Abstract
Globally, in 2017 35 million people were living with HIV (PLHIV) and 257 million had chronic HBV infection (HBsAg positive). The extent of HIV‐HBsAg co‐infection is unknown. We undertook a systematic review to estimate the global burden of HBsAg co‐infection in PLHIV. We searched MEDLINE, Embase and other databases for published studies (2002‐2018) measuring prevalence of HBsAg among PLHIV. The review was registered with PROSPERO (#CRD42019123388). Populations were categorized by HIV‐exposure category. The global burden of co‐infection was estimated by applying regional co‐infection prevalence estimates to UNAIDS estimates of PLHIV. We conducted a meta‐analysis to estimate the odds of HBsAg among PLHIV compared to HIV‐negative individuals. We identified 506 estimates (475 studies) of HIV‐HBsAg co‐infection prevalence from 80/195 (41.0%) countries. Globally, the prevalence of HIV‐HBsAg co‐infection is 7.6% (IQR 5.6%‐12.1%) in PLHIV, or 2.7 million HIV‐HBsAg co‐infections (IQR 2.0‐4.2). The greatest burden (69% of cases; 1.9 million) is in sub‐Saharan Africa. Globally, there was little difference in prevalence of HIV‐HBsAg co‐infection by population group (approximately 6%‐7%), but it was slightly higher among people who inject drugs (11.8% IQR 6.0%‐16.9%). Odds of HBsAg infection were 1.4 times higher among PLHIV compared to HIV‐negative individuals. There is therefore, a high global burden of HIV‐HBsAg co‐infection, especially in sub‐Saharan Africa. Key prevention strategies include infant HBV vaccination, including a timely birth‐dose. Findings also highlight the importance of targeting PLHIV, especially high‐risk groups for testing, catch‐up HBV vaccination and other preventative interventions. The global scale‐up of antiretroviral therapy (ART) for PLHIV using a tenofovir‐based ART regimen provides an opportunity to simultaneously treat those with HBV co‐infection, and in pregnant women to also reduce mother‐to‐child transmission of HBV alongside HIV.
Keywords: co‐infection, hepatitis B, HIV, systematic review, viral hepatitis
Abbreviations
- ART
antiretroviral therapy
- CHB
chronic hepatitis B
- CI
confidence interval
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- MSM
men who have sex with men
- PLHIV
people are living with HIV
- PWID
people who inject drugs
1. INTRODUCTION
Chronic hepatitis B (CHB) infection, defined as persistence of hepatitis B surface antigen (HBsAg), is a major public health problem resulting in an estimated 900 000 deaths in 2015.1, 2, 3, 4 Although HBV can be prevented with vaccination, in 2015, there were an estimated 257 million persons chronically infected.4 Between 20 and 30% of those with chronic infection develop complications, mainly cirrhosis and hepatocellular carcinoma (HCC).5 CHB accounts for 43% of cases of HCC and 40% of cirrhosis, with much higher proportions in lower middle‐income countries,4 and 5%‐10% of liver transplants in high‐income countries.6 Age is a key determinant of the risk of chronic infection: chronicity is common following acute infection in neonates (around 90%) and young children under the age of 5 years (20–60%), but occurs rarely (<5%) when infection is acquired in adulthood.7, 8 Worldwide, most persons with CHB were infected at birth or in early childhood.9 The highest prevalence of HBsAg (>5%) is in sub‐Saharan Africa, East Asia, parts of Balkans, the Pacific Islands and the Amazon Basin.10 Regional variation exists in the epidemiology of HBV: perinatal or horizontal transmission predominates in sub‐Saharan Africa and Asia, whereas in high‐income countries transmission is predominantly via injection drug use and high‐risk sexual behaviours.9, 11
As PLHIV live longer due to increased access to antiretroviral therapies, liver disease has emerged as a leading cause of death in PLHIV co‐infected with HBV or HCV.12, 13 Among people with HBsAg, co‐infection with HIV results in higher rates of chronicity and occult HBV (HBV‐DNA positivity in the absence of HBsAg), accelerated liver disease progression, higher liver‐related mortality and decreased treatment response.14, 15, 16, 17 Co‐infection with CHB also increases risk of hepatotoxicity from antiretroviral therapy (ART) three‐ to five‐fold,18, 19 and cross‐resistance between HIV and HBV drugs is common.20, 21 Fortunately, tenofovir, a drug commonly included in ART regimens, is also the most effective drug for long‐term treatment of HBV, leading to long‐term HBV viral suppression, reversal of cirrhosis and fibrosis, and reduction in HBV‐related mortality.22
There is a need to establish the global burden of HBsAg co‐infection among PLHIV, to characterize the most affected populations and geographical regions, and to inform national and regional screening programmes and clinical management. However, to date, no review has estimated the global burden of HBV co‐infection among PLHIV. Existing estimates suggest approximately 10% of PLHIV have chronic hepatitis B or 2‐4 million people, but were based on small numbers of studies with unclear methodology.17, 23, 24 Other reviews have focussed on specific regions25 or people who inject drugs (PWID).11, 26 We therefore undertook a global systematic review of the prevalence and burden of HBsAg in PLHIV.
2. METHODS
2.1. Search strategy and selection criteria
The systematic review was conducted alongside a companion review examining prevalence and burden of HIV‐HCV antibody co‐infection (consistent with current or past infection) which contains detailed description of the search and synthesis methods.27 The review was registered with the PROSPERO prospective register of systematic reviews (CRD42019123388).
In brief, we searched eight databases for material that reported prevalence of HBV and HIV, published between 1 January 2002 and 8 April 2018 following PRISMA guidelines.28 The searches were carried out in MEDLINE, EMBASE, CINAHL+, POPLINE, Africa‐wide Information, Global Health, Web of Science, and the Cochrane Library, Index Medicus of the Eastern Mediterranean Region, Index Medicus of the South‐East Asian Region, LILACS and Western Pacific Region Index Medicus. All English and non‐English language sources were included. The search terms used were as follows: ‘HIV OR Human immunodeficiency virus’ and ‘hepatitis‐B OR hepatitis C OR HBV OR HCV’ and ‘prevalen* OR inciden* OR seroprevalen* OR screening OR surveillance OR population* OR survey* OR epidem* OR data collection OR population sample* OR community survey* OR cohort OR cross‐sectional OR longitud* OR follow‐up’. Searches were tailored to the search functionality of each database. The reference lists of articles identified as reviews were screened for additional relevant sources.
We included papers with country‐level estimates of HBsAg co‐infection among an HIV population sample greater than 50, recruited based on their HIV‐positive status or other behavioural characteristics, such as injecting drug use. We excluded editorials or reviews containing no primary data, samples recruited based on their HBsAg status or HIV‐HBsAg‐positive status; studies based on self‐reported HIV or HBsAg status, hospital‐based studies, or in healthcare workers, organ or tissue donors, or from populations with other co‐morbidities such as persons with TB or mental illness, or undergoing interventions that put them at greater risk of co‐infection, including those receiving haemodialysis, those with haemophilia, cancer, cardiovascular disease, other co‐infections, kidney, liver or neurological diseases.
2.2. Screening and data extraction
Six reviewers (CF, CM, BM, AT, JS and JO) screened each record with a seventh reviewer (LP) consulted when there was no consensus. Data extracted included the following: study methods; field‐work dates; population; recruitment site; sample size; diagnostic assays used; and prevalence of co‐infection. For approximately 10% of included studies, data were double extracted by a second author (EG) to check the accuracy of data extraction.
2.3. Quality assessment
To address concerns of variable quality of studies in previous reviews, we assessed and rated the quality of included studies based on study design and assay quality (Supporting information S1). Studies with larger sample sizes, recruited from multiple sites, recording age and sex or HIV risk factors were scored higher (A); studies with >200 cases from >1 site, with some HIV risk factors recorded but not designed to measure prevalence was scored lower (B); and studies with <200 case from a single site with no risk factors recorded were scored lowest (C). HBsAg assay methods were rated from 0 where no assay type was specified, to up to 3 where a second confirmatory HBsAg assay was done, with or without a neutralisation step. Best estimates were selected for each population group per country based on the highest assay and study design score. Where multiple estimates were available, we applied decision rules to select the best estimate (Supporting information S1).
2.4. Classification of countries according to Global Burden of Disease region
Countries were initially grouped according to the 21 Global Burden of Disease regions, and these were then combined into ten regions to be consistent with previous published reviews on HIV, HBsAg and HCV burden.11, 27, 29
2.5. Definition of Population groups
We extracted data on risk behaviours associated with HIV and HBV transmission and populations were categorized according to their main HIV‐exposure categories. A general population sample was considered to be low‐risk and included samples of blood donors (unpaid), ante‐natal clinic attendees or general population and household surveys not recruited based on HIV‐positive status. Samples of PLHIV reporting heterosexual transmission as the main risk factor and HIV‐positive pregnant women were grouped together. We categorized study populations as PWID when >75% of the sample had current or past experience of injecting, and as men who have sex with men (MSM) when >50% reported main HIV exposure to be sex with men. The PWID and MSM population categories included studies of both known PLHIV as well as populations recruited based on their risk behaviour but where HIV testing was also done. Two other population groups included the following: high‐risk populations (PLHIV reporting any injecting drug use or sex between men (but ≤75% of the sample for PWID and ≤50% for MSM), sex workers; prison inmates, non‐injecting drug users, STI clinic attendees or a mixed population engaging in sexual and/or injecting risk behaviours but with ≤75% of the sample injecting); and children and young people (aged between two months and 17 years).
2.6. Data synthesis
We report HIV‐HBsAg co‐infection prevalence among six population groups (general population, heterosexual and pregnant women, PWID, MSM, children and young people, and other high‐risk populations) by country and region, reporting the best estimate and range for each country from all studies. Global and regional estimates of prevalence were derived from the median of the ‘best’ estimates for that region and presented alongside the interquartile range (IQR) of the best estimates. Data were entered into R (R Foundation for Statistical Computing, Vienna, Austria) to generate maps presenting country‐level HIV‐HBV co‐infection prevalence estimates.
Across these six populations, we also synthesized estimates of HBsAg prevalence in PLHIV and HIV‐negative populations where samples were recruited based on population characteristic rather than known HIV status and undertook a meta‐analysis of the odds of being HBsAg positive among HIV‐positive populations compared to HIV‐negative populations stratified by population group. A standard correction of 0.5 was added to all zero prevalence estimates using STATA 14.1 (Stata Corp). Odds ratios were calculated through a Mantel‐Haenszel method with a random‐effect model. Meta‐analyses of sub‐groups are presented as forest plots including the odds ratio and 95% confidence interval (CI).
Finally, we report global and regional estimates of burden of HBsAg co‐infection among PLHIV in 2017. Using number of persons with HIV infection by country and region estimated through the Spectrum model and reported by the Joint United Nations Programmes on HIV/AIDS (UNAIDS),30, 31 we applied median best estimates of HBsAg co‐infection prevalence among PLHIV not exposed via injecting drug use from all surveys included from the literature search for MSM, general population and HIV‐positive samples of pregnant women or those heterosexually exposed by regions, and overall. Median best estimates of HBsAg co‐infection prevalence among HIV‐positive PWID were applied to the distribution of PLHIV exposed via injecting drug use, as estimated by UNODC, across regions and overall.
2.7. Role of the funding source
The WHO commissioned this review for the purpose of informing the update of the WHO guidelines on testing for viral hepatitis.22, 23 The funder contributed to the data collection, analysis, interpretation and writing of the review. All authors had full access to the study data and share final responsibility for the findings submitted for publication. The full dataset and statistical source code used to generate estimates and select best estimates is available from the corresponding author on request.
3. RESULTS
Figure 1 summarizes the flow chart for the identification and selection of studies. From an overall 44 547 publication references, 475 papers/studies met the inclusion criteria resulting in 506 estimates of the prevalence of HIV‐HBsAg co‐infection across six population groups (general populations, PLHIV heterosexual and pregnant women, PWID, MSM, other high‐risk populations and children).
Figure 1.
Flow chart of included studies
3.1. Availability of studies and estimates by region and population group
Overall, 80 (41.0%) of 195 countries had estimates. One‐third (n = 28) of these countries were from sub‐Saharan Africa and 26.3% (n = 21) from Western/Central/Eastern Europe, with these regions having the highest proportion of countries with data (28/45 62.2% and 21/54 38.9%, respectively). The number and proportion of countries with estimates from other regions were as follows: North Africa and Middle East (7/21 33.3%); Latin America (9/36 25.0%); East Asia (2/2 100%); South and South‐East Asia (8/18 44.4%); Asia Pacific and Australasia (3/17 17.6%); and North America (2/2 100%).
Of the 506 co‐infection prevalence estimates, 45 were from general population samples (8.9% of estimates), 90 from PLHIV populations who were either pregnant women or where heterosexual transmission was the primary exposure (17.8% of estimates), 36 among PWID (7.1%), 70 among MSM (13.8%), 22 among children and young people, and 243 (48.0%) from high‐risk populations (202 from mixed PWID and MSM populations, five among sex workers, seven among prisoners and 29 from other high‐risk populations).
3.2. Rating of study quality and assay method
Very few estimates were rated ‘A’ in study design quality (15/506), based on large multisite surveys. 63.8% (323/506) of studies were rated ‘B’ in study design quality, based on data from more than one site with a sample size >200, and 33.2% as ‘C’ (168/506). Two‐fifths (n = 207) of estimates provided no information on type of HBsAg assay (rated 0), 23 estimates were based on a rapid test (rated 1), 218 used an HBsAg assay (any generation) with no confirmatory HBsAg assay (rated 2), and the remaining 58 used an HBsAg assay (any generation) with a confirmatory HBsAg assay (rated 3). Taking both assay and study design together, the highest rated study was among PWID in Vietnam33 (rated A3), with five studies (1.0%) having the next highest rating (A2). Most estimates were categorized as B0 (28.1%), B2 (27.3%), followed by C2 (14.8%) and C0 (11.1%). This information is summarized in Table S2.
3.3. Prevalence of HIV‐HBsAg co‐infection by population group
3.3.1. General population samples
The mid‐point prevalence of HBsAg co‐infection among 45 general population samples testing positive for HIV was 7.4% (IQR 1.4%‐15.7%) with country‐level prevalence estimates shown in Figure 2A. The highest prevalence was from West and Central Africa at 16.4% compared to 4.4% and 8.8% from South and East Africa, respectively. Very low prevalence was reported from East Asia (0.4%, one study) and Latin America (0.9%). There were no general population studies from Europe, North America or Asia Pacific/Australasia. All estimates are summarized in Table 1 with global prevalence maps shown in Figure 2.
Figure 2.
