Abstract
Tissue regeneration is widespread in the animal kingdom. To date, key roles for different molecular and cellular programs in regeneration have been described, but the ultimate blueprint for this talent remains elusive. In animals capable of tissue regeneration, one of the most crucial stages is wound healing, whose main goal is to close the wound and prevent infection. In this stage, it is necessary to avoid scar formation to facilitate the activation of the immune system and remodeling of the extracellular matrix, key factors in promoting tissue regeneration. In this review, we will discuss the current state of knowledge regarding the role of the immune system and the interplay with the extracellular matrix to trigger a regenerative response.
Keywords: extracellular matrix, immune system, regeneration, wound healing
1. INTRODUCTION
Tissue regeneration is widely distributed across Metazoa, and this capacity varies among highly related animals. Ambystoma mexicanum, Danio rerio, and Xenopus sp. are examples of animals that have the fascinating ability to regenerate appendages, such as limbs and fins, and organs, such as the heart and brain. However, even in very closely related species, differences in regenerative ability are observed. Newts, such as Notophthalmus viridescens, perform lens regeneration throughout their life, while the Mexican salamander A mexicanum can regenerate its lens only for a short time period during early development (developmental stages 44‐52).1, 2, 3 Some animals regenerate specific organs or tissues, while others, such as planarians, sea stars, and hydras, can rebuild the body axes from small fragments. For a long time, it was thought that adult mammals cannot regenerate; however, interesting research on deer clearly shows that they have the capacity to regenerate their antlers throughout life. 4 A more recent addition to the field of mammalian regeneration is the African spiny mouse, which has been shown to be capable of scar‐free skin regeneration. To gain more in‐depth knowledge of regenerative programs,5, 6 it will be important to perform more comparative cross‐species studies to gain a better understanding of the cellular and molecular programs that drive each specific type of tissue regeneration.
In all animals, the first event that occurs after trauma, independent of size, is wound closure; depending on the characteristics of the epithelium that grows during closure, a regenerative or reparative process (scar formation) will be triggered. 7 One aspect that is thought to define whether organisms have a regenerative or a reparative outcome is the immune response and the remodeling of the extracellular matrix (ECM). Animals that naturally regenerate tissues, such as A mexicanum, D rerio, and Acomys sp., share some similarities in the wound healing process, such as rapid re‐epithelization, a dampened immune system, delayed deposition of collagen, and remodeling of the ECM. 8
The immune systems of invertebrates and vertebrates (Figure 1) share two common components: innate and humoral responses. The innate immune response is composed of mainly phagocytic cells that trigger the initial injury response, while the humoral component is composed of different kinds of secreted molecules. 9 In invertebrates, because of the variety of ecological niches and body patterns, the composition of the innate immune system has become more diversified. 10 However, one core cell type is phagocytic cells, such as hemocytes or coelomocytes, which are also found in invertebrates. In vertebrates, the innate immune system includes a wide variety of cells, such as hematopoietic cells, leukocytes, and phagocytes. Vertebrates also have an adaptive immune system that includes B cells, the wide range of immunoglobulins they express, and T cells, which act mainly by T‐cell receptors that could mediate cell death. These cells help the immune system to retain a memory of previous infections and trigger a different set of inflammatory cytokines to modulate the response. 11 In organisms that lack an adaptive immune system (ie, invertebrates), humoral immune components, such as complement proteins, are an important front line of defense against microbes. In humans, the complement pathway consists of over 50 protein components that are largely synthesized and secreted into the blood by the liver, but other cell types, including fibroblasts, lymphocytes, adipocytes, and endothelial cells, have been shown to synthesize complement components as well.12, 13 In addition to complement proteins, other ancient components of the innate immune system that are present in invertebrates and vertebrates include the signaling pathway Toll‐to‐NF‐κB. 14 This pathway leads to the differential expression of genes involved in innate immunity, such as antimicrobial genes and complement genes, which are important components of the humoral response in invertebrates.9, 15, 16
FIGURE 1.
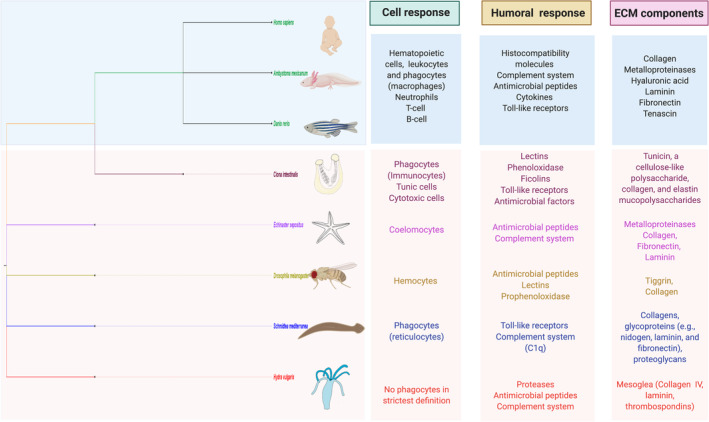
Comparative components of the immune system and the extracellular matrix (ECM) between some vertebrates and invertebrates. The immune systems of invertebrates and vertebrates have similarities between innate and humoral responses. The innate immune response involves mainly phagocytic cells and the humoral response of secreted molecules. One of the main ECM proteins in all these organisms is collagen, which is a key molecule that must be successfully remodeled to establish scar‐free wound healing
1.1. The ECM and regeneration
The ECM is a three‐dimensional network that supports cells and modulates important cellular processes such as proliferation, adhesion, migration, cell differentiation, and inflammation.17, 18, 19 The ECM is formed for a variety of macromolecules, such as collagen isoforms, fibronectin, tenascins, laminin, and glycosaminoglycans, which include heparin sulfate proteoglycans, chondroitin sulfate proteoglycans, dermatan sulphates, and hyaluronan (hyaluronic acid). These components are highly conserved during evolution. 20 The composition of the ECM can vary depending on the cellular context; for example, neurons are frequently found in a laminin‐ and fibronectin‐rich ECM, while muscle cells favor a collagen IV‐rich ECM but also one containing fibronectin. The specific composition of the ECM can play an important role in directing cell fate decisions.
In response to injury, some of the first events to occur include the activation of the immune systems and the upregulation of matrix metalloproteases (MMPs). MMPs are a large family of zinc‐dependent proteases that are important for the degradation of ECM after injury, and each MMP has substrate specificity. The progression of the response of cells to an injury signal and the final outcome are in some part controlled by the specific MMPs present in the wound bed and the duration of their activity. In regeneration‐competent organisms that heal scar free, the ECM undergoes a series of modifications to accomplish the goal of tissue regeneration, which is to regenerate a tissue with the same morphological and functional features as the uninjured tissue. 21
A key component of ECM breakdown is phagocytic macrophages, which have been reported to be essential to regeneration in several animals, including A mexicanum, 22 D rerio,23, 24, 25 and Acomys sp.26, 27 The inhibition of macrophage recruitment to the injury site has been shown to inhibit regeneration; however, the effect on ECM remodeling is less studied in vivo to date.
