Abstract
Background:
Systemic chemotherapy for pancreatic adenocarcinoma (PDAC) and cholangiocarcinoma (CC) with peritoneal metastases (PM) is affected by several pharmacological shortcomings and low clinical efficacy. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is expected to maximize exposure of peritoneal nodules to antiblastic agents. This study aims to evaluate safety and efficacy of PIPAC for PM of PDAC and CC origin.
Methods:
This is a retrospective analysis of consecutive PDAC and CC cases with PM treated with PIPAC at two European referral centers for peritoneal disease. We prospectively recorded from August 2016 to May 2019 demographic, clinical, surgical, and oncological data. We performed a feasibility and safety assessment and an efficacy analysis based on clinical and pathological regression.
Results:
Twenty patients with PM from PDAC (14) and CC (six) underwent 45 PIPAC administrations. Cisplatin–doxorubicin or oxaliplatin were administered to eight and 12 patients, respectively. We experienced one intraoperative complication (small bowel perforation) and 18 grade 1–2 postoperative adverse events according to Common Terminology Criteria for Adverse Events version 4.0. A pathological regression was recorded in 50% of patients (62% in the cisplatin–doxorubicin cohort and 42% in the oxaliplatin one). Median survival from the first PIPAC was 9.7 and 10.9 months for PDAC and CC, respectively.
Conclusion:
PIPAC resulted feasible and safe without relevant toxicity issues, with both cisplatin–doxorubicin and oxaliplatin. The pathological response observed supports the evidence of antitumoral activity. Despite the study limitations, these outcomes are encouraging, recommending PIPAC in prospective, controlled trials in the palliative setting or the first line chemotherapy for PM from PDAC and CC.
Keywords: aerosol chemotherapy, carcinomatosis, cholangiocarcinoma, locoregional chemotherapy, pancreatic cancer, peritoneal metastases, PIPAC
Introduction
Pancreatic adenocarcinoma (PDAC) and cholangiocarcinoma (CC) are both aggressive neoplasms carrying a high metastatic potential with a 5-year survival rate of 7% and 17%, respectively.1–3
Surgery represents the only curative alternative but only 15% of patients with PDAC4 and 30% with CC5–7 undergo primary tumor resection. Indeed, the vast majority of cases had already developed locally advanced disease, distant or peritoneal metastasis (PM) at the time of diagnosis. Furthermore, the recurrence rate is nearly 80% within the first 2 years after surgery, and about half of these patients show peritoneal relapse.8–10
Palliative systemic chemotherapy represents the standard treatment option in the case of PM from PDAC and CC but roughly reaches a median overall survival of 6–11 months with more than 5% of serious adverse events.11–13
The need to improve patients’ prognosis and quality of life prompted research efforts to develop new treatment alternatives. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) emerged in the last few years as a novel method of drug administration with encouraging results in the treatment of PM of several origins. Based on the locoregional administration of pressurized aerosol drugs, it maximizes exposure of peritoneal tumor implants to chemotherapy agents, with favorable pharmacokinetics and biodistribution, as demonstrated in several pre-clinical studies.14,15
Previous experiences on PIPAC for peritoneal diffusion from PDAC and CC reported a relevant anti-tumoral activity with reassuring safety and toxicity profiles with the combination of cisplatin and doxorubicin.13,16–18
Here we describe the preliminary experience on PIPAC with the combination of cisplatin and doxorubicin (PIPAC CD) or oxaliplatin (PIPAC Ox) for PM of pancreatic and biliary tract origin, from two European referral centers for peritoneal disease.
Methods
This study is a retrospective series of a prospectively maintained registry of consecutive patients affected by PM from PDAC and CC, attempting PIPAC treatment from August 2016 to May 2019. No endpoints were predefined. Patients were enrolled to receive three or more PIPAC cycles.
