Abstract
Sex hormone transition may trigger severe depressive episodes in some women. In order to map mechanisms related to such phenomena we developed a pharmacological preclinical human model using sex hormone manipulation with gonadotropin releasing hormone agonist (GnRHa) in a placebo‐controlled design. Here the findings from this model is synthesized and discussed in the context of related literature on hormonal contributions to reproductive mental health disorders. The GnRha model work points to an estradiol‐dependent depressive response in healthy women undergoing short‐term sex hormone manipulation with GnRHa, which is linked to serotonin transporter changes (a key regulator of synaptic serotonin), a disengagement of hippocampus, and overengagement of brain networks recruited when processing emotional salient information. Further, the GnRHa model suggest that key brain regions in the reward circuit are less engaged in positive stimuli when undergoing sex hormone manipulation, which may underlie anhedonia. Also, the work supports that enhanced sensitivity to estrogen signaling at the level of gene expression may drive increased risk for depressive symptoms when exposed to sex steroid hormone fluctuations. In conclusion, the GnRHa model work highlights the brain signatures of rapid and profound changes in sex steroid hormone milieu, which reflect plausible mechanisms by which risk for mood disorders works. This model points to the role of estrogen dynamics and sensitivity, and offers a rationale for personalized prevention in hormonal transition phases, for example pregnancy to postpartum transition, perimenopause, and hormone treatments, which now can move into clinical translation and ideally pave the way for protecting mental and cognitive health.
1. INTRODUCTION
Major depressive disorder (MDD) is expected to cause the highest ranking disability and burden of disease by 2030 (WHO, 2008) and strikingly affects twice as many women as men. Women are at a particularly heightened risk during hormonal transition phases such as during puberty (Thapar, Collishaw, Pine, & Thapar, 2012), across late pregnancy to postpartum (Munk‐Olsen, Laursen, Pedersen, Mors, & Mortensen, 2006) and perimenopause (Freeman, Sammel, Boorman, & Zhang, 2014). Hormone‐related risk mechanisms may even extend to exogenous hormone exposure (i.e., hormonal contraception) (Skovlund, Morch, Kessing, & Lidegaard, 2016).
MDD is a heterogeneous and complex disorder and depressive symptoms often occur transdiagnostically, for example in other disorders such as bipolar disorder, schizophrenia, neurodegenerative, and disorders. The etiological contributions to MDD are from a multitude of environmental and genetic factors, and their interplay, which indeed can be modified by steroid hormones. In line with this, steroid hormone dynamics play a prominent role specifically in MDD compared to other psychiatric disorders, that is schizophrenia, autism spectrum‐, bipolar disorder, and alcoholism, as recently emphasized by gene expression profiles in a large postmortem brain study (Gandal et al., 2018). Yet, the underlying risk and resilience mechanisms of MDD are far from clear and accordingly current preventive and treatment strategies are suboptimal. Identification of high‐risk individuals with distinct etiology and/or responsiveness to certain triggers or treatments is a strategy to stratify and assist build a rationale for personalized and precise treatment and prevention (Schumann et al., 2014). We and others propose that one such clinically important and distinct subgroup within MDD is women who are sensitive to hormonal transitions.
In this review, we synthesize and discuss findings from a preclinical human pharmacological sex hormone manipulation model we used in healthy women to provide unique insights to how sex hormone fluctuations may trigger depressive symptoms in some but not other women (Fisher et al., 2017; Frokjaer et al., 2015; Henningsson et al., 2015; Macoveanu et al., 2016; Mehta et al., 2018; Stenbaek, Budtz‐Jorgensen, Pinborg, Jensen, & Frokjaer, 2019; Stenbaek et al., 2016).
1.1. Epidemiology of depressive episodes during women's hormonal transitions
Strong epidemiological evidence suggests that women are at increased risk for depressive episodes in phases of life were endogenous sex steroid hormone milieu changes, such as across late pregnancy to postpartum or menopausal transition (Lokuge, Frey, Foster, Soares, & Steiner, 2011; Munk‐Olsen et al., 2006). This includes perinatal depression (PND), which according to DSM‐V criteria is defined as a depressive episode with onset during pregnancy or up to 4 weeks postpartum. Even though the current diagnostic classifications do not distinguish between antenatal and postnatal onset of PND, there is considerable evidence pointing to critical differences, for example in genetic risk factors, which are best characterized for postpartum onset (Elwood et al., 2019). Furthermore, depressive episodes manifesting later than 4 weeks postpartum may also have hormonal contributions in their pathophysiology and the risk for depressive episodes are increased up to 5 months postpartum (Munk‐Olsen et al., 2006). PND affects 10%–20% of postpartum mothers worldwide (Fellmeth, Fazel, & Plugge, 2017; Gavin et al., 2005; Howard et al., 2014). Notably, PND may not only affect new mothers but also can, especially if untreated, be adverse for their offspring in terms of infant language and early cognitive development (Evans et al., 2012) and future health (Pearson et al., 2013; Stein et al., 2014). PND frequently has an onset in late pregnancy (Meltzer‐Brody, Boschloo, Jones, Sullivan, & Penninx, 2013) and may worsen dramatically postpartum. Intriguingly, a large seminal Danish registry study demonstrated that for new mothers, the risk for developing a mental disorder which necessitates admission to psychiatric hospital or outpatient clinic peaks early postpartum at days 10 to 19 and for unipolar depression is sustained until 5 months after birth (Munk‐Olsen et al., 2006), which coincides with the dramatic postpartum decline in placenta‐produced hormones that are built up during pregnancy. However, PND frequently has an onset in late pregnancy. Likewise, non‐pathological manifestation of transient mental distress, postpartum blues, temporally coincides with the drop in placenta‐produced steroid hormones and heightens the risk for postpartum depressive episodes (O'Hara & Wisner, 2014). On the other hand, while new fathers also may experience depressive symptoms, severe adverse mental health responses to fatherhood that necessitates admission to psychiatric hospital are not increased 0–12 months postpartum (Munk‐Olsen et al., 2006). This emphasizes qualitative differences between mothers and fathers in the nature of postpartum/parenthood transition and its consequences, and further support hormonal contributions to perinatal mental disorders.
