Abstract
Atherosclerosis is considered an irreversible process, with crucial contribution of inflammation and immune cells. Impact of cancer immunotherapy on a partly immune-driven disease, such as atherosclerosis, is poorly understood, but preclinical models suggest its worsening on programmed death/ligand-1 (PD-1/PD-L1) inhibitors. In a previously reported cohort of 11 patients with non-small cell lung cancer (NSCLC) treated with nivolumab and pre-existing complicated atheromatous plaques, 3 patients had a dramatic radiologic reduction of aortic plaques while on nivolumab; of these 3, 2 died receiving no further treatment. The remaining patient was an 83-year-old woman with history of arterial hypertension and hypothyroidism who was diagnosed with locally advanced squamous NSCLC. At relapse, complicated aortic atheromatous plaques were demonstrated on scans. The patient was then treated with nivolumab obtaining stable disease at radiological assessment, which also demonstrated almost complete vanishing of aortic plaques. After relapse and interval treatment with chemotherapy, she experienced new development of aortic atheromatous plaques. At further relapse she received atezolizumab, which yielded disease response and new reduction in aortic plaques, until nearly complete resolution. The observation of a repeated improvement of atheromatous plaques on treatment with PD-1/PD-L1 inhibitors favors the protective role of T cells on atheromatous plaques that is impaired by PD-L1 expression by plaque-associated macrophages. Validation by independent and prospective observation is needed.
Keywords: atherosclerosis, immunotherapy, NSCLC, PD-1, PD-L1
Introduction
Non-small cell lung cancer (NSCLC) has the highest mortality rate among solid tumors, irrespective of gender.1 Immunotherapy with immune checkpoint inhibitors (ICIs) is meant to restore immune response against tumor cells. This approach improved survival and revolutionized treatment algorithm in different settings and in many cancer types, including NSCLC.2,3 Inhibition of binding of programmed cell death 1 (PD-1) to its ligand (PD-L1) is the most exploited immune-enhancing mechanism in NSCLC. Alongside improved outcome, ICIs introduced a new set of side effects because of their ability to hyperactivate the immune system, whose severity spans from mild to fatal.4,5
Atherosclerosis is a chronic multi-step inflammatory process characterized by deposition of lipids underneath the endothelium of medium-sized and large arteries. This process is led by high lipid plasma levels and activation of T cells and macrophages. The latter, in particular, phagocyte oxidized low-density lipoproteins (oxLDL), turning them into the classic ‘foam cells’.6 Atherosclerosis is largely considered a progressive phenomenon that eventually becomes irreversible; thus, available treatments to date, for example, acetylsalicylic acid and statins, are meant to prevent its progression, rather than revert it.
However, the contribution of inflammation in atherosclerosis is clinically relevant, considering the results obtained through targeting of the pro-inflammatory cytokine IL-1β with the monoclonal antibody canakinumab in the CANTOS trial in patients with previous myocardial infarction and high C-reactive protein levels.7
We previously reported a retrospective analysis on 38 NSCLC patients who received nivolumab, a fully human IgG4 anti-PD-1 monoclonal antibody, within the Italian Expanded Access Program (EAP) at our institution.8 We then identified 11 patients who presented atherosclerotic disease, characterized by the presence of pre-existing complicated aortic plaques, according to the assessment by two independent radiologists. All patients had a history of smoking, and had received a prior platinum-based chemotherapy. Of the 11 patients, 10 had at least one cardiovascular risk factor, for which only 1 patient was taking statins. Among these patients, three (27%) had a significant improvement in atherosclerotic plaques while on nivolumab. No correlation between plaque reduction and other variable was observed, also because of the small sample size, but all three patients had immune-related adverse events while on nivolumab. Two of these patients died without receiving further treatment. Here, we report follow up of the remaining patient. Written consent was obtained from the patient, while local Institutional Review Board approval was waived as the patient in the report is not identifiable.