Indication for PIPAC was given by a multidisciplinary tumor board for patients with pathologically proven PM from PDAC or CC. Inclusion criteria were Eastern Cooperative Oncology Group performance status (ECOG PS) inferior or equal to 2, absence of other distant metastases, at least one previous line of systemic chemotherapy for PM. PIPAC was considered in the presence of peritoneal disease progression under systemic chemotherapy but also in stable or responder patients at the end of the scheduled chemotherapy regimen. PIPAC was administered alone or in combination with second- or third-line metastatic systemic chemotherapy. Exclusion criteria were the contraindications of the procedure.19,20
This research obtained the approval of two Institutional Clinical Boards (Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy, ID: PipacPRO.964, and Institut du Cancer de Montpellier, Montpellier, France, ID: ICM-BCB 2019/10) according to the Helsinki Declaration from 1975. At the beginning of the therapeutic protocol, all subjects signed a specific institutional consent form allowing the collection of anonymized data for research and scientific purpose publishing.
The procedure is well standardized and was performed in both institutions as previously described.20
Chemotherapy agents considered for PIPAC administration were the combination of cisplatin 7.5 mg/m2 in 150 ml NaCl solution and doxorubicin 1.5 mg/m2 in 50 ml NaCl solution or oxaliplatin 92 mg/m2 in 200 ml of 5% glucose solution. Antiblastic agents were chosen based on previous drug exposure and response to therapy. Oxaliplatin was given in the case of a response to systemic folinic acid, flurouracil, oxaliplatin (FOLFOX) or folinic acid, flurouracil, irinotecan, oxaliplatin (FOLFIRINOX) or a poor response to gemcitabine ± cisplatin. Otherwise, PIPAC CD was preferred if a good response was documented with gemcitabine ± cisplatin or inadequate response or severe side effects to oxaliplatin-based systemic chemotherapy.
This retrospective analysis focused on the feasibility, safety and efficacy of PIPAC. For the feasibility assessment, we considered entry-related issues during laparoscopy, including access rate and entry complications, and the number of completed PIPAC cycles. Safety and toxicity were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Efficacy analysis was conducted by first to last procedure comparison in the population undergoing at least two PIPAC administrations. Efficacy was evaluated on ascites volume, peritoneal cancer index (PCI) and pathological response through the Peritoneal Regression Grading Score (PRGS),21 considering the mean and the highest score of biopsies. The rate of pathological regression was calculated on those who underwent at least two PIPAC cycles. Overall survival (OS) was computed from the date of both first PIPAC administration and peritoneal disease diagnosis in a Kaplan–Meier curve with SPSS Statistics, version 26.0 (IBM, NY, USA).
Results
A total of 20 patients affected by PDAC or CC underwent 45 PIPAC administrations. The cohort was composed of nine male and 11 female patients with a median age of 64 years (range 42–87). ECOG PS at the time of first PIPAC was 0 in four cases, 1 in nine cases, and 2 in the remaining seven cases.
Fourteen patients suffered from peritoneal spread from PDAC and six patients from CC. Fourteen patients suffered from metachronous disease: four patients underwent a Whipple operation, two a pancreatic tail resection, three a cholecystectomy, two a hepatic resection, one a cholecystectomy combined with hepatic resection, and two did not receive any primary tumor surgery. The remaining six patients with synchronous PM had no previous tumor-specific surgeries.
All patients underwent at least one line of metastatic systemic chemotherapy before PIPAC treatment. Chemotherapy regimens are listed in Table 1. In 11 cases, PIPAC was combined with systemic chemotherapy with a two-week interval before and 1 week after the PIPAC procedure. Consequently, there was an additional one-week delay between systemic chemotherapy administrations. The remaining nine patients underwent PIPAC as the only treatment. PIPAC Ox was administered in 12 cases and PIPAC CD in eight cases.
Table 1.
Patient demographics, surgical and oncological data.