Menopausal transition is another female life phase where ovarian sex steroid hormone levels fluctuate dramatically. At this time, women face about two to fourfold increased risk for depressive episodes (Bromberger et al., 2011; Freeman, Sammel, Liu, et al., 2004). Interestingly, the strongest risk factor for developing depressive symptoms across menopausal transition is fluctuation of estradiol around the women's own mean level (Freeman, Sammel, Lin, & Nelson, 2006). Notably, at the time the postmenopausal state is fully established and estradiol no longer fluctuates, that risk decreases (Freeman et al., 2014). Also, in PND, sensitivity to estradiol fluctuations seemingly is central to risk. This was demonstrated in a small (N = 8*2) but seminal study by Bloch et al. (2000) showing that women with a history of postpartum depression were differentially sensitive to mood‐destabilizing effects of ovarian steroids, that is estradiol and progesterone particularly in the withdrawal phase but also present in a hormone‐stimulated phase (modelling pregnancy). Recent studies have pointed to molecular mechanisms, in terms of gene expression and epigenetic modifications, of such sensitivity to ovarian hormone changes, in particular estrogen sensitivity (Guintivano, Arad, Gould, Payne, & Kaminsky, 2014; Mehta et al., 2014). Finally, the ovarian hormone sensitivity hypothesis is indirectly strengthened by observations in women with premenstrual dysphoric disorder (PMDD) who indeed develop depressive symptoms when exposed to ovarian steroid hormone replacement after a period of hormonal suppression (Schmidt et al., 2017), which again seem to involve epigenetic mechanisms related to estradiol (Marrocco et al., 2018).
With the pharmacological gonadotropin‐releasing hormone agonist (GnRHa) risk model we wanted to illuminate how hormone transitions increase the risk for triggering depressive episodes, and in particular we were interested in the role of estradiol dynamics. The GnRHa model setup we applied introduces a biphasic estradiol fluctuation (initially a stimulation and subsequently a suppression of the hypothalamus–pituitary–gonadal [HPG] axis), which enabled us to focus at the estradiol fluctuation‐driven changes and their coupling to early depressive‐like symptoms in certain susceptible women, a point of strength over previous study designs, which have more focused at the late suppression phase in recovered patients (Bloch et al., 2000).
2. THE GnRHa TRIAL SET‐UP
A detailed description of the trial and study population is available in Frokjaer et al. (2015). The study is registered at ClinicalTrials.gov (ID: NCT02661789). A brief overview is presented below.
2.1. GnRHa is a pharmacological tool to manipulate sex steroids
Pharmacologic intervention with continuous GnRHa, as can be obtained by an implant, induces a biphasic ovarian hormone response (Thomas, Jenkins, Lenton, & Cooke, 1986); after an initial stimulation of the HPG axis, pituitary GnRHa receptors desensitize, and consequently, ovarian sex steroid production is suppressed to menopausal levels within about 10 to 14 days and is sustained for 28 days. The GnRHa model thus mimics hormonal fluctuations and best matches the menopausal transition stage (Harlow et al., 2012) and reflects partly the physiological changes across the prepartum to postpartum transition where placenta‐produced hormones, including estradiol, built up in pregnancy decline rapidly from the high levels established during pregnancy.
2.2. Study participants
Sixty‐three healthy women (mean age 24.3 ± 4.9 years) were enrolled in this block randomized, placebo‐controlled, and double‐blinded intervention study. Two women could not complete the study program at follow‐up due to anovulation and pregnancy, respectively. Block randomization was performed to balance the distribution of 5‐HTTLPR genotype (LALA or not). All participants (mean age 24.3 ± 4.9 years) had regular menstrual cycles (duration 23–35 days). Participants were screened by face‐to‐face interview, gynecological ultrasound examinations, and blood tests to secure no significant neurological, psychiatric, endocrinological, or gynecological disorders.
2.3. Intervention
Baseline assessments were performed in the midfollicular phase (cycle day 6.6 ± 2.2) when ovarian hormone levels are most stable and time since the postovulatory estradiol drop is maximal. Contingent upon ovulation in their natural cycle, participants received a subcutaneous injection of a GnRHa implant (ZOLADEX, a biodegradable copolymer impregnated with 3.6 mg of goserelin; AstraZeneca, London, United Kingdom) (n = 30) or saline (n = 30), that is in the midluteal phase cycle day 22.6 ± 2.5, by a gynecologist not involved in any subsequent interaction with the participants, data collection, or analysis. This timing allowed a near‐identical timing of menstrual bleeding (placebo group) and withdrawal bleeding (GnRHa group), which enabled blinding. Follow‐up was placed post‐bleeding at a time point late enough to allow the GnRHa group to have entered their early ovarian suppression phase (16.2 ± 2.6 days after intervention). An overview of the timing of baseline, intervention, and follow‐up relative to the menstrual cycle is provided in Figure 1.
FIGURE 1.
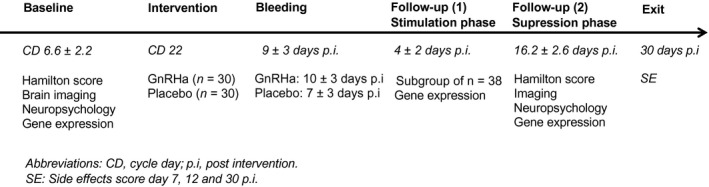
GnRHa model study design and timings
2.4. Outcomes
Data from the following domains of interest were collected at baseline and at follow‐up: (a) clinical outcomes (Hamilton depression rating scale (HDRS) of 17 items and self‐reported psychometrics), (b) serotonin transporter (SERT) brain binding ([11C]DASB PET‐scan), (c) cognitive processing of emotions (fMRI faces task), (d) reward processing (fMRI gambling task), (e) functional connectivity (resting‐state fMRI), (f) basic cognition (verbal affective memory and reaction time), and (g) gene expression and DNA methylation changes across intervention.
Also at baseline, self‐reported NEO Personality Inventory‐Revised personality trait scores were filled online to derive the neuroticism score. Neuroticism was considered relevant, since it is a robust risk factor for developing major depression that might interact with other risk factors in the interplay with markers of serotonin signaling as shown previously (Frokjaer, Vinberg, et al., 2010). Serial measurements of Profile of Mood States (POMS) were available across the intervention period as specified in Stenbaek et al. (2016).
For a subgroup of the latter 38 participants enrolled, an attempt was made to characterize the magnitude of ovarian hormone increase in the initial stimulatory phase of the GnRHa by adding hormones measurements at the day of intervention (midluteal phase) and 3 to 5 days after intervention (stimulated if GnRHa). At these time points, material for gene expression was also collected while DNA was only available from baseline and (late) follow‐up (Mehta et al., 2018).
3. CLINICAL OUTCOMES AND BRAIN SIGNATURES OF SEX ‐HORMONE MANIPULATIONS WITH GnRHa
The data presented below are published and discussed in detail in the corresponding original articles (Fisher et al., 2017; Frokjaer et al., 2015; Henningsson et al., 2015; Macoveanu et al., 2016; Mehta et al., 2018; Stenbaek et al., 2016, 2019). Here we provide a short synthesis and integrated discussion of the findings.
