Abstract
This narrative review aims to provide an overview of the current literature on the pharmacology, safety, efficacy and tolerability of intranasal esketamine, the S-enantiomer of ketamine, for the treatment of treatment-resistant depression (TRD). A literature search using Medline, Embase, PsycINFO and Cochrane Central was conducted (January 2000 to July 2019). Product information and www.clinicaltrials.gov were also reviewed. The literature search was limited to human studies published in English. Phase I, II, and III studies of intranasal esketamine for TRD were reviewed. About a third of patients with major depressive disorder fail to achieve remission despite treatment with multiple antidepressants. This article examines the trials that led to the approval of esketamine in the United States, as well as other recent studies of esketamine for TRD. The findings from limited phase III trials illustrate that intranasal esketamine is effective and safe in reducing depressive symptoms and achieving clinical response in patients with TRD. The optimum duration and frequency of use are not fully understood. Although the nasal spray is a convenient dosage form, its use in practice may be limited by cost and administrative regulation. While it may prove beneficial to many patients who suffer from TRD, further long-term data are required, along with comparative trials with the R-isomer (arketamine). In the interim, care and monitoring should be exercised in its use in clinical practice.
Keywords: antidepressant, esketamine, intranasal, major depressive disorder, R-ketamine, S-ketamine, TRD, treatment resistant depression
Plain language summary
Esketamine: new therapy for severe depression
Intranasal esketamine, the S-enantiomer of ketamine, was recently approved in the United States for the treatment of treatment-resistant depression (TRD). The findings from limited clinical trials indicate that intranasal esketamine is effective and safe in patients with TRD, although the optimum dose, duration and frequency of use are not fully understood. Its use in practice may be limited by cost and administrative regulation, and further long-term data are required.
Introduction
Major depressive disorder (MDD) is a common, debilitating and recurrent mental health disorder characterised by features including persistently low mood, anhedonia; altered appetite, weight, sleep and activity; guilt; feelings of worthlessness; and suicidality.1,2 MDD affects approximately 300 million people worldwide and has significant impact in terms of lack of productivity, impaired quality of life and increased mortality from suicide.3
Approximately one-third of patients with MDD do not respond to available antidepressants.4 Recent effectiveness trials indicated that only one-third of participants had achieved remission by the end of 12 weeks of initial antidepressant drug therapy, and this increased to a remission rate of 70% after four sequential antidepressants.5 Consequently, improving remission rates and reducing the latency period before the onset of drug action remain significant unmet clinical needs in the treatment of MDD.
Treatment-resistant depression (TRD) is the clinical term used to define inadequate response from two or more antidepressants with adequate dosing and duration in the subpopulation of patients with MDD.6 TRD is associated with an increased risk of subsequent relapse, hospitalisation and suicide.7,8 In the United States, higher costs of care and decreased work productivity are reported among patients with TRD, relative to treatment-responsive depression. A 2014 literature review found that TRD accounted for US$29–48 billion, in 2012, in total costs for managing depression, pushing up the total societal costs of MDD to between US$106–118 billion per annum.9
A rapidly acting new agent, the S-enantiomer of ketamine (also known as esketamine; delivered intranasally as Spravato™, Janssen), was approved on 5 March 2019 by the United States Food and Drug Administration (FDA), after showing effectiveness in patients with TRD. This narrative review provides an overview of the current literature on the pharmacology, chemistry, pharmacokinetics, clinical trial results, safety, efficacy and tolerability of intranasal esketamine as a novel drug for the treatment of TRD.
Data sources, extraction and selection
Medline, Embase, PsycINFO and Cochrane Central database searches (English only) were performed for the period January 2000 to May 2020 using the following keywords: esketamine, S-ketamine, Spravato, treatment-resistant depression, antidepressant and humans. Following the primary literature search to identify relevant papers, we carried out a citation analysis to identify potential studies pertinent to esketamine. The citation analysis was performed with the aid of Web of Science and Google Scholar to track prospective citing of references of selected articles. We also reviewed product information and https://www.clinicaltrials.gov for published and unpublished clinical research.
Articles included in the narrative review specifically discussed the use of intranasal esketamine for the treatment of TRD, clinical trials of this treatment, and/or adverse effects of treatment. Furthermore, the authors evaluated the included literature related to the chemistry, pharmacology and pharmacokinetics of esketamine. Review type articles and meeting abstracts identified from article references were also assessed. The primary search identified 457 articles.
Chemistry
Ketamine (or RS-ketamine) is a racemic mixture, containing equal parts of R-ketamine and S-ketamine (or esketamine). Esketamine is the generic name of the drug and its British Approved Name and International non-proprietary name is ‘esketamine hydrochloride’. It is also recognised as S(+)-ketamine, (S)-ketamine or (–)-ketamine, as well as by its developmental code name JNJ-54135419.10 The chemical name is (S)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride (Figure 1). Its molecular formula is C13H16ClNO.HCl and its molecular weight is 274.2 grams per mole.11 Esketamine hydrochloride is a white or almost white crystalline powder that is freely soluble in water and methanol, and soluble in ethanol.11 Intranasal esketamine represents a novel way of administering the agent and is marketed under the brand name ‘Spravato’ for use as an antidepressant for TRD.
Figure 1.
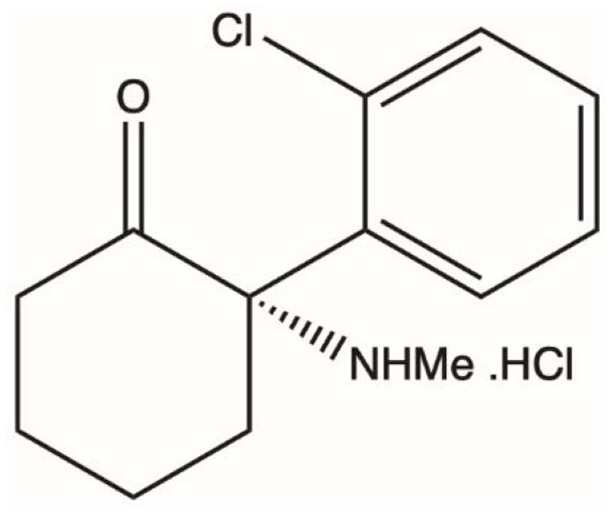
Chemical structure of esketamine.
Pharmacology
The N-methyl-d-aspartate (NMDA) receptor antagonist ketamine has shown rapid and sustained (2 months post-treatment) antidepressant effects in treatment-resistant patients with MDD.4,12 Esketamine was initially considered to be the more effective S-enantiomer of ketamine, having a higher affinity for the NMDA receptor, being about 2–4 times more potent than the R-enantiomer.13 The increasingly recognised potential role for R-ketamine (arketamine) as an antidepressant is discussed further below (under section Relevance to Patient Care and Clinical Practice).
The inhibitory constant (Ki) value of esketamine for the NMDA receptor ranges between 0.3 µM and 0.69 µM.14 The antidepressant properties of esketamine are not mediated by known mood-modulating pathways, such as the monoamine, gamma-aminobutyric acid (GABA), or opioid axes. The specific mechanism of action in depression has not been fully elucidated, although it is postulated that esketamine, like ketamine, is a non-selective, non-competitive antagonist that acts to block the NMDA receptor (an ionotropic glutamate receptor) on GABA interneurons and activate the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, thereby increasing neurotrophic signaling that restores synaptic function. An AMPA antagonist has been reported to block the antidepressant effects of both esketamine and R-ketamine.15
The action of esketamine on AMPA receptors may ultimately improve neural plasticity and synaptogenesis through signalling pathways resulting in enhanced brain-derived neurotrophic factor (BDNF) production, which is decreased in the prefrontal cortex and hippocampus in stress and depression.16 AMPA receptors initiate an intracellular cascade that leads to release of BDNF and stimulation of tropomyosin receptor kinase B (TrkB) that activates both mammalian target of rapamycin complex 1 (mTORC1) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling, resulting in increased synthesis of proteins required for synapse maturation and formation.16 Yang et al. demonstrated that mTORC1 signaling plays a role in the antidepressant effects of esketamine but not (R)-ketamine, and the MAPK/ERK signaling pathway plays a role in the antidepressant effects of (R)-ketamine but not esketamine.17
The rapid effects of esketamine, evident within about 1 week of initiating treatment, are likely related to its effect on synaptic potentiation,18 which may enhance the mitigation of negative thinking and the prevention of negative spirals of depressive thoughts. Evidence suggests that ketamine has a better direct stimulation effect on BDNF and mTORC1 than the present oral antidepressants, and this may elucidate the rapid onset of action.19 The mTORC1 is a prime signaling molecule that regulates protein synthesis, an essential component to induce synaptic potentiation and resultant antidepressant effects.20 The mTORC1 is also assumed to stimulate BDNF production via downstream modulation and subsequently increase brain plasticity through dendritic growth and improved synaptic transmission.21 According to Li et al.,22 the rapid action on synaptogenesis in the medial prefrontal cortex, via mTORC1 activation, plays a key role in mechanisms involving antidepressant effects. The role of mTORC1 signaling in the antidepressant effects of ketamine has been supported by successive preclinical and clinical studies.21,23,24 Theoretically, the immunosuppressant rapamycin has the potential to block the antidepressant effects of ketamine via mTORC1; however, there is preliminary evidence, requiring replication, that it may instead prolong its effect and increase the response rate.25
Pharmacokinetics
Much of the available pharmacokinetic information available is provided by the manufacturer, Janssen, based on 19 phase I, 3 phase II and 3 phase III studies (Table 1). While reported in the FDA application document26 and product information,11 much of these data could not be located in the peer-reviewed literature, limiting critical appraisal. Nasal esketamine has an estimated mean bioavailability of 48%,11 with hepatic first-pass avoided through this route. This is in contrast to oral esketamine, with a reported bioavailability of only 8–11%.27,28 There appears to be considerable inter-subject variation; a study from Japan of three healthy participants found a similar mean nasal bioavailability value of 46.4%, following a 25 mg dose of ketamine, but with a standard deviation (SD) of 12.9%.29 The absorption also clearly depends on the administration technique and on the presence of nasal pathology. Some of the clinical studies leading to registration excluded potential participants on the basis of nasal abnormalities that could impede absorption.4,30,31 There is also speculation that drug passage via the trigeminal or olfactory nerves facilitates drug entry into the central nervous system,32–34 in addition to systemic delivery via local capillary bed absorption. The time to maximum plasma concentration (Tmax) was estimated at 20–40 min following the final spray administration.11 This is consistent with a Japanese study (n = 3 healthy volunteers) in which the Tmax was 17.5 min (SD = 5 min).29 There is inter- and intra-subject variation in both the maximum concentration (Cmax; 27% and 15%, respectively) and total area under the curve (AUC; 66% and 10%, respectively). The Cmax and AUC increase was less than two-fold when increasing the dose from 28 mg to 56 mg, but was approximately proportional when increasing from 56 mg to 84 mg.11
Table 1.
