Abstract
Background. In low-income countries, preterm nutrition is often inadequately addressed. The aim of the study was to assess the patterns of feeding and associated clinical outcomes of preterm neonates admitted to neonatal intensive care units in Ethiopia. Method. This was a multicenter, prospective study. Infants’ clinical characteristics at birth, daily monitoring of feeding history, and weight measurements were collected. An outcome assessment was completed at 28 days. Result. For this analysis, 2560 infants (53% male) were eligible. The mean (SD) gestational age was 33.1 (2.2) weeks. During the hospital stay the proportion of infants on breast milk only, preterm formula, term formula, and mixed feeding was 58%, 27.4%, 1.6%, and 34.1%, respectively. Delay in enteral feeding was associated with increased risk of death (odds ratio [OR] = 1.92, 95% confidence interval [CI] = 1.33-2.78; P < .001) and (OR = 5.06, 95% CI = 3.23-7.87; P < .001) for 1 to 3 and 4 to 6 days of delay in enteral feeding, respectively, after adjusting for possible confounders. The length of delay in enteral feeding was associated with increased risk of hypoglycemia (OR = 1.2, 95% CI = 1.1-1.2; P = .005). The mortality rate was lower in hospitals providing preterm formula more often (P = .04). Half of the infants continued losing weight at the time of discharge. Conclusion. Delayed enteral feeding significantly increases the risk of mortality before discharge and hypoglycemia in preterm infants in resource-limited settings. Ensuring adequate nutritional support of preterm infants is highly needed.
Keywords: prematurity, infant feeding, preterm nutrition, neonatal mortality, low- and middle-income countries
Introduction
Complications of preterm birth are responsible for the largest proportion of neonatal deaths in the world, accounting for 35% of the world’s 3.1 million neonatal deaths per year.1 Worldwide, 15 million preterm births occur every year, with those less than 32 weeks of gestation at the highest risk of morbidity and mortality.2 Undernutrition in preterm infants is associated with serious consequences such as increased mortality and long-term neurodevelopmental, metabolic, and growth disorders.3 Undernutrition largely affects the brain, resulting in poor brain growth and neurodevelopmental delay.4 Regardless of the degree of prematurity, early postnatal growth (ie, during hospitalization) has been associated with neurological and cognitive outcomes in infancy and preschool-age.5 Premature infants are prone to nutrient deficiencies due to inadequate stores, inability to feed adequately, and digest due to immaturity of the digestive system, while optimal nutrition of preterm infants is expected to result in growth similar to that of normally growing fetuses of the same gestational age.6
In recent years, the nutritional support for preterm infants has been improved. Parenteral nutrition, enriched preterm formula, and fortification of human milk have been proven to be critically important for preterm infants admitted to neonatal intensive care units (NICUs).7 Unfortified human breast milk, the only option available in many low-income countries, does not contain adequate nutrients for the growth and development of preterm infants with the small volumes they can take in.8-10 Human breast milk alone does not contain the required protein, energy, minerals, vitamins, and trace elements to promote growth and development of preterm infants.11 Slower growth rates, including that of the head circumference, have been observed in preterm babies fed only on human milk as compared with those on fortified human milk.12
The advantage of starting small amount of enteral feeding earlier as a means of preparing the immature intestine for full enteral feeding has been recognized recently.10 However, the nutritional assessment of preterm infants is challenging. For example, the body weight may vary based on fluid status, and changes in linear growth take time. Moreover, assessing body composition has usually been limited to research settings, and the biochemical nutritional assessments are not well established for clinical use.13 Currently, in most settings in resource-limited countries, there is inevitable suboptimal feeding of preterm infants. This contributes significantly to the incidence of neonatal morbidity and mortality. Addressing preterm nutrition is vital, as there is an obvious need to reduce nutritional deficiencies in these susceptible infants.14 Thus, we aimed to assess feeding patterns, extent of nutritional problems, and associated clinical outcomes using data from a study on illness among preterm infants in Ethiopia.
Methods
Our primary objective was to assess patterns of feeding of preterm neonates admitted to NICUs and associated clinical outcomes. The specific objectives were to investigate the type of feeding of preterm infants and associated clinical outcomes; to assess the time of initiation of enteral feeding; to assess the association of length of delay in enteral feeding (duration of nil per os [NPO] “nothing through the mouth”) and clinical outcomes; and to review the weight changes of preterm infants during their hospital stay and finally to assess breast feeding prevalence at discharge.