3.1. Summary of the GnRHa findings
As summarized in Table 1, GnRHa intervention induced depressive symptoms that approached the level of a mild depressive state in about 13% of the healthy women who participated in the study, while the remaining participants experienced more subtle or no depressive symptoms (Frokjaer et al., 2015). This number aligns well with the known frequencies of PND of around 10%–20% (Fellmeth et al., 2017; Gavin et al., 2005; Howard et al., 2014). Interestingly, only by observer‐dependent methods (semi‐structured Hamilton 17‐item interview) were the increased levels of depressive symptoms evident (Stenbaek et al., 2016). However, self‐reported serial day‐to‐day changes in total mood disturbances (by POMS) showed labile mood in the GnRHa group only in women with elevated levels of mood disturbances at baseline (Stenbaek et al., 2016). Further analyses showed that mood disturbances were most pronounced during day 0 to 5 corresponding to the early stimulation phase of the intervention and was dependent on neuroticism levels such that most extreme neuroticism scores (high or low) were most sensitive to GnRHa intervention relative to placebo (Stenbaek et al., 2019).
TABLE 1.
Main findings from the GnRHa clinical trial
| Article | N | Main outcomes | Main findings |
|---|---|---|---|
| Frokjaer et al. (2015) | 30 GnRHa |
Changes from baseline in:
|
GnRHa induced depressive symptoms (HDRS 17‐item) in about 13% relative to placebo |
| 30 placebo | |||
| No main effects of GnRHa versus placebo on serotonin transporter BPnd | |||
| The emergence of depressive symptoms was associated with both increased serotonin transporter binding in neocortex from baseline and with the magnitude of estradiol decrease | |||
| Henningsson et al. (2015) | 26 GnRHa |
Emotional faces fMRI
|
No main effects of GnRHa versus placebo |
| 29 placebo | Women who displayed larger GnRHa‐induced increase in depressive symptoms had a larger increase in both negative and positive emotion‐elicited activity in insula (anterior) | ||
| Macoveanu et al. (2016) | 26 GnRHa | Gambling fMRI, brain activation related to reward | GnRHa reduced activation reward‐related activation of amygdala, relative to placebo |
| 29 placebo | |||
| Stenbæk et al. (2016) | 31 GnRHa |
|
GnRHa was associated with slower reaction time and more labile mood relative to placebo |
| 30 placebo | |||
| No effects of GnRHa were seen on affective verbal memory | |||
| Fisher et al. (2017) | 29 GnRHa |
rs‐fMRI: functional connectivity (rs‐FC) for
|
No main effects of GnRHa versus placebo |
| 29 placebo | Women who displayed larger GnRHa‐induced increase in depressive symptoms had an increased amygdala–right temporal cortex rs‐FC and decreased hippocampus–cingulate rs‐FC | ||
| Mehta (2019) |
|
Of the a priori defined PND predictive set of 116 genes, 19% were differentially expressed post‐GnRHa and 49% were differentially methylated relative to placebo | |
| Within the GnRHa group, a large proportion of PND genes were significantly associated (gene expression; DNA methylation) with changes in depressive symptoms (28%; 66%), estradiol levels (49%; 66%), and neocortex serotonin transporter binding (8%; 45%) between baseline and later follow‐up | |||
| Stenbaek, Budtz‐Jorgensen, Pinborg, Jensen, and Frokjaer (2019) | 28 GnRHa |
|
GnRHa with and without concomitant infertility‐related stress heightened total mood disturbances most pronounced at the early stimulatory phase day 0–5 post‐GnRHa in a manner dependent on neuroticism scores |
| 27 placebo | |||
| 37 IVF‐GnRHa |
Molecular brain imaging data showed that depressive responses to GnRHa were coupled to increases in SERT binding in neocortex suggesting a transiently reduced synaptic serotonin level and suppressed serotonin signaling (Frokjaer et al., 2015). Functional MRI with tasks probing emotional face processing pointed to a coupling between depressive responses to GnRHa and an increased involvement of anterior insula in processing emotions irrespective of emotion valence (Henningsson et al., 2015). Furthermore, resting‐state fMRI suggested that GnRHa‐induced depressive symptoms are coupled to an overengagement of amygdala and a disengagement of hippocampus in non‐goal‐oriented cognitive processes (Fisher et al., 2017). Also, task‐based fMRI using a gambling paradigm showed that GnRHa reduces brain responses to reward (Macoveanu et al., 2015). Specifically, the amygdala, which putatively helps encode the stimulus reward value in reward processing and plays a key role in reward learning, was less engaged in processing positive of stimuli pre‐ to post‐GnRHa relative to placebo. Finally, in a very recent study we showed that an a priori defined set of gene transcripts, which were differentially expressed in third trimester in women who later developed PND with postpartum onset (Mehta et al., 2014), was also associated with depressive responses to GnRHa in a manner dependent on estradiol changes and SERT changes (Mehta et al., 2018).
4. DISCUSSION
4.1. Estradiol, serotonin brain signaling and brain function
Our GnRHa data provide direct evidence for sex hormone manipulation to trigger depressive symptoms in healthy volunteers. The depressive symptoms were subtle except in two to three participants who met the clinical criteria of a mild depressive state. The emergence of depressive symptoms was coupled to increases in SERT binding (which lowers synaptic serotonin), and was dependent on the biphasic estradiol fluctuation (Frokjaer et al., 2015). This implies transiently compromised serotonin signaling in the mechanisms by which sex steroid hormone fluctuations provoke depressive symptoms in susceptible individuals. Serotonergic brain signaling is ostensibly disturbed in individuals with MDD also with postpartum onset and traditionally constitutes a key target for pharmacological treatment (di Scalea & Wisner, 2009); however, treatment success is disappointing (Rush et al., 2006). Sex hormones target serotonergic neurons and shape the adult female brain during hormonal transition periods (Barth, Villringer, & Sacher, 2015). Thus it is likely that serotonin signaling is affected during changes in sex steroid hormone milieu. In particular, estradiol potently affects the key features of the serotonin signaling system (Bethea, Lu, Gundlah, & Streicher, 2002), that is synthesis, degradation, postsynaptic receptor distribution, including induction of the main regulator of synaptic serotonin, the SERT (Lu, Eshleman, Janowsky, & Bethea, 2003; Suda, Segi‐Nishida, Newton, & Duman, 2008; Sumner et al., 2007). Also, importantly seminal studies have pointed to a role of increased enzymatic degradation of monoamines in the brain (MAO‐A activity) in postpartum depressive symptoms (Sacher et al., 2010, 2015), which would further tend to compromise not only serotonergic signaling but also other monoamines, that is dopamine and noradrenaline. Pharmacological treatments of depressive episodes occurring in relation to peripartum traditionally target SERT, that is selective serotonin reuptake inhibitors (SSRIs; Cooper, Willy, Pont, & Ray, 2007). Nevertheless, our GnRHa results and that of others (Dowlati et al., 2017) support that the brain architecture of hormonal transition includes key targetable features beyond serotonin that ostensibly contributes to an increased risk for depressive episodes most likely linked to the estradiol withdrawal phase.