Case report
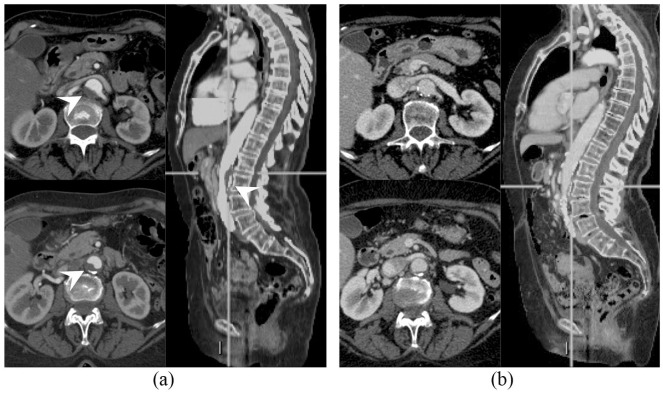
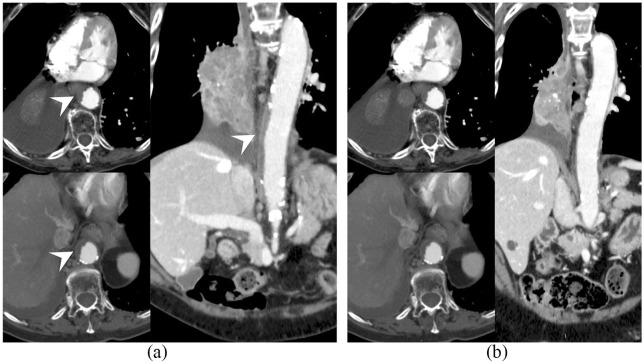
The patient was an 83-year-old woman, who is a former heavy smoker. Her past medical history was notable for a long history of arterial hypertension, on pharmacological treatment with calcium channel blockers and angiotensin-converting enzyme inhibitors, and for autoimmune thyroiditis that had occurred 10 years ago and eventually resulted in hypothyroidism on hormone replacement therapy. The patient was diagnosed in July 2014 with stage IIIB squamous NSCLC and received sequential chemotherapy (carboplatin and paclitaxel) and radiation therapy, achieving nearly complete response. At 5 months after the end of treatment, an 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scan showed disease relapse in a single supraclavicular lymph node that was treated with definitive radiation therapy (45 grays in 18 fractions). However, at the subsequent response evaluation after 3 months, the patient developed a more extensive relapse (lung and mediastinal lymph nodes). She was then enrolled in the nivolumab Italian EAP, which was available at the time. The patient stayed on nivolumab for 2 years, achieving stable disease as best objective response. The treatment was well-tolerated saving grade 2 hypothyroidism flare, which required increase of the hormone replacement therapy dose. Before treatment with nivolumab, a CT scan demonstrated a complicated atheromatous plaque in the abdominal aorta wall (Figure 1a), that almost completely resolved while on nivolumab (Figure 1b), as previously reported.8 However, disease eventually progressed (lung and thoracic lymph nodes) so that carboplatin and paclitaxel chemotherapy was delivered, given the long time that had elapsed since the previous chemotherapy treatment. After five chemotherapy courses and initial partial response, the disease progressed again and the patient was enrolled in a phase IV clinical trial of atezolizumab, a humanized Fc-optimized anti-PD-L1 monoclonal antibody (TAIL study, NCT03285763). A core biopsy performed on one of the progressing supraclavicular lymph nodes demonstrated nodal metastasis consistent with squamous NSCLC and PD-L1 immunohistochemistry staining (SP263 clone, Ventana) in 30% of tumor cells. At the time atezolizumab was started, a computed tomography (CT) scan demonstrated the appearance of a new complicated atheromatous plaque in the thoracic aorta (Figure 2a). Interestingly, after 4 months of atezolizumab, the thoracic aorta plaque considerably improved again until nearly complete resolution (Figure 2b). In addition, partial response of disease was observed (Figure 3) so the patient is still on treatment. Atezolizumab was again well-tolerated but a grade 2 hypothyroidism flare [thyroid-stimulating hormone (TSH) level increased from 1.03 to 5.51 µU/ml and positive anti-thyroglobulin antibodies titer] required increase of patient’s hormone replacement therapy, as during the previous nivolumab course.
Figure 1.
Abdominal aorta atheromatous plaque (arrowhead) at (a) nivolumab start and (b) resolution after treatment.
Figure 2.
Thoracic aorta atheromatous plaque (arrowhead) at (a) atezolizumab start and (b) resolution after 4 months of treatment.
Figure 3.