Summary of pharmacokinetic parameters.11.
| Absorption | Distribution | Metabolism | Elimination |
|---|---|---|---|
|
Bioavailability: ~48% Tmax: ~20–40 min |
Volume: ~709 l (based on intravenous administration) Protein binding: ~43–45% |
Main initial metabolite, nor-esketamine (less active than parent drug) Cytochrome P450 iso-enzyme mediated-metabolism: 2D6 and 3A4, with some input from 2C9 and 2C19 |
Mean terminal half-life: ~7–12 h Urinary: metabolites, 78% Faeces: metabolites, <2% |
Tmax, time to maximum plasma concentration.
The volume of distribution is estimated at 709 l, following intravenous (IV) administration. Esketamine is 43–45% plasma protein-bound. It is metabolised primarily to the pharmacologically active but more polar S-norketamine, which has a brain:plasma ratio 4–6 times lower than the parent drug.11 This metabolism is via cytochrome P450 isoenzymes CYP2D6 and 3A4, with some input from 2C9 and 2C19. S-norketamine undergoes subsequent CYP- and glucuronidation metabolism.11 These metabolic pathways prompt consideration of CYP-mediated drug interactions, though none of the combinations studied so far has indicated a clinically significant drug interaction with intranasal administration of esketamine. A study of 11 healthy volunteers found that concomitant administration of oral rifampicin (600 mg), compared with placebo, with IV esketamine (0.1 mg/kg) decreased the AUC of esketamine by 14% (p = 0.005), while the relative decrease was 86% for oral esketamine (0.3 mg/kg).28 This was similar to that seen when esketamine was administered IV at 20 mg/70kg/h or 40 mg/70kg/h for 2 h, following 5 days of oral rifampicin dosed at 600 mg daily. Among 20 participants, the AUC for esketamine was 10% lower and nor-esketamine 50% lower for those taking rifampicin than those taking a placebo.35 A CYP2D6 inhibitor (ticlopidine) conversely slowed metabolism of IV esketamine, though any relevance to the intranasal preparation is unclear.36 An in vitro study of esketamine showed modest induction of CYP2D6 and 3A4.11
The mean terminal half-life for esketamine ranges between 7 and 12 h, though there is a period of rapid decline in plasma concentrations over 2–4 h following the Cmax. Very little esketamine is recovered unchanged in the urine, while the metabolites are primarily (78%) renally cleared, with less than 2% recovery of a radio-labelled dose in the faeces.11 There is some indication of increased Cmax and AUC among Japanese versus Caucasian individuals (point estimate for both ~1.4×), and of increased AUC for Chinese versus Caucasian individuals (point estimate ~1.3×), though altered dosing is not recommended based on ethnic group. The Cmax and AUC were also higher for older (aged 75–85 years) than younger (25–54 years) patients (point estimate ~1.6× and ~1.4×, respectively).11 Patients above 65 years were commenced on a starting dose of 28 mg, rather than 56 mg in the clinical trials.37 There is no reported evidence of between-sex pharmacokinetic differences or by total body weight (39–170 kg assessed). There is no experience among patients on dialysis or among patients with severe (Child-Pugh Class C) liver disease.11
TRD treatment options
While a range of antidepressant and antipsychotic drugs could be used, only one product is specifically approved in the United States for the management of TRD: olanzapine in a fixed-dose combination with fluoxetine (Symbyax).38 One of the limitations of this combination therapy is the long ‘time to effect’ as compared with intranasal esketamine. Non-pharmacological options for TRD include electro-convulsive therapy (ECT) and transcranial magnetic stimulation, but these require specialised staff and facilities. In the case of ECT this includes access to appropriate anaesthetic expertise.38 If not previously tried, depression-focussed psychotherapy may be added to the existing pharmacological treatment but is not considered a standalone therapy for TRD.39 In 2005, the FDA approved vagus nerve stimulation (VNS) for the management of TRD; however, the mechanism of action is poorly understood.40 Deep brain stimulation (DBS)41 is another neurostimulation technique that avoids reliance on conventional pharmacological therapy.
According to the Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline,42 it is recommended to make adjustments if no improvement has been seen within 2–4 weeks of initiation of an antidepressant. In cases of acute suicide risk, this time period may be unsafely long, making faster-acting agents appealing.
Transition from ketamine to esketamine
The history of ketamine begins with phencyclidine, which was first synthesised in 1956.43 In 1970, ketamine, a non-selective NMDA receptor antagonist, was approved by the FDA to be used as an anaesthetic drug.44 Its half-life is 2.5 h and is metabolised to norketamine and dehydro-norketamine via cytochrome P450 iso-enzymes. Recently, the use of intravenous (IV) ketamine has increased (off-label) for the treatment of TRD. However, the optimal dose of IV ketamine for TRD is not well established. A small randomised trial that compared different doses of ketamine suggested that the preferred dose may be 0.5 mg/kg of body weight.45 Berman et al. first reported that a single IV infusion of ketamine (0.5 mg/kg over 40 min) exhibited a rapid and robust antidepressant response in patients with MDD.46 Several studies have reported the rapid and sustained efficacy of ketamine in MDD, including in patients with TRD.47,48 In a double-blind, placebo-controlled study of repeat IV ketamine infusion (0.5 mg/kg, either two or three times weekly for up to 4 weeks) in patients with TRD, Singh et al. found both regimens were well tolerated and showed consistent antidepressant effects.49 On the other hand, ketamine is known for its abuse potential and profound adverse effects, such as psychotomimetic symptoms, neurotoxicity, cognitive impairment and hypertension, precluding its routine use in clinical practice for depression.
In addition to IV infusion, ketamine is also available in other forms and can be administered by various routes, such as intramuscular, nasal, oral, rectal, sublingual and subcutaneous.50,51 Due to extensive first-pass metabolism, the oral bioavailability of ketamine varies from 17% to 29%.50 This pharmacokinetic profile led to the development of an intranasal formulation (8–45% bioavailability).50 Intranasal ketamine (50 mg) was well tolerated without any serious psychomimetic or dissociative effects, and showed improved depressive symptoms within 24 h compared with placebo in patients with MDD, who had failed at least one prior antidepressant trial.52
As aforementioned, ketamine is a racemic mixture of R- and S-enantiomers. Evidence from relatively old studies in humans showed that IV esketamine had better analgesic, intraoperative amnesia and anaesthetic properties, with less drowsiness, lethargy, cognitive impairment and psychotic emergent reactions, than the racemic mixture and R-enantiomer.53 Hence, interest in the potential efficacy of ketamine in MDD focused on esketamine. Administration of IV esketamine involves inherent difficulties for both patients and clinicians. The oral administration yields a low bioavailability of around 20%, which stimulated the development of intranasal esketamine.11,50
Having discussed the transition from ketamine to esketamine, the possibility of a transition from the clinical use of esketamine to R-ketamine (arketamine) as an antidepressant is mentioned below (see section Relevance to Patient Care and Clinical Practice).
Esketamine nasal spray (Spravato)
The FDA-approved esketamine intranasal spray (single-use) is convenient for outpatients – relative, for example, to ECT – and maximises access to therapy.26 The Spravato single-use device delivers two sprays (one each nostril) with a total of 32.3 mg of esketamine hydrochloride, which is equivalent to 28 mg of esketamine in 0.2 ml of a clear, colourless aqueous solution of pH 4.5. Inactive ingredients in Spravato are citric acid monohydrate, edetate disodium, sodium hydroxide and water for injection. The recommended storage temperature is between 20° and 25°C (68° to 77°F).11
Each device contains two sprays, one for each nostril and a ‘full device’ is indicated by two green dots. One spray into each nostril is required for a 28 mg dose, and the device should not be primed before use. The patient should blow their nose before using the device, which they then administer in a semi-reclined position. A 5-min rest period is recommended before administering a subsequent device, to maximise absorption. Two devices are required for a 56 mg dose, and three devices for an 84 mg dose. The maximum single dose studied in any clinical trial was 84 mg twice weekly.54–56 For acute management of TRD, the recommended dose of esketamine nasal spray is twice weekly (56 mg on day 1 followed by either 56 mg or 84 mg subsequently) for 4 weeks during treatment induction, reducing to once weekly (56 mg or 84 mg) for another 4 weeks and then, beyond 8 weeks, once weekly or every alternative week (56 mg or 84 mg) during ongoing maintenance therapy.26 According to the manufacturer, it is intended to be used under the direct supervision of a health care professional, making intranasal esketamine more complex to administer and monitor than oral antidepressants.
