A surge of patients with coronavirus disease 2019 (COVID-19) presenting to New York City hospitals in March 2020 led to a sharp increase in blood culture utilization, which overwhelmed the capacity of automated blood culture instruments. We sought to evaluate the utilization and diagnostic yield of blood cultures during the COVID-19 pandemic to determine prevalence and common etiologies of bacteremia and to inform a diagnostic approach to relieve blood culture overutilization. We performed a retrospective cohort analysis of 88,201 blood cultures from 28,011 patients at a multicenter network of hospitals within New York City to evaluate order volume, positivity rate, time to positivity, and etiologies of positive cultures in COVID-19.
KEYWORDS: COVID-19, SARS-CoV-2, bacteremia, blood culture, sepsis
ABSTRACT
A surge of patients with coronavirus disease 2019 (COVID-19) presenting to New York City hospitals in March 2020 led to a sharp increase in blood culture utilization, which overwhelmed the capacity of automated blood culture instruments. We sought to evaluate the utilization and diagnostic yield of blood cultures during the COVID-19 pandemic to determine prevalence and common etiologies of bacteremia and to inform a diagnostic approach to relieve blood culture overutilization. We performed a retrospective cohort analysis of 88,201 blood cultures from 28,011 patients at a multicenter network of hospitals within New York City to evaluate order volume, positivity rate, time to positivity, and etiologies of positive cultures in COVID-19. Ordering volume increased by 34.8% in the second half of March 2020 compared to the level in the first half of the month. The rate of bacteremia was significantly lower among COVID-19 patients (3.8%) than among COVID-19-negative patients (8.0%) and those not tested (7.1%) (P < 0.001). COVID-19 patients had a high proportion of organisms reflective of commensal skin microbiota, which, when excluded, reduced the bacteremia rate to 1.6%. More than 98% of all positive cultures were detected within 4 days of incubation. Bloodstream infections are very rare for COVID-19 patients, which supports the judicious use of blood cultures in the absence of compelling evidence for bacterial coinfection. Clear communication with ordering providers is necessary to prevent overutilization of blood cultures during patient surges, and laboratories should consider shortening the incubation period from 5 days to 4 days, if necessary, to free additional capacity.
INTRODUCTION
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across New York City in March 2020 led to an unprecedented strain on hospital resources, including shortages of beds, ventilators, personal protective equipment, and diagnostic materials such as laboratory reagents and nasopharyngeal swabs (1–3). The surge of febrile patients to our network of hospitals in New York City led to a sudden and dramatic increase in the number of blood cultures received in our laboratories, which overwhelmed the capacity of our automated blood culture instruments.
While blood cultures are an essential tool for the diagnosis and management of bloodstream infections among patients presenting to the emergency department and among inpatients, data are lacking on their utility for patients with suspected or confirmed coronavirus disease 19 (COVID-19). While many patients with severe COVID-19 are treated with empirical antibiotics for potential bacterial coinfections, the rate of bacteremia among these patients is unknown, and the benefit of empirical antibiotic therapy is unproven. Frequent ordering of blood cultures for patients with COVID-19 may overwhelm a laboratory’s capacity to perform and process these tests, which may negatively impact the overall benefit of testing for the entire medical center. Therefore, we sought to evaluate both the utilization and diagnostic yield of blood cultures during a surge of COVID-19 patients presenting to our hospitals, including positivity rates for patients with and without COVID-19, as well as the most common causes of bacteremia among COVID-19 patients. We also present strategies for diagnostic stewardship to mitigate challenges that may arise from a surge of blood culture orders.
MATERIALS AND METHODS
Study design.
A retrospective cohort study was conducted on patients with blood cultures performed at NewYork-Presbyterian Hospitals located throughout New York City from 1 January 2020 to 31 March 2020. Corresponding data from 1 January 2019 to 31 March 2019 were collected to establish a seasonal historic baseline of blood culture ordering and positivity. Records were extracted from the laboratory information system (Cerner Millennium, Cerner, North Kansas City, MO) using a Cerner Command Language query and included information on performing facility, SARS-CoV-2 reverse transcription-PCR (RT-PCR) result, blood culture result, organism(s) identified, and blood culture collection date and time. After the study period concluded, the blood culture incubation period was reduced from 5 days to 4 days to free additional space on the instruments. In a subset of patients for whom data were available, the interval from time of blood culture collection to time of Gram stain was used to calculate the time to blood culture positivity during the study period, which was used to examine the predicted effect of this intervention.