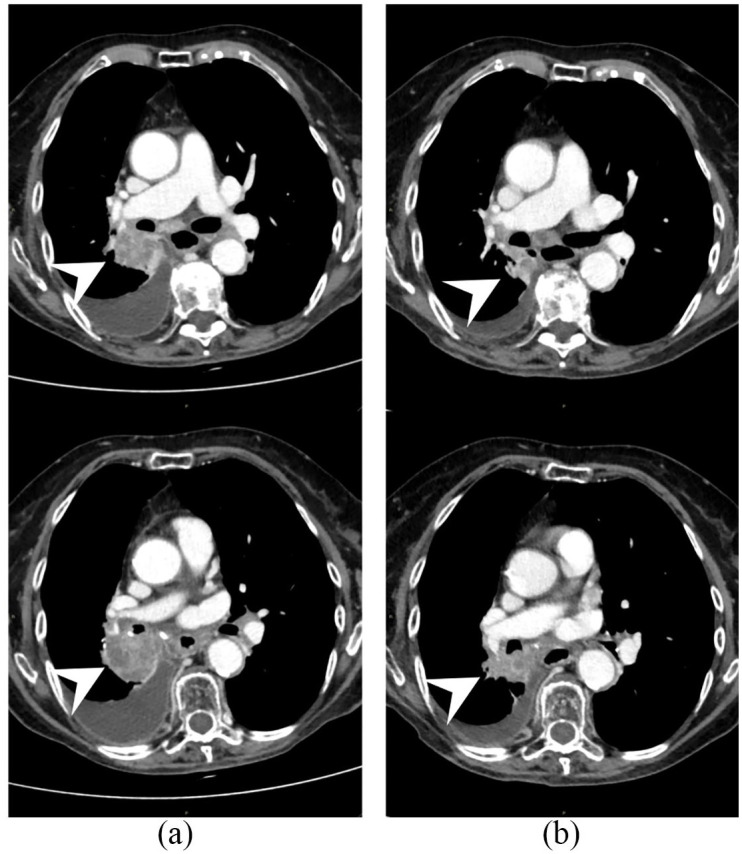
Pathologic soft tissue at right hilum (arrowhead) at (a) atezolizumab start and (b) its reduction after 4 months of treatment consisting with disease response.
Discussion
The immune system plays a pivotal role in atherosclerosis.6 The interplay among immune cells and the complex cytokine network that regulates their differentiation and behavior have different and opposite effects on atheromatous plaque formation and progression. Moreover, the effect of some cells, for example, dendritic cells, can vary depending on plaque microenvironment and in early versus advanced plaque. Costimulatory and coinhibitory molecules are integrated in this network and modulate activity of the different cell populations.
The regression of complicated atheromatous plaques on anti-PD-1 and anti-PD-L1 treatment is opposed to what would be expected based on available preclinical data.
In fact, data from mouse models have shown that the interaction between PD-1 and PD-L1 promotes T cell tolerance towards vascular wall antigens,9 and disruption of the PD-1/PD-L1 signal accelerates atheromatous plaque formation.10 These data outline PD-1/PD-L1 as a protective pathway on atheromatous plaques, and suggest that anti-PD-1/PD-L1 ICIs could worsen atherosclerosis. Moreover, PD-1 expressed on human CD-8+ T cells acts synergistically with other immune-checkpoints molecules, such as Tim-3, in reducing pro-atherogenic cytokines (e.g. IFN-γ and TNF-α) while increasing anti-atherogenic ones (e.g. IL-10).11 These data seem consistent with the case of a patient with metastatic giant cell bone tumor receiving pembrolizumab, a humanized IgG4 anti-PD-1 monoclonal antibody, who suffered from two episodes of non-ST elevated myocardial infarction 2 months apart.12 The latter authors raised the possibility of a role of pembrolizumab treatment in accelerating coronary plaque growth or rupture due to the short time that had elapsed between the two events. However, this patient also had multiple cardiovascular risk factors (hypertension, diabetes and smoking habit) and experienced the second coronary event while off the dual antiplatelet therapy that had been started right after previous stent positioning. Dual antiplatelet therapy was held to perform a liver biopsy, scheduled due to elevation in liver function tests, which eventually demonstrated drug-induced liver injury and primary biliary cholangitis.
Macrophages associated with atheromatous plaques in human arteries release pro-inflammatory cytokines, namely IL-1β and IL-6, that enhance innate immunity, and express high levels of PD-L1, that inhibit T cell response.13 Hypothesizing an immune-suppressive microenvironment, T cells from blood of patients with coronary artery disease (CAD) were exposed to nonself antigens, such as varicella-zoster virus (VZV) antigens, and found to response poorly, but responsiveness was enhanced in the presence of anti-PD-L1 antibodies. VZV infection reactivation and CAD had also been correlated since CAD patients show increased risk for VZV infection reactivation,14 and patients who develop VZV infection reactivation are at higher risk of cardiovascular events for 2 years.15
Macrophages in human arteries hamper the protective role of T-cells on the atheromatous plaques through expression of PD-L1.13 As we reported previously,8 in noncancer patients with atheromatous plaques, strong PD-L1 expression was observed only on dendritic cells of complicated plaques. All these data taken together seem to suggest that the atheromatous plaque formation process in mice is different from that in humans, where T cells fulfill tissue-protective functions, which are controlled by PD-1-derived signals.16 This leads to the hypothesis that some aspects of atheromatous plaque formation in humans might not be reproducible in mouse models.