| Pt. nr. | Age (years) | Primary tumor | Primary tumor site | Primary tumor to PM (months) | Primary tumor surgery | Previous chemotherapy lines and radiotherapy | Combined systemic chemotherapy | Drugs | First PIPAC ECOG PS | Survival from first PIPAC (months) | Survival from PM (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | CC | Gallbladder | 2.1 | Cholecystectomy | GEMOX | None | Oxa | 1 | 19.3 | 28.9 |
| 2 | 80 | CC | Gallbladder | 42.3 | Cholecystectomy + hepatic resection | GEM | None | Oxa | 2 | 10.9 | 12.3 |
| 3 | 73 | PDAC | Head | 2.8 | – | GEM –> FOLFIRINOX –>PACLITAXEL | TAXOL | Cis + Dox | 1 | 16.3 | 27.0 |
| 4 | 52 | PDAC | Head | 34.7 | Whipple Operation | FOLFIRINOX–> GEM –> FOLFOX | FOLFIRI | Cis + Dox | 2 | 10.0 | 18.2 |
| 5 | 78 | PDAC | Body–tail | Synchronous | None | GEM + NAB-PACLITAXEL | GEM + NAB-PACLITAXEL | Oxa | 1 | 7.8 | 11.0 |
| 6 | 55 | PDAC | Head | Synchronous | None | FOLFIRINOX | FOLFIRI | Cis + Dox | 0 | 10.0 | 21.6 |
| 7 | 43 | PDAC | Body–tail | Synchronous | None | GEM + NAB-PACLITAXEL | FOLFOX | Oxa | 1 | 5.8 | 22.0 |
| 8 | 61 | PDAC | Head | 16.8 | Whipple Operation | GEM –> RT | FOLFIRI | Oxa | 2 | 9.3 | 10.6 |
| 9 | 64 | PDAC | Body–tail | 18.0 | Pancreatic tail resection | GEM | FOLFIRI | Oxa | 1 | 6.9 | 7.5 |
| 10 | 87 | PDAC | Head | 12.9 | None | Capecitabine | Capecitabine | Oxa | 1 | 14.9 | 16.2 |
| 11 | 55 | PDAC | Body–tail | Synchronous | None | FOLFOX | None | Oxa | 1 | 1.7 | 9.0 |
| 12 | 70 | PDAC | Body–tail | Synchronous | None | GEM + NAB-PACLITAXEL | FOLFOX | Oxa | 1 | 9.7 | 17.9 |
| 13 | 60 | PDAC | Head | 11.2 | Whipple Operation | GEM –> FOLFOX | None | Oxa | 2 | 16.0 | 40.9 |
| 14 | 66 | PDAC | Body–tail | 8.2 | Pancreatic tail resection | GEM –> RT | None | Cis + Dox | 2 | 3.4^ | 13.0^ |
| 15 | 58 | PDAC | Head | 14.3 | Whipple Operation | FOLFIRI + GEM –> RT | GEM + NAB-PACLITAXEL | Cis + Dox | 0 | 9.9 | 16.1 |
| 16 | 64 | CC | Gallbladder | 10.4 | Cholecystectomy | GEM + Cis | None | Cis + Dox | 0 | 8.2* | 9.0* |
| 17 | 63 | PDAC | Head | Synchronous | None | GEM | GEM | Oxa | 2 | 1.7 | 7.0 |
| 18 | 42 | CC | Biliary tract | 6.0 | Hepatic resection | GEM | None | Cis + Dox | 0 | 6.8* | 15.3* |
| 19 | 64 | CC | Biliary tract | 6.0 | Hepatic resection | GEM + Cis | None | Oxa | 2 | 6.8* | 11.7* |
| 20 | 69 | CC | Gallbladder | 29.0 | Cholecystectomy | GEM + Cis | None | Cis + Dox | 1 | 1.4 | 11.9 |
Lost at follow-up.
Alive.
CC, cholangiocarcinoma; Cis, cisplatin; Dox, doxorubicin; ECOG PS, Eastern Cooperative Oncology Group performance status; GEM, gemcitabine; GEMOX, gemcitabine + oxaliplatin; Oxa, oxaliplatin; FOLFIRINOX, folinic acid, flurouracil, irinotecan, oxaliplatin; FOLFOX, folinic acid, flurouracil, oxaliplatin; FOLFIRI, folinic acid, flurouracil, irinotecan; PDAC, pancreatic adenocarcinoma; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PM, peritoneal metastases; Pt. nr., patient number; RT, radiotherapy.
The median PCI at the time of the first PIPAC was 20 for PDAC and 14 for CC (Table 2). High volume ascites (>2000 ml) was evident in six patients. Two more patients had low volume ascites (less than 1000 ml), while the remaining 12 patients had minimal (less than 100 ml) or no ascites at all. All six patients with high volume ascites received only one PIPAC cycle.