4.2. Neuroprotective properties of estradiol and estradiol sensitivity
We and others have shown that estradiol affects critical domains and key brain regions (e.g., the hippocampus) known to be dysfunctional in MDD (Barth et al., 2015, 2016; Comasco et al., 2014) which putatively is linked to neuroprotective features of estradiol. Such loss of neuroprotection may play a critical role specifically in estradiol withdrawal phases, as for example postpartum. Specifically, the GnRHa model highlights key regions in the reward circuit that are less engaged in response to positive stimuli when undergoing sex hormone manipulation as imaged in the ovarian hormone suppression phase, which may drive anhedonia in depressive episodes triggered by hormonal transitions. Also, in the same model, we have shown a disengagement of hippocampus (Fisher et al., 2017), and overengagement of brain networks recruited when processing emotional salient information (Henningsson et al., 2015).
Sex steroid hormones support neuroprotection through processes potentially driven by gene transcription and epigenetic mechanisms and are likely moderated by serotonergic brain signaling. Such steroid hormone‐driven processes may explain why pregnancy reorganizes brain structures in ways that, in healthy conditions, may prepare the brain for motherhood (Hoekzema et al., 2016). In particular, estrogen affects brain structure and function, including synaptic remodeling and neurogenesis (Yankova, Hart, & Woolley, 2001) and hippocampal plasticity (Barth et al., 2016). Estrogen replacement appears to have neuroprotective properties in animal models of early menopause (Sohrabji, 2005) and affects the primary serotonin receptor subtype 2A in brain cortex (Frokjaer, Erritzoe, et al., 2010; Kugaya et al., 2003; Moses‐Kolko et al., 2003) and the SERT (Comasco, Frokjaer, & Sundstrom‐Poromaa, 2014; Lu et al., 2003), which are key regulatory proteins in the system and markers of serotonergic wiring. Further human studies support a temporary neuroprotective effect of hormonal replacement in early menopause as reflected by increased hippocampal volumes (Lord, Buss, Lupien, & Pruessner, 2008) and improved cognitive function (i.e., verbal memory; Amin et al., 2006). Likewise, in healthy pregnancy, higher levels of estradiol are related to better verbal memory, which on the contrary is not the case for pregnant women who develop depressive symptoms (Hampson et al., 2015). Notably, a recent clinical trial supports that estradiol replacement in perimenopause protects against depressive episodes relative to placebo (Gordon et al., 2018); however no tools are yet available to personalize or time such preventive strategies during life course.
In PND, recent data suggest that estradiol sensitivity predisposes women to PND, which can be demonstrated with proxy markers for estrogen sensitivity derived from blood of pregnant women (i.e., DNA methylation Guintivano et al., 2014 and gene expression Mehta et al., 2014), thus constituting a candidate biomarker. Further strengthening the estradiol sensitivity hypothesis and the candidate biomarker, RNA and DNA material from the GnRHa study (Mehta et al., 2018) points to a link between these gene transcript PND markers (Mehta et al., 2014) and estradiol manipulation, which intriguingly predicts depressive responses. Importantly, this backtranslation from clinical PND biomarkers in pregnant cohorts to the GnRHa sex hormone manipulation preclinical human model further substantiates the estradiol sensitivity hypothesis of depressive episodes triggered by hormonal transitions across reproductive female life. Finally, the fact that we now can demonstrate an overlap between changes in gene expression and DNA methylation and psychometrics between the GnRHa elicited patterns and the independent clinical cohort of women with moderate to severe depressive episodes postpartum further validates the psychopathological importance of the phenomena triggered by the GnRHa manipulation. It also support GnRHa as a means of modelling mechanisms by which hormonal transition can trigger depressive episodes in certain sensitive women.
5. CLINICAL PERSPECTIVES AND FUTURE DIRECTIONS
Additional studies are needed to translate findings from the GnRHa model to clinically relevant groups of women. It is not yet known if disturbed serotonin signaling (Frokjaer et al., 2015), brain network recruitment (Henningsson et al., 2015; Macoveanu et al., 2016; Stenbaek et al., 2016), and functional connectivity (Fisher et al., 2017) translate to women who are sensitive to hormonal transitions, for example across the transition from natural pregnancy to early postpartum in women with a history of PND. This will be a critically needed step toward informing a stratified setup for prevention and clinical management of perinatal depression and to validate potential biomarkers, that is of estradiol sensitivity that may help identify such subgroups of women.
To counterbalance risk contributions from postpartum withdrawal from estradiol, transdermal estradiol has been suggested as a promising treatment option for PND (Moses‐Kolko, Berga, Kalro, Sit, & Wisner, 2009) supported by a convergence of epidemiological, preclinical, and clinical research, that is robust and rapid response to estradiol in some pilot PPD trials, few side effects, and minimal breast milk passage to the infant (Pinheiro, Bogen, Hoxha, & Wisner, 2016). However, a pilot clinical trial attempting to evaluate the effectiveness was disappointing (Wisner et al., 2015). Intriguingly, estradiol was administered at a late time point (up to 13 weeks postpartum) where a depressive episode had already been established (Wisner et al., 2015). It is not known if short‐term estradiol applied in the immediate postpartum, as a preventive strategy in a selected high‐risk group of women, may disrupt early risk mechanisms and protect maternal brain health. Other promising new PND treatments include allopregnanolone analogues, which have shown clinical efficacy; however, notably, at the same time, a large placebo effect was observed (Meltzer‐Brody et al., 2018). Importantly, robust evidence support heterogeneity of depressive episodes across the perinatal period (Putnam et al., 2017), which needs to be embraced by researchers and clinicians to fully exploit windows of opportunity for personalized prevention and treatment for the disorder(s) (Galea & Frokjaer, 2019). Notably, the GnRHa model suggests that both an increase in estradiol, which is pronounced in late pregnancy, and a subsequent withdrawal as seen postpartum contribute critically to offset brain biology and trigger depressive symptoms in susceptible women (Fisher et al., 2017; Frokjaer et al., 2015; Henningsson et al., 2015; Macoveanu et al., 2016; Stenbaek et al., 2016). Importantly, this may explain why the risk for depressive symptoms peaks in the early postpartum phase, that is, day 10–19 (Munk‐Olsen et al., 2006) where carryover effects from late pregnancy and effects of postpartum hormonal withdrawal add up. Again this may be worsened by the effects of MAO‐A activity further depleting monoamines, including serotonin in the early postpartum (Sacher et al., 2010, 2015). Intriguingly, this also raises the hypothesis that perinatal depression with onset in late pregnancy indeed have critical contributions from disturbed serotonin signaling that, however, may not be fully compensated by SSRI treatment when entering the postpartum withdrawal phase.