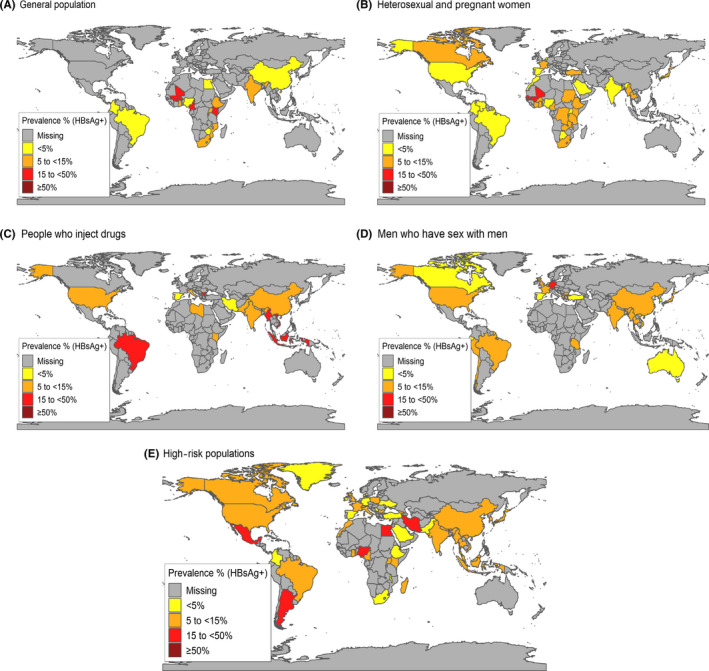
Best estimates of hepatitis B Surface Antigen co‐infection prevalence among samples of A, General population B, Heterosexual and pregnant women. C, People who inject drugs; D, Men who have sex with men; and E, Other high‐risk individuals.
Table 1.
Summary of global HIV‐HBsAg co‐infection prevalence estimate in general population sample, heterosexual and pregnant PLHIV, PWID and MSM
| General Population | Heterosexual and pregnant women | PWID | MSM | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total studies | Best estimate | Total studies | Best estimate | Total studies | Best estimate | Total studies | Best estimate | ||||||||||||||||||
| Country | n | Rangeb | % | S | n | Year | n | Rangeb | % | S | n | Year | n | Rangeb | % | S | n | Year | N | Rangeb | % | S | n | Year | |
| West and Central Africa | |||||||||||||||||||||||||
| Burkina Faso68, 69, 70 | 2 | 0.5‐17.0 | 17.0 | B3 | 761 | 2010 | 1 | 6.1 | 6.1 | C3 | 115 | 2009 | |||||||||||||
| Cameroon71, 72, 73, 74, 75, 76, 77, 78 | 2 | 4.2‐17.3 | 17.3 | B3 | 301 | 2013 | 6c | 8.3‐14.6 | 11.8 | B3 | 212 | 2010 | |||||||||||||
| Cote D'Ivoire79, 80 | 2c | 9.0‐12.7 | 12.7 | C2 | 495 | 2006 | |||||||||||||||||||
| Congo81 | 1 | 7.7 | 7.7 | B2 | 209 | 2008 | |||||||||||||||||||
| Equatorial Guinea82 | 1 | 15.7 | 15.7 | B3 | 230 | 2013 | |||||||||||||||||||
| Gambia83 | 1 | 12.2 | 12.2 | C0 | 572 | 2009 | |||||||||||||||||||
| Ghana84, 85, 86, 87, 88, 89 | 4 | 2.4‐18.7 | 6.0 | C2 | 168 | 2007 | 2 | 10.8‐11.0 | 11.0 | C2 | 155 | 2010 | |||||||||||||
| Guinea‐Bissau90 | 1 | 16 | 16.0 | B1 | 576 | 2011 | |||||||||||||||||||
| Mali91, 92 | 1 | 25.3 | 25.3 | C2 | 518 | 2002 | 1 | 22 | 22.0 | B2 | 242 | 2004 | |||||||||||||
| Nigeria93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 | 8 | 0.0‐19.8 | 1.3 | B2 | 174 | 2016 | 12c | 2.0‐11.9 | 4.2 | B2 | 2391 | 2011 | |||||||||||||
| Senegal113 | 1 | 16.8 | 16.8 | C2 | 363 | 2002 | |||||||||||||||||||
| Total a | 18 | 6.0‐17.3 | 16.4 | 28 | 7.7‐16.0 | 12.0 | |||||||||||||||||||
| South Africa | |||||||||||||||||||||||||
| Botswana114, 115 | 2 | 0.0‐5.3 | 0.0 | B2 | 1995 | 2006 | |||||||||||||||||||
| Lesotho116 | 1 | 5.5 | 5.5 | B2 | 205 | 2007 | |||||||||||||||||||
| South Africa117, 118, 119, 120, 121, 122 | 1 | 7.4 | 7.4 | B3 | 215 | 2009 | 5c | 2.1‐7.4 | 7.4 | B3 | 189 | 2013 | |||||||||||||
| Zimbabwe123 | 1 | 1.4 | 1.4 | C2 | 74 | 2005 | |||||||||||||||||||
| Total a | 2 | 1.4‐7.4 | 4.4 | 8 | 0.0‐7.4 | 5.5 | |||||||||||||||||||
| East Africa | |||||||||||||||||||||||||
| Comoros124 | 1 | 8 | 8.0 | C0 | 50 | 2002 | |||||||||||||||||||
| Djibouti125 | 1 | 9.7 | 9.7 | C3 | 175 | 2000 | |||||||||||||||||||
| Ethiopia126, 127, 128, 129, 130, 131, 132, 133 | 4 | 0.7‐61.4 | 7.9 | B2 | 101 | 2013 | 4 | 3.0‐6.1 | 5.0 | C3 | 400 | 2011 | |||||||||||||
| Kenya36, 134, 135, 136, 137, 138 | 1 | 20.9 | 6.8 | B1 | 267 | 2007 | 4 | 5.2‐6.9 | 6.1 | B2 | 378 | 2007 | 1 | 13.9 | 13.9 | C2 | 72 | 2010 | |||||||
| Malawi139, 140, 141 | 3c | 5.0‐16.9 | 8.7 | C3 | 309 | 2009 | |||||||||||||||||||
| Mozambique142, 143 | 2 | 10.1‐13.8 | 13.8 | C0 | 58 | 2009 | |||||||||||||||||||
| Rwanda144, 145 | 1 | 5.7 | 5.7 | C2 | 384 | 2001 | 1c | 2.4 | 2.4 | C3 | 85 | 2004 | |||||||||||||
| Tanzania146, 147 | 1c | 6.2 | 6.2 | C0 | 17 539 | 2011 | 1 | 9.2 | 9.2 | B0 | 65 | 2007 | |||||||||||||
| Uganda145, 148, 149, 150, 151, 152 | 4 | 4.6‐8.3 | 6.0 | B2 | 72 | 1999 | 2c | 4.6‐4.9 | 4.9 | C3 | 164 | 2004 | |||||||||||||
| Zambia153 | 1 | 9.9 | 9.9 | C2 | 323 | 2008 | |||||||||||||||||||
| Total a | 13 | 6.0‐13.8 | 8.8 | 17 | 4.9‐8.4 | 6.1 | 1 | 13.9 | 13.9 | 1 | 9.2 | 9.2 | |||||||||||||
| N Africa & Middle East | |||||||||||||||||||||||||
| Egypt154 | 1 | 3.4 | 3.4 | C2 | 59 | 2015 | |||||||||||||||||||
| Iran (Islamic Republic of)35, 155, 156, 157, 158 | 5 | 2.1‐44.2 | 2.1 | B3 | 888 | 2007 | |||||||||||||||||||
| Lebanon | |||||||||||||||||||||||||
| Libya159 | 1 | 5.1 | 5.1 | B0 | 294 | 2010 | |||||||||||||||||||
| Morocco160 | 1c | 2.4 | 2.4 | C2 | 1120 | 2015 | |||||||||||||||||||
| Saudi Arabia161 | 1 | 3.4 | 3.4 | C1 | 234 | 2010 | |||||||||||||||||||
| Sudan162, 163 | 2 | 11.7‐14.5 | 11.7 | B3 | 358 | 2012 | |||||||||||||||||||
| Totala | 1 | 3.4 | 3.4 | 4 | 2.4‐11.7 | 3.4 | 6 | 2.1‐5.1 | 3.6 | ||||||||||||||||
| West, Central and East Europe | |||||||||||||||||||||||||
| Belgium164, 165 | 3 | 0.0‐6.1 | 6.1 | B0 | 3081 | 2009 | |||||||||||||||||||
| Bulgaria166 | 1 | 16.9 | 16.9 | B2 | 359 | 2011 | 1 | 9.7 | 9.7 | B2 | 1087 | 2011 | |||||||||||||
| Denmark | |||||||||||||||||||||||||
| France167, 168, 169, 170, 171 | 2c | 6.0‐6.9 | 6.9 | B0 | 6548 | 2013 | 2 | 5.0‐8.8 | 8.8 | B2 | 2351 | 2002 | |||||||||||||
| Georgia | |||||||||||||||||||||||||
| Germany169, 172, 173, 174 | 4 | 1.7‐25.4 | 25.4 | B3 | 1843 | 2012 | |||||||||||||||||||
| Greece175, 176 | 2 | 6.0‐6.5 | 6.0 | A2 | 1729 | 2003 | |||||||||||||||||||
| Ireland | |||||||||||||||||||||||||
| Italy177, 178 | 2 | 3.5‐7.0 | 7.0 | C2 | 173 | 2006 | |||||||||||||||||||
| Moldova | 1 | 11.0 | 11.0 | C3 | 113 | 2009 | |||||||||||||||||||
| Netherlands179, 180, 181, 182 | 1c | 4.9 | 4.9 | B0 | 1546 | 2008 | 2 | 5.2‐7.7 | 5.2 | A2 | 12 800 | 2012 | |||||||||||||
| Poland | |||||||||||||||||||||||||
| Portugal183 | 1 | 4.1 | 4.1 | C0 | 343 | 2005 | |||||||||||||||||||
| Romania | |||||||||||||||||||||||||
| Spain184, 185, 186, 187, 188 | 1 | 2.3 | 2.3 | B0 | 741 | 2006 | 1 | 3.9 | 3.9 | B0 | 821 | 2015 | 4 | 4.3‐10.9 | 4.3 | B2 | 392 | 2011 | |||||||
| Serbia | |||||||||||||||||||||||||
| Slovenia | |||||||||||||||||||||||||
| Switzerland | |||||||||||||||||||||||||
| Turkey189, 190, 191 | 2 | 7.1‐11.4 | 11.4 | C3 | 70 | 2007 | 1 | 4.0‐4.0 | 4.0 | B0 | 55 | 2009 | |||||||||||||
| United Kingdom192, 193 | 2 | 2.1‐5.3 | 5.3 | A0 | 25 486 | 2012 | |||||||||||||||||||
| Ukraine | |||||||||||||||||||||||||
| Total a | 6 | 4.9‐7.1 | 5.9 | 6 | 4.1‐11.0 | 7.0 | 21 | 5.2‐8.8 | 6.0 | ||||||||||||||||
| East Asia | |||||||||||||||||||||||||
| China34, 194, 195, 196, 197, 198, 199 | 1 | 0.4 | 0.4 | C0 | 275 | 2017 | 5 | 1.1‐59.6 | 11.8 | B3 | 498 | 2014 | 1 | 14.2‐14.2 | 14.2 | B0 | 532 | 2010 | |||||||
| China, province of Taiwan200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217 | 1 | 12.8 | 12.8 | B2 | 105 | 2005 | 6 | 17.4‐20.0 | 18.9 | A2 | 301 | 2010 | 13 | 3.5‐22.0 | 3.5 | C3 | 523 | 2012 | |||||||
| Total a | 1 | 0.4 | 0.4 | 1 | 12.8 | 12.8 | 11 | 11.8‐18.9 | 15.4 | 14 | 3.5‐14.2 | 8.8 | |||||||||||||
| South and South‐East Asia | |||||||||||||||||||||||||
| Cambodia | |||||||||||||||||||||||||
| India218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235 | 6 | 0.0‐8.3 | 8.3 | B2 | 121 | 2017 | 8 | 0.0‐7.1 | 3.4 | B2 | 3142 | 2012 | 2 | 11.9‐12.9 | 12.9 | B2 | 595 | 2014 | 2 | 3.2‐7.9 | 7.9 | B2 | 1178 | 2002 | |
| Indonesia236, 237 | 2 | 7.0‐17.6 | 17.6 | B3 | 74 | 2009 | |||||||||||||||||||
| Malaysia | |||||||||||||||||||||||||
| Myanmar34, 238, 239 | 2 | 8.0‐9.0 | 8.0 | C1 | 122 | 2009 | 2 | 10.2‐41.9 | 41.9 | B2 | 86 | 2009 | 1 | 13.4 | 13.4 | C0 | 176 | 2012 | |||||||
| Pakistan240, 241 | 2 | 6.0‐37.0 | 6.0 | C3 | 100 | 2013 | |||||||||||||||||||
| Thailand242, 243, 244 | 2 | 9.0‐11.9 | 9.0 | B2 | 416 | 2008 | 1 | 5.6 | 5.6 | B2 | 215 | 2014 | |||||||||||||
| Vietnam33, 245, 246 | 2 | 9.4‐16.3 | 16.3 | A3 | 849 | 2009 | 1 | 13.2 | 13.2 | C0 | 153 | 2014 | |||||||||||||
| Total a | 6 | 8.3 | 8.3 | 12 | 3.6‐9.2 | 5.9 | 10 | 12.9‐17.6 | 16.3 | 5 | 6.7‐13.3 | 10.6 | |||||||||||||
| Asia Pacific & Australasia | |||||||||||||||||||||||||
| Australia247, 248, 249, 250 | 4 | 3.4‐6.3 | 4.9 | B2 | 1719 | 2001 | |||||||||||||||||||
| Japan251, 252, 253, 254, 255, 256 | 1 | 5.4 | 5.4 | B2 | 166 | 2002 | 6 | 6.3‐17.9 | 7.2 | B3 | 817 | 2013 | |||||||||||||
| South Korea257 | 1 | 5 | 5.0 | B0 | 541 | 2013 | |||||||||||||||||||
| Total a | 1 | 5.4 | 5.4 | 11 | 4.9‐7.2 | 5.0 | |||||||||||||||||||
| Latin America (Central, South America & Caribbean) | |||||||||||||||||||||||||
| Argentina | |||||||||||||||||||||||||
| Brazil258, 259, 260, 261, 262, 263, 264, 265, 266, 267, 268, 269, 270, 271 | 3 | 0.5‐1.0 | 0.5 | B2 | 186 | 2012 | 6c | 0.5‐3.3 | 2.3 | C3 | 130 | 2008 | 1 | 27.3 | 27.3 | B2 | 205 | 2003 | 5 | 2.9‐31.0 | 8.2 | B2 | 170 | 2000 | |
| Chile272 | 1 | 6.1 | 6.1 | B0 | 395 | 2007 | |||||||||||||||||||
| Colombia273, 274, 275 | 1 | 1.2 | 1.2 | C3 | 247 | 2009 | 2 | 2.9‐3.3 | 3.3 | B2 | 275 | 2010 | |||||||||||||
| Ecuador276 | 1 | 4.0 | 4.0 | C0 | 50 | 2012 | |||||||||||||||||||
| Haiti277 | 1c | 2.4 | 2.4 | B3 | 123 | 2012 | |||||||||||||||||||
| Peru278 | 1 | 9.5 | 9.5 | B0 | 338 | 2003 | |||||||||||||||||||
| Venezuela279, 280 | 2 | 3.1‐11.8 | 3.1 | C3 | 418 | 2008 | |||||||||||||||||||
| Total a | 4 | 0.5‐1.1 | 0.9 | 11 | 2.4‐3.2 | 2.8 | 1 | 27.3 | 27.3 | 8 | 5.1‐8.9 | 7.2 | |||||||||||||
| North America | |||||||||||||||||||||||||
| Canada154, 281 | 1c | 5.6 | 5.6 | C0 | 142 | 2010 | 1 | 2.8 | 2.8 | B2 | 294 | 2012 | |||||||||||||
| USA282, 283, 284, 285, 286, 287, 288, 289, 290, 291 | 1c | 2.9 | 2.9 | B2 | 1500 | 1995 | 1 | 7.0 | 7.0 | C0 | 3987 | 2001 | 9 | 1.8‐9.3 | 5.5 | B2 | 816 | 2006 | |||||||
| Total a | 2 | 2.9‐5.6 | 4.2 | 1 | 7.0 | 7.0 | 10 | 2.8‐5.5 | 4.2 | ||||||||||||||||
| Global total a | 45 | 1.4‐15.7 | 7.4 | 90 | 3.4‐11.0 | 6.1 | 36 | 6.0‐16.9 | 11.8 | 70 | 5.0‐9.2 | 6.1 | |||||||||||||
Abbreviation: S, Study quality score.