To understand the interplay between the ECM and the immune system, in vitro models and mouse models of wound healing have mainly been used. Data from both of these systems have begun to shed light on the different factors that are secreted by macrophages in the wound bed to trigger the activation of genes important for the modulation of the ECM, including transforming growth factor beta (TGFβ), MMPs, elastin microfibril interfacer (EMILIN), and a range of cytokines. 28 Additionally, the type of hyaluronic acid (high molecular weight vs low molecular weight) may modify macrophages to generate an anti‐inflammatory or pro‐inflammatory response. 29 The ECM component heparan sulfate has been reported to be an important modulator of concentration gradients to regulate the migration of leukocytes by the control of chemokines in mammals.30, 31
The interactions between the ECM and the immune system have been studied mainly in models related to mammalian tissues; however, how this interplay between the ECM and the immune system works in highly regenerative organisms across the tree of life is far less understood. Here, we will review the state of knowledge of the role of the immune system and ECM in different research organisms that are used in the regeneration field in comparison to scar‐prone mammals.
2. THE IMMUNE SYSTEM AND WOUND HEALING INTERACTIONS IN INVERTEBRATES
2.1. Cnidarian
Cnidaria diverged from bilaterians ~650 million years ago, giving them a unique position on the tree of life as a sister group to bilateria.32, 33 Considering the vast evolutionary distance between cnidaria and humans, genomic sequencing efforts revealed an unanticipated degree of complexity in the genomes of cnidarians.34, 35 For example, the starlet sea anemone Nematostella vectensis is steadily rising to prominence as a genetically tractable organism for research on development and regeneration.36, 37 The Nematostella genome is more similar to the human genome in size and content than Drosophila melanogaster or Caenorhabditis elegans and harbors a near complete complement of most gene families, including Wnt and a proto‐Hox cluster that seems to function analogously to bilaterian Hox genes.38, 39, 40, 41, 42, 43
It has been suggested that the main modulators of the immune system in cnidarians are proteases, serine protease inhibitors, antimicrobial proteins, and the complement system. 44 One of the signaling pathways that has been reported as crucial for the wound healing phase in N. vectensis is MAPK (ERK) signaling; inhibition of this pathway after injury leads to dramatic wound healing defects. 39 The MAPK pathway plays several roles in the modulation of the immune system. It is involved in the induction of pro‐inflammatory mediators such as cytokines and chemokines; in other research organisms, this pathway is triggered by pattern recognition receptors (PRRs) expressed in immune cells. 45 However, cnidarians do not have specialized immune cells, 14 and how MAPK is triggered to allow the expression of immune‐related genes is unknown.
In hydra, which is a very well‐established research model in the tissue regeneration field, the molecular signatures activated during the wound healing phase are consistently upregulated regardless of where along the body the regenerative program is started (eg, head, foot, and whole body). 46 Wenger et al reported approximately 1000 transcripts related to the immune response as genes involved in the remodeling of the ECM. 47 Some of those genes are evolutionarily conserved immune‐related genes. 47 One example is heat shock proteins (HSPs), which are upregulated during wound healing in hydra. HSP genes have the capacity to indirectly modulate the NF‐kB pathway, inducing an innate response in invertebrates and acquired immunity in vertebrates. 48 In the context of hydra regeneration, HSPs have been reported to be upregulated very early after amputation, suggesting a role in wound closure. 47 Additionally, after injury, the immune response of hydra includes proteolytic components, such as calpain‐15, which could trigger the activation of the NF‐kB pathway, allowing the production of different cytokines that may activate the remodeling of the ECM, allowing the recruitment of migratory cells to the wound via this pathway. 49
A primitive mechanism of immunity is antimicrobial peptides (AMPs). During hydra regeneration, some genes classified as AMPs are upregulated, such as hydramacin (specific hydra AMP). 47 To date, few connections have been described between AMPs and the ECM. Capodici et al reported that cathepsin G, an AMP module, may be involved in the degradation of collagen. 50 The ECM of hydra shares similar macromolecules to those expressed in vertebrates, such as collagen IV, laminin, heparan sulfate proteoglycan, and fibronectin‐like molecules. 51 Moreover, the ECM of hydra has multiple pores that act as a means of communication between the two cell layers (endoderm and ectoderm). 52 It is well known that the ECM in hydra has important roles in general morphogenesis, pattern formation and cell differentiation processes, and it is thought that the recovery of the ECM after an injury is a key aspect of regeneration in hydra and probably in other cnidarians such as N. vectensis. 51
2.2. Tunicates
Tunicates are members of the phylum Chordata belonging to the urochordates, a subphylum that occupies a key phylogenetic position because they are one of the closest related species to vertebrates 53 and are a key research organism for regenerative and developmental studies. 54 Among tunicates, solitary, and colonial ascidians have been most extensively studied in the context of innate immunity. 55 The adult animal is covered by a flexible ECM structure made up primarily of cellulose called the tunic. The tunic is developmentally derived from the ectoderm and represents the first barrier against infection. Damage to the tunic can lead to death, indicating an essential role in the health of the organism. 56 During the remodeling phase after a wound, the tunic matrix is remodeled by immune cells, such as degranulating cells. 57
Interestingly, mutual association between bacteria and ascidians has been reported.57, 58 This may involve secreted active products, such as antimicrobial proteins, that function together with phagocytic cells as a part of the innate immune system. 59 The AMP may also have a role in the initial wound healing response. 57 However, the complexity of this interaction of the microbiome modulating the immune response and ECM matrix during the wound healing phase in other organisms is poorly understood.
In addition, during wound healing in tunicates, an innate immune system that involves a humoral and cell‐mediated response protects the organism and helps heal the wound. 57 These cells include hemocytes, whose primary function is phagocytosis of pathogens. In mollusks, which also have hemocytes, these cells have been reported to undergo a phenotype switch during wound healing and adopt a fibroblast‐like phenotype. 60
2.3. Echinoderms
Echinoderms represent a significant phylogenetic point that could help to infer both the early evolution of bilaterian immunity and the development of the vertebrate adaptive immune system. 61 During the wound healing phase in starfish and brittle stars, components of the immune system, such as the proteins Ese‐fib‐like (starfish) and Afi‐ficolin (brittle stars), are upregulated after injury. 62 In this organism, the hematopoietic tissue has been described as the coelomic epithelium that secretes coelomocytes. 63 These are important modulators of wound healing, as they migrate to the wound site to form a clot and play a role in the modulation of the ECM. 64 The coelomic epithelium may be involved in the production of fibrinogen‐like proteins, such as Ese‐fib‐like, which is a fibrinogen‐related domain (FReD)‐containing protein. 62
Interestingly, the Afi‐ficolin protein also contains a FReD, and in vertebrates and invertebrates, ficolin is an important lectin in the innate immune response.65, 66 In general, FReD proteins have been reported in many metazoans, from porifera to mammals, and have a variety of biological functions, including roles in the innate immune response and in wound repair.65, 66, 67 In mammals, such as humans, this domain is present in fibrinogen‐related proteins (FRePs), which include different ECM molecules, such as tenascin, fibrinogen, angiopoietin, and ficolin, with important roles in regenerative responses.68, 69 The wide distribution of these types of proteins in the tree of life is an example of the interplay between the immune response and ECM during wound healing (Figure 2).
FIGURE 2.