Risk mechanisms for developing depression identified by the GnRHa model may identify key features of a clinical relevant “hormone‐sensitive subgroup” of the broader category of MDD. Recent evidence from studies across menopausal transition supports the notion that hormonal transitions may cause depressive symptoms in hormone‐sensitive individuals: Estradiol fluctuations around menopausal transition are associated with first‐time onset of MDD (Freeman et al., 2006) and appears to be preventable by hormonal replacement (Gordon et al., 2018). Another remarkable and recent register‐based finding, which again links exposure to hormonal transitions with depression, has shown that starting on hormonal contraceptive is associated with an increased risk of developing a depressive episode (Skovlund et al., 2016; Zettermark, Perez Vicente, & Merlo, 2018). This finding has been replicated in an independent prospective cohort study (de Wit et al., 2019). It remains unclear why the use of oral contraceptives increases the risk of a depressive episode in some women, including to what extent suppression of endogenous estradiol may play a role, and if these women can be identified by risk markers.
The GnRHa model does not align as well with sex hormone dynamics putatively underlying premenstrual dysphoric mood disorder (PMDD) since the disorder seem to be linked to sensitivity to high premenstrual levels of the active metabolite of progesterone, allopreganolone (Lanza di Scalea & Pearlstein, 2019), that most likely does not vary substantially with short‐term GnRHa manipulation. However, allopregnanolone also may be linked to serotonin brain signaling (Sundstrom Poromaa et al., 2018). Accordingly, patients with PMDD appear to respond particularly well to cyclic SSRI treatment administered prior to menses and similar to inhibition of allopregnanolone (Bixo et al., 2017). Indeed, progesterone and estradiol dynamics may interact in their effects on mental health; for example as shown in women with borderline personality disorder high levels of progesterone may render a woman more sensitive to estradiol deviations (lower than the women usual mean) and elicit emotional and mood instability, impulsivity, irritability, and aggressive behaviors (Eisenlohr‐Moul, DeWall, Girdler, & Segerstrom, 2015), which also characterize PMDD. Those sex steroid hormone interactions are not captured with the GnRHa model, which also highlights why these complex phenomena also need to be studied in clinical cohorts.
Future studies evaluating preventive strategies in women at high risk for depressive episodes triggered by hormonal transitions and candidate biomarkers such as estrogen sensitivity, which may help stratify risk, are warranted. Pharmacologically, such strategies may target serotonergic signaling in late pregnancy and/or estradiol replacement in the immediate postpartum. Other strategies may include dietary supplies that can lower MAO‐A activity (Dowlati et al., 2017). In perimenopause, preventive strategies could include a precision medicine approach to identifying women who may benefit from hormonal replacement in combination with antidepressant treatments. Also in a long‐term perspective it needs to be determined if estrogen sensitivity markers based on gene transcription profiles or epigenetics (Guintivano et al., 2014; Mehta et al., 2014, 2018) can work outside of the highly stimulated state of late pregnancy, for example via estrogen or steroid hormone‐stimulated assays and whether such markers also identify women at risk for depressive episodes when exposed to other hormonal transitions; however, this is so far unexplored.
Clearly, joint efforts to facilitate replications across data sets and sites will be needed to validate potential risk stratification and biomarker tools (Freeman, Sammel, Rinaudo, & Sheng, 2004; Guintivano, Manuck, & Meltzer‐Brody, 2018) and to optimize risk and disease management to support mental health including affective cognitive functions, that is, across perimenopause and peripartum. Ideally, advancing the understanding of hormonal contributions to depressive episodes may also help fight stigma and be useful in psychoeducation to support patient engagement in preventive initiatives and treatment compliance; however, this remains to be tested in clinical trials.
6. CONCLUSION
The GnRHa model, given its placebo‐controlled design and through modeling the contributions from a biphasic estradiol fluctuation, allowed us to isolate the estradiol fluctuation‐driven changes and their coupling to early depressive‐like symptoms in certain susceptible women, a point of strength over previous study designs, which have focused at the late suppression phase.
Taken together the GnRHa model provides an important source of insights into the ways by which sex hormone fluctuations can trigger depressive episodes of great translational value. The model supports that both an increase in estradiol and a subsequent withdrawal contribute critically to brain architecture of risk for depression in susceptible women who display estradiol sensitivity at a molecular level. However, the relative contributions from estradiol increase phases and subsequent dramatic decreases are not clarified and, accordingly, not yet exploited in current risk or disease management.
Our data point to a distinct pathophysiology of depressive episodes related to hormonal transitions. If better understood, and if bridging with observations in clinical cohorts, this may provide a starting point for actual preventive and personalized treatment strategies of relevant intensity to be tested in future studies.
CONFLICT OF INTEREST
VGF declares that she has received honorarium as consultant for SAGE therapeutics.
Supporting information
Transparent Peer Review Report
Frokjaer VG. Pharmacological sex hormone manipulation as a risk model for depression. J Neurosci Res. 2020;98:1283–1292. 10.1002/jnr.24632
Edited by Cristina Ghiani. Reviewed by Lauren Osborne, Sophie Schweizer, and Julia Sacher.
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.24632.