Because of the high relative incidence of dissociation and sedation associated with intranasal esketamine, the FDA label has a boxed warning for sedation and dissociation, and recommends that patients should be monitored for at least 2 h after the administration of the drug.57 This requirement and the associated funding implications might hinder the adoption of esketamine.58 The medication is available only through a restricted Risk Evaluation and Mitigation Strategy (REMS) program due to the concerns around sedation, and potential misuse and abuse for its dissociative and hallucinogenic effects.11 REMS is a medication safety program introduced by the FDA in 2007, to support the safe use of certain prescription medications with serious safety concerns and to help ensure that the benefits of these medications outweigh their risks.26
The cost for esketamine in the United States market (under brand name Spravato) is US$590 for a 56 mg dose (two devices) and US$885 for 84 mg (three devices);59 the cost for the first month of treatment would be approximately US$4800–6800, while it would be approximately US$1200–3600 per month during maintenance therapy.
Clinical trials
Phase I studies
A phase I randomised, double-blind, placebo-controlled, two-period crossover study [ClinicalTrials.gov identifier: NCT02094378] from the Netherlands evaluated the effects of cognitive function associated with intranasal esketamine 84 mg in 24 healthy adults aged 19–49 years.60 A significant difference in transient decline of cognitive functioning was observed at 40 min post-dose compared with baseline; however, no significant difference was evident between the groups at 2 h. In this trial, the common adverse effects included dizziness (67%), nausea (38%), disturbed attention (29%) and fatigue (29%), of which, most were considered mild in severity.60 While these data do not relate to TRD, there is relevance to safety.
Relevant phase II and phase III clinical trials of intranasal esketamine are listed in Table 2, and the published results of trials are outlined in Table 3.
Table 2.
Major randomised trials on esketamine from https://www.clinicaltrials.gov.
| Trial name, year | Esketamine strength | Age and population | Design and study focus | Treatment duration | Intervention(s) and comparator(s) | Sample size/ characteristics | Key outcomes |
|---|---|---|---|---|---|---|---|
| Phase I | |||||||
| DriveSaFe261
[ClinicalTrials.gov identifier: NCT02228239], 2014 |
Fixed esketamine dose 84 mg | Adults (21– 60 years) Healthy participants Body mass index 18–30 Bodyweight ⩾45 kg Normal cardiac function Valid driving license for more than 3 years |
Safety focus Randomised, double-blind placebo-controlled, 3-way crossover study |
7-week study • Screening phase (between 21 days and 1 day prior to the first dose administration) • 3-way crossover double-blind, single-dose treatment phase (45 days) • Follow-up phase (7–10 days after last dose administration) |
• Treatment A (esketamine 84 mg intranasal and 1 placebo capsule) • Treatment B (placebo intranasal and 1 mirtazapine 30 mg capsule) • Treatment C (placebo intranasal and placebo capsule) |
N = 26 | SDLP |
| Phase II | |||||||
| SYNAPSE10
[ClinicalTrials.gov identifier: NCT01998958], 2014–2015 |
Flexible esketamine doses (14 mg, 28 mg, 56 mg, 84 mg) | Adults (20–64 years) MDD, failed ⩾2ADs, IDS-C30 ⩾ 34 |
Safety focus Randomised, 2-panel, double-blind placebo-controlled study |
Four phases for Panel A and Panel B • 4-week screening phase • 1–15 days double-blind treatment phase • Optional open-label treatment phase (panel A: Day 15–74; Panel B: Day 15–25) • 8-week post-treatment (follow-up) phase |
• Esketamine 14 mg • Esketamine 28 mg • Esketamine 56 mg • Esketamine 84 mg • Placebo (comparator) |
N = 108 | MADRS change |
| SUI200162,63
[ClinicalTrials.gov identifier: NCT02133001], 2014–2016 |
Fixed esketamine dose 84 mg | Adult 18–64 Years MDD with suicidal ideation |
Safety focus Randomised, double-blind placebo-controlled study |
Approximately 81 days per participant • Screening phase (1-day before the study commences) • Double-blind treatment phase (day 1–25) • Follow-up phase (Day 26–81) |
• Esketamine 84 mg • Placebo |
N = 66 | MADRS change |
| Phase III | |||||||
| TRANSFORM-164,65
[ClinicalTrials.gov identifier: NCT02417064], 2015–2018 |
Fixed esketamine dose (56 mg or 84 mg) |
Adult 18–64 Years MDD IDS-C30 ⩾ 34 |
Efficacy and safety focus Randomised, double-blind, active-controlled, multicentre study |
• 4–7 weeks screening/prospective observation phase • 4-weeks double-blind induction phase • 24 weeks follow-up |
• Intervention: esketamine 56 mg (n = 115) or 84 mg (n = 116) intranasally twice per week • Comparator: Matching placebo given intranasally twice per week (n = 113) |
N = 344 Mean age: 47 Current episode duration (years): 3.9 MADRS mean: 37.5 Past failures of ⩾ 3 ADs: 40% |
MADRS change Clinical remission Clinical response |
| TRANSFORM-254–56
[ClinicalTrials.gov identifier: NCT02418585], 2015–2017 |
Flexible esketamine dose (56 mg or 84 mg) | Adult 18–64 Years MDD IDS-C30 ⩾ 34 |
Efficacy and safety focus Randomised, double-blind, active-controlled, multicentre study |
• 4–7 weeks screening/prospective observation phase • 4-weeks double-blind induction phase • 24 weeks follow-up |
• Intervention: esketamine 56 mg or 84 mg intranasally twice per week (n = 114) • Comparator: matching intranasal placebo (n = 109) |
N = 224 Mean age: 46 Current episode duration (years): 2.2 MADRS mean: 37 Past failures of ⩾ 3 ADs: 36% |
MADRS change Clinical remission Clinical response |
| TRANSFORM-366 [ClinicalTrials.gov identifier: NCT02422186], 2015–2017 | Flexible esketamine dose (28 mg or 56 mg or 84 mg) |
Adult ⩾65 Years MDD IDS-C30 ⩾ 31 |
Efficacy and safety focus Randomised, double-blind, active-controlled, multicentre study |
• 4–7 weeks screening/prospective observation phase • 4-weeks double-blind induction phase • 2-week follow-up |
• Intervention: esketamine 28 mg, 54 mg, or 84 mg intranasally twice per week (n = 72) • Comparator: matching intranasal placebo (n = 65) |
N = 137 Mean age: 70 Current episode duration (years): 4.1 MADRS mean: 35 Past failures of ⩾3 ADs: 39% |
MADRS change Clinical remission Clinical response |
| SUSTAIN-167
[ClinicalTrials.gov identifier: NCT02493868], 2015–2018 |
Flexible esketamine dose (56 mg or 84 mg) | Adult 18–64 Years MDD Response to esketamine MADRS ⩾28 at original screening IDS-C30 ⩾ 34 |
Efficacy focus Randomised withdrawal double-blinded, relapse prevention study |
• 4–7 weeks screening/prospective observation phase for direct-entry participants • 4-week open-label induction phase for direct-entry participants • 12 weeks optimisation phase (open-label for direct-entry participants and double-blind for transferred-entry participants) • Maintenance phase (double-blind, variable duration) • 2-week follow-up phase |
• Intervention: Induction: esketamine 56 mg or 84 mg intranasally twice per week for 4 weeks • Optimisation: intranasal esketamine 56 mg or 84 mg once every week for 4 weeks, then once every week or every other week • Maintenance: intranasal esketamine once every week or every other week (n = 152) • Comparator: matching intranasal placebo (n = 145) |
N=705 Mean age: 48 Current episode duration: NR Past AD failures: NR Stable remitters, MADRS mean: 37.5 Stable responders, MADRS mean: 39.5 |
Time to relapse |
| SUSTAIN-268,69
[ClinicalTrials.gov identifier: NCT02497287], 2015–2017 |
For 18 to 64 years old: Flexible esketamine dose (56 mg or 84 mg) For ⩾65 years old: Flexible esketamine dose (28 mg, 56 mg or 84 mg) |
18 Years and older Direct-entry, 18–64 years (n = 691) Transfer-entry, ⩾65 years (n = 111) MDD MADRS ⩾22 |
Long-term safety and efficacy focus Open-label, 52-week safety study |
• 4-week screening phase • 4-week induction phase (open-label) • 48-week optimisation/ maintenance phase (open-label) • 4-week follow-up phase |
• Intervention: Induction: esketamine 28 (for ⩾ 65 years), 56 mg or 84 mg intranasally twice per week • Optimisation and maintenance: intranasal esketamine once every week or every other week |
N = 802 MADRS mean: 16.4 Stable remitters 58.2% Stable responders (76.5%) 55 (6.9%) patients experienced serious TEAEs |
TEAEs, nasal tolerability and cognitive tests, MADRS total score, response rate and remission rate over time |
| SUSTAIN 370
[ClinicalTrials.gov identifier: NCT02782104], 2016–2022* |
Flexible dosing | Adult 18 years and older MDD Patients entered from other esketamine studies |
Long-term safety and tolerability focus Uncontrolled, open-label, long-term extension study |
Up to 63 months • 4-week Induction phase (if applicable) • Variable, open-label optimisation/maintenance phase |
• Intervention: Induction: esketamine 24 mg, 56 mg, or 84 mg intranasally twice per week for 4 weeks • Optimisation: esketamine intranasally once weekly (same dose as induction) for 4 weeks • Maintenance: esketamine intranasally once weekly or once every other week |
N = 1,150 | TEAE Safety outcome up to ~5 years |
| Aspire I71
[ClinicalTrials.gov identifier: NCT03039192), 2017–2018 |
Fixed esketamine dose 84 mg | Adults (18–64 years) MDD MADRS > 28 |
Efficacy and safety focus Randomised, double-blind placebo-controlled study |
• Screening phase (48 h prior to day 1 of dose administration) • Double-blind treatment phase for 25 days • Follow-up phase for 65 days |
• Intervention: esketamine 84 mg intranasally twice per week • Comparator: matching placebo given intranasally twice per week |
N = 226 | MADRS change CGI-SS-R change |
| Aspire II72
[ClinicalTrials.gov identifier: NCT03097133), 2017–2019* |
Fixed esketamine dose 84 mg | Adults (18–64 years) MDD MADRS > 28 |
Efficacy and safety focus Randomised, double-blind placebo-controlled study |
4-week | • Intervention: esketamine 84 mg intranasally twice per week • Comparator: matching placebo given intranasally twice per week |
N = 230 | MADRS change CGI-SS-R change |
| ESKETINTRD300673
[ClinicalTrials.gov identifier: NCT03434041), 2018–2021* |
Flexible dosing esketamine 56 mg or 84 mg | Adults (18–64 years) MDD MADRS ⩾28 |
Efficacy focus Randomised, double-blind, active-controlled, multicentre study | 4-week | • Intervention: esketamine 56 mg or 84 mg intranasally twice per week • Comparator: matching placebo given intranasally twice per week |
N = 234 | MADRS Onset of clinical response; response rate at week 4; remission rate at week 4; sustained remission at week 8 |
| [ClinicalTrials.gov identifier: NCT02918318]74
2016–2020* |
Fixed esketamine dose (28 mg, 56 mg and 84 mg) |
Adult 20–64 years MDD MADRS ⩾28 |
Efficacy focus Randomised, double-blind placebo-controlled study |
4-week | • Esketamine 28 mg versus 56 mg versus 84 mg versus placebo | N = 183 | Change from baseline MADRS at 4 weeks |
AD, antidepressant; CGI-SS-R, Clinical Global Impression - Severity of Suicidality – Revised; IDS-C30, Inventory of Depressive Symptoms-Clinician rated, 30-item total score; MADRS, Montgomery-Asberg depression rating scale; MDD, major depressive disorder; NR, not reported; RCT, randomised controlled trial; SDLP, standard deviation of lateral position; TEAEs, treatment emergent adverse events.