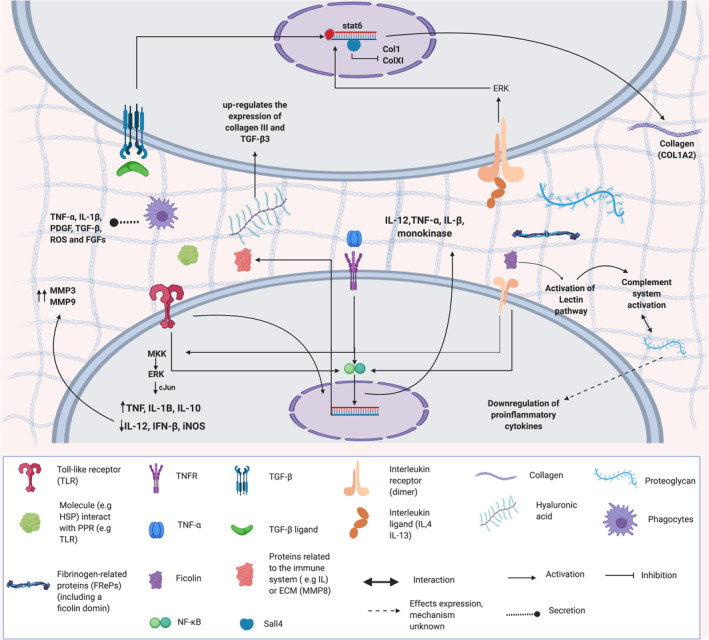
The interplay between the immune system and the extracellular matrix. Summary of the different molecules of the immune system and the extracellular matrix that can act as regulators or activators of signaling pathways. The interactions between these two systems play a crucial role in the response to injury, and many of these pathways are conserved between invertebrates and vertebrates
In sea cucumbers, which also excel at tissue regeneration,70, 71 coelomocytes may act as an important modulator of the immune response and wound repair. As in hydra, the activation of HSPs during the wound healing phase in sea cucumbers is an important modulator of the wound response. HSP70, which is expressed by coelomocytes and plays a role in cellular differentiation and migration, may exert a protective function against wounds and environmental stresses such as temperature stress, acidic pH levels and microbial infections. 72 The link between HSP and the modulation of ECM proteins is poorly understood. However, in mammals, HSP70 is known to be an important modulator of ECM in the context of muscle repair. 73
2.4. Planaria
Planarians are freshwater flatworms in the Platyhelminthes phylum of organisms and possess the outstanding ability to regenerate an entire animal from a small piece of tissue. The impressive regenerative capabilities of planarians are due to the pro‐regenerative response of endogenous stem cells called neoblasts. 74 The two most commonly studied species of planarians are Schmidtea mediterranea and Dugesia japonica, both of which express genes involved with innate immunity, including various PRRs, TLR signaling, selectins, and complement components. 75 Apart from playing a role in mediating the innate immune response, both RIG‐1 (retinoic acid‐inducible gene I) and CTL (C‐type lectin‐like protein) are upregulated during regeneration. However, their precise role in tissue regeneration is not well characterized. The knockdown of CTL results in delayed wound healing after decapitation, but whether this inhibits head regeneration is not clear. 76 RIG‐1 knockdown leads to downregulation of antimicrobial and antiviral genes, but whether this results in regenerative defects has not been reported. 77
Furthermore, in planarians undergoing regeneration, proteins of the complement system, such as the C1q‐like domain, are upregulated 6 hr post amputation. 78 In humans, it has been reported that proteoglycans, important components of the ECM, interact with C1q‐like, and this interaction could lead to a downregulation of pro‐inflammatory cytokines. 79
Apart from the innate signaling pathways, planarians also harbor a cellular component of their innate immune system. Gut epithelial cells, which represent a major barrier tissue, are highly phagocytic. 80 Additionally, nongut mesenchymal cells, called reticulocytes, are known to phagocytose Escherichia coli and migrate to injury sites within 10 hours of injury.81, 82, 83 Presumably, reticulocytes function to remove pathogens at an injury site and could function to promote tissue remodeling. The transcription factor FoxF‐1 has been recently implicated in the maintenance of cathepsin+ phagocytic cells, but whether these correspond to reticular cells is not clear. 84 Cathepsin is a protease that functions in phagolysosomes to aid in the destruction of phagocytosed pathogens and is broadly expressed in pigment cells, glia, and undetermined mesenchymal cells in planaria.40, 84, 85 Additionally, cathepsin is considered an important modulator during wound healing by the degradation of ECM components.50, 86
2.5. Drosophila as a model to study wound healing
D melanogaster has long been a workhorse for genetic discovery. The relative ease of laboratory husbandry, rapid life cycle, and vast genetic toolkit have highlighted this organism as a premier research organism in which to investigate the genetic basis of development and disease. 87 Similar to other invertebrates, Drosophila lacks an adaptive immune system but utilizes an extensive cellular and molecular repertoire in its innate immune system. Indeed, many of the cellular and molecular responses to wounding are conserved between Drosophila and mammals. 88 Larval Drosophila has an open circulatory system consisting of a liquid component called hemolymph, similar to human plasma, and a cellular component called hemocytes, which play an important role in embryonic development, wound detection, and healing, as well as pathogen clearance.88, 89, 90, 91, 92, 93
Epithelial wounding in embryonic and larval Drosophila is a widely used injury model to investigate the cellular, molecular, and genetic underpinnings of wound healing. Injury results in the activation of signaling cascades that appear to be largely evolutionarily conserved between worms, flies, fish, amphibians, and human cells. Initial injury leads to the formation and rapid propagation of a calcium wave through the damaged epithelium, leading to a burst in reactive oxygen species (ROS).89, 94, 95, 96, 97 The resulting increase in ROS is critical for the recruitment and trafficking of various leukocytes, such as neutrophils and macrophages, to the lesion.23, 98, 99, 100 Following fin amputation in zebrafish, the ability of leukocytes to respond to increased hydrogen peroxide is dependent on the ROS‐sensitive Src family kinase Lyn. 97 Similarly, in flies, the Lyn homologue Src42a is required for hemocyte migration to the injury. 101
While it is clear that injury‐induced signals such as ROS and other DAMPs are important for leukocyte homing to injuries, whether these factors are truly acting as chemoattractants or rather a permissive signal that sensitizes leukocytes to additional recruitment signals is not clear. While hydrogen peroxide is necessary for hemocyte migration to injuries, 98 it likely acts as a permissive cue that sensitizes hemocytes to the wound attractant. This function (hemocyte migration) could be related to the capacity of hematocytes to produce laminins for their migration using autocrine mechanisms. 102 It will be interesting in the future to see if this same signaling kinetics applies to vertebrates such as zebrafish, which are similarly amenable to live‐cell imaging approaches.