Table 2.
Perioperative outcomes.
| Variable | Value |
|---|---|
| Number of PIPAC | 45 |
| Number of patients per PIPAC cycle | |
| I PIPAC | 20 |
| II PIPAC | 11 |
| III PIPAC | 7 |
| IV PIPAC or more | 4 |
| Access failures | 0/45 (0%) |
| Operative time, min | 95 (71–137) |
| Intraoperative complications | 1*/45 (2%) |
| Overall postoperative morbidity/mortality (CTCAE version 4.0) | |
| Grade 1–2 | 18/45 (40%) |
| Grade ⩾ 3 | 0/45 (0%) |
| Post-operative stay, days ±SD | 2 ± 0.7 |
| Histological tumor response, PRGS | 10/20 (50%) |
| Oxaliplatin | 5/12 (42%) |
| Cisplatin + doxorubicin | 5/8 (62%) |
Values are presented as median (range) or number (%).
Small bowel perforation.
CTCAE, Common Terminology Criteria for Adverse Events version 4.0; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PRGS, Peritoneal Regression Grading Score.
The median procedure time was 95 min (range 71–137 min). The abdominal cavity was accessible in all patients. On laparoscopic entry, there was one (patient 15) small bowel perforation due to adhesions, which was repaired with interrupted suture and did not prevent PIPAC administration. Eighteen grade 1 or 2 CTCAE (nausea or mild abdominal pain) complications occurred in 45 PIPAC procedures (40%), but there were no major postoperative adverse events (grade 3–4 CTCAE). All patients were discharged on the first or second postoperative day, except for one, who left the hospital on the third postoperative day.
Eleven patients (55%) underwent more than one PIPAC and were therefore available for efficacy analysis: seven patients completed three cycles (35%), four patients four or more cycles (one patient underwent seven PIPAC administrations). The main reason for discontinuing the PIPAC treatment schedule after the first PIPAC was rapid clinical deterioration (seven cases). One patient was lost to follow-up after 3 months from the first PIPAC. The majority (6/9) of those receiving only one cycle suffered from high volume ascites and had ECOG PS of 2. Along with all PIPAC cycles, PCI decreased in four patients, increased in five patients and remained stable in the other two patients. Concerning ascites, there were only two new-onsets at the third PIPAC cycle.
According to the PRGS score, a pathological regression was recorded in 10 patients and stable disease in one more patient. Thus, considering those who underwent at least two PIPAC cycles, the pathological response rate was 90%, in particular 83% for the oxaliplatin group and 100% for the cisplatin–doxorubicin one. Whereas in the overall population, the pathological regression rate was 50% in both the PDAC (7/14) and CC (3/6) patients. Response rates in the PIPAC Ox and PIPAC CD cohorts were 42% and 62%, respectively.
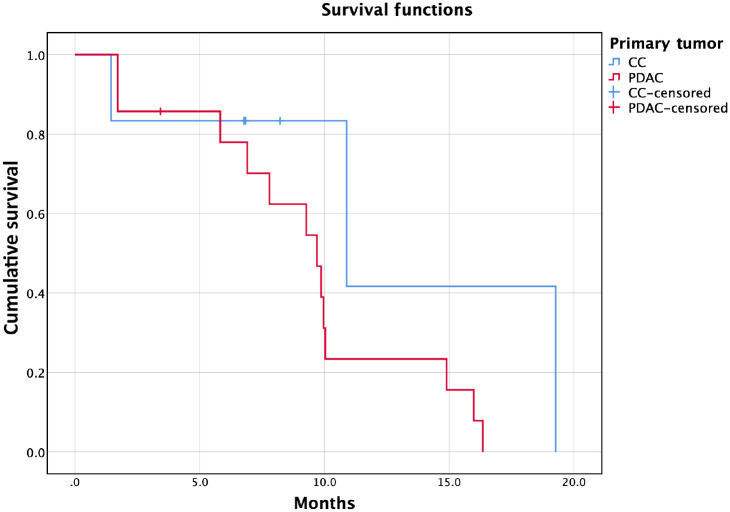
The median OS from the first PIPAC was 9.7 months for PDAC and 10.9 months for CC (Figure 1), while the median OS from PM diagnosis was 16.2 months for PDAC and 12.3 months for CC (three patients are still alive in the CC cohort). Five patients survived up to almost 2 years from PM diagnosis. Concerning survival analysis based on drug exposure, the median OS from the first PIPAC was 10.0 months for PIPAC CD and 9.3 months for PIPAC Ox.
