LETTER
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing coronavirus disease 2019 (COVID-19), was reported for the first time in Wuhan (Hubei Province, China) in December 2019 (1, 2) and has become a major public health concern all over the world. Early diagnosis is crucial for patient management and outbreak control. Most tests currently used for the detection of SARS-CoV-2 rely on viral RNA amplification by using real-time PCR (RT-PCR) and require a few hours before result release. Hence, highly sensitive immunological diagnostic methods that directly detect viral antigens in clinical samples would be very helpful for rapid and accurate diagnosis of COVID-19.
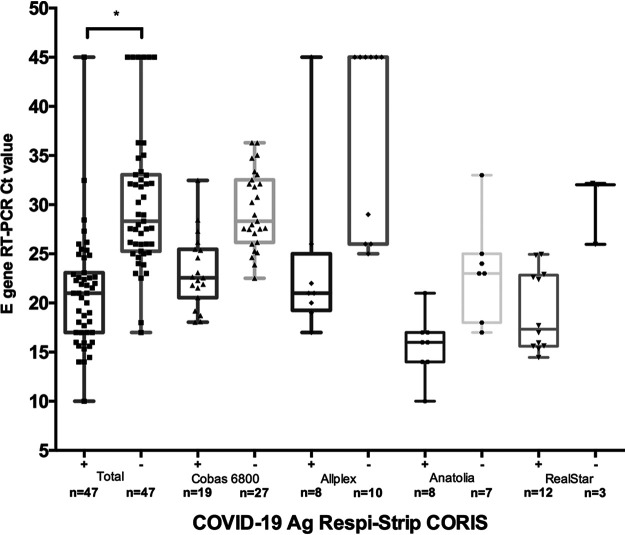
Here, we evaluated a rapid diagnostic test, COVID-19 Ag Respi-Strip (Coris BioConcept, Gembloux, Belgium), for detection of the SARS-CoV-2 antigen in nasopharyngeal secretions. The assay is ready to use and based on a nitrocellulose membrane technology with colloidal gold nanoparticles sensitized with monoclonal antibodies directed against highly conserved SARS-CoV-2 nucleoprotein antigens. We compared this test with RT-PCR, the current reference assay in virology laboratories of three university hospital groups from Assistance-Publique-Hôpitaux de Paris (APHP) (Saint-Antoine-Tenon-Trousseau, Saint-Louis-Lariboisière, and Kremlin Bicêtre-Paul Brousse). Different RT-PCR methods were used (RealStar [Altona Diagnostics], Bosphore novel coronavirus (2019-nCoV) detection kit [Anatolia Geneworks], Cobas 6800 [Roche], Allplex 2019 novel CoV assay [Seegene]). All assays amplify the SARS-CoV-2 E gene. Cycle threshold (CT) values were recorded. Nasopharyngeal samples were tested prospectively within a few hours after collection and without any cooling or freezing step, from 1 April to 15 April 2020. Swabs were collected in various transport media (COPAN’s UTM [3 ml], Virocult [1 ml], ESwab Amies [1 ml], 4MRT [3 ml], 0.9% NaCl buffer, and cobas [Roche]). The first four samples collected in cobas medium tested gave invalid results. We therefore excluded such samples from the study. Our analysis included 138 nasopharyngeal samples, of which 94 (68.8%) were positive for SARS-CoV-2 by RT-PCR. Compared to that of RT-PCR, the specificity of the test was 100% (95% confidence intervals [95% CI], 91.8 to 100). Among the 94 RT-PCR-positive samples, the rapid test detected only 47 specimens, resulting in a sensitivity of 50.0% (95 CI, 39.5 to 60.5). In nine positive and eight negative tests, control lines were barely visible. Medians of E gene CT values differed significantly between positive (median = 21; interquartile range [IQR], 17.0 to 23.0) and negative (median = 28.3; IQR, 25.6 to 33.0) antigenic test results (P < 0.0001) (Fig. 1). A study conducted by the manufacturer mentioned a sensitivity of 76.7% for samples positive with a CT value under 25 (3). In our study, the test had a sensitivity of 82.2% for CT values under 25.
FIG 1.
COVID-19 Ag Respi-Strip (Coris) results according to real-time PCR CT values. All cycle threshold values of E gene real-time PCR-positive assays are shown for positive and negative COVID-19 Ag Respi-Strip assay results. Results gathering CT values for all real-time PCR-positive assays are depicted by squares. CT values between samples positive or negative for the antigenic assay are significantly different (* indicates a P value of <0.0001). CT values corresponding to the Cobas 6800, Allplex, Anatolia, and RealStar assays are depicted by triangles, diamonds, circles, and upside-down triangles, respectively.
In our study, the COVID-19 Ag Respi-Strip (Coris) had a sensitivity of 50% compare to that of RT-PCR. The test was more sensitive for high viral loads and might perhaps be used for patients within a few days after symptom onset, when the load in the upper respiratory tract is at its peak. Considering COVID-19’s current low prevalence of 0.19% in France, prospective studies should be conducted to determine the best settings for its implementation.
REFERENCES
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens P, De Voe N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, Van den Wijngaert S, Monteil V, Melin P, Stoffels K, Yin N, Mileto D, Delaunoy S, Magein H, Lagrou K, Bouzet J, Serrano G, Wautier M, Leclipteux T, Van Ranst M, Vandenberg O, LHUB-ULB SARS-CoV-2 Working Diagnostic Group. 8 May 2020. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front Med https://www.frontiersin.org/articles/10.3389/fmed.2020.00225/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Medicine&id=551560. [DOI] [PMC free article] [PubMed]