Totals are derived from median of best estimates scored with interquartile range of best estimates.
Range is presented for country‐level estimates and interquartile range for regional and global totals. All best estimates are selected according to the decision rules in Text Box S2.
Denotes prevalence (total) derived from samples of PLHIV pregnant women among the population group PLHIV (heterosexual and pregnant women) including: Cameroon (2) 9.3%,74 14.6%73; Cote D’Ivoire (1) 9.0%80; Nigeria (1) 4.2%105; South Africa (3) 6.2%,120 3.4%,118 2.1%117; Malawi (1) 8.7%141; Rwanda (1) 2.4%145; Tanzania (1) 6.2%147; Uganda (1) 4.9%145; Brazil (5) 1.9%,261 0.5%,266 2.3%,260 0.9%,262 1.2%268; USA (1) 2.9%290; Canada (1) 5.6%154; Haiti (1) 2.4%277; France (2) 6.0%,170 6.9%168; the Netherlands (1) 4.9%,180 and Morocco (1).
3.3.2. Heterosexual and pregnant women
The mid‐point prevalence of HBsAg co‐infection among 90 studies in PLHIV heterosexual or pregnant women was 6.1% (IQR 3.4%‐11.0%) with country‐level prevalence estimates shown in Figure 2B. Prevalence was higher in East Asia (12.8%, one study), West and Central Africa (12.0%), and lowest in Latin America (2.8%), North Africa and the Middle East (3.4%) and North America (4.2%). Among this population, there were 23 estimates for pregnant women from 15 countries (see footnote to Table 1 for country‐specific estimates). The mid‐point prevalence of HBsAg co‐infection among 67 studies in heterosexual PLHIV was 8.0% (IQR 5.0%‐11.8%) compared to 4.6% (2.4%‐5.9%) among PLHIV pregnant women (data not shown).
3.3.3. People who inject drugs
The mid‐point prevalence among PWID based on 36 studies was 11.8% (IQR 6.0%‐16.9%) with country‐level prevalence estimates shown in Figure 2C. The highest prevalence was observed in Latin America (27.3%, one study), South and South‐East Asia (16.3%), East Asia (15.4%), and the lowest in North Africa and the Middle East (3.6%). Some single studies reported very high prevalence, including China (59.6%),34 Iran (44.2%)35 and Myanmar (41.9%).34 There was only one study from sub‐Saharan Africa (Kenya) which reported a prevalence of 13.9%.36
3.3.4. Men who have sex with men
The mid‐point prevalence among MSM was 6.1% (IQR 5.0%‐9.2%) based on 70 studies with country‐level prevalence estimates shown in Figure 2D. Prevalence was highest in South and South‐East Asia (10.6%), West and Central Africa (9.2%, one study), followed by East Asia (8.8%) and Latin America (7.2%). Lower prevalences of around 4%‐6% were reported from Asia Pacific/Australasia, Europe and North America. There were no studies from East Africa, South Africa or North Africa and the Middle East.
3.3.5. Other high‐risk groups
In addition to the main HIV‐exposure categories, there were 243 estimates from samples of PLHIV engaging in mixed sexual and/or injecting risk behaviours (Table S3) with country‐level prevalence estimates shown in Figure 2E. The point prevalence was 6.4% (IQR 4.3%‐9.6%) and was similar across the regions, except for East Asia (15.6%), and West and Central Africa (10.6%).
3.3.6. Children and young people
There were 22 estimates of HIV‐HBsAg co‐infection among children and young people from 13 countries (Table S3). The mid‐point prevalence was 6.8% (IQR 2.5‐10.0). Prevalence ranged from 2% to 20% across six estimates from Nigeria37, 38, 39, 40, 41, 42 and between 0% and 20.5% in South Africa.43, 44, 45, 46 Extremely high prevalence (43%‐46%) was observed in Romania in two studies47, 48 where HIV infection was acquired nosocomially prior to 1995. A high prevalence (32.6%) was also found in Thailand among young people perinatally infected with HIV (mean age 14 years).49 Prevalence was lower (~2%) among samples in India, Malawi, Poland, Spain and the United States,50, 51, 52, 53, 54 among HIV‐positive paediatric patients. Prevalence was higher in Benin (9.6%), Rwanda (6.8%), Tanzania (7%) and Zambia (10.4%) among children <16 years recruited through HIV clinics.48, 55, 56, 57 HIV‐exposure categories were not consistently reported.
3.3.7. Odds of HBsAg positivity among PLHIV compared to HIV‐negative persons
Overall, when we compared HBsAg estimates from 25 598 HIV‐positive with 286 121 HIV‐negative individuals, we found increased odds for HBsAg positivity among all HIV‐positive population groups compared to HIV‐negative populations (OR = 1.42; 95% CI = 1.10‐1.83) although there was a high degree of heterogeneity (I squared = 95.3%, P < .001). Odds of HBsAg were highest among HIV‐positive children (OR = 4.61; 95% CI = 1.80‐11.84), and there was a borderline statistically significant increase among MSM (2.69; 95% CI 0.71‐10.25), but not with other population groups. This is summarized in Figure 3 and Table S4.
Figure 3.
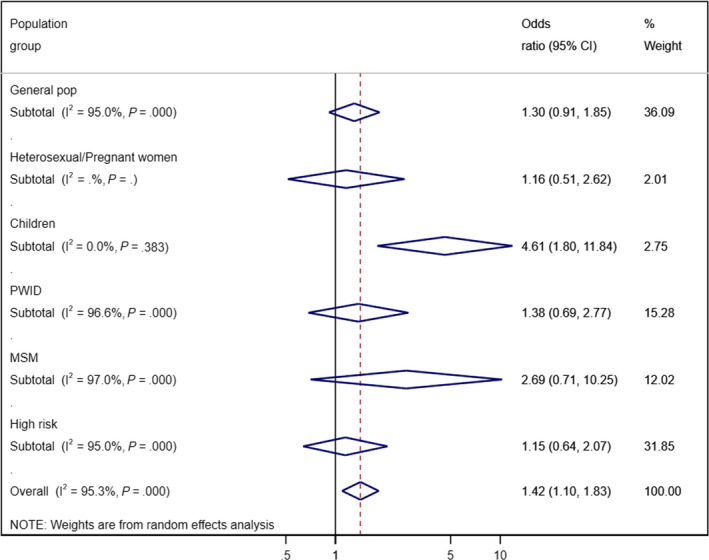
Forest plot showing meta‐analysis of odds of HBsAg infection in HIV‐positive populations versus HIV‐negative populations
3.3.8. Global burden of HBV co‐infection among PLHIV
Based on 2017 UNAIDS estimates of the number of PLHIV and PWID infected with HIV by region, we estimate that there are 2 653 300 (IQR = 1 970 300‐4 238 300) cases of HBsAg co‐infection among PLHIV globally. Only 3% of cases (n = 142 000; IQR = 124 400‐154 700) are among HIV‐positive PWID. This equates to a global prevalence of HBsAg co‐infection among PLHIV of 7.6% (IQR 5.6%‐12.1%). Sub‐Saharan Africa has the largest burden of HIV‐HBsAg co‐infection representing 69% of the total number of cases, followed by South‐East Asia (12%) and Latin America (6%). All other regions account for <5% each (Table 2).
Table 2.
Global estimates of HBsAg infection among People living with in 2017 HIV by global burden of disease region
| Region | PLHIV (excluding PWID) | PLHIV PWID | Total PLHIVa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLHIV | HBsAg Co‐infection | PLHIV | HBsAg Co‐infection | PLHIV | HBsAg Co‐infection | |||||
| n | Median Prevalence (IQR)b | Estimates (IQR) | n | % PWIDc | Median Prevalence (IQR)f | Estimates (IQR) | n | Estimates (range) | Region | |
| Africa (South, West, East, Central) | 23 965 000 | 8.0 (5.9‐13.3) | 1 905 200 (1 405 400‐3 177 800) | 41 700 | 0.2% | 13.9e | 5800e | 24 006 700 | 1 911 000 (1 411 200‐3 183 600) | 69% |
| North Africa and Middle East | 182 040 | 3.4 (2.9‐7.6) | 6200 (5200‐13 800) | 37 100 | 17% | 3.6 (2.1‐5.1) | 1300 (800‐1900) | 219 140 | 7500 (6000‐15 700) | 1% |
| Europe (West, Central) | 839 730 | 6.0 (4.9‐8.8) | 50 400 (41 300‐73 900) | 32 000 | 4% | 5.5 (4.0‐12.0) | 1800 (1300‐3800) | 871 730 | 52 200 (42 600‐77 700) | 2% |
| Eastern Europe/ CAR | 979 140 | 6.1 (4.0‐9.9)d | 59 700 (39 200‐96 900) | 451 000 | 32% | 11.0e | 49 600e | 1 430 140 | 109 300 (88 800‐146 500) | 4% |
| East Asia | 769 500 | 8.1 (1.9‐13.5) | 62 400 (14 700‐103 700) | 162 300 | 17% | 15.4 (11.8‐18.9) | 25 000 (19 200‐30 700) | 931 800 | 87 400 (33 900‐134 400) | 3% |
| South and South‐East Asia | 3 729 800 | 8.1 (6.7‐11.1) | 303 300 (251 400‐414 000) | 322 000 | 8% | 16.3 (12.9‐17.6) | 52 300 (41 500‐56 600) | 4 051 800 | 355 600 (292 900‐470 600) | 12% |
| Western Pacific (Asia Pacific, Australasia) | 118 500 | 5.2 (4.9‐6.3) | 6200 (5900‐7500) | 1200 | 1% | 11.8 (6.0‐16.9)d | 100 (100‐200) | 119 700 | 6300 (6000‐7700) | 0% |
| Latin America (South, Central America, Caribbean) | 2 108 900 | 3.2 (2.3‐6.1) | 67 200 (48 500‐128 600) | 5000 | 0.2% | 27.3e | 1400e | 2 113 900 | 68 600 (49 900‐130 000) | 6% |
| North America | 1 209 700 | 4.2 (2.8‐5.6) | 50 700 (34 300‐67 400) | 66 300 | 5% | 7.0e | 4700e | 1 276 000 | 55 400 (39 000‐72 100) | 4% |
| Total | 33 902 310 | 6.1 (4.0‐9.9) | 2 511 300 (1 845 900‐4 083 600) | 1 118 600 | 3% | 11.8 (6.0‐16.9) | 142 000 (124 400‐154 700) | 35 020 910 | 2 653 300 (1 970 300‐4 238 300) | 100% |
Estimates of persons living with HIV in each country were measured through Spectrum and published by UNAIDS and UNODC.
Median prevalence and IQRs are calculated across the best estimates for all population groups (except PWID estimates) and countries in each region (for regional estimates) or globally (for 'Total' estimates).
Proportion of HIV cases among PWID.
No regional estimate available, so global median used as a proxy.
Only one country estimate available, therefore no IQR presented.
Median prevalence and IQRs are calculated across the best PWID estimates for each country in each region (for regional estimates) or globally (for 'Total' estimates)
4. DISCUSSION
This is the first systematic review to provide global, regional and country estimates of prevalence and burden of HBsAg positivity among PLHIV across six population sub‐groups; complementing a companion review on HIV‐HCV antibody co‐infection.27 We estimate a global prevalence of 7.6% (IQR 5.6%‐12.1%) or 2.7 million (IQR 2.0‐4.2 million) cases of HIV‐HBsAg co‐infection. The greatest burden (69% of all cases; 1.9 million) is in sub‐Saharan Africa where there is the largest number of PLHIV. This is followed by 12% (355 600) in South and South‐East Asia and 6% (68 800) in Latin America.
HBsAg prevalence was broadly similar across different HIV‐positive population groups, with a prevalence of 6%‐7% reported among general population samples, heterosexually exposed or pregnant women, children, MSM and high‐risk populations. Only among PWID was prevalence higher at 11.8%. PWID accounted for only 3% of the global co‐infected population, but a much higher proportion in Eastern Europe (45% of cases). We were limited in our ability to make regional comparisons across sub‐populations because only two regions (sub‐Saharan Africa and South and South‐East Asia) had general population data, and there were little data among high‐risk populations in sub‐Saharan Africa. The most comprehensive data from different regions was among heterosexual or pregnant PLHIV. The prevalence was highest in West and Central Africa (12.0%), and East Asia (12.8%) and lowest in Latin America (2.8%) and North America (4.2%).
Our global estimate of burden of HIV‐HBsAg co‐infection is broadly consistent with previous estimates of 2‐4 million.24, 25, 58, 59 A review of HIV‐HBsAg co‐infection in sub‐Saharan Africa found a mean prevalence of 12.5% among HIV‐positive cohorts, slightly higher than we found, although that study did not disaggregate by HIV‐exposure category making comparisons challenging.25 Our findings broadly reflect existing data on the main routes of transmission of HBV infection. In sub‐Saharan Africa, HBV infection is predominantly acquired perinatally or in early childhood, leading to high rates of chronic infection.4, 60 The contribution of adult acquisition is low, as the majority are already chronically infected or immune. As a result, most people have already been HBV‐infected for many years by the time they are exposed to HIV in adulthood, which may explain why the prevalence is similar across different sub‐populations.9 In contrast, in other regions a higher prevalence occurs among PWID and MSM compared to the general population. This was particularly marked in Latin America, with co‐infection prevalence of 9.5% and 27% among PWID and MSM, respectively, compared to 0.9% in the general population. This is consistent with co‐transmission of HBV and HIV in adulthood in these settings, with much lower transmission of HBV in childhood.22 A significant proportion of cases in high risk populations may also represent acute infection, which will not lead to chronic infection.
This HIV‐HBsAg review and a companion review of HIV‐HCV antibody co‐infection (consistent with past or current infection)27 highlights important differences between the epidemiology of these co‐infections. Although the global prevalence and burden are similar (7.6% for HIV‐HBsAg compared to 6.2% for HIV‐HCV) antibody, almost three‐quarters of the global burden of HIV‐HBsAg co‐infection in 2017 is in sub‐Saharan Africa (1.91 million), 4‐5 times as many as HIV‐HCV co‐infections (429 600). In contrast, the greatest burden of HIV‐HCV co‐infection is in the concentrated epidemic settings of Central Asia and Eastern Europe among PWID, accounting for 27% of the HIV‐HCV burden (607 700). There is a much lower prevalence of HBsAg than HCV antibody among HIV‐positive PWID (11.8% vs 82%), accounting for 3% (142 000) of HIV‐HBsAg co‐infections but 59% (1.36 million) of HIV‐HCV co‐infections. Overall, we found less variability in HIV‐HBsAg prevalence between sub‐populations, and HIV infection was a less important risk factor for HBsAg positivity than for HCV antibody positivity.