Figure 1.
Kaplan–Meier survival curve from the first pressurized intraperitoneal aerosol chemotherapy. x-axis: survival in months; y-axis: cumulative survival. Red line: patients affected by pancreatic adenocarcinoma (PDAC). Blue line: patients affected by cholangiocarcinoma (CC).
Discussion
In this study, we report that PIPAC with cisplatin–doxorubicin or oxaliplatin is safe and has an antitumoral activity against peritoneal metastases of pancreatic and biliary tract origin.
Peritoneal metastases affect nearly 5–10% of patients suffering from PDAC and CC at diagnosis22 and 30–50% at the time of recurrence.23 These patients usually receive only systemic chemotherapy associated with poor efficacy and high toxicity profile.24
One of the critical flaws of systemic chemotherapy may be poor distribution into peritoneal nodules mainly due to scarce vascularization and high stromal fraction.25,26 In this sense, PIPAC emerged as a novel drug delivery system that is expected to overcome the mentioned drug-distribution limitations through aerosolization of antiblastic agents and high pressure obtained during laparoscopy. The possibility to repeat the procedure complies with multiple cycles of systemic chemotherapy administration and results as appealing to monitor treatment response. Furthermore, PIPAC seems a fertile field for personalized medicine, offering the chance to unveil the biological phenotype of survivor lines in PM through repeated biopsies.
Given the novelty of the procedure, PIPAC has gained increased interest. Phase II studies for gastrointestinal and gynecological cancers have already successfully tested its safety and antitumoral efficacy,14,27–30 while randomized phase II and phase III studies are awaited to explore its potential therapeutic effects.31–33 On the other hand, PIPAC investigation is currently at an early phase, as most published studies belong to IDEAL stages 0 and 1.34 Indeed, several issues should be addressed to clarify optimal doses, exposure times, intra-abdominal pressures, cycle intervals, combination with systemic chemotherapy regimens, and implementation of other drugs.
The dosage of the cisplatin and doxorubicin association has been investigated in a dose-escalation trial determining that the optimal doses for cisplatin and doxorubicin are 10.5 mg/m2 and 2.1 mg/m2 respectively.29 In the present cohort, cisplatin and doxorubicin were administered according to previously approved dosage, since new doses have been implemented afterwards.
To date, case series on PIPAC for PDAC and CC reported only the cisplatin–doxorubicin association.13,16–18 This is the first report on PIPAC Ox administration for PM of pancreatic and biliary tract origin. On the basis of several clinical trials, oxaliplatin is considered an active drug in pancreatic cancer systemic treatment. It is currently used in association with irinotecan, fluorouracil and levofolinic acid in the FOLFIRINOX regimen as first-line chemotherapy,11 or in association with fluorouracil and folinic acid (FOLFOX or OFF regimen) as a second-line treatment after gemcitabine-based first-line systemic chemotherapy.35 It is also administered in combination with gemcitabine or fluorouracil for biliary tract cancer treatment.36,37 Although the locoregional use of oxaliplatin has raised some concerns about toxicity, especially abdominal pain and hypersensitivity,38,39 a recent retrospective multicenter cohort study reported safety and efficacy profiles comparable to those of other drugs.40 In this study oxaliplatin was preferred over the cisplatin–doxorubicin association in more than half of patients, based on previous drug exposure and response to therapy.