ongoing trial.
Table 3.
Published trials on esketamine nasal spray.
| Author, year, country | Design and sample size | Age and study duration, randomisation | Gender (% female) | Inclusion criteria | Phases | Intervention(s) | Comparator(s) | Key outcome measures/findings | Study type and comments |
|---|---|---|---|---|---|---|---|---|---|
| van de Loo et al.,75 2017, Netherlands | Phase I, double-blind, placebo-controlled, randomised, three-way crossover study, N = 24 | 21–60 years, 100-km on-road driving test 8 h after administration, double-dummy technique | 50.0 | Valid driving license for more than 3 years. Have driven at least 5000 km in the past year. Normal visual acuity. Body mass index 18–30 kg/m2 Bodyweight: ⩾45kg |
• Subjects were trained once to obtain baseline performance on the driving test | Intranasal esketamine (84 mg) | Intranasal placebo | SDLP that is, the weaving of the car. No significant difference in driving performance was observed 8 h after administering intranasal esketamine (84 mg) or placebo. |
Safety and efficacy of on-road driving performance |
| Morrison et al.,60 2018, Netherlands [ClinicalTrials.gov identifier: NCT02094378] |
Phase I, randomised, double-blind, placebo-controlled, 2-way crossover study, N = 24 | 19–49 years June 2014 to August 2014 Computer-generated randomisation |
50.0 | Body mass index 18–30 kg/m2
Body weight: ⩾45kg |
• Screening phase (up to 3 weeks), • Double-blind treatment phase (2 weeks) • Post treatment (follow-up) phase (1-week) |
Treatment sequence 1 (n = 12): intranasal esketamine 84 mg Treatment sequence 2 (n = 12): intranasal placebo |
Treatment sequence 1: intranasal placebo Treatment sequence 2: intranasal esketamine 84 mg |
Primary: five tests of Cogstate® computerised test battery assessed at 1 h pre-dose and 40 min, 2, 4, and 6-hour post-dose. Secondary: Mental Effort Scale, KSS, and safety and tolerability (TEAEs, C-SSRS, MOAA/S, BPRS+, and CADSS) |
Safety and efficacy of intranasal esketamine on cognitive functioning in healthy participants. Esketamine was associated with cognitive performance decline |
| Targum et al.,30 2019, United States. Results from SYNAPSE trial10 |
Phase II, double-blind, two-panel, placebo-controlled, delayed-start pilot study, N = 14 | 20–64 years 28 January 2014 to 25 September 2015 Blinded computer-generated randomisation algorithm selected seven patients at the three participating trial sites who were randomly assigned to placebo nasal spray and seven patients assigned to one of three different esketamine doses for the MADRS recordings. |
NR | MDD TRD, defined as an inadequate response to at least two adequate AD treatments of which at least one documented failure. The AD that participants had been receiving immediately before study entry was continued unchanged |
• Screening phase • Double-blind treatment phase (days 1–15) composed of two 1-week periods (Period 1, Period 2) • Optional open-label treatment (days 15–74) with tapering of intranasal dosing frequency • Post-treatment follow-up phase (8 weeks) |
Esketamine 28, 56, or 84 mg (n = 7) | Placebo nasal spray (n = 7) | TEAEs Site-based MADRS CADSS Blinded remote ratings (without the likelihood of functional unblinding) were comparable with site-based ratings of the efficacy of esketamine nasal spray. |
Safety and efficacy Comparison of site-based MADRS scores to remote, site-independent scores by blinded raters. Change in baseline MADRS score was significantly higher in all three esketamine-assigned treatment groups compared with the placebo group after one week of treatment and revealed a significant ascending dose-response relationship. |
| Popova et al.,76 2019. Six sites in the Czech Republic, nine in Germany, seven in Poland, seven in Spain, and 10 in the United States. Results from TRANSFORM-2 trial54–56 |
Phase III, randomised, double-blind, active-controlled, multicentre study, N = 223 | 18–64 years Between August 2015 and November 2017 Computer-generated randomisation |
61.9 | Single-episode (⩾2 years) or recurrent MDD without psychotic features. TRD, defined as a nonresponse to an adequate trial of at least two ADs in the current episode. |
4-week screening and prospective observation phase 4-week treatment phase during which participants received a new oral AD combined with either esketamine nasal spray or placebo nasal spray. Post-treatment follow-up phase of up to 24 weeks. |
Esketamine (56 mg or 84 mg) nasal spray administered twice weekly, each combined with a newly initiated open-label oral AD administered daily. (n = 114) | Placebo nasal spray administered twice weekly, each combined with a newly initiated open-label oral AD administered daily. (n = 109) | Change in MADRS score Clinical response (defined as a ⩾50% reduction in MADRS). Clinical response (MADRS score ⩽12). Changes in the Sheehan Disability Scale and PHQ-9 scores. Change in MADRS score with esketamine plus AD was significantly higher (difference of least square means −4.0, SE 1.69, 95% CI 27.31–20.64) than with AD plus placebo at day 28, and clinically meaningful improvement was observed in the esketamine plus AD arm at earlier time points. |
Compared the efficacy and safety of switching patients with TRD from an ineffective AD to flexible dosed esketamine nasal spray plus a newly initiated AD versus placebo nasal spray plus a newly initiated AD. SSRI (escitalopram or sertraline) or an SNRI (duloxetine or venlafaxine extended-release) was provided as open-label AD. |
| Daly et al.,4 2018, 13 sites in the United States, one site in Belgium. Results from SYNAPSE trial10 |
Phase II, double-blind, doubly randomised, delayed-start, placebo-controlled study (14 sites), N = 67 | 20–64 years 28 January 2014, to 25 September 2015 Computer-generated randomisation |
NR | Diagnosis of MDD TRD, defined as an inadequate response to 2 or more AD with at least one inadequate response in the current depression episode |
Screening Double-blind treatment (days 1–15), composed of two 1-week periods. Optional open-label treatment (days 15–74). Post-treatment follow-up (8 weeks). |
Period 1:
Esketamine 28 mg ((n = 11) Esketamine 56 mg (n = 11) Esketamine 84 mg (n = 12) Period 2: Esketamine 28 mg (n = 8) Esketamine 56 mg (n = 9) Esketamine 84 mg (n = 5) |
Period 1:
Placebo (n = 33) Period 2: Placebo (n = 6) |
Change in MADRS score from baseline to day 8 CADSS BPRS Change from baseline in the MADRS total score was significantly higher in all three esketamine groups than in the placebo group after 1-week of treatment; the ascending dose-response relationship also was significant. |
Assessed the efficacy, safety, and dose-response of esketamine in patients with TRD. AD effect was rapid in onset and dose-related. The response appeared to persist for more than two months with a lower dosing frequency. |
| Canuso et al.,62 2018, United States. Results from SUI2001 trial62,63 | Double-blind, randomised, placebo-controlled, multicentre study (2 sites), N = 66 | 19–64 years June 2014 to February 2016 Computer-generated randomisation |
65.2 | Diagnosis of MDD | 24- to 48-hour screening 4 weeks of double-blind treatment (days 1–25) eight weeks of posttreatment follow-up (days 26–81). | Esketamine 84 mg administered twice weekly (n = 35) | Matching placebo administered twice weekly (n = 31) | Change in MADRS score from baseline to 4 h after the initial dose. CADSS Remission of depressive symptoms (MADRS score ⩽12). Significantly greater improvement in MADRS score was observed in the esketamine group compared with the placebo group at 4 h and at∼24 h but not at day 25. Significant improvement was also observed in the esketamine group on the MADRS suicidal thoughts item score at 4 h but not at 24 h or at day 25. |
Compared the efficacy of standard-of-care treatment plus intranasal esketamine or placebo for rapid reduction of symptoms of MDD, including suicidality, among individuals at imminent suicide risk. Standard-of-care AD medication was initiated or optimised for all participants on day 1. |
| Daly et al.,31 2019, United States, Canada, and Europe. Results from SUSTAIN-1 trial67 | Phase III, multicentre, double-blind, randomised withdrawal study in an outpatient setting (99 sites), N = 297 | 18–64 years 6 October 2015, to 15 February 2018 |
66.3 | Recurrent or single episode (⩾2 years) MDD A total score of 34 or higher on the Clinician-Rated Inventory of Depressive Symptomatology A total MADRS score of 28 or higher, indicating moderate to severe depression |
4-week screening and prospective observation phase (direct-entry patients only) 4-week open-label induction phase (direct-entry patients only); 12-week optimisation phase (open-label, direct entry patients or double-blind, transfer-entry patients) Maintenance phase (double-blind, randomised withdrawal, event-driven, variable duration) 2-week posttreatment follow-up phase |
Stable remission: Esketamine nasal spray (56 or 84 mg) twice weekly plus an oral AD administered daily (n = 90) Stable response: Esketamine nasal spray (56 or 84 mg) twice weekly plus an oral AD (n = 62) |
Stable remission: Matched placebo plus an oral AD administered daily (n = 86) Stable response: Matched placebo plus an oral AD (n = 59) |
Time to relapse C-SSRS CADSS BPRS Adverse events Stable remission, defined as MADRS score ⩽12 for ⩾3 of the last 4 weeks Stable response defined as ⩾50% reduction in MADRS score from baseline in the last 2-weeks of the optimisation phase. |