Laboratory methods.
Blood cultures were incubated on Bactec FX (Becton, Dickinson and Co., Franklin Lakes, NJ) or VersaTrek (Thermo Fisher Scientific, Inc., Waltham, MA) instruments for a maximum of 5 days. SARS-CoV-2 RT-PCR testing was performed in-house with the following assays: cobas SARS-CoV-2 (Roche Molecular Systems, Inc., Branchburg, NJ), Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA), RealStar SARS-CoV-2 (Altona Diagnostics USA, Inc., Plain City, OH), and a laboratory-developed test from the Wadsworth Center at the New York State Department of Health.
Participants.
A total of 88,201 blood cultures from 28,011 patients were included from the following hospitals within the NewYork-Presbyterian network: Columbia University Irving Medical Center (32,788 patients), Weill-Cornell Medical Center (26,794 patients), Allen Hospital (6,053 patients), Queens Hospital (16,913 patients), and Lower Manhattan Hospital (5,653 patients). Patients were stratified by SARS-CoV-2 RT-PCR result as positive, negative, or not tested. For the purposes of classifying blood cultures by SARS-CoV-2 RT-PCR status, we used the following criteria:
Blood cultures were labeled SARS-CoV-2 status positive if they were performed within 2 days of a positive SARS-CoV-2 RT-PCR result and considered positive for all subsequent blood cultures after a positive SARS-CoV-2 RT-PCR result.
Blood cultures were labeled SARS-CoV-2 status negative if they were was performed within 2 days of a negative SARS-CoV-2 RT-PCR result and considered negative for all subsequent blood cultures unless the patient had a subsequent positive SARS-CoV-2 RT-PCR result, at which point the status was changed to positive for any blood cultures performed within 2 days of the positive SARS-CoV-2 RT-PCR result.
All other blood cultures were labeled SARS-CoV-status not tested.
The 2-day interval was used to account for turnaround time from test ordering to SARS-CoV-2 test results as blood culture and SARS-CoV-2 RT-PCR tests ordered on the same day may have taken up to 2 days for the SARS-CoV-2 RT-PCR result to become available.
This study was approved by the Institutional Review Boards of Columbia University Irving Medical Center and Weill Cornell Medicine.
Data analysis.
All data analysis was performed with the R statistical language, version 3.6.3 (4). Blood culture volumes and positivity rates were calculated and graphed by day, with a moving regression line estimated by the default geom_smooth function of the ggplot2 R package, version 3.3.0. The regression lines were overlaid in the scatter plot to analyze directional trends.
Volumes and positivity rates were also stratified by SARS-CoV-2 RT-PCR result using the rules specified above and by various patient categories. Bacterial and fungal etiologies of blood cultures were also collected and stratified by SARS-CoV-2 RT-PCR result. Differences in continuous data between groups were assessed by one-way analysis of variance (ANOVA), whereas categorical data were analyzed by Pearson’s chi-square analysis.
RESULTS
Blood culture volumes and positivity rates.