Key strengths of our systematic review were the comprehensive search of published literature in English, French, Russian, Chinese, Portuguese, Arabic and Spanish; the stratification of co‐infection estimates for different population sub‐groups; and the large number of studies among heterosexually exposed and pregnant women living with HIV—the main source for regional estimates of HIV‐HBsAg co‐infection. Despite this, estimates were available for only 41% of countries, half being in sub‐Saharan Africa. Five regional prevalence estimates for different sub‐populations were based on data from a single country, possibly unrepresentative of the true regional profile. Few countries had data for all sub‐populations making regional comparisons difficult. We excluded studies that only presented regional‐level data, not disaggregated by country, to enable us to observe how prevalence varied by country and risk group within a region and to take account of the differing epidemiology of HIV at a country level. However, this resulted in the exclusion of large cohorts that aggregate across country and region. One major cohort that reports data across Europe, Israel and Argentina found a comparable prevalence of HIV‐HBsAg co‐infection (7.1%‐8.7%) with similar high prevalence observed in Argentina among PLHIV (exposure group not specified) to the range of prevalence we found among MSM.61, 62
The quality of studies was also variable. Few studies were based on large multisite surveys, with 40% of sero‐surveys being based on data from one city and fewer than 200 persons. In addition, over half of studies provided no details of the assay type and testing protocol; with much of the remainder using a recent HBsAg assay but without confirmatory HBsAg testing, possibly overestimating the infected population. In general, WHO does not recommend the use of a second confirmatory HBsAg assay for diagnosis of CHB infection (23). We also did not exclude populations based on receiving ART, with few studies reporting the prevalence of ART making it difficult to adjust for the effects of treatment. The use of tenofovir‐based ART regimens may have reduced detection of HBsAg among some samples.63 Finally, we have not taken into account increases in both ART coverage and the use of tenofovir‐based ART regimens that may result in lower levels of co‐infection in later years.
Our findings have important programmatic implications. First, universal infant and perinatal HBV vaccination remains the key strategy for preventing mother‐to‐child transmission and controlling the HBV epidemic. Although high uptake of infant vaccination has been achieved, leading to substantial decreases in incidence in recent years, HBV birth‐dose vaccination is being implemented by less than half of countries, and only 9/48 of countries in Africa.4 Rates of adult vaccination also remain low, with <3% of countries routinely vaccinating high‐risk populations (PWID, MSM, sex workers and prisoners).22 Our findings highlight the importance of targeting PLHIV, especially high‐risk groups and children for testing, catch‐up HBV vaccination and other preventative interventions.64 Second, the global scale‐up of HIV treatment for PLHIV using a tenofovir‐based ART regimen represents a major opportunity for achieving global targets towards hepatitis B elimination in PLHIV, by simultaneously treating those who have chronic and HIV co‐infection so reducing mother‐to‐child transmission of HBV alongside HIV.65 ART coverage is now approximately 50% in most countries and encouragingly coverage in eastern and southern Africa is higher than the global average,66 with 60% of persons on ART receiving a tenofovir‐based regimen.67 Our findings clearly show the need to scale‐up tenofovir‐based ART to address HIV‐HBsAg co‐infection, particularly focusing on sub‐Saharan Africa.
CONFLICTS OF INTEREST
No conflicts of interest to declare.
AUTHOR CONTRIBUTORS
PE conceived the study proposal. LP, PV and PE developed the overall methods for use in the report. LP developed the methodology and oversaw the search and data extraction for the report. CM developed and conducted the literature search. LP, CF, AT, JO, JS, BM and EG extracted data. LP, PE, HR and EG developed the quality assessment tool. LP and PV developed the analysis technique. LP and CF generated regional and global prevalence estimates, which were reviewed by PE, PV, HR and KS. KS generated the global burden of disease estimates. LP and PE led the writing of the manuscript; LP, PV and CF commented and contributed text. AT generated the maps.
DISCLAIMER
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated, including UNAIDS and the WHO.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank Jane Falconer (Information Services Librarian at the LSHTM) for her assistance with the search strategy and Yvan Hutin for inputs on the manuscript.
Platt L, French CE, McGowan CR, et al. Prevalence and burden of HBV co‐infection among people living with HIV: A global systematic review and meta‐analysis. J Viral Hepat. 2020;27:294–315. 10.1111/jvh.13217
Easterbrook and Vickerman are joint last author.
Funding information
WHO and National Institute for Health Research Health Protection Research Unit in Evaluation of Interventions.
REFERENCES
- 1. Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350(11):1118‐1129. [DOI] [PubMed] [Google Scholar]
- 2. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507‐539. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organisation . Progress report on HIV, viral hepatitis and sexually transmitted infections, 2019. Geneva, Switzerland: World Health Organisation; 2019. [Google Scholar]
- 4. World Health Organisation . Global Hepatitis report 2017. Geneva, Switzerland: World Health Organisation; 2017. [Google Scholar]
- 5. Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23(1):47‐58. [DOI] [PubMed] [Google Scholar]
- 6. Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting: a European and an American perspective. Liver Transplant. 2005;11(7):716‐732. [DOI] [PubMed] [Google Scholar]
- 7. Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253(1337):197‐201. [DOI] [PubMed] [Google Scholar]
- 8. Ott JJ, Stevens GA, Wiersma ST. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infect Dis. 2012;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edmunds WJ, Medley GF, Nokes DJ, O'Callaghan CJ, Whittle HC, Hall AJ. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiol Infect. 2009;117(2):313‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546‐1555. [DOI] [PubMed] [Google Scholar]
- 11. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Easterbrook P, Sands A, Harmanci H. Challenges and priorities in the management of HIV/HBV and HIV/HCV coinfection in resource-limited settings. Semin Liver Dis. 2012;32(2):147‐57. [DOI] [PubMed] [Google Scholar]
- 13. Weber R, Sabin CA, Friis‐Moller N, et al. Liver‐related deaths in persons infected with the human immunodeficiency virus: the DAD study. Arch Intern Med. 2006;166(15):1632‐1641. [DOI] [PubMed] [Google Scholar]
- 14. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a study of 150 homosexual men. J Infect Dis. 1989;160(4):577‐582. [DOI] [PubMed] [Google Scholar]
- 15. Colin JF, Cazals‐Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29(4):1306‐1310. [DOI] [PubMed] [Google Scholar]
- 16. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. Aids. 1997;11(5):597‐606. [DOI] [PubMed] [Google Scholar]
- 17. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV‐1, hepatitis B virus, and risk of liver‐related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921‐1926. [DOI] [PubMed] [Google Scholar]
- 18. Puoti M, Spinetti A, Ghezzi A, et al. Mortality for liver disease in patients with HIV infection: a cohort study. J Acquir Immune Defic Syndr. 2000;24(3):211‐217. [DOI] [PubMed] [Google Scholar]
- 19. Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48(2):353‐367. [DOI] [PubMed] [Google Scholar]
- 20. Benhamou Y, Bochet M, Thibault V, et al. Long‐term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus‐infected patients. Hepatology. 1999;30(5):1302‐1306. [DOI] [PubMed] [Google Scholar]
- 21. Zöllner B, Petersen J, Puchhammer‐Stöckl E, et al. Viral features of lamivudine resistant hepatitis B genotypes A and D. Hepatology. 2004;39(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organisation . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva, Switzerland: World Health Organisation; 2015. [PubMed] [Google Scholar]
- 23. World Health Organisation . Guidelines on hepatitis B and C testing. Geneva, Switzerland: World Health Organisation; 2017. [Google Scholar]
- 24. Basnayake SK, Easterbrook PJ. Wide variation in estimates of global prevalence and burden of chronic hepatitis B and C infection cited in published literature. J Viral Hepat. 2016;23(7):545‐559. [DOI] [PubMed] [Google Scholar]
- 25. Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub‐Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta‐analysis. Int J Infect Dis. 2010;14(12):e1024‐e1031. [DOI] [PubMed] [Google Scholar]
- 26. Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733‐1745. [DOI] [PubMed] [Google Scholar]
- 27. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(7):797‐808. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333‐1342. [DOI] [PubMed] [Google Scholar]
- 30. UNAIDS . How AIDS changes everything. MDG 6: 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 31. UNODC . World Drug Report 2014. Vienna: United Nations Office on Drugs and Crime, 2015. [Google Scholar]
- 32. World Health Organisation . Guidelines for screening, treatment and care for persons with hepatitis C. Geneva, Switzerland: World Health Organisation; 2018. [Google Scholar]
- 33. Nadol P, O'Connor S, Duong H, et al. Findings from integrated behavioral and biologic survey among males who inject drugs (MWID) ‐ Vietnam, 2009–2010: evidence of the need for an integrated response to HIV, hepatitis B virus, and hepatitis C virus. PLoS ONE [Electronic Resource]. 2015;10(2):e0118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou YH, Liu FL, Yao ZH, et al. Comparison of HIV‐, HBV‐, HCV‐ and co‐infection prevalence between Chinese and Burmese intravenous drug users of the China‐Myanmar border region. PLoS ONE [Electronic Resource]. 2011;6(1):e16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alavi SM, Etemadi A. HIV/HBV, HIV/HCV and HIV/HTLV‐1 co infection among injecting drug user patients hospitalized at the infectious disease ward of a training hospital in Iran. Pak J Med Sci. 2007;23(4):510‐513. [Google Scholar]
- 36. Kibaya RM, Lihana RW, Kiptoo M, et al. Characterization of HBV among HBV/HIV‐1 Co‐infected injecting drug users from Mombasa, Kenya. Curr HIV Res. 2015;13(4):292‐299. [DOI] [PubMed] [Google Scholar]
- 37. Anigilaje EA, Olutola A. Human immunodeficiency virus and hepatitis C virus co‐infection among children in an antiretroviral therapy programme in Benue. Internet J Infect Dis. 2013;12(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ashir GM, Rabasa AI, Gofama MM, Bukbuk D, Abubakar H, Farouk GA. Study of hepatic functions and prevalence of hepatitis B surface antigenaemia in Nigerian children with human immunodeficiency virus infection. Niger J Med. 2009;18(3):260‐262. [DOI] [PubMed] [Google Scholar]
- 39. Davidson UN, Chidiebele NI, Josephine EI, et al. The prevalence liver function and immunologic status of children with HIV and hepatitis B virus coinfection in Enugu, Nigeria. Afr J Infect Dis. 2016;10(2):61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nwolisa E, Mbanefo F, Ezeogu J, Amadi P. Prevalence of Hepatitis B co‐infection amongst HIV infected children attending a care and treatment centre in Owerri, South‐eastern Nigeria. Pan Afr Med J. 2013;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sadoh AE, Sadoh WE, Iduoriyekemwen NJ. HIV co‐infection with hepatitis B and C viruses among Nigerian children in an antiretroviral treatment programme. S Afr J Child Health. 2011;5(1):7‐10. [Google Scholar]
- 42. Ubesie AC, Iloh KK, Eze CU, Iloh O, Ibeziako NS, Okoli C, et al.Clinical and Laboratory Profile of ARV Naive HIV Infected Children in the Era of Highly Active Anti‐retroviral Therapy in Enugu, South‐East Nigeria. Jul‐2014.