Efficacy of esketamine nasal spray plus an oral AD compared with an oral AD plus placebo nasal spray in delaying relapse of depressive symptoms. Continued treatment with esketamine and an AD demonstrated clinically meaningful and statistically significant superiority compared with AD and placebo in delaying relapse. |
| Fedgchin et al.,65 2019, Belgium, Brazil, Canada, Estonia, France, Hungary, Mexico, Slovakia, and the United States. Results from TRANSFORM-1 trial64,65 |
Phase III, randomised, double-blind, active-controlled, multicentre study, N = 342 | 18–64 years September 2015 and February 2018 Computer-generated randomisation |
70.5 | Recurrent MDD or single-episode MDD (⩾2 years). A total MADRS score of 34 or higher, indicating moderate to severe depression. One AD with non-response (⩽25% improvement) in the current depressive episode. |
Three phases: 4-week screening/ prospective observation phase 4-week double-blind treatment phase Up to 24-week follow-up phase |
Esketamine 56 mg/oral AD (n = 115) Esketamine 84 mg/oral AD (n = 114) |
Oral AD/ placebo (n = 113) | Change in MADRS total score from baseline to day 28. | Efficacy of esketamine 84 mg/ AD compared with AD/ placebo did not achieve statistical significance and as a result, the study did not formally evaluate esketamine 56 mg/AD dosing. No new safety-related concerns were identified across the treatment groups. |
| Ochs-Ross et al.,77 2020, includes 13 countries. Results from TRANSFORM-3 trial66 |
Phase III, randomised, double-blind, active-controlled, multicentre study | ⩾65 years (mean age 70.0 (4.52)), August 2015 and August 2017, Computer-generated randomisation | 62.0 | Patients ⩾65 years old and diagnosed (DSM-5) with recurrent moderate to severe MDD without psychotic features. TRD, defined as no clinically meaningful improvement following treatment with ⩾2 different ADs. IDS-C30 total score of ⩾31 |
Three phases: 4-week screening/ prospective observational phase 4-week double-blind induction phase 2-week post-treatment follow-up phase assessing safety and tolerability, including potential withdrawal symptoms |
Flexibly-dosed esketamine nasal spray (28 mg, 56 mg, or 84 mg) dosed twice-weekly for 4 weeks and oral AD | Oral AD and placebo nasal spray | Changes in MADRS total score | Efficacy: Change in MADRS total score from baseline to day 28 did not achieve statistical significance Safety: TEAEs developed in 70.8% of intervention patients, and 60.0% of control group. Common TEAEs in the intervention group were dizziness (20.8%) and nausea (18.1%). |
| Wajs et al.,68 2020, includes 21 countries and 114 sites. Results from SUSTAIN-2 trial69 |
Phase III, open-label, multicentre study, N = 802 | 18 Years and older Direct-entry, 18–64 years Transfer-entry, ⩾65 years; mean age (SD), 52.2 (13.7) October 2015 and October 2017 |
62.6 | DSM-5 diagnosis of recurrent MDD or single episode (⩾2 years) MDD without psychotic features, nonresponse to ⩾2 oral AD and MADRS total score ⩾22 at screening | Four phases: 4-week screening phase 4-week induction phase Up to 48 weeks optimisation/ maintenance phase, 4-week follow-up phase |
Esketamine nasal spray (28-mg, 56-mg, or 84-mg) plus new oral AD (administered twice a week in a 4-week induction phase and weekly or every-other-week for patients who were responders and entered a 48-week optimisation/ maintenance phase) | No comparator (single group assignment) | TEAEs C-SSRS assessment Changes in MADRS total score |
Safety assessments of esketamine for TEAEs (common TEAEs were dizziness (32.9%), dissociation (27.6%), nausea (25.1%), and headache (24.9%)) Efficacy: MADRS total score reduced during the induction phase, and this reduction continued during the maintenance phase. |
AD, antidepressant; BPRS, Brief Psychiatric Rating Scale; CADSS, Clinician-Administered Dissociative States Scale; C-SSRS, Columbia Suicide Severity Rating Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; IDS-C30, Inventory of Depressive Symptoms-Clinician rated-30-item; KSS, Karolinska Sleepiness Scale; MADRS, Montgomery-Åsberg depression rating scale; MDD, major depressive disorder; MOAA/S, Modified Observer’s Assessment of Alertness/Sedation; NR, not reported; SDLP, standard deviation of lateral position; TEAEs, treatment-emergent adverse events; TRD, treatment-resistant depression.
Phase II studies
A randomised controlled trial (RCT) by Daly et al. (the SYNAPSE trial protocol) evaluated the efficacy and safety of intranasal esketamine as an adjunctive treatment for patients with TRD, for 130 days, with (i) screening, (ii) blinded treatment, (iii) open-label treatment and (iv) follow-up phases.4,10 Participants continued their existing antidepressant treatment during the study. TRD was defined as the failed response to two or more antidepressants, with at least one inadequate response for the current depression episode.4 However, about two-thirds of included patients had reported only a single trial of failed antidepressants in the current episode (in addition to one in a previous episode). Hence, the authors’ claim of patients with TRD is somewhat questionable, even though it was a proof-of-concept study to evaluate the dose-response relationship with intranasal esketamine. The combined data analysis from both 1-week periods showed that, after 1 week of treatment, the mean difference (change in Montgomery-Asberg Depression Rating Scale score; MADRS) between the esketamine dose (28 mg, 56 mg, and 84 mg) groups and the placebo group was statistically, as well as clinically, significant. The MADRS score is on a scale of 0–60, where 0 indicates an absence of depression symptoms. The least mean squares difference for period one and two – the blinded component of the study – was −4.2 [standard error (SE) 2.09, p = 0.02] for the 28 mg dose, –6.3 (SE 2.07, p = 0.001) for the 56 mg dose, and −9.0 (SE 2.13, p < 0.001) for the 84 mg dose. Moreover, the authors confirmed that there was evidence of sustained improvement on the Generalised Anxiety Disorder scale during the open-label phase, which lasted up to 9 weeks. Though the sample size was limited (only 67 participants were included in the efficacy and safety analyses) and the study duration was reasonably short, these data also suggest there is a beneficial effect of esketamine on anxiety symptoms.
With additional participants to those in the paper by Daly et al., in the full SYNAPSE trial the double-blind phase showed a decrease in depressive symptoms with varying doses of esketamine (14 mg, 28 mg, 56 mg and 84 mg) at 2 h, 24 h, 8 days, and 15 days from baseline when compared with placebo (plus an oral antidepressant).4,10 The trial included four parts: screening, double-blind treatment (two 1-week periods), optional open-label treatment, and post-treatment follow up. This dose-response study imparts further information about safety and tolerability when exposed to several fixed doses of short-term esketamine. However, the open-label phase (optional for those who completed the double-blind phase) response rate on day 74 showed 65% of participants achieved a ⩾50% decrease in MADRS score whereas 32% had achieved a MADRS total score of ⩽10 on day 74, suggesting ‘no to mild depression’. When compared with placebo, a large decrease in MADRS total score was seen with the 84 mg dose [−9.0 (2.13), p < 0.001]. However, the 28 mg dose [−4.2 (2.09), p = 0.02] elicited a lower response. During the 8-week follow-up phase, results indicated that 56% of participants consistently exhibited a ⩾50% reduction in MADRS total score.
Phase III studies
The FDA application was approved based on five phase III trials in patients with TRD.54–56,64–69 Of these, three studies54–56,64,65,66 were for short-term use, one on withdrawal maintenance of effect,31,67 and one on long-term safety.68,69 Another ongoing, long-term (5-year) safety (phase III) continuation trial (SUSTAIN-3) is intended to determine treatment-emergent adverse events (TEAE) in patients aged 18 years and above.70 In the majority of the phase III trials, TRD was defined as nonresponse (⩽25% improvement according to clinical judgement) to more than two oral antidepressants managing depression at a therapeutic dose for at least a 6-week period. The FDA reported that, across the phase III trials, around 33–40% of randomised patients had a failed therapy using ⩾4 antidepressant medications.26
The overall findings demonstrated that treatment with intranasal esketamine in combination with a new oral antidepressant was more effective than placebo plus an oral antidepressant, and associated with a rapid reduction of depressive symptoms and delayed time to relapse from symptoms of depression.76,78 Consistent with the short-term esketamine studies, the long-term safety study showed that the esketamine doses studied were generally well tolerated, with no new safety concerns with dosing up to 52 weeks.68 A phase III study by Popova et al.76 demonstrated a rapid (within 24–48 h) improvement in depressive symptoms with esketamine nasal spray in combination with an oral antidepressant compared with placebo plus an antidepressant, in 223 adult patients with TRD. Partly because the effect size was relatively small (difference of least squares means for MADRS of −4 after 28 days), it is questionable whether efficacy was truly demonstrated.