3. VERTEBRATE REGENERATION AND THE IMMUNE SYSTEM: INSIGHTS FROM THE AXOLOTL AND AFRICAN SPINY MOUSE
3.1. Wound healing and the immune system in nonregenerative mammals
Full‐thickness skin wounds in mammals result in complex cascades of cell‐cell, cell‐ECM, and growth factor signaling that most often result in scar formation. Wounding disrupts blood vessel networks, leading to the activation of various clotting factors, infiltration of platelets, and ultimately formation of a fibrin clot.8, 103, 104 The fibrin clot fulfills several functions during mammalian wound healing: (a) it provides structural support for the migration of various local and recruited cell types, such as keratinocytes or leukocytes and (b) it helps to potentiate growth factor signaling, such as PDGF, VEGF, bFGF, and TGF‐beta.105, 106 The activation of cells to fill in the missing tissue after injury is thought to involve an epithelial to mesenchymal transition, an event that may also involve activation of the immune system, reviewed in Reference 107. Mechanical disruption of the skin and the resulting cellular and molecular events stimulates an inflammatory response. Neutrophils are the first cell type to infiltrate the wound bed and function to kill microorganisms, clear debris, and release various cytokines that recruit other leukocytes. Specifically, macrophages are the next inflammatory cell type to arrive at the wound bed. Macrophages secrete various cytokines, including TNF‐α, IL‐1β, PDGF, TGF‐β, and FGFs, which further regulate the inflammatory response after wounding but also stimulate the formation of new tissue called granulation tissue.8, 103, 104 Ultimately, mammalian wound healing results in the formation of scar tissue due to excessive collagen deposition and a failure to remodel collagen.8, 103, 104 Additionally, mammals fail to regenerate skin appendages such as hair follicles or sebaceous glands, which results in functional impairment.
Classical studies in the 1970s aimed to evaluate the effects of neutrophils and macrophages during wound healing in guinea pigs. Interestingly, antisera depletion of neutrophils largely had no effect on wound healing; however, depletion of macrophages with antisera and steroids led to defects in wound healing.108, 109 Collectively, these results suggest that macrophages are necessary for wound healing and that neutrophils may not play an appreciable role.
Scar‐free wound healing occurs in fetal mammals in the absence of an inflammatory response and in postnatal mice lacking macrophages and functional neutrophils, suggesting that scar formation results from robust wound‐induced inflammation. 110 However, these studies do not provide insight into the physiological role of the innate immune system during adult wound healing.
Macrophage depletion during the early or middle stages of wound healing affected the formation of granulation tissue, neovasculogenesis, and transition of granulation tissue into scar tissue. However, macrophage depletion during the late phase of wound healing did not affect the overall process. 111 Interestingly, a similar result has been observed in axolotl limb regeneration, where the early depletion of macrophages results in a lack of regeneration but later depletion has no significant impact on regeneration. 22 These studies suggest that different immune cells are important at select timepoints after injury.
Interestingly, myeloid‐specific IL‐4 receptor α signaling results in the secretion of RELM‐α, which stimulates the expression of the collagen crosslinking enzyme Plod2 in fibroblasts and contributes to fibrillary collagen remodeling in scar tissue. 112 Additionally, defects in insulin/IGF‐1 receptor signaling, specifically in myeloid cells, decreased granulation tissue formation but did not affect overall wound healing. 113 However, insulin/IGF‐1 receptor signaling is critical for the normal epidermal‐dermal inflammatory response to detergent treatment (SDS) and UV B exposure. 113 Collectively, these studies support a role for the immune system in regulating the mammalian response to wound healing. However, the genetic or conditional ablation of various components of the innate immune system does not (a) result in scar‐free wound healing or (b) appear to affect the regeneration of skin appendages, such as hair follicles or sebaceous glands. Therefore, it is unlikely that a binary relationship exists between the presence or absence of immune cell subtypes and scar‐free wound healing.
Other important key factors in scar‐free wound healing in fetal mammals are attenuated biochemical stress, the potential role of stem cells, growth factors and the protein conformation of the ECM. The key components of the ECM in fetal scar‐less wounds are type III collagen and hyaluronan.110, 114 Collagen III confers a small‐fiber structure on the ECM of the wound, which allows the reticular deposition of fiber in the wound, resulting in a more flexible wound bed. Additionally, the increased level of hyaluronan upregulates the expression of collagen III and TGF‐β. It has been reported that TGF‐β is an important modulator of the scar phenotype, as TGF‐β1 and TGF‐β2 are the principal isoforms expressed in postnatal scar wounds, while no expression of TGF‐β3 is detected. 114 As we learn more about the pathways that drive scar‐free wound healing in regeneration‐competent animals, it will be interesting to determine which TGF forms and interacting partners are key to this outcome.
3.2. Scar‐free wound healing in the African spiny mouse
While most mammals have poor regenerative ability after wounding, African spiny mice (Acomys spp.) are able to regenerate their skin in a scar‐free manner and replace skin appendages, such as hair follicles. 6 In the wild, it is com to find spiny mice in various states of regeneration, as they release their back skin and fur relatively easily. This is thought to be a defense mechanism against predation that makes it easier for the mouse to escape if caught. Remarkably, the mouse is able to regenerate the lost tissue scar free and regenerate hair follicles de novo through the activation of Wnt and BMP signaling. 6 Initial comparative gene expression studies suggest that Acomys spp. have a dampened inflammatory response compared to Mus musculus after wounding. 26 In terms of macrophage profiles, Acomys spp. and M musculus display a marked difference; MI macrophages are mainly present in the scar‐prone M musculus, while M2 phenotypes are present in the scar‐free Acomys spp., which has a more pronounced pro‐regenerative cytokine profile. 26 The differences in the macrophage profiles may generate different ECM microenvironments. COLXII and COLXIV are present at much higher levels in M musculus than in African spiny mice. Additionally, Acomys spp. have higher expression of proteases and collagen remodeling proteins, which help to successfully remodel the ECM after a wound. One of these proteins is CTHRC1, which helps to reduce the expression levels of collagen I. 115 In humans, it has been shown that the activation of CTHRCI, which is modulated by M2 macrophages, promotes wound healing by the regulation of TGF‐β, a key pathway involved in ECM remodeling.116, 117
In addition to scar‐free skin regeneration, Acomys is also able to regenerate after a 4 mm ear hole punch, which Mus is unable to fully close.27, 118 Following an ear hole punch in Mus, there is a large, rapid influx of CD11b + myeloid cells; an influx of myeloid cells occurs in Acomys, but to a much lesser degree. 27 Further characterization revealed that neutrophils made up a majority of the infiltrating myeloid cells in Mus, and there was no significant difference in the proportion of macrophages between the two species; however, the key difference may lie in the subtypes of macrophages present in the wound area. 118 Additionally, following injury in Acomys, there is a strong ROS burst, which was postulated to be generated by infiltrating macrophages. Comparatively, a strong ROS burst was absent in Mus. 27 The functional significance of the difference in ROS levels in Acomys compared to Mus was not determined. However, ear hole punch regeneration is dependent on macrophages, as the ablation of macrophages by the injection of clodronate‐containing liposomes results in regenerative defects. 27 Cell ablation with clodronate indiscriminately ablates cells with phagocytic activity, but it is presumed that regenerative defects are primarily due to macrophage depletion. Whether macrophage ablation was sufficient to affect ROS levels after injury remains to be determined.