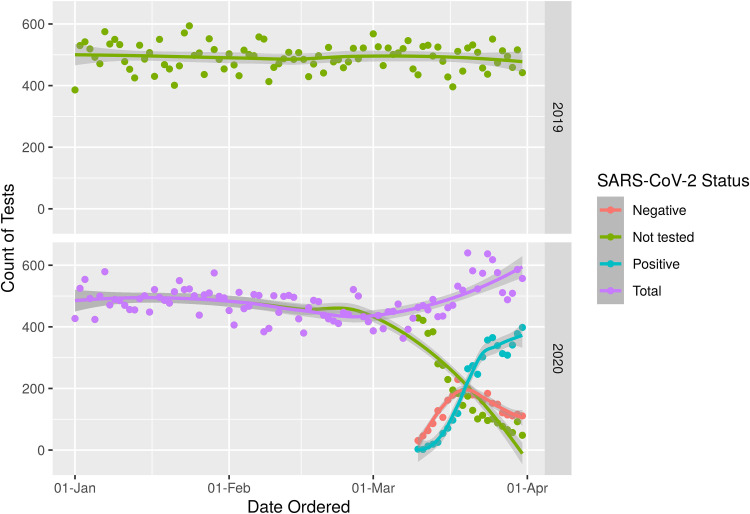
During the study period, blood culture volumes rose substantially during the month of March 2020 (Fig. 1). Overall, 8,784 blood cultures were performed during the second half of March 2020, representing a 34.8% increase from the first half of the month. Patients who were positive for SARS-CoV-2 accounted for the majority of the increased orders of blood cultures. Notably, the increased ordering among COVID-19 patients was not primarily attributable to repeated ordering as 48.2% of COVID-19 patients had more than 2 blood culture sets drawn, whereas blood cultures were ordered for 66.2% of COVID-19-negative patients and 62.1% of patients not tested for SARS-CoV-2 (P < 0.001) (see Tables S1 and S2 in the supplemental material).
FIG 1.
Number of blood cultures ordered by day in 2019 and 2020. Blood culture groups are identified according to the legend on the figure.
The blood culture positivity rate was significantly lower for patients that tested positive for SARS-CoV-2 (3.8%) than for patients that tested negative for SARS-CoV-2 (8.0%) or for patients that were not tested (7.1%, P < 0.001) (Table S3). As additional COVID-19 patients presented to the hospital throughout the month of March 2020, the overall rate of blood culture positivity decreased from 6.5% in the first half of March 2020 to 5.6% in the second half of the month as the number of negative blood cultures increased from 6,097 to 8,295.
Etiologies of bacteremia.
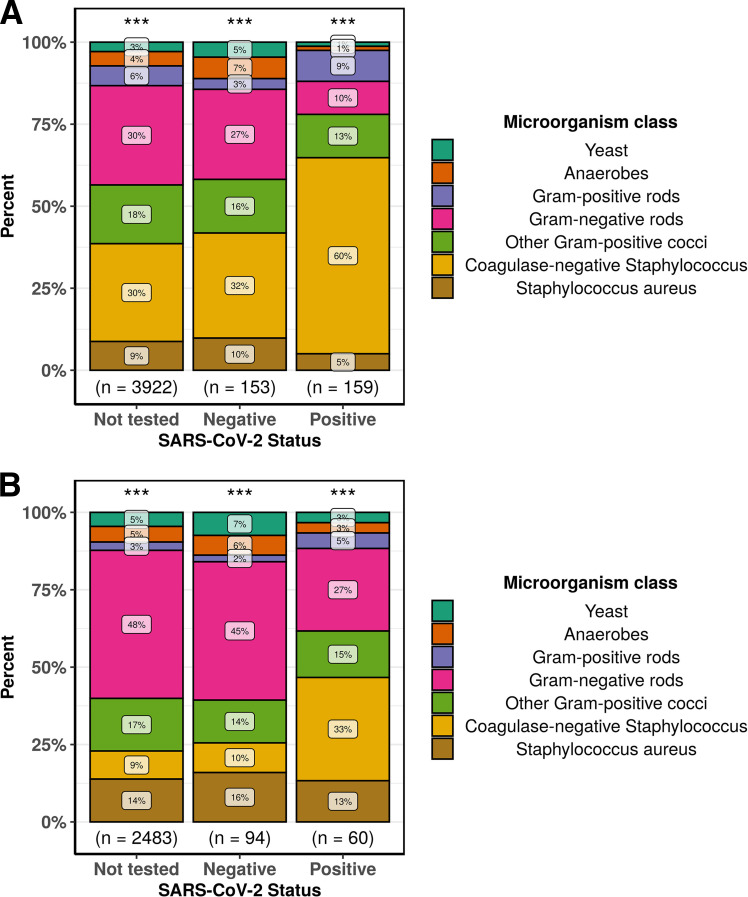
Among patients with positive blood cultures, COVID-19 patients had a significantly higher proportion of cultures that likely represented contamination with normal skin microbiota than all other groups (Fig. 2). Organisms were labeled as likely contaminants if they were isolated only once per patient and belonged to groups generally defined as commensal skin microbiota (5). Coagulase-negative Staphylococcus species accounted for 59.7% of all positive cultures among COVID-19 patients in contrast to 32.0% among patients that tested negative for SARS-CoV-2 and 29.8% among patients that were not tested