Study Setting and Design
This was a hospital-based multicenter prospective study, known as the Study of Cause of Illness and Death of Preterm infants in Ethiopia (SIP). SIP was conducted in 5 teaching hospitals in Ethiopia. The methodology paper and primary outcomes have been published.15,16
The study participants for this analysis included a subgroup from the main study. Specifically, we included only preterm infants with a gestational age (GA) of less than 37 completed weeks admitted to one of the study hospitals with a minimum duration of hospital stay of 3 days in order to have sufficient time to measure undernutrition. Finally, only those infants whose parents gave informed consent for study participation were included.
Study Procedures
The clinical status of the infants, their general condition, vital signs, and oxygen saturation were monitored twice a day. Revisions of the diagnosis and pertinent investigations, assessment of the type and method of feeding, and weight measurements were done or collected daily. Specific clinical diagnoses of the infants were made with a combination of clinical data and laboratory results, and final diagnosis of cause of death for those who died was determined with additional data from a postmortem autopsy, among those with a completed autopsy. The assessment of the infants’ GA was based on 3 methods: last menstrual period, physical examination using the New Ballard Score, and prenatal ultrasound when available.
Ethical Approval and Informed Consent
The study was conducted after ethical approval was obtained from Addis Ababa University College of Health Sciences Institutional Review Board (Ethics ID: AAUMF 03-008), and LMU Institutional Review Board (Ethics ID: 19-649). An information sheet and written consent for participation in the study was provided to potential participants in the 2 commonly spoken local languages. Only data from the women and infants of women who provided informed consent were included. Procedures were generally performed according to the national NICU management protocol.17
Statistical Analyses
Data were analyzed using SPSS version 23 and R software. Continuous variables were calculated as means and standard deviations, and other results were presented as the mean difference, with the calculated 95% confidence interval (CI), odds ratio (OR), and a P value. Binary logistic regression was done to investigate the associations of different variables related to the outcomes studied.
Results
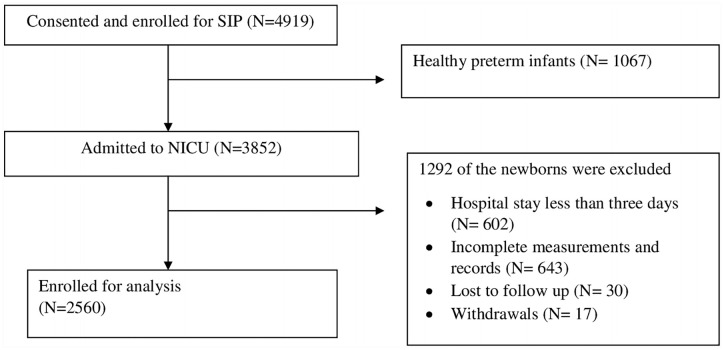
Figure 1 and Table 1 show study participants selection and infants’ characteristics. Out of a total of 2560 infants, 1225 (48%) were females and 1335 (52%) were males. During the first 24 hours, the majority of the infants, 1938 (76%), were kept NPO (nothing by mouth), and 559 (22%) were kept NPO for the subsequent 3 days after admission, getting only 10% dextrose intravenously. Overall, the infants were kept NPO 26.8% of the time they were in the hospital NICU. The percent of time the infants were kept NPO increased with lower GA. Similarly, 24.9% of those who had birth weight less than 1500 g were kept NPO for more than 3 days compared with 13.2% of the infants who weighed greater than 1500 g (P < .001).
Figure 1.
Flow chart of recruitment of study subjects.
Table1.
Clinical Characteristics of the Preterm Infants.
| Variables | Values |
|---|---|
| Maternal age, mean (SD) | 25.9 (5.4) |
| Gender | |
| Male, n (%) | 1335 (52) |
| Gestational age (weeks), n (%) | |
| <28 | 80 (3) |
| 28 to32 | 508 (20) |
| 32 to 34 | 1159 (45) |
| 35 to <37 | 813 (32) |
| Birth weight (g), n (%) | |
| <1000 | 150 (5.9) |
| 1000 to <1500 | 728 (28) |
| 1500 to <2000 | 1049 (41) |
| ≥2000 | 591 (23) |
| Missing | 42 (2) |
| Newborn major diagnosis, n (%) | |
| Respiratory distress syndrome | 1405 (55) |
| Sepsis | 918 (36) |
| Pneumonia | 72 (3) |
| Necrotizing enterocolitis | 103 (4) |
| Perinatal asphyxia | 145 (6) |
| Hypothermia | 1383 (54) |
| Total duration of hospital stay, mean days (SD) | 10.0 (7) |
Abbreviation: SD, standard deviation.