3.3. Nonmammalian vertebrate scar‐free wound healing
While mammals largely fail to heal wounds scar free, fish, frogs, and salamanders do regenerate full‐thickness wounds scar free.7, 8, 94, 104 Furthermore, these nonmammalian models successfully regenerate various skin appendages, such as gland cells. These observations highlight the importance of studying wound healing across various species to better understand the molecular mechanisms that support scar‐free wound healing. While frogs largely lose the ability to regenerate after metamorphosis, wound healing studies conducted across species and life stages suggest that aspects of skin regeneration persist past metamorphosis. Scar‐free skin regeneration occurs in froglets in the apparent absence of an inflammatory response, suggesting that this process could be similar to fetal wound healing in mammals.7, 100, 104, 119 However, in postmetamorphic frogs, various inflammatory cell types arrive at the wound bed within 24 hours of injury. 120 Specifically, macrophages, neutrophils, and lymphocytes were observed throughout the wound bed and were immunopositive for various pro‐inflammatory molecules, such as TNFα, iNOS, and TGFβ. 120 Interestingly, adult Lithobates catesbeiana (American bullfrogs) regenerate skin exocrine glands that lack ducts, suggesting that these structures are not functional. 121 However, adult Lithobates sphenocephalus (southern leopard frogs) and Xenopus mulleri regenerate exocrine glands and ducts, suggesting species‐specific differences in regenerative capabilities. 104 Whether these differences reflect variations in immune cell trafficking or cytokine profiles remains an interesting question.
Salamanders are able to undergo scar‐free wound healing at various life stages. 122 Full‐thickness excisional wounds are completely regenerated by 80 days post wounding in adult axolotls. 122 Even animals that were forced to metamorphose after thyroxine injections are able to regenerate scar free. Metamorphosed animals do take longer to regenerate, partially due to an extended re‐epithelialization phase and increased duration of ECM deposition). The difference in regenerative rate could be due to altered expression profiles of various MMPs and/or earlier leukocyte infiltration. 122 During wound repair in larval zebrafish, the crucial role of MMP9 during collagen reorganization has been reported to be critical for acute and chronic wound repair, showing how the NF‐κB signaling pathway drives the expression of MMP9 in the epithelium during caudal fin regeneration. 123
During limb regeneration in salamanders, MMPs play crucial roles during the formation, maintenance, and growth of the regenerative blastema.124, 125 MMPs may have dual roles: they are expressed during blastema formation when the cells are starting to dedifferentiate and later during blastema outgrowth. It has been postulated that MMPs could help to prevent scar formation by remodeling the ECM and creating a permissive environment for blastema cell proliferation and migration. 125 Similarly, a dual role for the ECM during muscle regeneration has been reported where hyaluronic acid, tenascin, laminin, and fibronectin modulate myoblast identity during limb regeneration. During the early stages of limb regeneration, hyaluronic acid and tenascin help keep myoblasts in an undifferentiated state, while during later stages, laminin and fibronectin are upregulated and promote myoblast differentiation. 68 This dynamic expression of ECM‐related genes during the regenerative process could also be related to the dynamic changes in the immune response, since both processes share similar signaling pathways, such as TGF‐β,126, 127 and it is known that MMPs can modulate the immune response (eg, MMP7). 128 This interplay between the ECM and the immune system has been explored in other biological contexts, such as skin diseases, ischemic brain injuries, and response to infection.129, 130, 131 It would be interesting to further investigate the interplay in the context of scar‐free wound healing between different organisms.
Scar‐free wound healing in axolotls seems to be characterized by the early deposition of type III collagen, which is replaced by type I collagen at later time points. 122 A recent study identified the transcription factor Sall4 as a critical regulator of scar‐free wound healing in axolotl. Specifically, Sall4 functions to regulate the timing of collagen deposition by directly regulating the expression of type I and type XII collagens. 132 The precise role of type XII collagen during scar‐free wound healing is not well understood. However, type XII collagen is also highly upregulated during spinal cord regeneration in zebrafish. 133 Whether Sall4 is expressed in leukocyte subtypes or regulates the inflammatory response to wounding in axolotls is not clear.
3.4. The immune system during complex tissue regeneration
While it is clear that the immune system plays a critical role in scar‐free wound healing, its function during complex tissue regeneration has only recently been addressed. Axolotl limb and heart regeneration is dependent on macrophages, as depletion of phagocytic cells with clodronate liposomes immediately prior to injury leads to regenerative defects.22, 134 Specifically, during limb regeneration, macrophage depletion leads to altered expression dynamics of various pro‐ and anti‐inflammatory cytokines. 22 However, macrophage depletion once the blastema has already formed results in largely normal limb regeneration. 22 This suggests that the injury‐induced inflammatory response is essential for blastema formation. Consistent with this hypothesis, early blastema progenitors upregulate IL‐8 expression, which is necessary for the recruitment of myeloid cells, including macrophages, into the regenerating limb. 135 IL‐8 knockdown or pharmacologic inhibition of its receptor leads to decreased myeloid cell migration, defective blastema formation, and defects in regeneration. 135
Macrophage ablation after cryoinjury to the axolotl heart results in increased fibrosis and reduced functional recovery. The increase in fibrosis is likely due to dysregulated expression of various collagens, MMPs, and collagen remodeling enzymes. 134 Whether macrophages play a key role in regulating the cytokine profile during heart regeneration, as they do during limb regeneration, remains an interesting question.
Similar to axolotls, zebrafish are able to functionally regenerate heart tissue after injury. 136 This is in contrast to a closely related fish, the medaka, which is unable to regenerate after heart injury. A comparative approach revealed that the immune response to injury in medaka is dampened compared to that in zebrafish and consists mostly of neutrophil infiltration. 137 Ablation of macrophages in zebrafish resulted in regenerative defects, while activation of the innate immune system in medaka by poly(I:C) injection resulted in an enhanced regenerative response to injury. Interestingly, macrophage ablation in zebrafish led to sustained neutrophil presence at the injury site, suggesting macrophage‐dependent mechanisms facilitating neutrophil clearance. 137 Whether neutrophils play a functional role during axolotl heart and limb regeneration and whether there is similar cross talk between macrophages and neutrophils is not clear. Additionally, in the context of zebrafish fin regeneration, macrophages are reported to be crucial during the wound healing and blastema formation stages. 24
4. FUTURE DIRECTIONS
The crosstalk between the immune system and ECM in the context of scar‐free wound healing unquestionably plays an important role in enabling a regenerative program. However, our knowledge about the secreted factors from resident cells in the wound, the cells that migrate to the wound, such as immune cells, and the local environment that helps to remodel the ECM is in its infancy. In addition we still have much to learn in nonregenerative vs regenerative animals about the interplay of the ECM, EMT, and the immune system. 107
Studying wound healing in a wide range of different research organisms may help understand the cellular and molecular programs that drive the wound healing response. This could lead to a more in‐depth understanding of how interactions between an ancestral immune system and ECM have evolved to drive scar‐free wound healing and the regulation of complex tissue regeneration in some species.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The research in the Echeverri lab is supported NIH NCID R01 to Karen Echeverri and start‐up funds from the MBL. Keith Z. Sabin has been supported by an NIH T32 GM113846 grant.