for SARS-CoV-2 in 2020 (P < 0.001). Corynebacterium species, Bacillus species, and Micrococcus species were also seen more frequently among COVID-19 patients (Data Set S1). When potential contaminants were excluded, the rate of bacteremia for COVID-19 patients decreased to 1.6%, which was significantly lower than the rate of bacteremia, excluding contaminants, among COVID-19-negative patients (5.9%) and during the same period in 2019 (5.7%, P < 0.001) (Table S4). The most common causes of true bacteremia among COVID-19 patients were Escherichia coli (16.7%), Staphylococcus aureus (13.3%), Klebsiella pneumoniae (10.0%), and Enterobacter cloacae complex (8.3%) (Data Set S1). None of these pathogens were overrepresented among COVID-19 patients compared to levels for the other groups.
FIG 2.
Frequency of microorganisms identified from positive blood cultures stratified by SARS-CoV-2 status. Each microorganism was counted once per patient, and microorganisms are grouped as indicated. (A) All microorganisms isolated were counted. (B) Likely skin contaminants were excluded. ***, P < 0.001 (Pearson’s chi-square test).
Incubation period.
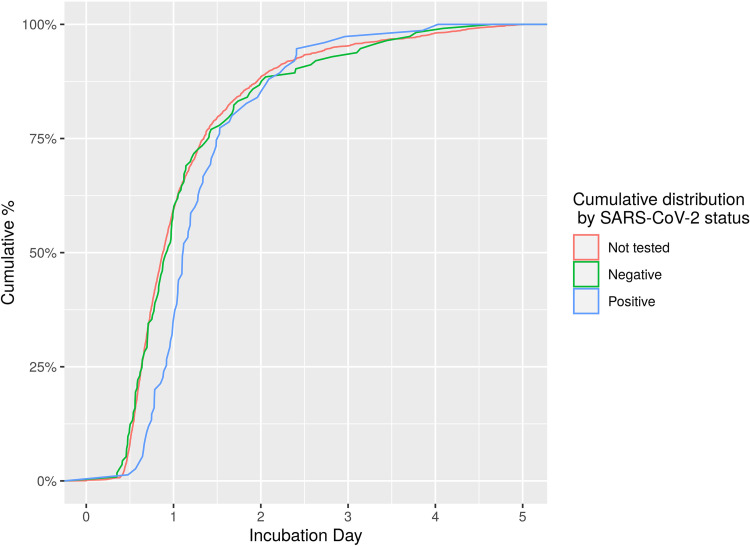
Among the subset of 1,859 positive blood cultures for which the incubation period could be reliably assessed, the vast majority (88.2%) signaled positive within 1 to 2 days of incubation, with an additional 7.0% signaling positive on day 3 and 3.0% signaling positive on day 4 (Fig. 3 and Table 1). Only 1.8% of all blood cultures signaled positive on day 5, many of which yielded normal skin microbiota (Table S5). Among COVID-19 patients, 97.3% of positive cultures signaled positive within 3 days of incubation, with one culture positive on the fourth day for Cutibacterium acnes and one culture positive on the fifth day for Candida albicans.
FIG 3.
Time to positivity of positive blood cultures stratified by SARS-CoV-2 status. Time to positivity was calculated from the time of collection to the first positive signal as recorded by the Gram stain date and time. The cumulative distribution of positivity over time is represented.
TABLE 1.