REFERENCES
- Amin, Z. , Gueorguieva, R. , Cappiello, A. , Czarkowski, K. A. , Stiklus, S. , Anderson, G. M. , … Epperson, C. N. (2006). Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology, 31, 2489–2497. 10.1038/sj.npp.1301114 [DOI] [PubMed] [Google Scholar]
- Barth, C. , Steele, C. J. , Mueller, K. , Rekkas, V. P. , Arelin, K. , Pampel, A. , … Sacher, J. (2016). In‐vivo dynamics of the human hippocampus across the menstrual cycle. Scientific Reports, 6, 32833 10.1038/srep32833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, C. , Villringer, A. , & Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9, 37 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea, C. L. , Lu, N. Z. , Gundlah, C. , & Streicher, J. M. (2002). Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinology., 23, 41–100. 10.1006/frne.2001.0225 [DOI] [PubMed] [Google Scholar]
- Bixo, M. , Ekberg, K. , Poromaa, I. S. , Hirschberg, A. L. , Jonasson, A. F. , Andreen, L. , … Backstrom, T. (2017). Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010)‐A randomized controlled trial. Psychoneuroendocrinology, 80, 46–55. 10.1016/j.psyneuen.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Bloch, M. , Schmidt, P. J. , Danaceau, M. , Murphy, J. , Nieman, L. , & Rubinow, D. R. (2000). Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry, 157, 924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Bromberger, J. T. , Kravitz, H. M. , Chang, Y. F. , Cyranowski, J. M. , Brown, C. , & Matthews, K. A. (2011). Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN). Psychological Medicine, 41, 1879–1888. 10.1017/S003329171100016X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco, E. , Frokjaer, V. G. , & Sundstrom‐Poromaa, I. (2014). Functional and molecular neuroimaging of menopause and hormone replacement therapy. Frontiers in Neuroscience, 8, 388 10.3389/fnins.2014.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, W. O. , Willy, M. E. , Pont, S. J. , & Ray, W. A. (2007). Increasing use of antidepressant in pregnancy. American Journal of Obstetrics and Gynecology, 196, 544–545. [DOI] [PubMed] [Google Scholar]
- de Wit, A. E. , Booij, S. H. , Giltay, E. J. , Joffe, H. , Schoevers, R. A. , & Oldehinkel, A. J. (2019). Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry, 77(1), 52 10.1001/jamapsychiatry.2019.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Scalea, T. L. , & Wisner, K. L. (2009). Pharmacotherapy of postpartum depression. Expert Opinion on Pharmacotherapy, 10, 2593–2607. 10.1517/14656560903277202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati, Y. , Ravindran, A. V. , Segal, Z. V. , Stewart, D. E. , Steiner, M. , & Meyer, J. H. (2017). Selective dietary supplementation in early postpartum is associated with high resilience against depressed mood. Proceedings of the National Academy of Sciences of the United States of America, 114, 3509–3514. 10.1073/pnas.1611965114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr‐Moul, T. A. , DeWall, C. N. , Girdler, S. S. , & Segerstrom, S. C. (2015). Ovarian hormones and borderline personality disorder features: Preliminary evidence for interactive effects of estradiol and progesterone. Biological Psychology, 109, 37–52. 10.1016/j.biopsycho.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood, J. , Murray, E. , Bell, A. , Sinclair, M. , Kernohan, W. G. , & Stockdale, J. (2019). A systematic review investigating if genetic or epigenetic markers are associated with postnatal depression. Journal of Affective Disorders, 253, 51–62. 10.1016/j.jad.2019.04.059 [DOI] [PubMed] [Google Scholar]
- Evans, J. , Melotti, R. , Heron, J. , Ramchandani, P. , Wiles, N. , Murray, L. , & Stein, A. (2012). The timing of maternal depressive symptoms and child cognitive development: A longitudinal study. Journal of Child Psychology and Psychiatry, 53, 632–640. 10.1111/j.1469-7610.2011.02513.x [DOI] [PubMed] [Google Scholar]
- Fellmeth, G. , Fazel, M. , & Plugge, E. (2017). Migration and perinatal mental health in women from low‐ and middle‐income countries: A systematic review and meta‐analysis. BJOG: An International Journal of Obstetrics and Gynaecology, 124, 742–752. 10.1111/1471-0528.14184 [DOI] [PubMed] [Google Scholar]
- Fisher, P. M. , Larsen, C. B. , Beliveau, V. , Henningsson, S. , Pinborg, A. , Holst, K. K. , … Frokjaer, V. G. (2017). Pharmacologically induced sex hormone fluctuation effects on resting‐state functional connectivity in a risk model for depression: A randomized trial. Neuropsychopharmacology, 42, 446–453. 10.1038/npp.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, E. W. , Sammel, M. D. , Boorman, D. W. , & Zhang, R. (2014). Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry, 71, 36–43. 10.1001/jamapsychiatry.2013.2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, E. W. , Sammel, M. D. , Lin, H. , & Nelson, D. B. (2006). Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry, 63, 375–382. 10.1001/archpsyc.63.4.375 [DOI] [PubMed] [Google Scholar]
- Freeman, E. W. , Sammel, M. D. , Liu, L. , Gracia, C. R. , Nelson, D. B. , & Hollander, L. (2004). Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry, 61, 62–70. 10.1001/archpsyc.61.1.62 [DOI] [PubMed] [Google Scholar]
- Freeman, E. W. , Sammel, M. D. , Rinaudo, P. J. , & Sheng, L. (2004). Premenstrual syndrome as a predictor of menopausal symptoms. Obstetrics and Gynecology, 103, 960–966. 10.1097/01.AOG.0000124804.81095.7f [DOI] [PubMed] [Google Scholar]
- Frokjaer, V. G. , Erritzoe, D. , Juul, A. , Nielsen, F. A. , Holst, K. , Svarer, C. , … Knudsen, G. M. (2010). Endogenous plasma estradiol in healthy men is positively correlated with cerebral cortical serotonin 2A receptor binding. Psychoneuroendocrinology, 35, 1311–1320. 10.1016/j.psyneuen.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Frokjaer, V. G. , Pinborg, A. , Holst, K. K. , Overgaard, A. , Henningsson, S. , Heede, M. , … Knudsen, G. M. (2015). Role of serotonin transporter changes in depressive responses to sex‐steroid hormone manipulation: A positron emission tomography study. Biological Psychiatry, 78, 534–543. 10.1016/j.biopsych.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Frokjaer, V. G. , Vinberg, M. , Erritzoe, D. , Baare, W. , Holst, K. K. , Mortensen, E. L. , … Knudsen, G. M. (2010). Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology, 35, 1129–1137. 10.1038/npp.2009.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea, L. A. M. , & Frokjaer, V. G. (2019). Perinatal depression: Embracing variability toward better treatment and outcomes. Neuron, 102, 13–16. 10.1016/j.neuron.2019.02.023 [DOI] [PubMed] [Google Scholar]