The resolution of complicated atheromatous plaques on treatment with both nivolumab and atezolizumab in this patient might suggest that the PD-1/PD-L1 interaction might hamper the T cell potentially protective action on atheromatous plaques. Several confounding factors may have affected this observation. For example, the tumor-related factors or the response to treatment (prolonged radiological stable disease on nivolumab and partial response on atezolizumab) might have played a role in plaque changes. It should also be considered that the observed phenomenon could have been an isolated idiosyncratic event in this patient. In addition, the possible contribution of immune-mediated adverse event occurrence should also be considered, since it can reflect an abnormal immune-system activation. If improvement of complicated plaques while on anti-PD-1/PD-L1 ICIs can be confirmed by independent researchers from other groups (e.g. by radiological imaging revision of clinical trial patients), it might be questioned whether the mouse models in use are adequate to represent the relationship between the immune system and atheromatous plaque, at least in theory, and if ICIs might be investigated as atherosclerosis treatments.
Conclusion
In conclusion, we observed repeated atheromatous plaque vanishing in a NSCLC patient during treatment with both nivolumab and atezolizumab. In our Center, two other patients have previously shown reduction of atheromatous plaque while on nivolumab. The interactions among cancer, atherosclerosis, and immune system are far from being comprehensively understood.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Giuseppe Lamberti  https://orcid.org/0000-0003-3069-7630
https://orcid.org/0000-0003-3069-7630
Francesco Gelsomino  https://orcid.org/0000-0002-9204-1728
https://orcid.org/0000-0002-9204-1728
Contributor Information
Giuseppe Lamberti, Medical Oncology, Policlinico S.Orsola-Malpighi, University of Bologna, Bologna, Italy.
Francesco Gelsomino, Medical Oncology Unit, Policlinico S.Orsola-Malpighi, Via P. Albertoni, 15, Bologna, 40138, Italy.
Stefano Brocchi, Radiology Unit, Policlinico S.Orsola-Malpighi, Bologna, Italy.
Antonio Poerio, Radiology Unit, Policlinico S.Orsola-Malpighi, University of Bologna, Bologna, Italy.
Barbara Melotti, Medical Oncology Unit, Policlinico S.Orsola-Malpighi, Bologna, Italy.
Francesca Sperandi, Medical Oncology Unit, Policlinico S.Orsola-Malpighi, Bologna, Italy.
Mauro Gargiulo, Vascular Surgery, Policlinico S.Orsola-Malpighi, University of Bologna, Bologna, Italy.
Claudio Borghi, Internal Medicine, Policlinico S.Orsola-Malpighi, University of Bologna, Bologna, Italy.
Michelangelo Fiorentino, Pathological Anatomy Unit, Maggiore Hospital, Bologna, Italy.
Andrea Ardizzoni, Medical Oncology, Policlinico S.Orsola-Malpighi, University of Bologna, Bologna, Italy; Medical Oncology Unit, Policlinico S.Orsola-Malpighi, Bologna, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-small cell lung cancer - Version 5, https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. (2019, accessed 21 December 2019).
- 4. Abdel-Wahab N, Alshawa A, Suarez-Almazor ME. Adverse events in cancer immunotherapy. Adv Exp Med Biol 2017; 995: 155–174. [DOI] [PubMed] [Google Scholar]
- 5. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors. JAMA Oncol 2018; 4: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legein B, Temmerman L, Biessen EAL, et al. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci 2013; 70: 3847–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 8. Gelsomino F, Fiorentino M, Zompatori M, et al. Programmed death-1 inhibition and atherosclerosis: can nivolumab vanish complicated atheromatous plaques? Ann Oncol 2018; 29: 284–286. [DOI] [PubMed] [Google Scholar]
- 9. Gotsman I, Grabie N, Dacosta R, et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 2007; 117: 2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bu D, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 2011; 31: 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu MK, Wang SC, Dai YX, et al. PD-1 and tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS One 2015; 10: e0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwan JM, Cheng R, Feldman LE. Hepatotoxicity and recurrent NSTEMI while on pembrolizumab for metastatic giant cell bone tumor. Am J Med Sci 2019; 357: 343–347. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe R, Shirai T, Namkoong H, et al. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J Clin Invest 2017; 127: 2725–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ke CC, Lai HC, Lin CH, et al. Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: a population-based study in Taiwan. PLoS One 2016; 11: e0146750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu P, Lin CL, Sung FC, et al. Increased risk of cardiovascular events in patients with herpes zoster: a population-based study. J Med Virol 2014; 86: 772–777. [DOI] [PubMed] [Google Scholar]
- 16. Weyand CM, Berry GJ, Goronzy JJ. The immunoinhibitory PD-1/PD-L1 pathway in inflammatory blood vessel disease. J Leukoc Biol 2018; 103: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]