. Peffault de la Tour, O. Peyrony, B. Plaud, M. Salmona, J. Saussereau, and J. Soret.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Tang Y-W, Schmitz JE, Persing DH, Stratton CW. 2020. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol 58:e00512-20. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petherick A. 2020. World Report: developing antibody tests for SARS-CoV-2. Lancet 395:1101–1102. doi: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, Sandt CE, Jia X, Nicholson S, Catton M, Cowie B, Tong SYC, Lewin SR, Kedzierska K. 2020. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 26:453–410. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, Jerome KR, Mathias PC, Greninger AL. 7 May 2020. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, Pan W, Zhan Z, Chen L, Ye F. 27 February 2020. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pallett S, Denny S, Patel A, Charani E, Mughal N, Stebbing J, Davies G, Moore L. 2020. Point-of-care serological assays for SARS-CoV-2 in a UK hospital population: potential for enhanced case. ResearchSquare (preprint). doi: 10.21203/rs.3.rs-28006/v1. [DOI] [PMC free article] [PubMed]