During the hospital stay, 1485 (58%) of the infants were on exclusive breast feeding, while 201 (8%) were not given any enteral feeding at all; of the rest of the infants, 701 (27.4%), 41 (1.6%), and 874 (34.1%) received preterm formula, term formula, and mixed feeding (infants on more than one type of feeding), respectively. The pattern of feeding varied significantly by hospital. Preterm formula was given to more than one third of the infants admitted to the 3 hospitals located in Addis Ababa, while in 2 hospitals outside Addis Ababa (Jimma University Medical Center and Gonder University Hospital) only 3% to 6% of the infants were given preterm formula. Similarly, exclusive breastfeeding occurred in 69% and 80% of the infants in these 2 hospitals, while the infants in the other hospitals in Addis Ababa had 50% or less exclusive breast feeding. The in-hospital neonatal mortality rate observed in this study was also significantly different by hospital (P = .004). The lowest mortality rate (15.6%) was seen in the hospital with the highest rate of preterm formula feeding (54%; Table 2). At the time of discharge, the majority of infants (83%) was on breast milk feeding, while 14.9%, 5%, and 1.2% were given preterm formula, term formula, and mixed feeding, respectively.
Table 2.
Feeding Pattern and Outcome of Study Participants in Each Hospital, N (%).
| Hospitals | N (%) | Exclusive breast feeding | Preterm formula | Term formula | Mixed feeding | No enteral feeding | Outcome |
|
|---|---|---|---|---|---|---|---|---|
| Alive | Died | |||||||
| Tikur Anbessa Hospital | 659 (25.7) | 330 (50.1) | 223 (33.8) | 14 (2.1) | 260 (39.5) | 69 (10.5) | 530 (80.4) | 129 (19.6) |
| Ghandi Memorial Hospital | 276 (10.8) | 102 (37.0) | 148 (53.6) | 7 (2.8) | 162 (58.7) | 12 (4.3) | 233 (84.4) | 43 (15.6) |
| St Paul Hospital | 741 (28.9) | 368 (49.7) | 286 (38.6) | 11 (1.5) | 324 (43.7) | 49 (6.6) | 566 (76.4) | 175 (23.6) |
| Gondar University Hospital | 674 (26.3) | 540 (80.1) | 38 (5.6) | 4 (0.6) | 73 (10.8) | 61 (9.1) | 533 (79.1) | 141 (20.9) |
| Jimma University Medical Center | 210 (8.0) | 145 (69.0) | 6 (2.9) | 5 (2.4) | 55 (26.2) | 10 (4.8) | 150 (71.4) | 60 (28.6) |
| Total | 2560 (100.0) | 1485 (58.0) | 701 (27.4) | 41 (1.6) | 874 (34.1) | 201 (7.9) | 2012 (78.6) | 548 (21.4) |
Logistic regression analysis was done to determine the relationship of delayed enteral feeding to the risk of death in the hospital. Delay in enteral feeding by 1 to 3 days was associated with twice the risk of death (OR = 1.92, 95% CI = 1.33-2.78), while being kept NPO for 4 to 6 days was associated with a much higher risk of death (OR = 5.06, 95% CI = 3.23-7.87, P < .001), after adjusting for possible confounders, including GA, birth weight, and for the most common causes of death such as respiratory distress syndrome, neonatal infection, perinatal asphyxia, and hypothermia. Similarly, we found a statistically significant association between length of duration of NPO and hypoglycemia (OR = 1.2, 95% CI = 1.1-1.2; P = .005). We did not find a statistically significant association between risk of infection and types of feeding (Table 3).
Table 3.