Proportion of positive cultures identified by Gram stain at days 1 to 5 of culture, stratified by SARS-CoV-2 status
| Incubation day | No. (%) of positive cultures by SARS-CoV-2 statusa
|
|||
|---|---|---|---|---|
| Not tested (n = 1,672) | Negative (n = 112) | Positive (n = 75) | Total (n = 1,859) | |
| 1 | 993 (59.4) | 65 (58.0) | 26 (34.7) | 1,084 (58.3) |
| 2 | 487 (29.1) | 32 (28.6) | 37 (49.3) | 556 (29.9) |
| 3 | 113 (6.8) | 7 (6.2) | 10 (13.3) | 130 (7.0) |
| 4 | 48 (2.9) | 6 (5.4) | 1 (1.3) | 55 (3.0) |
| 5 | 31 (1.9) | 2 (1.8) | 1 (1.3) | 34 (1.8) |
P = 0.002, Pearson’s chi-square test.
DISCUSSION
Beginning in March 2020, a surge of COVID-19 patients presenting to our network of hospitals in New York City, the current epicenter of the global COVID-19 pandemic (6), led to a dramatic increase in the utilization of blood cultures. In patients presenting with severe febrile illness, blood cultures are essential in ruling out bacterial infection and guiding appropriate antibiotic utilization. However, we found a very low rate of bacteremia among patients diagnosed with COVID-19, implying a remarkably low diagnostic yield of blood cultures for COVID-19 patients. The proportion of positive blood cultures that yielded contaminants was also significantly higher among COVID-19 patients, which is likely attributable to overordering in a population with a low rate of true bacteremia. When likely contaminants were excluded, COVID-19 patients had bacteremia rates that were less than one-third of the baseline rate from 2019. Among COVID-19 patients with true bacteremia, the distribution of clinically important organisms was similar to that of patients without COVID-19. Together, these data demonstrate that bloodstream infections appear to be very rare for COVID-19 patients and suggest that empirical antibiotics may not be useful in the absence of compelling evidence of an accompanying bacterial infection. Notably, we did not evaluate other bacterial infections such as bacterial pneumonia although other studies have shown low levels of procalcitonin among COVID-19 patients, arguing that bacterial superinfection may be uncommon (7–9). Low rates of bacteremia have also been found among patients with other respiratory viral infections, including SARS (10, 11), respiratory syncytial virus (RSV) (12, 13), and influenza virus (14) although adults with severe influenza, particularly those who die, have been shown to have a higher prevalence of bacteremia (15).
As medical centers across the United States prepare for anticipated waves of COVID-19 patients, our data may be used to justify the judicious utilization of blood cultures to preserve the operational capacity of diagnostic laboratories and to promote antimicrobial stewardship efforts to reduce unnecessary antibiotic administration. During the initial phase of the COVID-19 surge in mid-March 2020, long-standing order sets for sepsis and official hospital guidance promoted the default ordering of blood cultures as part of the initial workup for patients with suspected COVID-19 being admitted to the hospital. However, these order sets and guidance documents had unintended consequences as the ordering of blood cultures exceeded the capacity of our automated instruments, requiring additional staff to manually process these cultures at a time when staffing and supplies were already constrained. Our experience should serve as a caution to other medical centers that overordering of blood cultures during patient surges can overwhelm laboratory capacity and may negatively impact the quality of results for all patients.
As a harm reduction measure, we decreased the incubation period of blood cultures from 5 days to 4 days after the study period concluded, which freed additional space to allow for timely processing of incoming cultures. Our data demonstrate that this intervention likely had little to no adverse effect on patient care as all but one of the COVID-19 patients with positive cultures during the study period signaled positive within 4 days, and only 1.8% of all positive cultures signaled positive on the fifth day, many of which were positive for normal skin microbiota. Previous studies have also shown that decreasing the incubation of blood cultures to 4 days (16) or even 3 days (17, 18) has minimal effect on positivity rates, particularly for clinically significant bacteria.
To our knowledge, this study is the first to examine blood culture utilization for COVID-19 patients. The inclusion of over 88,000 patient cultures is a major strength of the study design, as is the multicenter analysis from a wide geographic catchment area in New York City, which increases the generalizability of our results. Limitations of the study include paucity of data on other bacterial coinfections and lack of data on patient antibiotic utilization to demonstrate how blood culture utilization impacted therapy.