Feasibility and safety outcomes of our series do not differ from those coming from phase II studies on ovarian and gastric cancer, or from series on pancreatic and biliary tract cancer using PIPAC CD.13,16–18 We experienced no access failures and PIPAC was feasible in all attempted cases. However, half of the patients underwent two cycles and only one-third completed three cycles. The main reason for discontinuing PIPAC treatment was rapid clinical deterioration. Previous studies on PIPAC for PDAC and CC13,16,17 described similar dropout rates, and probably a more accurate selection of patients could help to identify those able to receive three cycles. Concerning safety, we experienced one intraoperative small bowel perforation, which was less than 1 cm in size and was repaired by laparoscopy, so that it did not prevent PIPAC administration. Postoperative complications were self-limiting or addressed by oral drug administration in both PIPAC CD and PIPAC Ox cohorts. No case of severe abdominal pain occurred. Toxicity was not a concern, as we did not observe any renal, hepatic or bone marrow impairment.
In our study, we assessed efficacy in patients undergoing more than one PIPAC comparing ascites, PCI and PRGS from the first to the last PIPAC procedure, notwithstanding the heterogeneity of data. These criteria had already been used in previous studies.16,17
Despite previous mentions of ascites control as a potential PIPAC indication, in our series, all patients with high volume ascites (>2000 ml) and six out of seven patients with ECOG PS 2 underwent only one PIPAC cycle. Possibly, in the case of pancreatic and biliary tract cancer, high volumes of free peritoneal fluid may reflect a very late stage disease, probably unresponsive to treatment. Although these data are preliminary, and ascites do not represent a contraindication ‘per se’, in the presence of high volume ascites, the indication should be considered with care. One of the main challenges when dealing with PM is the response to therapy assessment. Indeed, radiological imaging performs poorly on measurable peritoneal nodules volume reduction41 while PCI evaluation, conceived as a preoperatory cytoreduction predictor, it is not reliable on sclerosis and fibrotic nodules.21 The PRGS system proposed and validated by Solass et al.42 may represent a valid tool for antitumoral efficacy evaluation and has gained broad acceptance. We registered a pathological response in the overall population according to PRGS in 50% of cases of PDAC and CC. Such data are in line with those reported in the literature. From a recent pooled analysis,43 response rates for PDAC and CC were 45% and 37%, respectively. Of course, the pathological response rate could be underestimated in the overall population, as the PRGS is unknown for those receiving just one PIPAC. Since 11 out of 14 patients affected by PDAC underwent a combined treatment, both systemic and locoregional therapy may contribute to the observed antitumoral efficacy and thus, concerning PDAC, our findings should not be considered conclusive on the causal role of PIPAC. On the other hand, none of the CC patients had systemic chemotherapy during the study period, and the pathological response was only attributable to PIPAC. Antitumoral efficacy did not significantly differ when comparing PIPAC Ox and PIPAC CD patients, with pathological response rates of 42% and 62%, respectively.
Standard systemic chemotherapy for both PDAC and CC with PM yields median survival of 6–11 months with severe side effects.11,12 In our series, systemic chemotherapy followed by PIPAC administrations brought a median OS from PM diagnosis of 12–16 months. Furthermore, PIPAC was well tolerated, as we experienced no serious adverse events. The median OS from the first PIPAC of 9–10 months, in line with previous experiences,13,16,17 seems encouraging as we always administered PIPAC in the second-line treatment for metastatic disease. In a similar salvage setting, Takahara et al. reported a median OS of 4.8 months for gemcitabine-refractory pancreatic cancer with malignant ascites treated with a combination chemotherapy of intravenous–intraperitoneal paclitaxel and S-1.44 It is impossible to infer from the present study whether our survival outcomes are attributable to PIPAC or to patient selection but they represent a promising avenue for future research.
Further caution should be used in drawing conclusions, bearing in mind the retrospective nature of the study, the small number of patients, and the possibility of selection bias associated with the exclusion of worse cases.
In spite of the limitations mentioned above, our study corroborates published data on the safety and efficacy of PIPAC and explores both the use of PIPAC CD and PIPAC Ox for PM of pancreatic and biliary tract origin.