Duration of NPO and Associated Neonatal outcome.
| NPO daysa | Outcome |
Crude odds ratio |
Adjusted odds ratiob |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alive, n (%) | Died, n (%) | P | OR | 95% CI |
P | OR | 95% CI |
|||
| Lower | Upper | Lower | Upper | |||||||
| <1 day | 476 (90.5) | 50 (9.5) | — | — | — | — | — | — | — | — |
| 1-3 days | 1269 (79.6) | 325 (20.4) | .000 | 2.44 | 1.78 | 3.34 | .001 | 1.92 | 1.33 | 2.78 |
| 4-6 days | 201 (59.1) | 139 (40.9) | .000 | 6.58 | 4.58 | 9.46 | .000 | 5.06 | 3.26 | 7.87 |
| 7-9 days | 44 (71.0) | 18 (29.0) | .000 | 3.90 | 2.09 | 7.25 | .017 | 2.70 | 1.19 | 6.11 |
| ≥10 days | 22 (57.9) | 16 (42.1) | .000 | 6.92 | 3.42 | 14.04 | .000 | 6.33 | 2.48 | 16.18 |
Abbreviations: NPO, nil per os; OR, odds ratio; CI, confidence interval.
Days the infants were given 10% dextrose only without enteral feeding.
Adjusted for birth weight, gestational age, and diagnoses such as respiratory distress syndrome neonatal infection, perinatal asphyxia, and hypothermia.
Nearly half (47%) of the infants studied continued losing weight at the time of discharge or death, while only 22% of them regained their birth weight. The mean (SD) percentage weight loss and gain observed among the groups were 10.2% (8.6) and 13.0% (17.0), respectively. For those infants who stayed in the hospital for greater than or equal to 14 days, the mean weight at the 7th and 14th days across the GA classifications was found to be less than the mean birth weight. However, for these infants, the mean weight at the time of discharge was slightly higher than the mean birth weight (Table 4).
Table 4.
Change in Mean Weight of Preterm Infants Who Had Been in the Hospital for 14 Days and More (N = 579), Mean ± SD.
| GA (in weeks) | N | Days in NICU stay |
|||
|---|---|---|---|---|---|
| At birth | 7th day | 14th day | Day of discharge | ||
| <28 | 24 | 1145 ± 290 | 1131 299 | 1130 ± 320 | 1261 ± 285 |
| 28 to 31 | 157 | 1381 ± 273 | 1328 ± 283 | 1357 ± 280 | 1442 ± 286 |
| 32 to 34 | 284 | 1612 ± 360 | 1523 ± 335 | 1543 ± 351 | 1619 ± 344 |
| 35 to <37 | 114 | 1869 ± 369 | 1778 ± 377 | 1807 ± 387 | 1872 ± 396 |
Abbreviations: SD, standard deviation; GA, gestational age; NICU, neonatal intensive care unit.
Discussion
The objective of preterm infant nutritional support is to achieve a rate of growth comparable to that found in normally growing fetuses of similar GA. However, that goal is not attained in most of settings, even including high-resource settings where parenteral nutrition and human milk fortification is available.6 Preterm infants face increased risk of death,18 and survivors are at continued risk of growth restriction, unless provided with adequate nutrients required for growth. There is also an increased nutritional demand for preterm infants related to the serious illness they often suffer.19 In low-income countries, where there are high rates of preterm birth,20 poorly equipped NICUs and a shortage of trained health workers, the nutritional needs of preterm infants are often neglected. In our study, we found that none of the infants received parenteral nutrition or breast milk fortification.
The rate of breast feeding in this study is comparable to findings from NICUs in Europe and the United States, which ranged from 19% to 75% among mothers who gave birth to preterm infants.21,22 Despite the recent study findings and the World Health Organization recommendation to give human milk fortifiers to very low birth weight (VLBW) infants who fail to gain weight while on adequate breast milk feeding,23-25 none of the infants were given human milk fortifier. This is likely due to the fact that the Ethiopian national guidelines had not yet been revised, and human milk fortifiers were not available in the study NICUs.