In summary, we observed an overutilization of blood cultures during a surge of COVID-19 patients to our network of medical centers in New York City and found a very low rate of bloodstream infections among COVID-19 patients. This overutilization was mitigated through a 4-day incubation with likely minimal impact on patient care. Clear communication with ordering providers and hospital leadership regarding the low yield of blood cultures is a necessary step to mitigate overordering and to preserve laboratory functionality during these periods. Laboratories should also consider reducing the incubation period of blood cultures from 5 days to 4 days to further increase their capacity.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the efforts of the NewYork-Presbyterian Laboratory Information Team, in particular, Kelvin Espinal, Dennis Camp, Yingzhe Kuang, and Bulent Oral, for designing queries and providing data extracts.
J.J.C. received research support from Roche Diagnostics. We have no other conflicts to report.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Fomsgaard AS, Rosenstierne MW. 2020. An alternative workflow for molecular detection of SARS-CoV-2 - escape from the NA extraction kit-shortage, Copenhagen, Denmark, March 2020. Euro Surveill 25:2000398 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.14.2000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton PN, Bond KC. 9 April 2020. COVID-19 and risks to the supply and quality of tests, drugs, and vaccines. Lancet Glob Health doi: 10.1016/S2214-109X(20)30136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranney ML, Griffeth V, Jha AK. 2020. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med 382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 4.R Core Team. 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org. [Google Scholar]
- 5.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.New York City Department of Health and Mental Hygiene. 21 April 2020. COVID-19: data. https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed 21 April 2020.
- 7.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Plebani M. 2020. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Plebani M. 3 March 2020. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 10.Leong HN, Earnest A, Lim HH, Chin CF, Tan C, Puhaindran ME, Tan A, Chen MI, Leo YS. 2006. SARS in Singapore–predictors of disease severity. Ann Acad Med Singapore 35:326–331. [PubMed] [Google Scholar]
- 11.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, HKU/UCH-SARS Study Group. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine DA, Platt SL, Dayan PS, Macias CG, Zorc JJ, Krief W, Schor J, Bank D, Fefferman N, Shaw KN, Kuppermann N, Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. 2004. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics 113:1728–1734. doi: 10.1542/peds.113.6.1728. [DOI] [PubMed] [Google Scholar]
- 13.Greenes DS, Harper MB. 1999. Low risk of bacteremia in febrile children with recognizable viral syndromes. Pediatr Infect Dis J 18:258–261. doi: 10.1097/00006454-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Leung CH, Tseng HK, Wang WS, Chiang HT, Wu AY, Liu CP. 2014. Clinical characteristics of children and adults hospitalized for influenza virus infection. J Microbiol Immunol Infect 47:518–525. doi: 10.1016/j.jmii.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Yeh CY, Wang FD, Chuang YC, Yang CJ, Huang SF, Weng WS, Liaw CH, Sheng WH. 2018. Clinical outcomes and prognostic factors of patients with severe influenza receiving intravenous peramivir salvage therapy in intensive care units. J Microbiol Immunol Infect 51:697–704. doi: 10.1016/j.jmii.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Doern GV, Brueggemann AB, Dunne WM, Jenkins SG, Halstead DC, McLaughlin JC. 1997. Four-day incubation period for blood culture bottles processed with the Difco ESP blood culture system. J Clin Microbiol 35:1290–1292. doi: 10.1128/JCM.35.5.1290-1292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourbeau PP, Foltzer M. 2005. Routine incubation of BacT/ALERT FA and FN blood culture bottles for more than 3 days may not be necessary. J Clin Microbiol 43:2506–2509. doi: 10.1128/JCM.43.5.2506-2509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourbeau PP, Pohlman JK. 2001. Three days of incubation may be sufficient for routine blood cultures with BacT/Alert FAN blood culture bottles. J Clin Microbiol 39:2079–2082. doi: 10.1128/JCM.39.6.2079-2082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.