In three similarly designed, short-term use trials, TRANSFORM-1,64,65 and TRANSFORM-2 were conducted in patients aged 18–64 years,54–56 whereas TRANSFORM-3 was conducted in patients aged 65 years and older.66 Among these trials, dosing of esketamine (4-week randomised, placebo-controlled phase) was undertaken only after a 4-week prospective screening and observational phase, where patients remained on the same oral antidepressants in order to develop an additional failure of non-response (defined as ⩽25% improvement in the MADRS total score). The MADRS total score at week 4 was assessed as the primary efficacy outcome measure in all three trials. The majority of the patients in TRANSFORM-1 and -254–56,64 achieved a clinical response (at least 50% decrease in the MADRS total score from baseline) but not clinical remission (MADRS ⩽12) at 4 weeks. A larger proportion of older patients in the esketamine arm of the TRANSFORM-3 trial also achieved clinical response (23.6% versus 12.3%) and clinical remission (15.3% versus 6.2%) compared with placebo.66 Using the minimal data from two phase III trials (TRANSFORM 1 and 2), a meta-analysis was performed and the findings favoured greater improvement in MADRS score for esketamine plus an antidepressant, compared with placebo plus antidepressant (mean difference: –3.84; 95% CI: –6.29, –1.39).79 The minimum clinically important difference (MCID) estimates for MADRS varied from 1.6 to 1.9.80,81
Another randomised withdrawal trial was designed to assess primarily relapse prevention (SUSTAIN-1)31,67 by recruiting patients either from two other phase III trials (TRANSFORM-1 or -2) or direct entry to the trial from patients who met the same inclusion and screening criteria. The trial involved a 4-week induction period during which patients received twice-weekly intranasal esketamine (56 mg or 84 mg) in combination with a newly-initiated oral antidepressant. This was continued with a 12-week optimisation phase for responders (receiving the same dose of esketamine plus newly-initiated oral antidepressant) with less frequent esketamine dosing, followed by a 48-week maintenance phase. During the maintenance phase, patients who were stable remitters (MADRS score ⩽12 in weeks 12–16) or stable responders (⩾50% reduction in MADRS score from baseline but without achieving remission) were separately randomised to either continue with esketamine nasal spray plus oral antidepressant at the same dose, or switch to placebo plus an oral antidepressant. The primary outcome was time-to-relapse in patients with stable remission, and the key secondary outcome was the time to relapse in patients with stable response.31,67 There was a decrease in risk of relapse by 51% among patients (receiving esketamine and antidepressant) who achieved stable remission and 70% risk reduction among those who achieved a stable response compared with the antidepressant and placebo group.31
Safety
The most commonly observed adverse reactions (incidence greater than 5% and at least twice that of placebo plus oral antidepressants) from esketamine are dizziness, sedation, nausea, vertigo, anxiety, hypoesthesia, lethargy, vomiting, hypertension and ‘feeling drunk’.57 Intranasal esketamine was associated with a transient 7–9 mm Hg increase in systolic blood pressure and 4–6 mm Hg increase in diastolic blood pressure, both of which peaked at 40 min post-dose and returned to baseline within 2 h.11 The highest mean increases from baseline during both study periods (two periods each of 1 week) were observed in the 84-mg esketamine group.4 Notably, a small subset of treated patients (8–17%) reported clinically significant hypertension, within a range of 40 mm Hg increase in systolic blood pressure and/or 25 mm Hg diastolic blood pressure.11 Suicidal thoughts were found to be decreased significantly with an esketamine 84 mg dose at 4 h, but not at 24 h or 25 days.62 In terms of pharmacodynamic drug–drug interactions, central nervous system depressants (benzodiazepines, opioids, alcohol) may increase sedation, while psychostimulants (amphetamines, modafinil, methylphenidate, armodafinil) and monoamine oxidase inhibitors (selegiline, phenelzine) may induce hypertension.11
An open-label, long-term, multicentre trial of esketamine (SUSTAIN-2)68,69 was intended primarily to evaluate the long-term safety of esketamine in 603 patients who had responded during a 4-week induction phase. The trial consisted of a 4-week screening phase, 4-week induction phase, 48-week maintenance phase (responders in induction phase only), and a 4-week follow-up phase to determine TEAEs. At least one TEAE was reported among 86% of the study participants during the maintenance phase, most of which occurred on dosing days and resolved on the same day. A total of 68 serious TEAEs were reported among 55 (6.9%) patients, in addition to two reported deaths during the maintenance phase; one due to acute cardiac and respiratory failure and the other due to suicide.68 The investigators claimed that both deaths were considered either doubtfully related, or not related, to esketamine. A total of 10% of participants discontinued the use of esketamine due to TEAEs, where 6.8% withdrew from treatment during the induction phase and 3.8% in the maintenance phase; indicating the dose was tolerable and without reports of new safety concerns.68,69 Another phase III trial reported that the adverse effects (dissociation, vertigo, dysgeusia, dizziness and nausea) appeared shortly after dosing and settled by 1.5 h post-dosing.76
Caution is warranted if driving after receiving esketamine, given the potential for dissociative and sedative side effects.26 In a small, double-blinded, randomised phase I study (n = 24), investigators found no significant difference in driving performance measured by the weaving of a car 8 h after administering intranasal esketamine (84 mg) in healthy participants (both male and female).75 Mirtazapine (oral, 30 mg) was used as a positive control, and it showed a significant detrimental impact on driving.75 From the aforementioned study, the generalisability of driving performance to chronic esketamine users is questionable; further information closer to dosing time would also be useful. Another double-blinded crossover study is evaluating the human abuse potential of intranasal esketamine doses 84 mg and 112 mg compared with racemic IV ketamine in nondependent, recreational polydrug users, assessed by a change in abuse potential based on a Visual Analogue Scale.82 The study is also measuring pharmacokinetic parameters as secondary outcomes (Tmax, AUC, half-life, clearance, volume of distribution and Cmax).82
The opioid properties of esketamine need to be further assessed when considering how best to use it safely.83 In a rodent pain study, Pacheco et al. reported that ketamine exerts a central antinociceptive effect through the endogenous release of opioids that act on the μ and δ (but not the κ) receptors.84 However, ketamine tends to reduce the risk of tolerance with opioids and, at anaesthetic doses, it further reduces post-surgery opioid use, likely through its NMDA antagonism effects.85,86 Because of the addictive potential through endogenous opioid release, use of esketamine in real-world settings needs close monitoring and further evaluation to assess and prevent drug abuse or misuse. After administration of intranasal esketamine, patients can rapidly experience significant dissociation and sedation, which may contribute to abuse. There are currently limited long-term safety data and there are no published data on the risk of abuse or misuse for esketamine, when used in the treatment of depression.
Relevance to patient care and clinical practice
Among long-term studies, a higher proportion of patients responded to treatment doses of 84 mg, and about one-third of patients responded to 56 mg, with doses administered either weekly or fortnightly.87 The overall results from different trials show that intranasal esketamine reduces suicidal risk and symptoms of depression, as measured through a reduced MADRS score.56,68,77 Clinical trials indicate that the use of intranasal esketamine in the geriatric population is safe and effective.77 However, with insufficient data, the dose adjustments for patients with hepatic or renal impairment remain unknown. Currently, the use of intranasal esketamine has been not studied in people less than 18 years of age, or among pregnant or lactating women. The product information states it should not be used in pregnancy or breastfeeding.11 Furthermore, esketamine is contraindicated for use in patients with aneurysmal vascular disease, arteriovenous malformation, a history of intracerebral haemorrhage or hypersensitivity.11 A Canadian Health Technology Assessment mentioned the off-label potential of esketamine for treating MDD with imminent risk of suicide and treatment-resistant bipolar depression, in addition to its intended use for unipolar TRD, although to date there are no available RCT data.88
Though esketamine is available in a convenient dosage form (nasal spray), the FDA requires it to be administered under direct supervision by a healthcare professional, with monitoring afterwards. This could potentially challenge the accessibility of therapy and might require repeat visits to the clinic on a weekly/fortnightly basis. If long-term or short-term use of esketamine is associated with driving impairment, then patients might find it challenging to visit the clinic subject to limited public transportation. Policies should be made to ensure patients do not self-administer if the medication is available through a pharmacy, and there should be provision for supervision with each patient.
The practice relevance of duration and frequency of intranasal esketamine use is not fully understood. Considering the rapid relapse and potential suicide risk in TRD, it is difficult to know what to recommend to clinicians regarding whether they should continue to use the agent beyond an acute course, for how long and in which patients, and how the therapy should be ceased. The similar pharmacological profile to ketamine and therefore potential for abuse and misuse means that, while efficacy and effectiveness are of course important, facilitating the appropriate cessation of treatment also warrants consideration. This has been discussed by the FDA and remains an important issue.57 Concerns have been raised about both the opioid properties of esketamine and the possible risk imposed by the reliance induced by rapid symptom relief followed by rapid relapse.83,89
Commonly, for MDD, the initial treatment may show a lack of effect or may cause intolerable side effects and switching to an alternative therapy is common.90 Therefore, conducting a trial might require dose adjustments of antidepressant and approximately 6–12 weeks to assess response. It is challenging for a patient to remain on antidepressant therapy long enough for an adequate trial, especially if some side effects or symptoms are incapacitating. Consequently, the definition of TRD is challenging because it includes not only the number of unique treatments undertaken but also whether the length of each medication trial was adequate to observe a clinical response.