The mortality rate of the infants in this study was very high (21.4%), and the risk of death of infants who were kept NPO longer while only getting 10% glucose was even higher. One in 5 of the infants received only 10% glucose intravenously, without enteral feeding for the first 3 days of admission. Glucose provides only 20% of an infant’s energy requirement and has no protein. Several randomized controlled trials have shown the benefits of combined early parenteral and enteral nutritional support, without increased risk of adverse clinical outcomes.26
Minimal postnatal weight loss is expected in the first week of life, due to contraction of the extracellular compartment. However, exaggerated weight loss occurs in infants whose energy intake is inadequate.27 At the end of the first week, more than half of the infants had a mean weight loss of 10% and only 30% of them gained weight by the second week. The initial postnatal weight loss observed in this study is comparable to a report of Shaffer et al,28 which found a weight loss of 7.9% to 14.6% in the first few days. However, in our study, a significant proportion of the infants did not gain weight as expected even in the second week or thereafter. Recent studies show the benefits of early and enhanced nutrition of preterm infants in terms of improving neonatal outcomes and prevention of long-term complications, shorter hospital stay, better weight gain, and same or less risk of infection including necrotizing eneterocolitis.6
Preterm formula was given more often in the hospitals located in the capital city of Addis Ababa. This could be due to the influence of pharmaceutical companies’ advertisement of the preterm formula in the capital city compared with the other regions. Though the hospitals had similar settings, the mothers in hospitals located in Addis Ababa could have had better income to afford the preterm formula. Lower mortality rates were seen in the hospitals providing preterm formula more often compared with those who had higher exclusive breast feeding rates. This is probably due to improved nutritional support as preterm formulas contain approximately 3.5 to 3.6 g/kg/day of protein when adequate energy is provided (120 kcal/kg/day). This is within the range of recommended daily protein intake (3.5-4.5 g/kg/day).29 In addition to the improved nutrient content of the preterm formula, early feeding, and avoidance of unnecessary IV fluids and starvation may have contributed for the lower mortality rather than the preterm formula itself.
A limitation of the study was the fact that we did not capture the volumes of the feeds and the time infants achieved full enteral feeding. Preterm infants often start enteral feeding gradually based on the infants’ general condition and tolerance, while receiving 10% dextrose intravenously in addition to the small amount of enteral feeding they received.
Conclusion
Unlike the general recommendation to initiate early enteral feeding, a considerable number of the infants were kept NPO in the first few days, receiving only maintenance fluid. This was associated with increased risk of death and development of hypoglycemia. We noted that the feeding patterns varied among the hospitals. A low death rate was observed in the hospital providing preterm formula feeding. Association of preterm formula feeding with improved survival may be only because earlier feeding is more easily achieved when formula feeding is used, as mothers often have difficulty to produce and express enough milk. In our opinion, the Ethiopian National Guideline on preterm nutrition should be revised, to promote more practices proven to be beneficial for survival and growth of the infants. These practices include the advancement of enteral feeding, the use of parenteral nutrition, human milk fortifiers, mixed feeding using preterm formula and breast milk, and enteral additives.
Acknowledgments
We would like to acknowledge all the study staff who contributed to the data collection and data entry, families of the study participants, and administrators of the respective hospitals for facilitation of the logistics.
Footnotes
Author Contributions: The study was conceptualized by NW, LMM, MS, and OG and reviewed by RLG, AM, EMM, BW, and AKN. The following authors contributed to data acquisition: LMM, AM, BW, AD, EMM, ZT, GM, AKN, EMM, and NW. ZTB contributed to data management, data analysis, and interpretation. NW drafted the manuscript, and RLG, LMM, AM, BW, AD, EMM, ZT, GM, ZTB, AKN, MS, and OG contributed to the writing and reviewing the manuscript. All authors have approved the final draft of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was fully funded by Bill & Melinda Gates Foundation.