- 43. Beghin JC, Ruelle J, Sokal E, et al. Effectiveness of the South African expanded program of immunization against hepatitis B in children infected with human immunodeficiency virus‐1 living in a resource‐limited setting of KwaZulu‐Natal. J Med Virol. 2017;89(1):182‐185. [DOI] [PubMed] [Google Scholar]
- 44. Chotun N, Nel E, Cotton MF, Preiser W, Andersson MI. Hepatitis B virus infection in HIV‐exposed infants in the Western Cape, South Africa. Vaccine. 2015;33(36):4618‐4622. [DOI] [PubMed] [Google Scholar]
- 45. Jooste P, van Zyl A, Adland E, et al. Screening, characterisation and prevention of Hepatitis B virus (HBV) co‐infection in HIV‐positive children in South Africa. J Clin Virol. 2016;85:71‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mdlalose N, Parboosing R, Moodley P. The prevalence of hepatitis B virus infection in HIV‐positive and HIV‐negative infants: KwaZulu‐Natal, South Africa. Afr J Lab Med. 2016;5(1):a283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arbune M, Benea OE. Particularities of HBV‐HIV co‐infection in the youth from galafi. J Gastrointestin Liver Dis. 2012;21:56. [Google Scholar]
- 48. Ruta SM, Matusa RF, Sultana C, et al. High prevalence of hepatitis B virus markers in Romanian adolescents with human immunodeficiency virus infection. MedGenMed. 2005;7(1):68. [PMC free article] [PubMed] [Google Scholar]
- 49. Aurpibul L, Lumbiganon P, Kolasaraksa P, et al. HIV and Hepatitis B coinfection among perinatally HIV‐infected Thai adolescents. Pediatr Infect Dis J. 2012;31(9):943‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alvarez‐Uria G, Manoranjan M, Raghavakalyam P, Naik PK. Gender differences, routes of transmission, socio‐demographic characteristics and prevalence of HIV related infections of adults and children in an HIV cohort from a rural district of India. Infect Dis Rep. 2012;4(1):66‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dapena M, Jimenez B, Noguera‐Julian A, et al. Metabolic disorders in vertically HIV‐infected children: future adults at risk for cardiovascular disease. J Pediatr Endocrinol. 2012;25(5–6):529‐535. [DOI] [PubMed] [Google Scholar]
- 52. Pokorska‐Spiewak M, Stanska‐Perka A, Popielska J, et al. Prevalence and predictors of liver disease in HIV‐infected children and adolescents. Sci Rep. 2017;7:12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toussi SS, Abadi J, Rosenberg M, Levanon D. Prevalence of hepatitis B and C virus infections in children infected with HIV. Clin Infect Dis. 2007;45(6):795‐798. [DOI] [PubMed] [Google Scholar]
- 54. Varo R, Buck WC, Kazembe PN, Phiri S, Andrianarimanana D, Weigel R. Seroprevalence of CMV, HSV‐2 and HBV among HIV‐infected Malawian children: a cross‐sectional survey. J Trop Pediatr. 2016;62(3):220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. D'Almeida M, Adedemy JD, Agossou J, et al. Frequency of HIV and viral hepatitis B co‐infection in children aged 1 to 15 years attended in a hospital environment in Parakou (Benin). Curr Pediatr Res. 2015;19(2):81‐89. [Google Scholar]
- 56. Mutwa PR, Boer KR, Rusine JB, et al. Hepatitis B virus prevalence and vaccine response in HIV‐infected children and adolescents on combination antiretroviral therapy in Kigali, Rwanda. Pediatr Infect Dis J. 2013;32(3):246‐251. [DOI] [PubMed] [Google Scholar]
- 57. Peebles K, Nchimba L, Chilengi R, Moore CB, Mubiana‐Mbewe M, Vinikoor MJ. Pediatric HIV‐HBV coinfection in Lusaka, Zambia: prevalence and short‐term treatment outcomes. J Trop Pediatr. 2015;61(6):464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stabinski L, O'Connor S, Barnhart M, Kahn RJ, Hamm TE. Prevalence of HIV and hepatitis B virus co‐infection in sub‐Saharan Africa and the potential impact and program feasibility of hepatitis B surface antigen screening in resource‐limited settings. J Acquir Immune Defic Syndr. 1999;2015(68 Suppl 3):S274‐S285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49(5 Suppl):S138‐S145. [DOI] [PubMed] [Google Scholar]
- 60. Barth RE, Huijgen Q, Tempelman HA, Mudrikova T, Wensing AM, Hoepelman AI. Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol. 2011;83(6):929‐934. [DOI] [PubMed] [Google Scholar]
- 61. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19(6):593‐601. [DOI] [PubMed] [Google Scholar]
- 62. Soriano V, Mocroft A, Peters L, et al. Predictors of hepatitis B virus genotype and viraemia in HIV‐infected patients with chronic hepatitis B in Europe. J Antimicrob Chemother. 2010;65(3):548‐555. [DOI] [PubMed] [Google Scholar]
- 63. Amini A, Varsaneux O, Kelly H, et al. Diagnostic accuracy of tests to detect hepatitis B surface antigen: a systematic review of the literature and meta‐analysis. BMC Infect Dis. 2017;17(Suppl 1):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. World Health Organisation . Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: 2016. [PubMed] [Google Scholar]
- 65. World Health Organisation . Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva, Switzerland: World Health Organisation; 2017. [PubMed] [Google Scholar]
- 66. World Health Organisation . Prevent HIV, treat and test all. Geneva, Switzerland: World Health Organisation; 2017. [Google Scholar]
- 67. World Health Organisation, UNICEF, UNAIDS . Global update on HIV treatment 2013: Results, impact and opportunities. Geneva, Switzerland: World Health Organisation; 20131‐126. [Google Scholar]
- 68. Kirakoya‐Samadoulougou F, Sanou M, Samadoulougou S, et al. High seroprevalence of hepatitis B virus and hepatitis C virus among human immunodeficiency virus carriers in blood donors of Burkina Faso: A need for their screening before HARRT therapy. J Viral Hepat. 2014;21(7):e52‐e63. [DOI] [PubMed] [Google Scholar]
- 69. Simpore J, Ilboudo D, Karou D, et al. Prevalence of HHV‐8 infections associated with HIV, HBV and HCV in pregnant women in Burkina Faso. J Med Sci. 2006;6(1):93‐98. [Google Scholar]
- 70. Ilboudo D, Simpore J, Ouermi D, et al. Towards the complete eradication of mother‐to‐child HIV/HBV coinfection at Saint Camille Medical Centre in Burkina Faso, Africa. Braz J Infect Dis. 2010;14(3):219‐224. [DOI] [PubMed] [Google Scholar]
- 71. Ankouane F, Noah DN, Atangana MM, Simo RK, Guekam PR, Sida MB. Seroprevalence of hepatitis B and C viruses, HIV‐1/2 and syphilis among blood donors in the Yaounde Central Hospital in the centre region of Cameroon. Transfus Clin Biol. 2016;23(2):72‐77. [DOI] [PubMed] [Google Scholar]
- 72. Rodgers MA, Vallari AS, Harris B, et al. Identification of rare HIV‐1 Group N, HBV AE, and HTLV‐3 strains in rural South Cameroon. Virology. 2017;504:141‐151. [DOI] [PubMed] [Google Scholar]
- 73. Dionne‐Odom J, Mbah R, Rembert NJ, et al. Hepatitis B, HIV, and syphilis seroprevalence in pregnant women and blood donors in cameroon. Infect Dis Obstet Gynecol. 2016;2016:4359401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kfutwah AK, Tejiokem MC, Njouom R. A low proportion of HBeAg among HBsAg‐positive pregnant women with known HIV status could suggest low perinatal transmission of HBV in Cameroon. Virol J. 2012;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Laurent C, Bourgeois A, Mpoudi‐Ngole E, et al. High rates of active hepatitis B and C co‐infections in HIV‐1 infected Cameroonian adults initiating antiretroviral therapy. HIV Med. 2010;11(1):85‐89. [DOI] [PubMed] [Google Scholar]
- 76. Salpini R, Fokam J, Ceccarelli L, et al. High burden of HBV‐infection and atypical HBV strains among HIV‐infected Cameroonians. Curr HIV Res. 2016;14(2):165‐171. [DOI] [PubMed] [Google Scholar]
- 77. Zoufaly A, Onyoh EF, Tih PM, Awasom CN, Feldt T. High prevalence of hepatitis B and syphilis co‐infections among HIV patients initiating antiretroviral therapy in the north‐west region of Cameroon. Int J STD AIDS. 2012;23(6):435‐438. [DOI] [PubMed] [Google Scholar]
- 78. Fomulu NJ, Morfaw FLI, Torimiro JN, Nana P, Koh MV, William T. Prevalence, correlates and pattern of Hepatitis B among antenatal clinic attenders in Yaounde‐Cameroon: Is perinatal transmission of HBV neglected in Cameroon? BMC Pregnancy Childbirth. 2013;13:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. N'Dri‐Yoman T, Anglaret X, Messou E, et al. Occult HBV infection in untreated HIV‐infected adults in Cote d'Ivoire. Antivir Ther. 2010;15(7):1029‐1034. [DOI] [PubMed] [Google Scholar]
- 80. Rouet F, Chaix ML, Inwoley A, et al. HBV and HCV prevalence and viraemia in HIV‐positive and HIV‐negative pregnant women in Abidjan, Cote d'Ivoire: the ANRS 1236 study. J Med Virol. 2004;74(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 81. Kabinda JM, Katchunga BP. Viral hepatitis B and C in individuals infected with human immunodeficiency virus in Bukavu (South‐Kivu), Democratic Republic of Congo. [French]. Les hepatites virales B et C chez les porteurs du virus de l'immunodeficience humaine a Bukavu (Sud‐Kivu), Republique democratique du Congo. J Africain d'Hepato‐Gastroenterologie. 2010;4(4):230‐235. [Google Scholar]
- 82. Xie D, Li J, Chen J, et al. Seroprevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and Treponema pallidum infections among blood donors on Bioko Island, Equatorial Guinea. PLoS ONE. 2015;10(10):e0139947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jobarteh M, Malfroy M, Peterson I, et al. Seroprevalence of hepatitis B and C virus in HIV‐1 and HIV‐2 infected Gambians. Virol J. 2010;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Amidu N, Owiredu W, Addai‐Mensah O, Alhassan A, Quaye L, Batong B. Seroprevalence and Risk Factors for Human Immunodeficiency Virus, Hepatitis B and C Vi‐ruses Infections among Blood Donors at the Bolgatanga Regional Hospital in Bolgatanga, Ghana. Ghana Sci Assoc J. 2010;12(1):77‐88. [Google Scholar]
- 85. Cho Y, Bonsu G, Akoto‐Ampaw A, et al. The prevalence and risk factors for hepatitis B surface Ag positivity in pregnant women in eastern region of Ghana. Gut Liv. 2012;6(2):235‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lokpo SY, Dakorah MP, Norgbe GK, et al. The burden and trend of blood‐borne pathogens among asymptomatic adult population in Akwatia: a retrospective study at the. St. Dominic Hospital, Ghana. J Trop Med. 2017;2017:3452513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jolly PE, Shuaib FM, Jiang Y, et al. Association of high viral load and abnormal liver function with high aflatoxin B1‐albumin adduct levels in HIV‐positive Ghanaians: preliminary observations. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(9):1224‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Obuseh FA, Jolly PE, Kulczycki A, et al. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: a cross‐sectional study. J Int AIDS Soc. 2011;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walana W, Ahiaba S, Hokey P, et al. Sero‐prevalence of HIV, HBV and HCV among blood donors in the Kintampo municipal hospital, Ghana. Br Microbiol Res J. 2014;4(12):1491‐1499. [Google Scholar]
- 90. Langhoff Honge B, Jespersen S, Medina C, et al. Hepatitis B and Delta virus are prevalent but often subclinical co‐infections among HIV infected patients in Guinea‐Bissau, West Africa: A cross‐sectional study. PLoS ONE. 2014;9(6):e99971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tounkara A, Sarro YS, Kristensen S, et al. Seroprevalence of HIV/HBV coinfection in Malian blood donors. J Int Assoc Physicians AIDS Care (Chic Ill). 2009;8(1):47‐51. [DOI] [PubMed] [Google Scholar]
- 92. Dao S, Ba A, Doumbia S, Bougoudogo F. Co‐infection hepatitis B and C (HBV and HCV) in HIV patients in urban area in Mali. Medecine d'Afrique Noire. 2007;54(10):485‐488. [Google Scholar]
- 93. Adewumi MO, Donbraye E, Sule WF, et al. HBV infection among HIV‐infected cohort and HIV‐negative hospital attendees in South Western Nigeria. Afr J Infect Dis. 2015;9(1):14‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ajayi BB, Ajayi OD, Hamidu I, et al. Seroprevalence of some sexually transmitted infections among antenatal attendees in university of Maiduguri teaching hospital, Maiduguri‐Nigeria. Ann Biol Res. 2013;4(2):141‐145. [Google Scholar]
- 95. Mabayoje VO, Oparinde DP, Akanni EO, Taiwo SS, Muhibi MA, Adebayo TO. Seroprevalence of hepatitis B and C and of human immunodeficiency virus among blood donors in south‐west Nigeria. Br J Biomed Sci. 2007;64(4):177‐179. [DOI] [PubMed] [Google Scholar]
- 96. Nwogoh B, Ikpomwen OD, Isoa EM. Donor blood procurement and the risk of transfusion transmissible viral infections in a tertiary health facility in South‐South Nigeria. Niger Med J. 2011;52(4):227‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Okonkwo UC, Okpara H, Otu A, et al. Prevalence of hepatitis B, hepatitis C and human immunodeficiency viruses, and evaluation of risk factors for transmission: report of a population screening in Nigeria. S Afr Med J. 2017;107(4):346‐351. [DOI] [PubMed] [Google Scholar]
- 98. Oronsaye FE, Oronsaye JI. Prevalence of HIV‐positives and hepatitis B surface antigen‐positives among donors in the University of Benin Teaching Hospital, Nigeria. Trop Doct. 2004;34(3):159‐160. [DOI] [PubMed] [Google Scholar]
- 99. Takalmawa HU, Emokpae MA, Abubakar AG, Kwaru AH. Prevalence of Hepatitis B Surface Antigen and human immunodeficiency virus antibodies among blood donors in Aminu Kano Teaching Hospital, Kano, Nigeria, 1996–2001. Hamdard Medicus. 2004;47(2):54‐57. [Google Scholar]
- 100. Jombo GTA, Egah DZ, Banwat EB. Hepatitis B virus and human immunodeficiency virus co‐infection in Zawan community of Plateau State. J Med Trop. 2005;7(1):21‐26. [Google Scholar]
- 101. Lesi OA, Kehinde MO, Oguh DN, Amira CO. Hepatitis B and C virus infection in Nigerian patients with HIV/AIDS. Niger Postgrad Med J. 2007;14(2):129‐133. [PubMed] [Google Scholar]
- 102. Salami TAT, Babatope IO, Adewuyi GM, Samuel SO, Echekwube PO. Hepatitis B and HIV co‐infection‐experience in a rurul/suburban health center in Nigeria. J Microbiol Biotechnol Res. 2012;2(6):841‐844. [Google Scholar]
- 103. Opara MI, Ogbebor VO, Fasasi MA, et al. Incidences of hepatitis B and syphilis co‐infection with HIV in antiretroviral treatment‐nave adult patients attending APIN clinic at a University Teaching hospital in Lagos, Nigeria. J AIDS Clin Res. 2013;4(1):1‐4. [Google Scholar]
- 104. Erhabor O, Opurum H, Ejele OA, Nwauche CA, Akani CI. HIV sero‐discordance among Nigerian couples: challenges and controversies. Niger Med Pract. 2005;48(3):62‐66. [Google Scholar]
- 105. Ezechi OC, Kalejaiye OO, Gab‐Okafor CV, et al. Sero‐prevalence and factors associated with Hepatitis B and C co‐infection in pregnant Nigerian women living with HIV Infection. Pan Afr Med J. 2014;17(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Otegbayo JA, Taiwo BO, Akingbola TS, et al. Prevalence of hepatitis B and C seropositivity in a Nigerian cohort of HIV‐infected patients. Ann Hepatol. 2008;7(2):152‐156. [PubMed] [Google Scholar]