Conclusion
PIPAC resulted feasible in our cohort of patients affected by late-stage PDAC and CC, but only one-third completed the three-cycles course. All patients with high volume ascites underwent only one cycle; thus, indication in these cases should be considered with care. PIPAC is a safe procedure without relevant toxicity issues, both with cisplatin–doxorubicin and with oxaliplatin. We documented a pathological response in 50% of patients, which did not significantly differ in the PIPAC CD (62%) and PIPAC Ox (42%) cohorts. These results should be handled with caution since the clinical benefit of pathological regression has not been proven yet. Besides the limitations inherent to a retrospective study, the median survival from the first PIPAC of 9–10 months is encouraging considering the end-stage of our cohort. More efforts should be made to improve the poor survival of these patients and PIPAC may be worth considering for prospective, controlled trials in the palliative setting or at the time of the first metastatic line.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Carlo Alberto Schena  https://orcid.org/0000-0003-1136-1103
https://orcid.org/0000-0003-1136-1103
Contributor Information
Andrea Di Giorgio, Foundation Policlinico Universitario A. Gemelli - IRCCS, Peritoneum and Retroperitoneum Surgery, Roma, Lazio, Italy.
Olivia Sgarbura, Department of Surgical Oncology, Montpellier Cancer Institute, Montpellier, Languedoc-Roussillon, France.
Stefano Rotolo, Department of Surgical, Oncological and Oral Sciences (Di.Chir.On.S.), University of Palermo, Via del Vespro, 129, Palermo, 90127, Sicilia, Italy; Foundation Policlinico Universitario A. Gemelli – IRCCS, Peritoneum and Retroperitoneum Surgery, Largo A. Gemelli, 8, Rome 00168, Italy.
Carlo Alberto Schena, Foundation Policlinico Universitario A. Gemelli – IRCCS, General Surgery Unit, Roma, Lazio, Italy.
Cinzia Bagalà, Foundation Policlinico Universitario A. Gemelli – IRCCS, Division of Medical Oncology, Roma, Lazio, Italy.
Frediano Inzani, Foundation Policlinico Universitario A. Gemelli – IRCCS, Anatomic Pathology Unit, Roma, Lazio, Italy.
Andrea Russo, Foundation Policlinico Universitario A. Gemelli – IRCCS, Institute of Intensive Care Medicine and Anesthesiology, Roma, Lazio, Italy.
Vito Chiantera, Division of Gynecologic Oncology, University of Palermo, Palermo, Sicilia, Italy.
Fabio Pacelli, Foundation Policlinico Universitario A. Gemelli - IRCCS, Peritoneum and Retroperitoneum Surgery, Roma, Lazio, Italy.
References
- 1. Ducreux M, Caramella C, Hollebecque A, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26(Suppl. 5): v56–v68. [DOI] [PubMed] [Google Scholar]
- 2. Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2014. Ann Oncol 2014; 25: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 3. Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: results of EUROCARE-5. Eur J Cancer 2015; 51: 2169–2178. [DOI] [PubMed] [Google Scholar]
- 4. Schneider G, Siveke JT, Eckel F, et al. Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005; 128: 1606–1625. [DOI] [PubMed] [Google Scholar]
- 5. Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012; 61: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 6. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014; 383: 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009; 69: 259–270. [DOI] [PubMed] [Google Scholar]
- 8. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–579. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto T, Yagi S, Kinoshita H, et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol 2015; 21: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg 2005; 390: 94–103. [DOI] [PubMed] [Google Scholar]
- 11. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 12. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015; 20: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horvath P, Beckert S, Struller F, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of pancreas and biliary tract cancer. Clin Exp Metastasis 2018; 35: 635–640. [DOI] [PubMed] [Google Scholar]
- 14. Tempfer CB, Hilal Z, Dogan A, et al. Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal metastasis. Eur J Surg Oncol 2018; 44: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 15. Weinreich J, Struller F, Sautkin I, et al. Chemosensitivity of various peritoneal cancer cell lines to HIPEC and PIPAC: comparison of an experimental duplex drug to standard drug regimens in vitro. Invest New Drugs 2019; 37: 415–423. [DOI] [PubMed] [Google Scholar]