ORCID iDs: Netsanet Workneh Gidi  https://orcid.org/0000-0002-7213-8178
https://orcid.org/0000-0002-7213-8178
Amha Mekasha  https://orcid.org/0000-0002-0066-0100
https://orcid.org/0000-0002-0066-0100
Matthias Siebeck  https://orcid.org/0000-0001-5290-5344
https://orcid.org/0000-0001-5290-5344
Lulu M. Muhe  https://orcid.org/0000-0002-2776-9923
https://orcid.org/0000-0002-2776-9923
References
- 1. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(1 suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384:189-205. [DOI] [PubMed] [Google Scholar]
- 3. Strydom K, Van Niekerk E, Dhansay MA. Factors affecting body composition in preterm infants: assessment techniques and nutritional interventions. Pediatr Neonatol. 2017;60:121-128. [DOI] [PubMed] [Google Scholar]
- 4. Ehrenkranz RA. Nutrition, growth and clinical outcomes. World Rev Nutr Diet. 2014;110:11-26. [DOI] [PubMed] [Google Scholar]
- 5. Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899-e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hay WW., Jr. Aggressive nutrition of the preterm infant. Curr Pediatr Rep. 2013;1:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tonkin EL, Collins CT, Miller J. Protein intake and growth in preterm infants: a systematic review. Glob Pediatr Health. 2014;1:2333794X14554698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enweronu-Laryea CC, Aryee IN, Adei EA. Severe acute malnutrition in very low birth weight preterm infants. JPEN J Parenter Enteral Nutr. 2012; 36:354-357. [DOI] [PubMed] [Google Scholar]
- 9. Jeong E, Jung YH, Shin SH, et al. The successful accomplishment of nutritional and clinical outcomes via the implementation of a multidisciplinary nutrition support team in the neonatal intensive care unit. BMC Pediatr. 2016;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slusher TM, Vaucher YE, Zamora T, Curtis BA. Feeding and fluids in the premature and sick newborn in the low-middle income countries. Contemporary Pediatrics. doi: 10.5772/34879 https://www.intechopen.com/books/contemporary-pediatrics/feeding-and-fluids-in-the-premature-and-sick-newborn-in-the-low-middle-income-countries [DOI]
- 11. Jenness R. The composition of human milk. Semin Perinatol. 1979;3:225-239. [PubMed] [Google Scholar]
- 12. Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2016;(5):CD000343. [DOI] [PubMed] [Google Scholar]
- 13. Griffin IJ. Nutritional assessment in preterm infants. Nestle Nutr Workshop Ser Pediatr program. 2007;59:177-192. [DOI] [PubMed] [Google Scholar]
- 14. Ayede AI. Achieving optimal feeds for preterm babies, recommendations and realities in practice: Nigerian perspective. Ann Ib Postgrad Med. 2011;9:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhe LM, McClure EM, Mekasha A, et al. Prospective study of causes of illness and death in preterm infants in Ethiopia: the SIP study protocol. Reprod Health. 2018;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muhe LM, McClure EM, Nigussie AK, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7:e1130-e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Federal Ministry of Health of Ethiopia. Neonatal Intensive Care Unit (NICU) Management Protocol. Federal Ministry of Health of Ethiopia; 2014. [Google Scholar]
- 18. Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9:e1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Association of Pediatrics Committee on Nutrition: nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976-986. [PubMed] [Google Scholar]
- 20. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonet M, Blondel B, Agostino R, et al. Variations in breastfeeding rates for very preterm infants between regions and neonatal units in Europe: results from the MOSAIC cohort. Arch Dis Child Fetal Neonatal Ed. 2011;96:F450-F452. [DOI] [PubMed] [Google Scholar]
- 22. Merewood A, Brooks D, Bauchner H, MacAuley L, Mehta SD. Maternal birthplace and breastfeeding initiation among term and preterm infants: a statewide assessment for Massachusetts. Pediatrics. 2006;118:e1048-e1054. [DOI] [PubMed] [Google Scholar]
- 23. Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev. 2004;(1):CD000343. [DOI] [PubMed] [Google Scholar]
- 24. Mukhopadhyay K, Narang A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatr. 2007;44:286-290. [PubMed] [Google Scholar]
- 25. World Health Organization. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low- and Middle-Income Countries. World Health Organization; 2011. [PubMed] [Google Scholar]
- 26. Ehrenkranz RA. Early nutritional support and outcomes in ELBW infants. Early Hum Dev. 2010;86:21-25. [DOI] [PubMed] [Google Scholar]
- 27. Heimler R, Doumas BT, Jendrzejczak BM, Nemeth PB, Hoffman RG, Nelin LD. Relationship between nutrition, weight change, and fluid compartments in preterm infants during the first week of life. J Pediatr. 1993;122:110-114. [DOI] [PubMed] [Google Scholar]
- 28. Shaffer SG, Quimiro CL, Anderson JV, Hall RT. Postnatal weight changes in low birth weight infants. Pediatrics. 1987;79:702-705. [PubMed] [Google Scholar]
- 29. Hay WW., Jr. Optimizing protein intake in preterm infants. J Perinatol. 2009;29:465-466. [DOI] [PMC free article] [PubMed] [Google Scholar]