- Gandal, M. J. , Haney, J. R. , Parikshak, N. N. , Leppa, V. , Ramaswami, G. , Hartl, C. , … Geschwind, D. H. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science, 359, 693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, N. I. , Gaynes, B. N. , Lohr, K. N. , Meltzer‐Brody, S. , Gartlehner, G. , & Swinson, T. (2005). Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology, 106, 1071–1083. 10.1097/01.AOG.0000183597.31630.db [DOI] [PubMed] [Google Scholar]
- Gordon, J. L. , Rubinow, D. R. , Eisenlohr‐Moul, T. A. , Xia, K. , Schmidt, P. J. , & Girdler, S. S. (2018). Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: A randomized clinical trial. JAMA Psychiatry, 75, 149–157. 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano, J. , Arad, M. , Gould, T. D. , Payne, J. L. , & Kaminsky, Z. A. (2014). Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Molecular Psychiatry, 19, 560–567. 10.1038/mp.2013.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano, J. , Manuck, T. , & Meltzer‐Brody, S. (2018). Predictors of postpartum depression: A comprehensive review of the last decade of evidence. Clinical Obstetrics and Gynecology, 61(3), 591–603. 10.1097/GRF.0000000000000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, E. , Phillips, S. D. , Duff‐Canning, S. J. , Evans, K. L. , Merrill, M. , Pinsonneault, J. K. , … Steiner, M. (2015). Working memory in pregnant women: Relation to estrogen and antepartum depression. Hormones and Behavior, 74, 218–227. 10.1016/j.yhbeh.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, S. D. , Gass, M. , Hall, J. E. , Lobo, R. , Maki, P. , Rebar, R. W. , … de Villiers, T. J. (2012). Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Journal of Clinical Endocrinology and Metabolism, 97, 1159–1168. 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson, S. , Madsen, K. H. , Pinborg, A. , Heede, M. , Knudsen, G. M. , Siebner, H. R. , & Frokjaer, V. G. (2015). Role of emotional processing in depressive responses to sex‐hormone manipulation: A pharmacological fMRI study. Translational Psychiatry, 5, e688 10.1038/tp.2015.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema, E. , Barba‐Müller, E. , Pozzobon, C. , Picado, M. , Lucco, F. , García‐García, D. , … Vilarroya, O. (2016). Pregnancy leads to long‐lasting changes in human brain structure. Nature Neuroscience, 20, 287–296. 10.1038/nn.4458 [DOI] [PubMed] [Google Scholar]
- Howard, L. M. , Molyneaux, E. , Dennis, C. L. , Rochat, T. , Stein, A. , & Milgrom, J. (2014). Non‐psychotic mental disorders in the perinatal period. Lancet, 384, 1775–1788. 10.1016/S0140-6736(14)61276-9 [DOI] [PubMed] [Google Scholar]
- Kugaya, A. , Epperson, C. N. , Zoghbi, S. , van Dyck, C. H. , Hou, Y. , Fujita, M. , … Innis, R. B. (2003). Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. American Journal of Psychiatry, 160, 1522–1524. [DOI] [PubMed] [Google Scholar]
- Lanza di Scalea, T. , & Pearlstein, T. (2019). Premenstrual dysphoric disorder. Medical Clinics of North America, 103, 613–628. 10.1016/j.mcna.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Lokuge, S. , Frey, B. N. , Foster, J. A. , Soares, C. N. , & Steiner, M. (2011). Depression in women: Windows of vulnerability and new insights into the link between estrogen and serotonin. Journal of Clinical Psychiatry, 72, e1563–e1569. 10.4088/JCP.11com07089 [DOI] [PubMed] [Google Scholar]
- Lord, C. , Buss, C. , Lupien, S. J. , & Pruessner, J. C. (2008). Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiology of Aging, 29, 95–101. 10.1016/j.neurobiolaging.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Lu, N. Z. , Eshleman, A. J. , Janowsky, A. , & Bethea, C. L. (2003). Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular Psychiatry, 8, 353–360. 10.1038/sj.mp.4001243 [DOI] [PubMed] [Google Scholar]
- Macoveanu, J. , Henningsson, S. , Pinborg, A. , Jensen, P. , Knudsen, G. M. , Frokjaer, V. G. , & Siebner, H. R. (2015). Sex‐steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology, 41(4), 1057–1065. 10.1038/npp.2015.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoveanu, J. , Henningsson, S. , Pinborg, A. , Jensen, P. , Knudsen, G. M. , Frokjaer, V. G. , & Siebner, H. R. (2016). Sex‐steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology, 41, 1057–1065. 10.1038/npp.2015.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco, J. , Einhorn, N. R. , Petty, G. H. , Li, H. , Dubey, N. , Hoffman, J. , … McEwen, B. S. (2018). Epigenetic intersection of BDNF Val66Met genotype with premenstrual dysphoric disorder transcriptome in a cross‐species model of estradiol add‐back. Molecular Psychiatry, 25(3), 572–583. 10.1038/s41380-018-0274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, D. , Newport, D. J. , Frishman, G. , Kraus, L. , Rex‐Haffner, M. , Ritchie, J. C. , … Binder, E. B. (2014). Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychological Medicine, 44(11), 2309–2322. 10.1017/S0033291713003231 [DOI] [PubMed] [Google Scholar]
- Mehta, D. , Rex‐Haffner, M. , Sondergaard, H. B. , Pinborg, A. , Binder, E. B. , & Frokjaer, V. G. (2018). Evidence for oestrogen sensitivity in perinatal depression: Pharmacological sex hormone manipulation study. British Journal of Psychiatry, 215(3), 519–527. 10.1192/bjp.2018.234 [DOI] [PubMed] [Google Scholar]
- Meltzer‐Brody, S. , Boschloo, L. , Jones, I. , Sullivan, P. F. , & Penninx, B. W. (2013). The EPDS‐Lifetime: Assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Archives of Women's Mental Health, 16, 465–473. 10.1007/s00737-013-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer‐Brody, S. , Colquhoun, H. , Riesenberg, R. , Epperson, C. N. , Deligiannidis, K. M. , Rubinow, D. R. , … Kanes, S. (2018). Brexanolone injection in post‐partum depression: Two multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet, 392, 1058–1070. 10.1016/S0140-6736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- Moses‐Kolko, E. L. , Berga, S. L. , Greer, P. J. , Smith, G. , Cidis Meltzer, C. , & Drevets, W. C. (2003). Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertility and Sterility, 80, 554–559. 10.1016/S0015-0282(03)00973-7 [DOI] [PubMed] [Google Scholar]
- Moses‐Kolko, E. L. , Berga, S. L. , Kalro, B. , Sit, D. K. , & Wisner, K. L. (2009). Transdermal estradiol for postpartum depression: A promising treatment option. Clinical Obstetrics and Gynecology, 52, 516–529. 10.1097/GRF.0b013e3181b5a395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk‐Olsen, T. , Laursen, T. M. , Pedersen, C. B. , Mors, O. , & Mortensen, P. B. (2006). New parents and mental disorders: A population‐based register study. JAMA, 296, 2582–2589. 10.1001/jama.296.21.2582 [DOI] [PubMed] [Google Scholar]