Arenas Gómez CM, Sabin KZ, Echeverri K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Developmental Dynamics. 2020;249:834–846. 10.1002/dvdy.178
Funding information National Institute of Health, Grant/Award Numbers: T32 GM113846, NCID R01
REFERENCES
- 1. Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525‐549. [DOI] [PubMed] [Google Scholar]
- 2. Eguchi G, Eguchi Y, Nakamura K, Yadav MC, Millán JL, Tsonis PA. Regenerative capacity in newts is not altered by repeated regeneration and ageing. Nat Commun. 2011;2:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suetsugu‐Maki R, Maki N, Nakamura K, et al. Lens regeneration in axolotl: new evidence of developmental plasticity. BMC Biol. 2012;10(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li C, Zhao H, Liu Z, McMahon C. Deer antler – a novel model for studying organ regeneration in mammals. Int J Biochem Cell Biol. 2014;56:111‐122. [DOI] [PubMed] [Google Scholar]
- 5. Maden M, Brant JO. Insights into the regeneration of skin from Acomys, the spiny mouse. Exp Dermatol. 2019;28(4):436‐441. [DOI] [PubMed] [Google Scholar]
- 6. Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489(7417):561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murawala P, Tanaka EM, Currie JD. Regeneration: the ultimate example of wound healing. Semin Cell Dev Biol. 2012;23(9):954‐962. [DOI] [PubMed] [Google Scholar]
- 8. Erickson J, Echeverri K. Learning from regeneration research organisms: the circuitous road to scar free wound healing. Dev Biol. 2018a;433(2):144‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du Pasquier L. The immune system of invertebrates and vertebrates. Comp Biochem Physiol B: Biochem Mol Biol. 2001;129(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 10. Rowley AF, Powell A. Invertebrate immune systems–specific, quasi‐specific, or nonspecific? J Immunol. 2007;179(11):7209‐7214. [DOI] [PubMed] [Google Scholar]
- 11. Boehm T. Evolution of vertebrate immunity. Curr Biol. 2012;22(17):R722‐R732. [DOI] [PubMed] [Google Scholar]
- 12. Merle NS, Noe R, Halbwachs‐Mecarelli L, Fremeaux‐Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller DJ, Hemmrich G, Ball EE, et al. The innate immune repertoire in cnidaria—ancestral complexity and stochastic gene loss. Genome Biol. 2007;8(4):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchmann K. Evolution of innate immunity: clues from invertebrates via fish to mammals. Front Immunol. 2014;5:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller L, Fülöp T, Pawelec G. Immunosenescence in vertebrates and invertebrates. Immunity Ageing. 2013;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyd DF, Thomas PG. Towards integrating extracellular matrix and immunological pathways. Cytokine. 2017;98:79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartmana C, Isenberga B, Chuaa S, Wonga J. Extracellular matrix type modulates cell migration on mechanical gradients. Exp Cell Res. 2017;176(5):139‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parnaud G, Hammar E, Ribaux P, Donath MY, Berney T, Halban PA. Signaling pathways implicated in the stimulation of β‐cell proliferation by extracellular matrix. Mol Endocrinol. 2009;23(8):1264‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada S, Sugahara K, Özbek S. Evolution of glycosaminoglycans: comparative biochemical study. Commun Integr Biol. 2011;4(2):150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar‐free repair and regeneration: insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol. 2014;56:47‐55. [DOI] [PubMed] [Google Scholar]
- 22. Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110(23):9415‐9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morales RA, Allende ML. Peripheral macrophages promote tissue regeneration in zebrafish by fine‐tuning the inflammatory response. Front Immunol. 2019;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrie TA, Strand NS, Tsung‐Yang C, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development (Cambridge). 2014;141(13):2581‐2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simões FC, Cahill TJ, Kenyon A, et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun. 2020;11(1):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brant JO, Yoon JH, Polvadore T, Barbazuk WB, Maden M. Cellular events during scar‐free skin regeneration in the spiny mouse, Acomys. Wound Repair Regen. 2016;24(1):75‐88. [DOI] [PubMed] [Google Scholar]
- 27. Simkin J, Gawriluk TR, Gensel JC, Seifert AW. Macrophages are necessary for epimorphic regeneration in African spiny mice. Elife. 2017;6:e24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Etich J, Koch M, Wagener R, Zaucke F, Fabri M, Brachvogel B. Gene expression profiling of the extracellular matrix signature in macrophages of different activation status: relevance for skin wound healing. Int J Mol Sci. 2019;20(20):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1(7):481‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ori A, Wilkinson MC, Fernig DG. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J Biol Chem. 2011;286(22):19892‐19904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Proudfoot AEI, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100(4):1885‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunn SR. Immunorecognition and immunoreceptors in the Cnidaria. Invertebr Surv J. 2009;6(1):7‐14. [Google Scholar]
- 33. Hejnol A, Obst M, Stamatakis A, et al. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc Biol Sci. 2009;276(1677):4261‐4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman JA, Kirkness EF, Simakov O, et al. The dynamic genome of Hydra. Nature. 2010;464(7288):592‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Putnam NH, Srivastava M, Hellsten U, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317(5834):86‐94. [DOI] [PubMed] [Google Scholar]
- 36. Layden MJ, Rentzsch F, Röttinger E. The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. Wiley Interdiscip Rev Dev Biol. 2016;5(4):408‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rentzsch F, Technau U. Genomics and development of Nematostella vectensis and other anthozoans. Curr Opin Genet Dev. 2016;39:63‐70. [DOI] [PubMed] [Google Scholar]
- 38. Chourrout D, Delsuc F, Chourrout P, et al. Minimal ProtoHox cluster inferred from bilaterian and cnidarian Hox complements. Nature. 2006;442(7103):684‐687. [DOI] [PubMed] [Google Scholar]
- 39. DuBuc TQ, Stephenson TB, Rock AQ, Martindale MQ. Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. Nat Commun. 2018;9(1):2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finnerty JR, Martindale MQ. Ancient origins of axial patterning genes: Hox genes and ParaHox genes in the Cnidaria. Evol Dev. 1999;1(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 41. He S, Del Viso F, Chen C‐Y, Ikmi A, Kroesen AE, Gibson MC. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis . Science (New York, NY). 2018;361(6409):1377‐1380. [DOI] [PubMed] [Google Scholar]
- 42. Kusserow A, Pang K, Sturm C, et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433(7022):156‐160. [DOI] [PubMed] [Google Scholar]
- 43. Sullivan JC, Ryan JF, Mullikin JC, Finnerty JR. Conserved and novel Wnt clusters in the basal eumetazoan Nematostella vectensis . Dev Genes Evol. 2007;217(3):235‐239. [DOI] [PubMed] [Google Scholar]
- 44. Bosch TC, Augustin R, Anton‐Erxleben F, et al. Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev Comp Immunol. 2009;33(4):559‐569. [DOI] [PubMed] [Google Scholar]
- 45. Arthur JSC, Ley SC. Mitogen‐activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679‐692. [DOI] [PubMed] [Google Scholar]
- 46. Galliot B. Regeneration in Hydra. Chichester, England: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 47. Wenger Y, Buzgariu W, Reiter S, Galliot B. Injury‐induced immune responses in Hydra. Semin Immunol. 2014;26(4):277‐294. [DOI] [PubMed] [Google Scholar]