Ongoing research with arketamine (R-ketamine) will also influence the future place of esketamine in clinical practice. A number of animal studies have now indicated that arketamine has more potent and longer-lasting antidepressant effects than esketamine, with less psychotomimetic side effects.15,91–94
Recently, a small open-label pilot study of arketamine produced positive results.95 Seven female subjects with TRD received a single intravenous infusion of arketamine (0.5 mg/kg). The mean MADRS dropped from 30.7 before infusion to 10.4 after 1 day (mean difference: –20.3; 95% CI: –13.6, –27.0, p < 0.001), and dissociation was nearly absent. The mean difference in MADRS at 7 days was still 16.7. Human studies have also indicated that arketamine does not have psychotic effects and produces a state of relaxation, while esketamine has more dissociative effects.96 The slower elimination of arketamine, with a clearance approximately one-half that of esketamine and racemic ketamine,97 may also be advantageous.
In addition, S-norketamine, the active metabolite of esketamine, appears to have similar antidepressant potency to the parent drug, with less potential for psychotomimetic side effects, presumably because of less activity on NMDA receptors.98,99 It can activate BDNF and mTORC1, independently of AMPA receptors.98
Conclusion
For TRD, intranasal esketamine signifies an easier method of administration than IV administration of ketamine, with a rapid onset of action, reasonable bioavailability, and being more practical and less resource-demanding. However, the requirement for observed administration and post-dose monitoring introduces some implementation challenges. The findings from phase III trials illustrate that esketamine is promising among patients with TRD for symptom improvement and achieving clinical response. However, the current evidence provided by efficacy-related studies is sparse, especially in terms of dose-titration (e.g. if response wanes over time) and long-term safety and effectiveness.
Schatzberg highlighted that the FDA application included a drug relapse/discontinuation trial in the approval process,31 which he described as a ‘somewhat unusual’ approach to demonstrate proof of efficacy.83 As noted above, in that study, patients whose depression achieved stable remission or stable response after 16 weeks of initial treatment with esketamine nasal spray and an antidepressant had a significantly delayed time to relapse with continued treatment with intermittently administered esketamine plus an oral antidepressant, compared with those treated with oral antidepressant plus placebo nasal spray.31 Similar concerns have been raised by others, along with the issues of the definition of TRD used to include patients in the trials and that the participants were not required to have failed psychotherapy.100,101
TRD is a debilitating condition, with few treatment options. Further work on intranasal esketamine is needed to further elucidate the potential for abuse, and if, when and how to discontinue treatment, and to clarify cost-effectiveness across health systems deciding to approve its use. Given its rapid effect, it may also be considered as a bridging therapy in the future, while conventional oral antidepressants take effect.102 As with any new agent, caution should be applied by clinicians and attention paid to the REMS program requirements of the current FDA approval. With these caveats noted, esketamine nasal spray offers an innovative and interesting new option for TRD. Time will tell whether it becomes supplanted by either arketamine or S-norketamine.
Footnotes
Author contributions: All authors substantially contributed to the planning of the manuscript, critical revision of the draft and final approval of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gregory M Peterson  https://orcid.org/0000-0002-6764-3882
https://orcid.org/0000-0002-6764-3882
Contributor Information
Mohammed S Salahudeen, School of Pharmacy and Pharmacology, College of Health and Medicine, University of Tasmania, 8 Churchill Avenue, Hobart, Tasmania, Australia.
Cameron M Wright, School of Pharmacy and Pharmacology, College of Health and Medicine, University of Tasmania, Hobart, Tasmania, Australia; Health Systems and Health Economics, School of Public Health, Faculty of Health Sciences, Curtin University, Perth, Western Australia, Australia.
Gregory M Peterson, School of Pharmacy and Pharmacology, College of Health and Medicine, University of Tasmania, Private Bag 26 UTAS, HOBART, TAS 7001, Australia; Faculty of Health, University of Canberra, Bruce, ACT, Australia.
References
- 1. Lam RW, McIntosh D, Wang J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1. Disease burden and principles of care. Can J Psychiatry 2016; 61: 510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 3. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- 4. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 2018; 75: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 6. Gaynes BN, Asher G, Gartlehner G, et al. Technology assessment program. project ID. PSYT0816. Rockville, MD: Agency for Healthcare Research and Quality, https://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id105TA.pdf (2018, accessed 6 May 2020). [Google Scholar]
- 7. Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry 2011; 72: e18. [DOI] [PubMed] [Google Scholar]
- 8. Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry 2017; 74: 9–10. [DOI] [PubMed] [Google Scholar]
- 9. Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv 2014; 65: 977–987. [DOI] [PubMed] [Google Scholar]
- 10. Janssen Research & Development, LLC. NCT01998958. A study to evaluate the safety and efficacy of intranasal esketamine in treatment-resistant depression (SYNAPSE). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT01998958 (2019, accessed 6 May 2020). [Google Scholar]
- 11. SPRAVATO™ (esketamine) nasal spray. Full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf (2019, accessed 6 May 2020).
- 12. Dang YH, Ma XC, Zhang JC, et al. Targeting of NMDA receptors in the treatment of major depression. Curr Pharm Des 2014; 20: 5151–5159. [DOI] [PubMed] [Google Scholar]
- 13. Moaddel R, Abdrakhmanova G, Kozak J, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol 2013; 698: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacological Rev 2018; 70: 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 2015; 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang C, Ren Q, Qu Y, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 2018; 83: 18–28. [DOI] [PubMed] [Google Scholar]
- 18. Cornwell BR, Salvadore G, Furey M, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 2012; 72: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 2013; 18: 595–606. [DOI] [PubMed] [Google Scholar]
- 20. Zanos P, Gould TD. Intracellular signaling pathways involved in (S)- and (R)-Ketamine antidepressant actions. Biol Psychiatry 2018; 83: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ignacio ZM, Reus GZ, Arent CO, et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 2016; 82: 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li N, Lee B, Liu R-J, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 2011; 61: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 24. Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 2014; 29: 419–423. [DOI] [PubMed] [Google Scholar]
- 25. Abdallah CG, Averill LA, Gueorguieva R, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 2020; 45: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Center for Drug Evalulation and Research. FDA briefing document: Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee Meeting. Silver Spring, MD: U.S. Food and Drug Administration (FDA), https://www.fda.gov/media/121376/download (2019, accessed 6 May 2020). [Google Scholar]
- 27. Fanta S, Kinnunen M, Backman JT, et al. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur J Clin Pharmacol 2015; 71: 441–447. [DOI] [PubMed] [Google Scholar]
- 28. Peltoniemi MA, Saari TI, Hagelberg NM, et al. Rifampicin has a profound effect on the pharmacokinetics of oral S-ketamine and less on intravenous S-ketamine. Basic Clin Pharmacol Toxicol 2012; 111: 325–332. [DOI] [PubMed] [Google Scholar]
- 29. Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos 2003; 24: 37–43. [DOI] [PubMed] [Google Scholar]
- 30. Targum SD, Daly E, Fedgchin M, et al. Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment resistant depression. J Psychiatr Res 2019; 111: 68–73. [DOI] [PubMed] [Google Scholar]
- 31. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 2019; 76: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorne RG, Emory CR, Ala TA, et al. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res 1995; 692: 278–282. [DOI] [PubMed] [Google Scholar]
- 33. Thorne RG, Pronk GJ, Padmanabhan V, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004; 127: 481–496. [DOI] [PubMed] [Google Scholar]
- 34. Balin BJ, Broadwell RD, Salcman M, et al. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol 1986; 251: 260–280. [DOI] [PubMed] [Google Scholar]
- 35. Noppers I, Olofsen E, Niesters M, et al. Effect of rifampicin on S-ketamine and S-norketamine plasma concentrations in healthy volunteers after intravenous S-ketamine administration. Anesthesiology 2011; 114: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashraf MW, Peltoniemi MA, Olkkola KT, et al. Semimechanistic population pharmacokinetic model to predict the drug-drug interaction between S-ketamine and ticlopidine in healthy human volunteers. CPT Pharmacometrics Syst Pharmaco 2018; 7: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janssen Research & Development. Advisory Committee Briefing Document: Esketamine Nasal Spray for Patients with Treatment-resistant Depression, JNJ-54135419 (esketamine). 2019. [Google Scholar]
- 38. Papadimitropoulou K, Vossen C, Karabis A, et al. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: a systematic review and network meta-analysis. Curr Med Res Opin 2017; 33: 701–711. [DOI] [PubMed] [Google Scholar]
- 39. Trivedi RB, Nieuwsma JA, Williams JW., Jr. Examination of the utility of psychotherapy for patients with treatment resistant depression: a systematic review. J Gen Intern Med 2011; 26: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 2017; 14: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord 2014; 159: 31–38. [DOI] [PubMed] [Google Scholar]
- 42. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 2016; 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maddox VH, Godefroi EF, Parcell RF. The synthesis of phencyclidine and other 1-arylcyclohexylamines. J Med Chem 1965; 8: 230–235. [DOI] [PubMed] [Google Scholar]
- 44. Domino EF. Taming the Ketamine Tiger. Anesthesiology 2010; 113: 678–684. [DOI] [PubMed] [Google Scholar]
- 45. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 2020; 25: 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 47. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 49. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 2016; 173: 816–826. [DOI] [PubMed] [Google Scholar]
- 50. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci 2016; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peltoniemi MA, Hagelberg NM, Olkkola KT, et al. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet 2016; 55: 1059–1077. [DOI] [PubMed] [Google Scholar]
- 52. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 2014; 76: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol 2016; 6: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Janssen Research & Development, LLC. NCT02418585. A study to evaluate the efficacy, safety, and tolerability of flexible doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment resistant depression (TRANSFORM-2). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/ NCT02418585 (2018, accessed 6 May 2020). [Google Scholar]
- 55. Popova V, Daly EJ, Trivedi M, et al. Randomized, double-blind study of flexibly-dosed intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. Eur Neuropsychopharmacol 2019; 29(Suppl. 1): S35–S36. [Google Scholar]
- 56. Popova V, Daly E, Trivedi M, et al. S111. Randomized, double-blind study of flexibly-dosed intranasal esketamine plus oral antidepressant vs. active control in treatment-resistant depression. Biol Psychiatry 2018; 83: S390. [Google Scholar]
- 57. U.S. Food and Drug Administration. Briefing information for the Feb 12, 2019. Presented at the joint meeting of the Psychopharmacologic Drugs Advisory Committee (PDAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM), 12 February 2019, Silver Spring, MD. [Google Scholar]
- 58. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA 2019; 322: 1039–1040. [DOI] [PubMed] [Google Scholar]
- 59. Reuters. J&J prices ketamine-like depression treatment at $590–$885 for two doses, https://www.reuters.com/article/us-johnson-johnson-fda-pricing/jj-prices-ketamine-like-depression-treatment-at-590-885-for-two-doses-idUSKCN1QN2AX (2019, accessed 6 May 2020).