- 16. Khosrawipour T, Khosrawipour V, Giger-Pabst U. Pressurized intra peritoneal aerosol chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One 2017; 12: e0186709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graversen M, Detlefsen S, Bjerregaard JK, et al. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin Exp Metastasis 2017; 34: 309–314. [DOI] [PubMed] [Google Scholar]
- 18. Falkenstein TA, Goetze TO, Ouaissi M, et al. First clinical data of pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage therapy for peritoneal metastatic biliary tract cancer. Anticancer Res 2018; 38: 373–378. [DOI] [PubMed] [Google Scholar]
- 19. Alyami M, Hübner M, Grass F, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol 2019; 20: e368–e377. [DOI] [PubMed] [Google Scholar]
- 20. Hübner M, Grass F, Teixeira-Farinha H, et al. Pressurized intraperitoneal aerosol chemotherapy–practical aspects. Eur J Surg Oncol 2017; 43: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 21. Solass W, Sempoux C, Detlefsen S, et al. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016; 1: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sleeman JP. PIPAC puts pressure on peritoneal metastases from pancreatic cancer. Clin Exp Metastasis 2017; 34: 291–293. [DOI] [PubMed] [Google Scholar]
- 23. Sergeant G, Ectors N, Van Steenbergen W, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009; 35: 600–604. [DOI] [PubMed] [Google Scholar]
- 24. Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013; 31: 23–29. [DOI] [PubMed] [Google Scholar]
- 25. Lu Z, Wang J, Wientjes MG, et al. Intraperitoneal therapy for peritoneal cancer. Future Oncol 2010; 6: 1625–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanco A, Giger-Pabst U, Solass W, et al. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2013; 20: 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solaß W, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013; 20: 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tempfer CB, Giger-Pabst U, Seebacher V, et al. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol 2018; 150: 23–30. [DOI] [PubMed] [Google Scholar]
- 30. Grass F, Vuagniaux A, Teixeira-Farinha H, et al. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017; 104: 669–678. [DOI] [PubMed] [Google Scholar]
- 31. Bakrin N, Tempfer C, Scambia G, et al. PIPAC-OV3: a multicenter, open-label, randomized, two-arm phase III trial of the effect on progression-free survival of cisplatin and doxorubicin as pressurized intra-peritoneal aerosol chemotherapy (PIPAC) vs chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Pleura Peritoneum 2018; 3: 20180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goetze TO, Al-Batran SE, Pabst U, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naïve upper GI-adenocarcinomas with peritoneal seeding–a phase II/III trial of the AIO/CAOGI/ACO. Pleura Peritoneum 2018; 3: 20180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sgarbura O, Gourgou S, Tosi D, et al. MESOTIP: phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura Peritoneum 2019; 4: 20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tate SJ, Torkington J. Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open 2020; 4: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 36. Fiteni F, Nguyen T, Vernerey D, et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med 2014; 3: 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He S, Shen J, Sun X, et al. A phase II FOLFOX-4 regimen as second line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother 2014; 26: 243–247. [DOI] [PubMed] [Google Scholar]
- 38. Demtröder C, Solass W, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016; 18: 364–371. [DOI] [PubMed] [Google Scholar]
- 39. Siebert M, Alyami M, Mercier F, et al. Severe hypersensitivity reactions to platinum compounds post-pressurized intraperitoneal aerosol chemotherapy (PIPAC): first literature report. Cancer Chemother Pharmacol 2019; 83: 425–430. [DOI] [PubMed] [Google Scholar]
- 40. Sgarbura O, Hubner M, Alyami M, et al. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and well tolerated: a multicenter study. Eur J Surg Oncol 2019; 45: e60. [DOI] [PubMed] [Google Scholar]
- 41. Mazzei MA, Khader L, Cirigliano A, et al. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Imaging 2013; 38: 1422–1430. [DOI] [PubMed] [Google Scholar]
- 42. Solass W, Sempoux C, Carr NJ, et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 2019; 74: 1014–1024. [DOI] [PubMed] [Google Scholar]
- 43. Di Giorgio A, Abatini C, Attalla ME, et al. From palliation to cure: PIPAC for peritoneal malignancies. Minerva Med 2019; 110: 385. [DOI] [PubMed] [Google Scholar]
- 44. Takahara N, Isayama H, Nakai Y, et al. Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Invest New Drugs 2016; 34: 636–644. [DOI] [PubMed] [Google Scholar]