- 48. Colaco CA, Bailey CR, Walker KB, Keeble J. Heat shock proteins: stimulators of innate and acquired immunity. Biomed Res Int. 2013;2013:461230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fontenele M, Lim B, Oliveira D, et al. Calpain A modulates Toll responses by limited Cactus/IκB proteolysis. Mol Biol Cell. 2013;24(18):2966‐2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Capodici C, Berg RA. Cathepsin G degrades denatured collagen. Inflammation. 1989;13(2):137‐145. [DOI] [PubMed] [Google Scholar]
- 51. Sarras MP. Components, structure, biogenesis and function of the Hydra extracellular matrix in regeneration, pattern formation and cell differentiation. Int J Dev Biol. 2012;56(6‐8):567‐576. [DOI] [PubMed] [Google Scholar]
- 52. Shimizu H, Aufschnaiter R, Li L, et al. The extracellular matrix of hydra is a porous sheet and contains type IV collagen. Zoology (Jena, Germany). 2008;111(5):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439(7079):965‐968. [DOI] [PubMed] [Google Scholar]
- 54. Jeffery WR. The tunicate CIONA: a model system for understanding the relationship between regeneration and aging. Invertebr Reprod Dev. 2015;59(Suppl 1):17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Franchi N, Ballarin L. Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev Comp Immunol. 2014;46(2):430‐438. [DOI] [PubMed] [Google Scholar]
- 56. Cha IS, Castillo CS, Nho SW, Hikima J, Aoki T, Jung TS. Innate immune response in the hemolymph of an ascidian, Halocynthia roretzi, showing soft tunic syndrome, using label‐free quantitative proteomics. Dev Comp Immunol. 2011;35(8):809‐816. [DOI] [PubMed] [Google Scholar]
- 57. Di Bella MA, Carbone MC, De Leo G. Ultrastructural aspects of naturally occurring wound in the tunic of two ascidians: Ciona intestinalis and Styela plicata (Tunicata). Micron. 2015;69:6‐14. [DOI] [PubMed] [Google Scholar]
- 58. Kwan JC, Donia MS, Han AW, Hirose E, Haygood MG, Schmidt EW. Genome streamlining and chemical defense in a coral reef symbiosis. Proc Natl Acad Sci U S A. 2012;109(50):20655‐20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Franchi N, Ballarin L. Immunity in protochordates: the tunicate perspective. Front Immunol. 2017;8:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Franchini A, Ottaviani E. Repair of molluscan tissue injury: role of PDGF and TGF‐β. Tissue Cell. 2000;32(4):312‐321. [DOI] [PubMed] [Google Scholar]
- 61. Hibino T, Loza‐Coll M, Messier C, et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300(1):349‐365. [DOI] [PubMed] [Google Scholar]
- 62. Ferrario C, Ben Khadra Y, Czarkwiani A, et al. Fundamental aspects of arm repair phase in two echinoderm models. Dev Biol. 2018;433(2):297‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holm K, Dupont S, Sköld H, Stenius A, Thorndyke M, Hernroth B. Induced cell proliferation in putative haematopoietic tissues of the sea star, Asterias rubens (L.). J Exp Biol. 2008;211(Pt 16):2551‐2558. [DOI] [PubMed] [Google Scholar]
- 64. Gliński Z, Jarosz J. Immune phenomena in echinoderms. Archivum immunologiae et therapiae experimentalis. 2000;48(3):189‐193. [PubMed] [Google Scholar]
- 65. Fujita T. Evolution of the lectin–complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2(5):346‐353. [DOI] [PubMed] [Google Scholar]
- 66. Zuliani‐Alvarez L, Midwood KS. Fibrinogen‐related proteins in tissue repair: how a unique domain with a common structure controls diverse aspects of wound healing. Adv Wound Care. 2015;4(5):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Doolittle RF, McNamara K, Lin K. Correlating structure and function during the evolution of fibrinogen‐related domains. Protein Sci. 2012;21(12):1808‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Calve S, Odelberg SJ, Simon H‐G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344(1):259‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang TY, Wu CH, Wang MH, Chen BS, Chiou LL, Lee HS. Cooperative regulation of substrate stiffness and extracellular matrix proteins in skin wound healing of axolotls. Biomed Res Int. 2015;2015:712546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. García‐Arrarás JE, Estrada‐Rodgers L, Santiago R, Torres II, Díaz‐Miranda L, Torres‐Avillán I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea:Echinodermata). J Exp Zool. 1998;281(4):288‐304. [DOI] [PubMed] [Google Scholar]
- 71. Zhang X, Sun L, Yuan J, et al. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 2017;15(10):e2003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vazzana M, Siragusa T, Arizza V, Buscaino G, Celi M. Cellular responses and HSP70 expression during wound healing in Holothuria tubulosa (Gmelin, 1788). Fish Shellfish Immunol. 2015;42(2):306‐315. [DOI] [PubMed] [Google Scholar]
- 73. Hirunsai M, Srikuea R, Yimlamai T. Heat stress promotes extracellular matrix remodelling via TGF‐β1 and MMP‐2/TIMP‐2 modulation in tenotomised soleus and plantaris muscles. Int J Hyperthermia. 2015;31(4):336‐348. [DOI] [PubMed] [Google Scholar]
- 74. Reddien PW. The cellular and molecular basis for planarian regeneration. Cell. 2018;175(2):327‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peiris TH, Hoyer KK, Oviedo NJ. Innate immune system and tissue regeneration in planarians: an area ripe for exploration. Semin Immunol. 2014;26(4):295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gao L, Han Y, Deng H, et al. The role of a novel C‐type lectin‐like protein from planarian in innate immunity and regeneration. Dev Comp Immunol. 2017;67:413‐426. [DOI] [PubMed] [Google Scholar]
- 77. Li N, Li A, Zheng K, et al. Identification and characterization of an atypical RIG‐I encoded by planarian Dugesia japonica and its essential role in the immune response. Dev Comp Immunol. 2019;91:72‐84. [DOI] [PubMed] [Google Scholar]
- 78. Sandmann T, Vogg MC, Owlarn S, Boutros M, Bartscherer K. The head‐regeneration transcriptome of the planarian Schmidtea mediterranea . Genome Biol. 2011;12(8):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Groeneveld TWL, Oroszlán M, Owens RT, et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005;175(7):4715‐4723. [DOI] [PubMed] [Google Scholar]
- 80. Forsthoefel DJ, James NP, Escobar DJ, et al. An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Dev Cell. 2012;23(4):691‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morita M. Phagocytic response of planarian reticular cells to heat‐killed bacteria. Hydrobiologia. 1991;227:193‐199. [Google Scholar]
- 82. Morita M. Structure and function of the reticular cell in the planarian Dugesia‐Dorotocephala. Hydrobiologia. 1995;305(1‐3):189‐196. [Google Scholar]
- 83. Morita M, Best JB. Electron microscopic studies of planarian regeneration. II. Changes in epidermis during regeneration. J Exp Zool. 1974;187(3):345‐373. [DOI] [PubMed] [Google Scholar]
- 84. Scimone ML, Wurtzel O, Malecek K, et al. foxF‐1 controls specification of non‐Body wall muscle and phagocytic cells in planarians. Curr Biol. 2018a;28(23):3787‐3801.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Plass M, Solana J, Wolf FA, et al. Cell type atlas and lineage tree of a whole complex animal by single‐cell transcriptomics. Science. 2018;360(6391). 10.1126/science.aaq1723. [DOI] [PubMed] [Google Scholar]
- 86. Fonović M, Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta Gen Subj. 2014;1840(8):2560‐2570. [DOI] [PubMed] [Google Scholar]