- 107. Tremeau‐Bravard A, Ogbukagu IC, Ticao CJ, Abubakar JJ. Seroprevalence of hepatitis B and C infection among the HIV‐positive population in Abuja, Nigeria. Afr Health Sci. 2012;12(3):312‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Isoa EM, Obieche JC, Nwogoh B, Ikponmwen OD, Nwannadi AI. Hepatitis B Virus [HBV], Hepatitis C Virus [HCV] and Syphilis Co‐Infections among HIV Infected Patients at the University of Benin Teaching Hospital, Benin City. Ann Biomed Sci. 2012;11(1):65‐71. [Google Scholar]
- 109. Ejilemele AA, Nwauche CA, Ejele OA. Pattern of abnormal liver enzymes in HIV patients presenting at a Nigerian Tertiary Hospital. Niger Postgrad Med J. 2007;14(4):306‐309. [PubMed] [Google Scholar]
- 110. Eze EU, Onunu AN, Kubeyinje EP. Seroprevalence of hepatitis B virus among human immunodeficiency virus patients attending tertiary and secondary health facilities in Benin City Nigeria. J Med Biomed Res. 2007;6(1):19‐25. [Google Scholar]
- 111. Mbaawuaga EM. Studies on prevalence, co‐infection and associated risk factors of hepatitis B virus (HBV) and human immunodeficiency virus (HIV) in Benue State, Nigeria. Sex Transm Dis. 2014;41:S143. [Google Scholar]
- 112. Diwe CK, Okwara EC, Enwere OO, Azike JE, Nwaimo NC. Sero‐prevalence of hepatitis B virus and hepatitis C virus among HIV patients in a suburban University Teaching Hospital in South‐East Nigeria. Pan Afr Med J. 2013;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Diop‐Ndiaye H, Toure‐Kane C, Etard JF, et al. Hepatitis B, C seroprevalence and delta viruses in HIV‐1 Senegalese patients at HAART initiation (retrospective study). J Med Virol. 2008;80(8):1332‐1336. [DOI] [PubMed] [Google Scholar]
- 114. Patel P, Davis S, Tolle M, Mabikwa V, Anabwani G. Prevalence of hepatitis B and hepatitis C coinfections in an adult HIV centre population in Gaborone, Botswana. Am J Trop Med Hyg. 2011;85(2):390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid‐associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV‐infected adults in Botswana. Am J Respir Crit Care Med. 2010;182(2):278‐285. [DOI] [PubMed] [Google Scholar]
- 116. Rabenau HF, Lennemann T, Kircher C, et al. Prevalence‐ and gender‐specific immune response to opportunistic infections in HIV‐infected patients in Lesotho. Sex Transm Dis. 2010;37(7):454‐459. [DOI] [PubMed] [Google Scholar]
- 117. Diale Q, Pattinson R, Chokoe R, Masenyetse L, Mayaphi S. Antenatal screening for hepatitis B virus in HIV‐infected and uninfected pregnant women in the Tshwane district of South Africa. S Afr Med J. 2016;106(1):97‐100. [DOI] [PubMed] [Google Scholar]
- 118. Andersson MI, Maponga TG, Ijaz S, Theron G, Preiser W, Tedder RS. Cross sectional analysis of the prevalence and character of hepatitis B virus infection in HIV‐infected and HIV‐uninfected pregnant women in the western cape, South Africa. J Hepatol. 2012;56:S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thumbiran NV, Moodley D, Parboosing R, Moodley P. Hepatitis B and HIV co‐infection in pregnant women: indication for routine antenatal hepatitis B virus screening in a high HIV prevalence setting. S Afr Med J. 2014;104(4):307‐309. [DOI] [PubMed] [Google Scholar]
- 120. Burnett RJ, Ngobeni JM, Francois G, et al. Increased exposure to hepatitis B virus infection in HIV‐positive South African antenatal women. Int J STD AIDS. 2007;18(3):152‐156. [DOI] [PubMed] [Google Scholar]
- 121. Boyles TH, Cohen K. The prevalence of hepatitis B infection in a rural South African HIV clinic. S Afr Med J. 2011;101(7):470‐471. [PubMed] [Google Scholar]
- 122. Hoffmann CJ, Mashabela F, Cohn S, et al. Maternal hepatitis B and infant infection among pregnant women living with HIV in South Africa. J Int AIDS Soc. 2014;17:18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mavenyengwa RT, Moyo SR, Nordbo SA. Streptococcus agalactiae colonization and correlation with HIV‐1 and HBV seroprevalence in pregnant women from Zimbabwe. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):34‐38. [DOI] [PubMed] [Google Scholar]
- 124. Receveur MC, Coulaud X, Ali R, Gasnier O, Benoit‐Cattin T, Pettinelli ME. HIV in Mayotte, Indian ocean. Bull Soc Pathol Exot. 2003;96(3):238‐240. [PubMed] [Google Scholar]
- 125. Dray X, Dray‐Spira R, Bronstein JA, Mattera D. Prevalences of HIV, hepatitis B and hepatitis C in blood donors in the Republic of Djibouti. Med Trop (Mars). 2005;65(1):39‐42. [PubMed] [Google Scholar]
- 126. Wondimeneh Y, Alem M, Asfaw F, Belyhun Y. HBV and HCV seroprevalence and their correlation with CD4 cells and liver enzymes among HIV positive individuals at University of Gondar Teaching Hospital, Northwest Ethiopia. Virol J. 2013;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yami A, Alemseged F, Hassen A. Hepatitis B and C viruses infections and their association with human immunodeficiency virus: a cross‐sectional study among blood donors in Ethiopia. Ethiop J Health Sci. 2011;21:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Biadgo B, Shiferaw E, Woldu B, Alene KA, Melku M. Transfusion‐transmissible viral infections among blood donors at the North Gondar district blood bank, northwest Ethiopia: A three year retrospective study. PLoS ONE. 2017;12(7):e0180416 (no:pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tiruneh M. Seroprevalence of multiple sexually transmitted infections among antenatal clinic attendees in Gondar Health Center, northwest Ethiopia. Ethiop Med J. 2008;46(4):359‐366. [PubMed] [Google Scholar]
- 130. Manyazewal T, Sisay Z, Abegaz WE. Hepatitis B and hepatitis C viruses' infections among antiretroviral naive and experienced HIV co‐infected adults in Addis Ababa, Ethiopia. Int J Infect Dis. 2012;16:e96. [DOI] [PubMed] [Google Scholar]
- 131. Misganaw B. Prevalence of transfusion‐transmissible infections in donors to an Ethiopian blood bank between 2009 and 2013 and donation factors that would improve the safety of the blood supply in underdeveloped countries. Lab Med. 2016;47(2):134‐139. [DOI] [PubMed] [Google Scholar]
- 132. Tsegahun M, Zufan S, Sibhatu B, Woldaregay EA. Hepatitis B and hepatitis C virus infections among antiretroviral‐naive and ‐experienced HIV co‐infected adults. J Med Microbiol. 2014;63(5):742‐747. [DOI] [PubMed] [Google Scholar]
- 133. Balew M, Moges F, Yismaw G, Unakal C. Assessment of hepatitis B virus and hepatitis C virus infections and associated risk factors in HIV infected patients at Debretabor hospital, South Gondar, Northwest Ethiopia. Asian Pac J Trop Dis. 2014;4(1):1‐7. [Google Scholar]
- 134. Kim HN, Scott J, Cent A, et al. HBV lamivudine resistance among hepatitis B and HIV coinfected patients starting lamivudine, stavudine and nevirapine in Kenya. J Viral Hepat. 2011;18(10):E447‐E452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kerubo G, Khamadi S, Okoth V, et al. Hepatitis B, Hepatitis C and HIV‐1 Coinfection in Two Informal Urban Settlements in Nairobi, Kenya. [Erratum appears in PLoS One. 2015;10(7): e0133342 Note: Abdalla, Ziraba [correctd to Ziraba, Abdhalah]; PMID: 26192604]. PLoS ONE [Electronic Resource]. 2015;10(6):e0129247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ondondo R. A cross‐sectional survey of hepatitis B virus infections and natural immunity against hepatitis B virus infections among HIV discordant heterosexual couples in Kisumu, Kenya. Sex Transm Infect. 2011;87:A310‐A311. [Google Scholar]
- 137. Harania RS, Karuru J, Nelson M, Stebbing J. HIV, hepatitis B and hepatitis C coinfection in Kenya. AIDS. 2008;22(10):1221‐1222. [DOI] [PubMed] [Google Scholar]
- 138. Minniear TD, Morwabe A, Girde S, et al. Hepatitis B Virus Mother‐To‐Child‐Transmission Among HIV‐Infected Pregnant Women in Kenya. Topics Antiviral Med. 2014;22(e‐1):351‐352. [Google Scholar]
- 139. Sutcliffe S, Taha TE, Kumwenda NI, Taylor E, Liomba GN. HIV‐1 prevalence and herpes simplex virus 2, hepatitis C virus, and hepatitis B virus infections among male workers at a sugar estate in Malawi. J Acquir Immune Defic Syndr. 2002;31(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 140. Chasela CS, Kourtis AP, Wall P, et al. Hepatitis B virus infection among HIV‐infected pregnant women in Malawi and transmission to infants. J Hepatol. 2014;60(3):508‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Andreotti M, Pirillo MF, Liotta G, et al. The impact of HBV or HCV infection in a cohort of HIV‐infected pregnant women receiving a nevirapine‐based antiretroviral regimen in Malawi. BMC Infect Dis. 2014;14(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cunha L, Plouzeau C, Ingrand P, et al. Use of replacement blood donors to study the epidemiology of major blood‐borne viruses in the general population of Maputo, Mozambique. J Med Virol. 2007;79(12):1832‐1840. [DOI] [PubMed] [Google Scholar]
- 143. Stokx J, Gillet P, De Weggheleire A, et al. Seroprevalence of transfusion‐transmissible infections and evaluation of the pre‐donation screening performance at the Provincial Hospital of Tete, Mozambique. BMC Infect Dis. 2011;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ladner J, Leroy V, Simonon A, Karita E, Van de Perre P, Dabis F. Hepatitis B and HIV type 1 co‐infection in pregnant African women. Médecine et Maladies Infectieuses. 2002;32(7):396‐397. [Google Scholar]
- 145. Pirillo MF, Bassani L, Germinario EA, et al. Seroprevalence of hepatitis B and C viruses among HIV‐infected pregnant women in Uganda and Rwanda. J Med Virol. 2007;79(12):1797‐1801. [DOI] [PubMed] [Google Scholar]
- 146. Dahoma M, Johnston LG, Holman A, et al. HIV and related risk behavior among men who have sex with men in Zanzibar, Tanzania: results of a behavioral surveillance survey. Aids Behav. 2011;15(1):186‐192. [DOI] [PubMed] [Google Scholar]
- 147. Hawkins C, Christian B, Ye J, et al. Prevalence of hepatitis B co‐infection and response to antiretroviral therapy among HIV‐infected patients in Tanzania. AIDS. 2013;27(6):919‐927. [DOI] [PubMed] [Google Scholar]
- 148. Bwogi J, Braka F, Makumbi I, et al. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9(2):98‐108. [PMC free article] [PubMed] [Google Scholar]
- 149. Stabinski L, Reynolds SJ, Ocama P, et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011;16(3):405‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stabinski L, Reynolds SJ, Ocama P, et al. Hepatitis B virus and sexual behavior in Rakai, Uganda. J Med Virol. 2011;83(5):796‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hladik W, Dollard SC, Downing RG, et al. Kaposi's sarcoma in Uganda: risk factors for human herpesvirus 8 infection among blood donors. J Acquir Immune Defic Syndr. 2003;33(2):206‐210. [DOI] [PubMed] [Google Scholar]
- 152. Boon D, Redd AD, Laeyendecker O, et al. Hepatitis E virus seroprevalence and correlates of anti‐HEV IgG Antibodies in the Rakai District, Uganda. J Infect Dis. 2018;217(5):785‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kapembwa KC, Goldman JD, Lakhi S, et al. HIV, hepatitis B, and hepatitis C in Zambia. J Global Infect Dis. 2011;3(3):269‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yudin MH, Caprara D, MacGillivray J, Urquia M, Shah R. Are pregnancies among HIV‐positive pregnant women always complicated? Can J Infect Dis Med Microbiol. 2015; Conference: 24th Annual Canadian Conference on HIV/AIDS Research, CAHR 2015. Toronto, ON Canada. Conference Publication: (var.pagings). 26(SUPPL. SB):71B. [Google Scholar]
- 155. Mir‐Nasseri MM, Mohammadkhani A, Tavakkoli H, Ansari E, Poustchi H. Incarceration is a major risk factor for blood‐borne infection among intravenous drug users. Hepatitis Mon. 2011;11(1):19‐22. [PMC free article] [PubMed] [Google Scholar]
- 156. Ataei B, Tayeri K, Kassaian N, Farajzadegan Z, Babak A. Hepatitis B and C among patients infected with human immunodeficiency virus in Isfahan, Iran: Seroprevalence and associated factors. Hepat Mon. 2010;10(3):188‐192. [PMC free article] [PubMed] [Google Scholar]
- 157. Vaziri S, Mansouri F, Sayad B, Afsharian M, Janbakhsh A, Karami M. Hepatitis D virus infection among HIV‐HBV co‐infected patients in Kermanshah, West of Iran. Hepat Mon. 2008;8(4):252‐257. [Google Scholar]
- 158. Javad Zahedi M, Darvish Moghaddam S, Hayatbakhsh Abasi M, Parnian M, Shokoohi M. Hepatitis B, C virus co‐infection and behavioral risks in HIV‐positive patients in southern Iran. J Pak Med Assoc. 2014;64(2):134‐137. [PubMed] [Google Scholar]
- 159. Mirzoyan L, Berendes S, Jeffery C, et al. New evidence on the HIV epidemic in Libya: why countries must implement prevention programs among people who inject drugs. Jaids‐J Acquir Immune Defic Syndr. 2013;62(5):577‐583. [DOI] [PubMed] [Google Scholar]
- 160. Sbiti M, Khalki H, Benbella I, Louzi L. Seroprevalence of HBsAg in pregnant women in central Morocco. [French]. Pan Afr Med J. 2016;24;187. (no:pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Alhuraiji A, Alaraj A, Alghamdi S, Alrbiaan A, Alrajhi AA. Viral hepatitis B and C in HIV‐infected patients in Saudi Arabia. Ann Saudi Med. 2014;34(3):207‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mudawi H, Hussein W, Mukhtar M, et al. Overt and occult hepatitis B virus infection in adult Sudanese HIV patients. Int J Infect Dis. 2014;29:e65‐e70. [DOI] [PubMed] [Google Scholar]
- 163. Dirar H, Yassin MEM, Enan KA, Nafi M, Eldaif WA, Elkhidir IM. Sero‐prevalence of hepatitis B virus (HBsAg) and hepatitis C virus (anti‐HCV) among HIV‐infected in Sudan. J Biomed Pharmaceut Res. 2013;2(6):78‐81. [Google Scholar]
- 164. Verbrugge R, Van Beckhoven D, Sasse A. STI‐Surveillance within AIDS reference centres in Belgium ‐ High consistent sti incidence among HIV‐positive men having sex with men, 2008–2009. Sex Transm Infect. 2011;87:A137. [Google Scholar]
- 165. Dauby N, De Wit S, Delforge M, Necsoi VC, Clumeck N. Characteristics of non‐AIDS‐defining malignancies in the HAART era: a clinico‐epidemiological study. J Int AIDS Soc. 2011;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Alexiev I, Shankar A, Dimitrova R, et al. Origin and spread of HIV‐1 in persons who inject drugs in Bulgaria. Infect Genet Evol. 2016;46:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. D'Oliveira A Jr, Voirin N, Allard R, et al. Prevalence and sexual risk of hepatitis C virus infection when human immunodeficiency virus was acquired through sexual intercourse among patients of the Lyon University Hospitals, France, 1992–2002. J Viral Hepat. 2005;12(3):330‐332. [DOI] [PubMed] [Google Scholar]
- 168. Benhammou V, Tubiana R, Matheron S, et al. HBV and HCV infections in HIV‐infected pregnant women: Obstetrical outcomes. Topics Antiviral Med. 2016; Conference: 23rd Conference on Retroviruses and Opportunistic Infections, CROI 2016. United States. 24(E‐1):338. [Google Scholar]
- 169. Spinner CD, Boesecke C, Jordan C, et al. Prevalence of asymptomatic sexually transmitted infections in HIV‐positive men who have sex with men in Germany: results of a multicentre cross‐sectional study. Infection. 2018;46(3):341‐347. [DOI] [PubMed] [Google Scholar]