- 60. Morrison RL, Fedgchin M, Singh J, et al. Effect of intranasal esketamine on cognitive functioning in healthy participants: a randomized, double-blind, placebo-controlled study. Psychopharmacology 2018; 235: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Janssen Research & Development, LLC. NCT02228239. Study to assess the effects of esketamine on safety of on-road driving in healthy participants (DRiVESaFe). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02228239 (2019, accessed 6 May 2020). [Google Scholar]
- 62. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled Study. Am J Psychiatry 2018; 175: 620–630. [DOI] [PubMed] [Google Scholar]
- 63. Janssen Research & Development, LLC. NCT02133001. A double-blind study to assess the efficacy and safety of intranasal esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in participants who are assessed to be at imminent risk for suicide. ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02133001 (2019, Accessed 6 May 2020).
- 64. Janssen Research & Development, LLC. NCT02417064. A study to evaluate the efficacy, safety, and tolerability of fixed doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment-resistant depression (TRANSFORM-1). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02417064 (2019, accessed 6 May 2020). [Google Scholar]
- 65. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 2019; 22: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Janssen Research & Development, LLC. NCT02422186. A study to evaluate the efficacy, safety, and tolerability of intranasal esketamine plus an oral antidepressant in elderly participants with treatment-resistant depression (TRANSFORM-3). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02422186 (2018, accessed 6 May 2020). [Google Scholar]
- 67. Janssen Research & Development, LLC. NCT02493868. A study of intranasal esketamine plus an oral antidepressant for relapse prevention in adult participants with treatment-resistant depression (SUSTAIN-1). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02493868 (2019, accessed 6 May 2020). [Google Scholar]
- 68. Wajs E, Aluisio L, Holder R, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 2020; 81: 19m12891. [DOI] [PubMed] [Google Scholar]
- 69. Janssen Research & Development, LLC. NCT02497287. A long-term, safety and efficacy study of intranasal esketamine in treatment-resistant depression (SUSTAIN-2). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02497287 (2019, accessed 6 May 2020). [Google Scholar]
- 70. Janssen Research & Development, LLC. NCT02782104. A long-term safety study of intranasal esketamine in treatment-resistant depression (SUSTAIN-3). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02782104 (2019, accessed 6 May 2020). [Google Scholar]
- 71. Janssen Research & Development, LLC. NCT03039192. A study of the efficacy and safety of intranasal esketamine in the rapid reduction of symptoms of major depressive disorder, in adult at imminent risk for suicide (Aspire I). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT03039192 (2019, accessed 6 May 2020). [Google Scholar]
- 72. Janssen Research & Development, LLC. NCT03097133. A Study to Evaluate the Efficacy and Safety of Intranasal Esketamine in Addition to Comprehensive Standard of Care for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Adult Participants Assessed to be at Imminent Risk for Suicide (Aspire II). ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT03097133 (2019, accessed 6 May 2020). [Google Scholar]
- 73. Janssen Research & Development, LLC. NCT03434041. A study to evaluate the efficacy, pharmacokinetics, safety and tolerability of flexible doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment-resistant depression. ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT03434041 (2019, accessed 6 May 2020). [Google Scholar]
- 74. Janssen Research & Development, LLC. NCT02918318. A study to evaluate the efficacy, safety and tolerability of fixed doses of intranasal esketamine in japanese participants with treatment resistant depression. ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02918318 (2019, accessed 6 May 2020). [Google Scholar]
- 75. van de Loo A, Bervoets AC, Mooren L, et al. The effects of intranasal esketamine (84 mg) and oral mirtazapine (30 mg) on on-road driving performance: a double-blind, placebo-controlled study. Psychopharmacology 2017; 234: 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 2019; 176: 428–438. [DOI] [PubMed] [Google Scholar]
- 77. Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of intranasal esketamine plus an oral antidepressant in elderly patients with treatment-resistant depression - TRANSFORM-3. Am J Geriatr Psychiatry 2020; 28: 121–141. [DOI] [PubMed] [Google Scholar]
- 78. Daly E, Trivedi M, Janik A, et al. A Randomized withdrawal, double-blind, multicenter study of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. Presented at the Annual Meeting of the American Society of Clinical Psychopharmacology (ASCP), 29 May–1 June 2018, Miami. [Google Scholar]
- 79. Institute for Clinical and Economic Review. Esketamine for the treatment of treatment-resistant depression: effectiveness and value. Evidence report prepared for Midwest CEPAC in May 2019. https://icer-review.org/material/trd-evidence-report/ (2019, accessed 6 May 2020).
- 80. Duru G, Fantino B. The clinical relevance of changes in the Montgomery-Asberg depression rating scale using the minimum clinically important difference approach. Curr Med Res Opin 2008; 24: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 81. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014; 312: 1342–1343. [DOI] [PubMed] [Google Scholar]
- 82. Janssen Research & Development, LLC. NCT02682225. Crossover study to evaluate the abuse potential of intranasal esketamine compared to racemic intravenous ketamine in nondependent, recreational drug users. ClinicalTrials.gov. Bethesda, MD: U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/show/study/NCT02682225 (2019, accessed 6 May 2020). [Google Scholar]
- 83. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry 2019; 176: 422–424. [DOI] [PubMed] [Google Scholar]
- 84. Pacheco Dda F, Romero TR, Duarte ID. Central antinociception induced by ketamine is mediated by endogenous opioids and mu- and delta-opioid receptors. Brain Res 2014; 1562: 69–75. [DOI] [PubMed] [Google Scholar]
- 85. Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 1991; 251: 85–87. [DOI] [PubMed] [Google Scholar]
- 86. Jonkman K, Dahan A, van de Donk T, et al. Ketamine for pain. F1000Res 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bahr R, Lopez A, Rey JA. Intranasal esketamine (Spravato(TM)) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T 2019; 44: 340–375. [PMC free article] [PubMed] [Google Scholar]
- 88. Tibensky BN, de Leseleuc L, Perras C, et al. Esketamine for treatment-resistant depression. CADTH Issues in Emerging Health Technologies. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health Copyright (c) CADTH 2019, 2019, pp.1–17. [PubMed] [Google Scholar]
- 89. McShane R, Baldwin DS, McAllister-Williams RH, et al. Esketamine and the need for a new type of registry for drugs with abuse potential. Am J Psychiatry 2019; 176: 966. [DOI] [PubMed] [Google Scholar]
- 90. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 2012; 6: 369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 2014; 116: 137–141. [DOI] [PubMed] [Google Scholar]
- 92. Fukumoto K, Toki H, Iijima M, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 2017; 361: 9–16. [DOI] [PubMed] [Google Scholar]
- 93. Hashimoto K, Kakiuchi T, Ohba H, et al. Reduction of dopamine D2/ 3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 2017; 267: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chang L, Zhang K, Pu Y, et al. Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-keta-mine, (R)-ketamine and (S)-ketamine. Pharmacol Biochem Behav 2019; 181: 53–59. [DOI] [PubMed] [Google Scholar]
- 95. Leal GC, Bandeira ID, Correia-Melo FS, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci 2020. February 20. DOI: 10.1007/s00406-020-01110-5. [DOI] [PubMed] [Google Scholar]
- 96. Vollenweider FX, Leenders KL, Oye I, et al. Differential psycho-pathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 1997; 7: 25–38. [DOI] [PubMed] [Google Scholar]
- 97. Ihmsen H, Geisslinger G, Schüttler J. Stereoselective pharmacokinetics of ketamine: R(-)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther 2001; 70: 431–438. [DOI] [PubMed] [Google Scholar]
- 98. Yang C, Kobayashi S, Nakao K, et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry 2018; 84: 591–600. [DOI] [PubMed] [Google Scholar]
- 99. Hashimoto K, Yang C. Is (S)-norketamine an alternative antidepressant for esketamine? Eur Arch Psychiatry Clin Neurosci 2019; 269: 867–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019; 6: 977–979. [DOI] [PubMed] [Google Scholar]
- 101. Medscape Medical News. FDA’s rapid approval of esketamine for severe depression questioned, https://www.medscape.com/viewarticle/921248 (2019, accessed 6 May 2020).
- 102. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci 2019. November 19. DOI: 10.1007/s00406-019-01084-z. [DOI] [PubMed] [Google Scholar]