- 87. Hales KG, Korey CA, Larracuente AM, Roberts DM. Genetics on the fly: a primer on the drosophila model system. Genetics. 2015;201(3):815‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wood W, Martin P. Macrophage functions in tissue patterning and disease: new insights from the fly. Dev Cell. 2017;40(3):221‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23(5):424‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Razzell W, Wood W, Martin P. Swatting flies: modelling wound healing and inflammation in Drosophila. Dis Model Mech. 2011;4(5):569‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vlisidou I, Wood W. Drosophila blood cells and their role in immune responses. FEBS J. 2015;282(8):1368‐1382. [DOI] [PubMed] [Google Scholar]
- 92. Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster . J Cell Biol. 2006;173(3):405‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8(7):542‐551. [DOI] [PubMed] [Google Scholar]
- 94. Lisse TS, King BL, Rieger S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: implications toward conservation, migration and wound healing. Sci Rep. 2016;6(1):20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xu S, Chisholm AD. A Galphaq‐Ca(2)(+) signaling pathway promotes actin‐mediated epidermal wound closure in C. elegans . Curr Biol. 2011;21(23):1960‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yoo SK, Freisinger CM, LeBert DC, Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol. 2012;199(2):225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20(5):464‐470. [DOI] [PubMed] [Google Scholar]
- 99. Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue‐scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yokoyama H, Maruoka T, Aruga A, et al. Prx‐1 expression in Xenopus laevis scarless skin‐wound healing and its resemblance to epimorphic regeneration. J Invest Dermatol. 2011;131(12):2477‐2485. [DOI] [PubMed] [Google Scholar]
- 101. Evans IR, Rodrigues FS, Armitage EL, Wood W. Draper/CED‐1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol. 2015;25(12):1606‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sánchez‐Sánchez BJ, Urbano JM, Comber K, et al. Drosophila embryonic hemocytes produce laminins to strengthen migratory response. Cell Rep. 2017;21(6):1461‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Eming SA, Martin P, Tomic‐Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Seifert A, Maden M. New insights into vertebrate skin regeneration. Int Rev Cell Mol Biol. 2014;310:129‐169. [DOI] [PubMed] [Google Scholar]
- 105. Clark RA. Fibrin is a many splendored thing. J Invest Dermatol. 2003;121(5):xxi‐xxii. [DOI] [PubMed] [Google Scholar]
- 106. Rivera J, Lozano ML, Navarro‐Nunez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94(5):700‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Haensel D, Dai XA‐O. Epithelial‐to‐mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev Dyn. 2018;247(3):473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78(1):71‐100. [PMC free article] [PubMed] [Google Scholar]
- 109. Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51(8):2009‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Res C Embryo Today. 2012;96(4):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964‐3977. [DOI] [PubMed] [Google Scholar]
- 112. Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin‐4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity. 2015;43(4):803‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Knuever J, Willenborg S, Ding X, et al. Myeloid cell‐restricted insulin/IGF‐1 receptor deficiency protects against skin inflammation. J Immunol. 2015;195(11):5296‐5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Leung A, Crombleholme TM, Keswani SG. Fetal wound healing. Curr Opin Pediatr. 2012;24(3):371‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yoon JH, Cho K, Garrett TJ, Finch P, Maden M. Comparative proteomic analysis in scar‐free skin regeneration in Acomys cahirinus and scarring Mus musculus . Sci Rep. 2020;10(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. LeClair RJ, Durmus T, Wang Q, Pyagay P, Terzic A, Lindner V. Cthrc1 is a novel inhibitor of transforming growth factor‐beta signaling and neointimal lesion formation. Circ Res. 2007;100(6):826‐833. [DOI] [PubMed] [Google Scholar]
- 117. Qin S, Zheng J‐H, Xia Z‐H, Qian J, Deng C‐L, Yang S‐L. CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF‐β and notch pathways. Biomed Pharmacother. 2019;113:108594. [DOI] [PubMed] [Google Scholar]
- 118. Gawriluk TR, Simkin J, Thompson KL, et al. Comparative analysis of ear‐hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun. 2016;7:11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mescher AL, Neff AW, King MW. Inflammation and immunity in organ regeneration. Dev Comp Immunol. 2017;66:98‐110. [DOI] [PubMed] [Google Scholar]
- 120. Bertolotti E, Malagoli D, Franchini A. Skin wound healing in different aged Xenopus laevis . J Morphol. 2013;274(8):956‐964. [DOI] [PubMed] [Google Scholar]
- 121. Yannas IV, Colt J, Wai YC. Wound contraction and scar synthesis during development of the amphibian Rana catesbeiana . Wound Repair Regen. 1996;4(1):29‐39. [DOI] [PubMed] [Google Scholar]
- 122. Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar‐free healing in vertebrates. PLoS One. 2012;7(4):e32875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. LeBert DC, Squirrell JM, Rindy J, et al. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development (Cambridge, England). 2015;142(12):2136‐2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Santosh N, Windsor LJ, Mahmoudi BS, et al. Matrix metalloproteinase expression during blastema formation in regeneration‐competent versus regeneration‐deficient amphibian limbs. Dev Dyn. 2011;240(5):1127‐1141. [DOI] [PubMed] [Google Scholar]
- 125. Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol. 2005;279(1):86‐98. [DOI] [PubMed] [Google Scholar]
- 126. Li MO, Wan YY, Sanjabi S, Robertson A‐KL, Flavell RA. Transforming growth factor‐β regulation of immune responses. Annu Rev Immunol. 2006;24(1):99‐146. [DOI] [PubMed] [Google Scholar]
- 127. Munger JS, Sheppard D. Cross talk among TGF‐β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3(11):a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Parks WC, Wilson CL, López‐Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617‐629. [DOI] [PubMed] [Google Scholar]
- 129. Bhattacharjee O, Ayyangar U, Kurbet AS, Ashok D, Raghavan S. Unraveling the ECM‐immune cell crosstalk in skin diseases. Front Cell Dev Biol. 2019;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dzyubenko E, Manrique‐Castano D, Kleinschnitz C, Faissner A, Hermann DM. Role of immune responses for extracellular matrix remodeling in the ischemic brain. Ther Adv Neurol Disord. 2018;11:1756286418818092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tomlin H, Piccinini AM. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology. 2018;155(2):186‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Erickson JR, Gearhart MD, Honson DD, et al. A novel role for SALL4 during scar‐free wound healing in axolotl. NPJ Regen Med. 2016;1:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wehner D, Tsarouchas TM, Michael A, et al. Wnt signaling controls pro‐regenerative collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun. 2017;8(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Godwin JW, Pinto AR, Rosenthal NA. Chasing the recipe for a pro‐regenerative immune system. Semin Cell Dev Biol. 2017;61:71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tsai SL, Baselga‐Garriga C, Melton DA. Blastemal progenitors modulate immune signaling during early limb regeneration. Development. 2019;146(1):dev169128. [DOI] [PubMed] [Google Scholar]
- 136. Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188‐2190. [DOI] [PubMed] [Google Scholar]
- 137. Lai SL, Marin‐Juez R, Moura PL, et al. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife. 2017;6:e25605. [DOI] [PMC free article] [PubMed] [Google Scholar]