- O'Hara, M. W. , & Wisner, K. L. (2014). Perinatal mental illness: Definition, description and aetiology. Best Practice & Research Clinical Obstetrics & Gynaecology, 28, 3–12. 10.1016/j.bpobgyn.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, R. M. , Fernyhough, C. , Bentall, R. , Evans, J. , Heron, J. , Joinson, C. , … Lewis, G. (2013). Association between maternal depressogenic cognitive style during pregnancy and offspring cognitive style 18 years later. American Journal of Psychiatry, 170, 434–441. 10.1176/appi.ajp.2012.12050673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro, E. , Bogen, D. L. , Hoxha, D. , & Wisner, K. L. (2016). Transdermal estradiol treatment during breastfeeding: Maternal and infant serum concentrations. Archives of Women's Mental Health, 19, 409–413. 10.1007/s00737-015-0532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, K. T. , Wilcox, M. , Robertson‐Blackmore, E. , Sharkey, K. , Bergink, V. , Munk‐Olsen, T. , … Meltzer‐Brody, S. (2017). Clinical phenotypes of perinatal depression and time of symptom onset: Analysis of data from an international consortium. The Lancet Psychiatry, 4(6), 477–485. 10.1016/S2215-0366(17)30136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush, A. J. , Trivedi, M. H. , Wisniewski, S. R. , Nierenberg, A. A. , Stewart, J. W. , Warden, D. , … Fava, M. (2006). Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry, 163, 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Sacher, J. , Rekkas, P. V. , Wilson, A. A. , Houle, S. , Romano, L. , Hamidi, J. , … Meyer, J. H. (2015). Relationship of monoamine oxidase‐A distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology, 40, 429–435. 10.1038/npp.2014.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher, J. , Wilson, A. A. , Houle, S. , Rusjan, P. , Hassan, S. , Bloomfield, P. M. , … Meyer, J. H. (2010). Elevated brain monoamine oxidase A binding in the early postpartum period. Archives of General Psychiatry, 67, 468–474. 10.1001/archgenpsychiatry.2010.32 [DOI] [PubMed] [Google Scholar]
- Schmidt, P. J. , Martinez, P. E. , Nieman, L. K. , Koziol, D. E. , Thompson, K. D. , Schenkel, L. , … Rubinow, D. R. (2017). Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. American Journal of Psychiatry, 174, 980–989. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann, G. , Binder, E. B. , Holte, A. , de Kloet, E. R. , Oedegaard, K. J. , Robbins, T. W. , … Wittchen, H. U. (2014). Stratified medicine for mental disorders. European Neuropsychopharmacology, 24, 5–50. 10.1016/j.euroneuro.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Skovlund, C. W. , Morch, L. S. , Kessing, L. V. , & Lidegaard, O. (2016). Association of hormonal contraception with depression. JAMA Psychiatry, 73, 1154–1162. 10.1001/jamapsychiatry.2016.2387 [DOI] [PubMed] [Google Scholar]
- Sohrabji, F. (2005). Estrogen: A neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Annals of the New York Academy of Sciences, 1052, 75–90. 10.1196/annals.1347.006 [DOI] [PubMed] [Google Scholar]
- Stein, A. , Pearson, R. M. , Goodman, S. H. , Rapa, E. , Rahman, A. , McCallum, M. , … Pariante, C. M. (2014). Effects of perinatal mental disorders on the fetus and child. Lancet, 384, 1800–1819. 10.1016/S0140-6736(14)61277-0 [DOI] [PubMed] [Google Scholar]
- Stenbaek, D. S. , Budtz‐Jorgensen, E. , Pinborg, A. , Jensen, P. S. , & Frokjaer, V. G. (2019). Neuroticism modulates mood responses to pharmacological sex hormone manipulation in healthy women. Psychoneuroendocrinology, 99, 251–256. 10.1016/j.psyneuen.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Stenbaek, D. S. , Fisher, P. M. , Budtz‐Jorgensen, E. , Pinborg, A. , Hjordt, L. V. , Jensen, P. S. , … Frokjaer, V. G. (2016). Sex hormone manipulation slows reaction time and increases labile mood in healthy women. Psychoneuroendocrinology, 68, 39–46. 10.1016/j.psyneuen.2016.02.023 [DOI] [PubMed] [Google Scholar]
- Suda, S. , Segi‐Nishida, E. , Newton, S. S. , & Duman, R. S. (2008). A postpartum model in rat: Behavioral and gene expression changes induced by ovarian steroid deprivation. Biological Psychiatry, 64, 311–319. 10.1016/j.biopsych.2008.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, B. E. H. , Grant, K. E. , Rosie, R. , Hegele‐Hartung, C. , Fritzemeier, K.‐H. , & Fink, G. (2007). Raloxifene blocks estradiol induction of the serotonin transporter and 5‐hydroxytryptamine2A receptor in female rat brain. Neuroscience Letters, 417(1), 95–99. 10.1016/j.neulet.2007.02.039 [DOI] [PubMed] [Google Scholar]
- Sundstrom Poromaa, I. , Comasco, E. , Backstrom, T. , Bixo, M. , Jensen, P. , & Frokjaer, V. G. (2018). Negative association between allopregnanolone and cerebral serotonin transporter binding in healthy women of fertile age. Frontiers in Psychology, 9, 2767 10.3389/fpsyg.2018.02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar, A. , Collishaw, S. , Pine, D. S. , & Thapar, A. K. (2012). Depression in adolescence. Lancet, 379, 1056–1067. 10.1016/S0140-6736(11)60871-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, E. J. , Jenkins, J. , Lenton, E. A. , & Cooke, I. D. (1986). Endocrine effects of goserelin, a new depot luteinising hormone releasing hormone agonist. British Medical Journal, 293, 1407–1408. 10.1136/bmj.293.6559.1407-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2008). The global burden of disease: 2004 update. Geneva, Switzerland: Author. [Google Scholar]
- Wisner, K. L. , Sit, D. K. , Moses‐Kolko, E. L. , Driscoll, K. E. , Prairie, B. A. , Stika, C. S. , … Wisniewski, S. R. (2015). Transdermal estradiol treatment for postpartum depression: A pilot randomized trial. Journal of Clinical Psychopharmacology, 35, 389–395. 10.1097/JCP.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova, M. , Hart, S. A. , & Woolley, C. S. (2001). Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: A serial electron‐microscopic study. Proceedings of the National Academy of Sciences of the United States of America, 98, 3525–3530. 10.1073/pnas.051624598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettermark, S. , Perez Vicente, R. , & Merlo, J. (2018). Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: A pharmacoepidemiological study on 800 000 Swedish women. PLoS ONE, 13, e0194773 10.1371/journal.pone.0194773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparent Peer Review Report