- 170. Le MP, Charpentier C, Soulie C, et al. Safety, Efficacy, and PK of Atazanavir/Ritonavir (300/100 mg QD) in HIV+ Pregnant Women Cohort. Topics Antiviral Med. 2014;22(e‐1):465. [Google Scholar]
- 171. Le Moing V, Chene G, Spire B, Raffi F, Leport C. [Outcome of HIV‐infected patients after 5 years of antiretroviral therapy including a protease inhibitor: the Aproco/Copilote Cohort]. Presse Med. 2005;34(10 Suppl):1s31‐7. [PubMed] [Google Scholar]
- 172. Bruck S, Witte S, Brust J, et al. Hepatotoxicity in patients prescribed efavirenz or nevirapine. Eur J Med Res. 2008;13(7):343‐348. [PubMed] [Google Scholar]
- 173. Jansen K, Thamm M, Bock C‐T, et al. High prevalence and high incidence of coinfection with hepatitis B, hepatitis C, and syphilis and low rate of effective vaccination against hepatitis B in HIV‐positive men who have sex with men with known date of HIV seroconversion in Germany. PLoS ONE [Electronic Resource]. 2015;10(11):e0142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jansen K, Scheufele R, Bock C, et al. High prevalence of hepatitis B (HBV) coinfections, and low rate of effective HBV‐vaccination in msm with known date of HIV‐1 seroconversion in Germany. Sex Transm Infect. 2013;89:A209‐88. [Google Scholar]
- 175. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV‐infected individuals: a cohort study and meta‐analysis. Clin Infect Dis. 2009;48(12):1763‐1771. [DOI] [PubMed] [Google Scholar]
- 176. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. HIV/HBV co‐infection and rate of antiretroviral treatment change after highly active antiretroviral treatment initiation in a cohort of HIV‐infected patients in Greece. Int J STD AIDS. 2010;21(10):702‐707. [DOI] [PubMed] [Google Scholar]
- 177. Pontali E, Ferrari F. Prevalence of hepatitis B virus and/or hepatitis C virus co‐infections in prisoners infected with the Human Immunodeficiency Virus. Int J Prison Health. 2008;4(2):77‐82. [DOI] [PubMed] [Google Scholar]
- 178. De Socio GV, Bonfanti P, Ricci E, et al. Cholesterol levels in HIV‐HCV infected patients treated with lopinavir/r: results from the SCOLTA project. Biomed Pharmacother. 2008;62(1):16‐20. [DOI] [PubMed] [Google Scholar]
- 179. Guriev V, Spinu C. Evaluation of epidemiological particularities of HIV‐infection associated with viral hepatitis B and C in the Republic of Moldova. HIV AIDS Rev. 2010;9(4):97‐100. [Google Scholar]
- 180. Snijdewind IJ, Smit C, Godfried MH, et al. Hcv coinfection, an important risk factor for hepatotoxicity in pregnant women starting antiretroviral therapy. J Infect. 2012;64(4):409‐416. [DOI] [PubMed] [Google Scholar]
- 181. Kooij KW, Wit FWNM, van Zoest RA, et al. Liver fibrosis in HIV‐infected individuals on long‐term antiretroviral therapy: associated with immune activation, immunodeficiency and prior use of didanosine. Aids. 2016;30(11):1771‐1780. [DOI] [PubMed] [Google Scholar]
- 182. Zhang S, Av S, Kesselring A, et al. Risk of non‐AIDS‐defining events among HIV‐infected patients not yet on antiretroviral therapy. HIV Med. 2015;16(5):265‐272. [DOI] [PubMed] [Google Scholar]
- 183. Tovo CV, Santos DED, Mattos ÂZD, Almeida PRLd, Mattos AAD, Santos BR. Prevalência ambulatorial em um hospital geral de marcadores para hepatites B e C em pacientes com infecção pelo vírus da imunodeficiência humana. Arq Gastroenterol. 2006;43(2):73‐76. [DOI] [PubMed] [Google Scholar]
- 184. Collazos J, Valle‐Garay E, Carton JA, et al. Factors associated with long‐term CD4 cell recovery in HIV‐infected patients on successful antiretroviral therapy. HIV Med. 2016;17(7):532‐541. [DOI] [PubMed] [Google Scholar]
- 185. Tuma P, Pineda JA, Labarga P, et al. HBV primary drug resistance in newly diagnosed HIV‐HBV‐coinfected individuals in Spain. Antivir Ther. 2011;16(4):585‐589. [DOI] [PubMed] [Google Scholar]
- 186. Campo J, Cano J, del Romero J, Hernando V, Rodriguez C, Bascones A. Oral complication risks after invasive and non‐invasive dental procedures in HIV‐positive patients. Oral Dis. 2007;13(1):110‐116. [DOI] [PubMed] [Google Scholar]
- 187. Palacios R, Mata R, Aguilar I, et al. High seroprevalence but low incidence of HCV infection in a cohort of patients with sexually transmitted HIV in Andalusia, Spain. J Int Assoc Physicians AIDS Care (Chic Ill). 2009;8(2):100‐105. [DOI] [PubMed] [Google Scholar]
- 188. Quezada M, Martin‐Carbonero L, Soriano V, et al. Prevalence and risk factors associated with pulmonary hypertension in HIV‐infected patients on regular follow‐up. AIDS. 2012;26(11):1387‐1392. [DOI] [PubMed] [Google Scholar]
- 189. Sahin A, Sahin ST, Namiduru M, Karaoglan I, Bosnak V. Seroprevalence of hepatitis B and hepatitis C virus in HIV/AIDS patients at Gaziantep University. Mediterranean J Infect Microb Antimicrob. 2016;5:1‐5. [Google Scholar]
- 190. Ural S, Kaptan F, Turker N, Ormen B, El S, Coskun NA. Seroprevalance of hepatitis B virus and hepatitis C virus infections in human immunodeficiency virus‐infected patients. Hepatol Int. 2013;7:S275‐S276. [Google Scholar]
- 191. Alp E, Bozkurt I, Doganay M. Epidemiological and clinical characteristics of HIV/AIDS patients followed‐up in Cappadocia region: 18 years experience. Mikrobiyol Bul. 2011;45(1):125‐136. [PubMed] [Google Scholar]
- 192. Manavi K. Hepatitis B exposure, immunity and infection in newly diagnosed HIV infected men who have sex with men: A 10‐ year analysis. HIV Med. 2017; Conference: 23rd Annual Conference of the British HIV Association, BHIVA 2017. United Kingdom. 18(Supplement 1):38. [Google Scholar]
- 193. Thornton AC, Jose S, Bhagani S, et al. Hepatitis B, hepatitis C, and mortality among HIV‐positive individuals. AIDS. 2017;31(18):2525‐2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zhu L, Wang Y, Sun M, Hu W. HBV, HCV, and syphilis co‐infections among HIV positive blood donors in blood center of zhejiang province, china: A retrospective analysis. Vox Sang. 2017; Conference: 28th Regional Congress of the International Society of Blood Transfusion. China. 112(Supplement 2):113.. [Google Scholar]
- 195. Dong Y, Qiu C, Xia X, et al. Hepatitis B virus and hepatitis C virus infection among HIV‐1‐infected injection drug users in Dali, China: prevalence and infection status in a cross‐sectional study. Arch Virol. 2015;160(4):929‐936. [DOI] [PubMed] [Google Scholar]
- 196. Li JR, Gong RY, Tian KL, Wang J, Wang YX, Huang HJ. Study on the blood‐borne virus co‐infection and T lymphocyte subset among intravenous drug users. World J Gastroenterol. 2007;13(16):2357‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhang F, Zhou S, Chen X, et al. Seroprevalence of HBV and HCV among HIV‐infected adults in China according to route of HIV infection: High rate of HBV and HCV exposure. Future Virol. 2012;7(10):1015‐1020. [Google Scholar]
- 198. Kou H, Du X, Li Y, et al. Comparison of Nevirapine Plasma Concentrations between Lead‐In and Steady‐State Periods in Chinese HIV‐Infected Patients. PLoS ONE. 2013;8(1):e52950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zhou YH, Yao ZH, Liu FL, et al. High prevalence of HIV, HCV, HBV and co‐infection and associated risk factors among injecting drug users in Yunnan Province, China. PLoS ONE. 2012;7(8):e42937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sheng WH, Kao JH, Chen PJ, et al. Evolution of hepatitis B serological markers in HIV‐infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45(9):1221‐1229. [DOI] [PubMed] [Google Scholar]
- 201. Hsieh MH, Hsieh MY, Huang CF, et al. Anti‐HIV seropositivity was related to HBsAg seropositivity among injecting drug users in Taiwan. Kaohsiung J Med Sci. 2016;32(2):96‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lee HC, Ko NY, Lee NY, Chang CM, Ko WC. Seroprevalence of viral hepatitis and sexually transmitted disease among adults with recently diagnosed HIV infection in Southern Taiwan, 2000–2005: upsurge in hepatitis C virus infections among injection drug users. J Formos Med Assoc. 2008;107(5):404‐411. [DOI] [PubMed] [Google Scholar]
- 203. Chung‐Chih L, Wen‐Chun L, Chi‐Tai F, et al. Transmitted drug resistance of HIV‐1 strains among individuals attending voluntary counselling and testing in Taiwan. J Antimicrob Chemother. 2016;71(1):226‐234. [DOI] [PubMed] [Google Scholar]
- 204. Chang SY, Yang CL, Ko WS, et al. Molecular epidemiology of hepatitis D virus infection among injecting drug users with and without human immunodeficiency virus infection in Taiwan. J Clin Microbiol. 2011;49(3):1083‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cheng SH, Chiang SC, Hsieh YL, Chang YY, Liu YR, Chu FY. Gender difference in the clinical and behavioral characteristics of human immunodeficiency virus‐infected injection drug users in Taiwan. J Formos Med Assoc. 2007;106(6):467‐474. [DOI] [PubMed] [Google Scholar]
- 206. Liang SH, Lo YC, Lin HH, et al. Seroprevalence of viral hepatitis and infectious complications among human immunodeficiency virus‐infected injection drug users at a referral hospital. J Microbiol Immunol Infect. 2008;41(3):200‐208. [PubMed] [Google Scholar]
- 207. Liu W, Sun H, Lee N, et al. Multicentre study of prevalence of hepatitis B virus infection among persons born in the era of nationwide HBV Vaccination: Implication for booster vaccination for persons at risk for HIV transmission. Clin Microbiol Infect. 2012;18:87. [Google Scholar]
- 208. Chang SY, Chen MY, Lee CN, et al. Trends of antiretroviral drug resistance in treatment‐naive patients with human immunodeficiency virus type 1 infection in Taiwan. J Antimicrob Chemother. 2008;61(3):689‐693. [DOI] [PubMed] [Google Scholar]
- 209. Lee Y‐L, Lin K‐Y, Cheng C‐Y, et al. Evolution of hepatitis A virus seroprevalence among HIV‐positive adults in Taiwan. PLoS ONE [Electronic Resource]. 2017;12(10):e0186338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV‐infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50(3):781‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Tseng YT, Sun HY, Chang SY, et al. Seroprevalence of hepatitis virus infection in men who have sex with men aged 18–40 years in Taiwan. J Formos Med Assoc. 2012;111(8):431‐438. [DOI] [PubMed] [Google Scholar]
- 212. Tsai HC, Chen IT, Weng YW, et al. High rate of HIV‐1 drug resistance in treatment‐experienced patients in Southern Taiwan, 2009–2014. J Microbiol Immunol Infect. 2015;Conference: 7th International Congress of the Asia Pacific Society of Infection Control. Taipei Taiwan (Republic of China). Conference Publication: (var.pagings). 48(2 SUPPL. 1):S135. [Google Scholar]
- 213. Lai C‐C, Liu W‐C, Fang C‐T, et al. Transmitted drug resistance of HIV‐1 strains among individuals attending voluntary counselling and testing in Taiwan. J Antimicrob Chemother. 2016;71(1):226‐234. [DOI] [PubMed] [Google Scholar]
- 214. Wu P, Cheng C, Liu C, et al. Multicenter study of skin rashes and hepatotoxicity in antiretroviral‐naive HIV‐positive patients receiving non‐nucleoside reverse‐transcriptase inhibitor plus nucleoside reverse‐transcriptase inhibitors in Taiwan. PLoS ONE. 2017;12(2):e0171596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Yang JJ, Huang CH, Liu CE, et al. Multicenter study of trimethoprim/sulfamethoxazole‐related hepatotoxicity: Incidence and associated factors among HIV‐infected patients treated for Pneumocystis jirovecii pneumonia. PLoS ONE. 2014;9(9):e106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sun H, Cheng C, Lee N, et al. Seroprevalence of hepatitis B virus among adults at high risk for HIV transmission two decades after implementation of nationwide hepatitis B virus vaccination program in Taiwan. PLoS ONE. 2014;9(2):e90194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hsieh M, Tsai J, Hsieh M, et al. Hepatitis C virus infection among injection drug users with and without human immunodeficiency virus co‐infection. PLoS ONE. 2014;9(4):e94791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Verma S, Mahajan A, Singh JB, Sharma M. Clinical profile of HIV/AIDS patients in Jammu. JK Pract. 2007;14(2):79‐83. [Google Scholar]
- 219. Sawaithul VK, Ukey PM, Bobhate SK. Seroprevalence of HIV, HBV, HCV and Syphilis in blood donors of Central India. Biomed Res. 2006;17(2):139‐143. [Google Scholar]
- 220. Saini PA, Chakrabarti PR, Varma AV, Gambhir S, Tignath G, Gupta P. Hepatitis C virus: Unnoticed and on the rise in blood donor screening? A 5 years cross‐sectional study on seroprevalence in voluntary blood donors from central India. J Global Infect Dis. 2017;9(2):51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Arora DR, Gautam V, Sethi S, Arora B. A 16‐year study of HIV seroprevalence and HIV‐related diseases in a teaching tertiary care hospital in India. Int J STD AIDS. 2004;15(3):178‐182. [DOI] [PubMed] [Google Scholar]
- 222. Ankur G, Sapna G, Ankit L, Arti A. Very low prevalence of hepatitis B and C Co‐infection in HIV‐positive medical inpatients in a tertiary care hospital in Agra (UP), Northern India. Indian J Sex Transm Dis. 2012;33(2):147‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Nayak S, Kakkar B, Bajpai M. Trends in the prevalence of transfusion transmitted infections among blood donors in a tertiary care hospital in north India: Eight years' experience. Vox Sang. 2017;Conference: 28th Regional Congress of the International Society of Blood Transfusion. China. 112(Supplement 2):93‐94. [Google Scholar]
- 224. Anamika V, Ramavtar S, Pooja G. Prevalence of hepatitis B surface antigen (HBsAg) among HIV seropositive patients. J Basic Appl Sci. 2012;8(1):243‐246. [Google Scholar]
- 225. Raizada A, Dwivedi S, Bhattacharya S, Hepatitis B. hepatitis C and HIV co‐infection at an antiretroviral centre in Delhi. Trop Doc. 2011;41(3):154‐156. [DOI] [PubMed] [Google Scholar]
- 226. Solomon SS, Hawcroft CS, Narasimhan P, et al. Comorbidities among HIV‐infected injection drug users in Chennai, India. Indian J Med Res. 2008;127(5):447‐452. [PMC free article] [PubMed] [Google Scholar]
- 227. Dikshit B, Wanchu A, Sachdeva RK, Sharma A, Das R. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disord. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Singh SK, Singh S, Nath G, Srivastava MK. Co‐infection of hepatitis B virus and hepatitis C virus with human immunodeficiency virus infection: A cross‐sectional study. Indian J Sex Transm Dis. 2016;37(1):95‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ray Saraswati L, Sarna A, Sebastian MP, et al. HIV, Hepatitis B and C among people who inject drugs: high prevalence of HIV and Hepatitis C RNA positive infections observed in Delhi, India. BMC Public Health. 2015;15(1):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ketki J, Charoo H, Shalini M. Study of hepatitis B virus and hepatitis D virus infection in HIV infected patients and its correlation with CD4 counts in a tertiary care hospital. Int J Curr Microbiol Appl Sci. 2014;3(8):445‐452. [Google Scholar]
- 231. Parande MV, Mantur BG, Parande AM, et al. Seroprevalence of human immunodeficiency virus & hepatitis B virus co‐infection in Belgaum, southern India. Indian J Med Res. 2013;138(3):364‐365. [PMC free article] [PubMed] [Google Scholar]
- 232. Bisht TV, Kanetkar SR, Kumbhar SS, Chavan S. Seroprevalence of infectious markers among blood donors from blood bank of a tertiary care hospital. Indian J Hematol Blood Transf. 2013;29(4):391‐392. [Google Scholar]
- 233. Sharma DC, Rai S, Bharat S, et al. Transfusion Transmissible Infections among Blood Donors at the Blood Bank of Medical College of Gwalior: A 5 Year Study. Int Blood Res Rev. 2014;2:235‐246. [Google Scholar]
- 234. Premalatha E, Sharavanan TKV, Jayalakshmi G. Seroprevalence of hepatitis‐B and hepatitis‐C infection among HIV positive and HIV negative individuals in a Tertiary Care Hospital. Scholars J Appl Med Sci. 2014;2(5B):1596‐1600. [Google Scholar]
- 235. Mathai J, Sulochana PV, Satyabhama S, Nair PKR, Sivakumar S. Profile of transfusion transmissible infections and associated risk factors among blood donors of Kerala. Indian J Pathol Microbiol. 2002;45(3):319‐322. [PubMed] [Google Scholar]
- 236. Wisaksana R, Indrati AK, Fibriani A, et al. Response to first‐line antiretroviral treatment among human immunodeficiency virus‐infected patients with and without a history of injecting drug use in Indonesia. Addiction. 2010;105(6):1055‐1061. [DOI] [PubMed] [Google Scholar]
- 237. Utsumi T, Yano Y, Lusida MI, et al. Detection of highly prevalent hepatitis B virus co‐infection with HIV in Indonesia. Hepatol Res. 2013;43(10):1032‐1039. [DOI] [PubMed] [Google Scholar]
- 238. Sai Ko Ko Z, Tun ST, Thida A, et al. Prevalence of hepatitis C and B virus among patients infected with HIV: a cross‐sectional analysis of a large HIV care programme in Myanmar. Trop Doct. 2013;43(3):113‐115. [DOI] [PubMed] [Google Scholar]
- 239. Min Min W, Win A. Seroprevalence of HBsAg and anti‐HBs among people living with HIV/AIDS (PLWHA). Myanmar Health Sci Res J. 2010;22(2):129‐130. [Google Scholar]
- 240. Sana M, Muhammad I, Shah AMH, et al. Hepatitis B and C virus infections among human immunodeficiency virus‐infected people who inject drugs in Lahore, Pakistan. Viral Immunol. 2017;30(5):366‐370. [DOI] [PubMed] [Google Scholar]
- 241. Mansha S, Imran M, Shah AMU, et al. Hepatitis B and C virus infections among human immunodeficiency virus‐infected people who inject drugs in Lahore, Pakistan. Viral Immunol. 2017;30(5):366‐370. [DOI] [PubMed] [Google Scholar]
- 242. Lam CR, Holtz TH, Leelawiwat W, et al. Subtypes and risk behaviors among incident HIV cases in the Bangkok men who have sex with men cohort study, Thailand, 2006–2014. AIDS Res Hum Retroviruses. 2017;33(10):1004‐1012. [DOI] [PubMed] [Google Scholar]
- 243. Kiertiburanakul S, Chotiprasitsakul D, Atamasirikul K, Sungkanuparph S. Late and low compliance with hepatitis B serology screening among HIV‐infected patients in a resource‐limited setting: an issue to improve HIV care. Curr HIV Res. 2011;9(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 244. Tsuchiya N, Pathipvanich P, Rojanawiwat A, et al. Chronic hepatitis B and C co‐infection increased all‐cause mortality in HAART‐naive HIV patients in Northern Thailand. Epidemiol Infect. 2013;141(9):1840‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Rangarajan S, Colby DJ, Truong GL, et al. Factors associated with HIV RNA viral loads in ART‐naive patients: implications for treatment as prevention in concentrated epidemics. J Virus Erad. 2016;2(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ishizaki A, Tran VT, Nguyen CH, et al. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS ONE. 2017;12(6):e0179616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Falster K, Wand H, Donovan B, et al. Hospitalizations in a cohort of HIV patients in Australia, 1999–2007. AIDS. 2010;24(9):1329‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Jin F, Prestage GP, Zablotska I, et al. High rates of sexually transmitted infections in HIV positive homosexual men: data from two community based cohorts. Sex Transm Infect. 2007;83(5):397‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Lincoln D, Petoumenos K, Dore GJ, Australian HIVOD . HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med. 2003;4(3):241‐249. [DOI] [PubMed] [Google Scholar]
- 250. Cooley L, Ayres A, Bartholomeusz A, et al. Prevalence and characterization of lamivudine‐resistant hepatitis B virus mutations in HIV‐HBV co‐infected individuals. AIDS. 2003;17(11):1649‐1657. [DOI] [PubMed] [Google Scholar]
- 251. Kojima Y, Kawahata T, Mori H, et al. Identification of novel recombinant forms of hepatitis B virus generated from genotypes Ae and G in HIV‐1‐positive Japanese men who have sex with men. AIDS Res Hum Retroviruses. 2015;31(7):760‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Koike K, Kikuchi Y, Kato M, et al. Prevalence of hepatitis B virus infection in Japanese patients with HIV. Hepatol Res. 2008;38(3):310‐314. [DOI] [PubMed] [Google Scholar]
- 253. Gatanaga H, Ibe S, Matsuda M, et al. Drug‐resistant HIV‐1 prevalence in patients newly diagnosed with HIV/AIDS in Japan. Antivir Res. 2007;75(1):75‐82. [DOI] [PubMed] [Google Scholar]
- 254. Shibayama T, Masuda G, Ajisawa A, et al. Characterization of seven genotypes (A to E, G and H) of hepatitis B virus recovered from Japanese patients infected with human immunodeficiency virus type 1. J Med Virol. 2005;76(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 255. Kojima Y, Kawahata T, Mori H, et al. Prevalence and epidemiological traits of HIV infections in populations with high‐risk behaviours as revealed by genetic analysis of HBV. Epidemiol Infect. 2013;141(11):2410‐2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013;56(12):1812‐1819. [DOI] [PubMed] [Google Scholar]
- 257. Kim YC, Ahn JY, Kim JM, et al. Human immunodeficiency virus (HIV) and hepatitis virus coinfection among HIV‐infected Korean patients: The Korea HIV/AIDS cohort study. Infect Chemother. 2017;49(4):268‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Santos EA, Yoshida CF, Rolla VC, et al. Frequent occult hepatitis B virus infection in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2003;22(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 259. Sutmoller F, Penna TL, de Souza CT, Lambert J. Oswaldo Cruz Foundation STDHIVPG. Human immunodeficiency virus incidence and risk behavior in the 'Projeto Rio': results of the first 5 years of the Rio de Janeiro open cohort of homosexual and bisexual men, 1994–98. Int J Infect Dis. 2002;6(4):259‐265. [DOI] [PubMed] [Google Scholar]
- 260. Tornatore M, Goncalves CV, Bianchi MS, et al. Co‐infections associated with human immunodeficiency virus type 1 in pregnant women from southern brazil: high rate of intraepithelial cervical lesions. Mem Inst Oswaldo Cruz. 2012;107(2):205‐210. [DOI] [PubMed] [Google Scholar]
- 261. Kreitchmann R, Fuchs SC, Suffert T, Preussler G. Perinatal HIV‐1 transmission among low income women participants in the HIV/AIDS Control Program in Southern Brazil: a cohort study. BJOG. 2004;111(6):579‐584. [DOI] [PubMed] [Google Scholar]
- 262. Maia MMM, Lage EM, Moreira BCB, et al. Prevalence of congenital and perinatal infection in HIV positive pregnant in Belo Horizonte metropolitan region. Rev Bras Ginecol Obstet. 2015;37(9):421‐427. [DOI] [PubMed] [Google Scholar]
- 263. Marchesini AM, Pra‐Baldi ZP, Mesquita F, Bueno R, Buchalla CM. Hepatitis B and C among injecting drug users living with HIV in Sao Paulo, Brazil. Rev Saude Publica. 2007;41(Suppl 2):57‐63. [DOI] [PubMed] [Google Scholar]
- 264. Moura AA, de Mello MJ, Correia JB. Prevalence of syphilis, human immunodeficiency virus, hepatitis B virus, and human T‐lymphotropic virus infections and coinfections during prenatal screening in an urban Northeastern Brazilian population. Int J Infect Dis. 2015;39:10‐15. [DOI] [PubMed] [Google Scholar]
- 265. Goncalez TTG, Moreno ECM, Bolina‐Santos BSE, et al. HIV, hepatitis B, hepatitis C and HTLV co‐infection among blood donors at three large Brazilian blood centers. Vox Sang. 2015; Conference: 25th Regional Congress of the International Society of Blood Transfusion in Conjunction with the 33rd Annual Conference of the British Blood Transfusion Society. London United Kingdom. Conference Publication: (var.pagings). 109(SUPPL. 1):229‐230. [Google Scholar]
- 266. Joao EC, Calvet GA, Menezes JA, et al. Nevirapine toxicity in a cohort of HIV‐1‐infected pregnant women. Am J Obstetr Gynecol. 2006;194(1):199‐202. [DOI] [PubMed] [Google Scholar]
- 267. Queiroz NMB, Sampaio DDA, Santos EDS, Bezerra ACdS. Logistic model for determining factors associated with HIV infection among blood donor candidates at the Fundação HEMOPE. Rev Bras Hematol Hemoter. 2012;34(3):217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Brunini SM, Valadares J, Guimaraes LCC, Almeida BL, Souza CM. Prevalence of sexually transmitted infections among pregnant HIV‐positive in central‐west Brazil. Sex Transm Infect. 2015;Conference: STI and HIV World Congress 2015. Brisbane, QLD Australia. Conference Publication: (var.pagings). 91(SUPPL. 2):A214‐A215. [Google Scholar]
- 269. Mendes‐Correa MC, Pinho JR, Locarnini S, et al. High frequency of lamivudine resistance mutations in Brazilian patients co‐infected with HIV and hepatitis B. J Med Virol. 2010;82(9):1481‐1488. [DOI] [PubMed] [Google Scholar]
- 270. Alencar WK, Duarte PS, Waldman EA. Survival analysis of acquired immune deficiency syndrome patients with and without hepatitis C virus infection at a reference center for sexually transmitted diseases/acquired immune deficiency syndrome in Sao Paulo, Brazil. Braz J Infect Dis. 2014;18(2):150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Torres TS, Cardoso SW, Velasque LDS, et al. Aging with HIV: an overview of an urban cohort in Rio de Janeiro (Brazil) across decades of life. Braz J Infect Dis. 2013;17(3):324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Pérez CC, Cerón AI, Fuentes LG, et al. Coinfecciones por virus hepatitis B, virus hepatitis C, Treponema pallidum y Toxoplasma gondii en la cohorte de pacientes VIH positivos en control en la Pontificia Universidad Católica de Chile Hepatitis B, C, Treponema pallidum and Toxoplasma gondii co‐infections in HIV infected patients. Rev Méd Chile. 2009;137(5):641‐648. [PubMed] [Google Scholar]
- 273. Polo P, Castañeda C, Sierra M, Alvis N. Hepatitis B oculta en pacientes VIH positivos de una institución de salud en Barranquilla Colombia. Infectio. 2010;14(1):39‐46. [Google Scholar]
- 274. Bedoya JAP, Marquez MMC, Arias JAC. Seroprevalence of markers of transfusion transmissible infections in blood bank in Colombia. Rev Saude Publica. 2012;46(6):950‐959. [PubMed] [Google Scholar]
- 275. Bautista‐Amorocho H, Castellanos‐Dominguez YZ, Rodriguez‐Villamizar LA, Velandia‐Cruz SA, Becerra‐Pena JA, Farfan‐Garcia AE. Epidemiology, risk factors and genotypes of HBV in HIV‐infected patients in the northeast region of Colombia: High prevalence of occult hepatitis B and F3 subgenotype dominance. PLoS ONE. 2014;9(12):e114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sanchez‐Gomez A, Jacobson JO, Montoya O, et al. HIV, STI and behavioral risk among men who have sex with men in a setting of elevated HIV prevalence along Ecuador's Pacific Coast. AIDS Behav. 2015;19(9):1609‐1618. [DOI] [PubMed] [Google Scholar]
- 277. Tohme RA, Andre‐Alboth J, Tejada‐Strop A, et al. Hepatitis B virus infection among pregnant women in Haiti: a cross‐sectional serosurvey. J Clin Virol. 2016;76:66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lama JR, Agurto HS, Guanira JV, et al. Hepatitis B infection and association with other sexually transmitted infections among men who have sex with men in Peru. Am J Trop Med Hyg. 2010;83(1):194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Jaspe RC, Sulbaran YF, Loureiro CL, et al. Genetic diversity of hepatitis B virus and hepatitis C virus in human immunodeficiency virus type 1‐co‐infected patients from Venezuela. J Med Microbiol. 2014;63(Pt 8):1099‐1104. [DOI] [PubMed] [Google Scholar]
- 280. Araujo NM, Branco‐Vieira M, Silva ACM, et al. Occult hepatitis B virus infection in HIV‐infected patients: evaluation of biochemical, virological and molecular parameters. Hepatol Res. 2008;38(12):1194‐1203. [DOI] [PubMed] [Google Scholar]
- 281. Remis RS, Liu J, Loutfy MR, et al. Prevalence of sexually transmitted viral and bacterial infections in HIV‐positive and HIV‐negative men who have sex with men in Toronto. PLoS ONE. 2016;11(7):e0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Tedaldi EM, Absalon J, Thomas AJ, Shlay JC, van den Berg‐Wolf M. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: results of CPCRA 058 (FIRST Study). J Acquir Immune Defic Syndr. 2008;47(4):441‐448. [DOI] [PubMed] [Google Scholar]
- 283. Dutta A, Uno H, Holman A, Lorenz DR, Wolinsky SM, Gabuzda D. Long‐term nitrite inhalant exposure and cancer risk in MSM. AIDS. 2017;31(8):1169‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus‐infected subjects. J Infect Dis. 2003;188(4):571‐577. [DOI] [PubMed] [Google Scholar]
- 285. Saberi P, Ranatunga DK, Quesenberry CP, Silverberg MJ. Clinical implications of the nelfinavir‐proton pump inhibitor drug interaction in patients with human immunodeficiency virus. Pharmacotherapy. 2011;31(3):253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. King MD, Reznik DA, O'Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus‐associated oral warts among human immunodeficiency virus‐seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34(5):641‐648. [DOI] [PubMed] [Google Scholar]
- 287. Price H, Bansi L, Sabin CA, et al. Hepatitis B virus infection in HIV‐positive individuals in the UK collaborative HIV cohort (UK CHIC) study. PLoS ONE. 2012;7(11):e49314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Hoffmann CJ, Seaberg EC, Young S, et al. Hepatitis B and long‐term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23(14):1881‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kim HN, Crane HM, Rodriguez CV, et al. The role of current and historical alcohol use in hepatic fibrosis among HIV‐infected individuals. AIDS Behav. 2017;21(7):1878‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Tien PC, Kovacs A, Bacchetti P, et al. Association between syphilis, antibodies to herpes simplex virus type 2, and recreational drug use and hepatitis B virus infection in the Women's Interagency HIV Study. Clin Infect Dis. 2004;39(9):1363‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Butt ZA, Wilkins MJ, Hamilton E, Todem D, Gardiner JC, Saeed M. Hepatitis B and C co‐infection in HIV/AIDS population in the state of Michigan. Epidemiol Infect. 2013;141(12):2604‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


