Abstract
Central obesity may contribute to the development of hypertension in youths with diabetes. The SEARCH for Diabetes in Youth Study followed 1518 youths with type 1 diabetes (T1D) and 177 with type 2 diabetes (T2D) diagnosed when <20 years of age for incident hypertension. Incident hypertension was defined as blood pressure ≥95th percentile (or ≥130/80 mm Hg) or reporting antihypertensive therapy among those without hypertension at baseline. Poisson regression models were stratified by diabetes type and included demographic and clinical factors, clinical site, and waist‐to‐height ratio (WHtR). Youths with T2D were more likely to develop hypertension than those with T1D (35.6% vs 14.8%, P < .0001). For each 0.01 unit of annual increase in WHtR, adjusted relative risk for hypertension was 1.53 (95% CI 1.36‐1.73) and 1.20 (95% CI 1.00‐1.43) for youths with T1D and T2D, respectively. Effective strategies targeted toward reducing central obesity may reduce hypertension among youths with diabetes.
Keywords: adolescents, children, diabetes type 1, diabetes type 2, hypertension
1. INTRODUCTION
Between 2002 and 2012, the incidence of US youth‐onset (<20 years of age) type 1 increased by 1.4% annually (from 19.5 cases/100 000 youths per year to 21.7 cases/100 000 youths per year); the incidence of youth‐onset type 2 diabetes increased by 4.8% during the same time (from 9.0 cases/100 000 youths per year in 2002‐2003 to 12.5 cases/100 000 youths per year in 2011‐2012). 1 A similar trend has been observed worldwide. 2 , 3 , 4 The increase in type 1 and type 2 diabetes incidence affects in both sexes, and almost all racial/ethnic groups. 5 Adolescents and young adults with youth‐onset diabetes may experience complications such as kidney disease, retinopathy, and peripheral neuropathy after <10 years diabetes duration suggesting that early‐ versus late‐onset diabetes is particularly aggressive. 6 Cardiovascular disease and mortality are higher in patients with young‐onset type 2 than in type 1 diabetes, which is primarily driven by accompanying factors such as obesity and high blood pressure. 7 , 8 , 9 Comorbidities such as hypertension in youths and young adults with diabetes increase the risk for cardiovascular disease and diabetes‐related complications in adulthood. 6 The clustering of unfavorable cardiometabolic risk factors at a very early age contributes to a poor long‐term prognosis particularly in youth‐onset type 2 diabetes. However, our knowledge on the complications and progression of type 1 and type 2 diabetes in youths is still limited.
The SEARCH for Diabetes in Youth study (SEARCH) previously reported that the prevalence of high blood pressure is higher in youths and young adults with type 1 or type 2 diabetes compared to those without diabetes. 10 , 11 This may be explained in part by a higher body mass index (BMI) of youths and young adults with type 1 or type 2 diabetes compared to those without diabetes. 12 Compared to BMI, central obesity has been previously examined as a better predictor for hypertension in ethnically diverse populations of adults, 13 , 14 but this relationship is controversial in youth. 15 In youths with diabetes, central obesity is associated with greater arterial stiffness, independent of diabetes type. 11 Adjusting for central obesity but not BMI attenuated the difference in risk for arterial stiffness and hypertension for youths and young adults with type 1 versus type 2 diabetes to non‐significance—indicating that central obesity may be a better indicator for diabetes‐related complications. 6 Considering the implications for future risk of cardiovascular disease, a better understanding of how changes in central obesity and other risk factors are associated with risk for hypertension among youths and young adults with diabetes is necessary to reduce the future burden of the sequelae of diabetes diagnosed in childhood.
To expand on previous analyses on the prevalence of hypertension using cross‐sectional data in youths with type 1 or type 2 diabetes, 10 the objectives of the present study were to quantify the risk associated with increasing central obesity on the incidence of hypertension among youths and young adults with type 1 or type 2 diabetes.
2. PATIENTS AND METHODS
2.1. Study design and subjects
SEARCH is a prospective, multicenter study which identifies and follows youths diagnosed with diabetes prior to age 20 years from diverse racial and ethnic backgrounds. The SEARCH study design and methods have been previously described in detail. 6 Youths and young adults included in this analysis were diagnosed with diabetes in calendar years 2002‐2006 or 2008, completed a brief survey and participated in a baseline research visit after diagnosis (mean of 9.2 months [SD, 6.4], Figure 1A). Participants were later invited for a SEARCH outcome visit between 2011 and 2015 at which time diabetes‐related complications and comorbidities were assessed. The study was approved by institutional review board(s) with jurisdiction. For each visit, participants ≥18 years of age and the parent/guardian of participants <18 years of age provided consent and participants <18 years of age provided assent.
Figure 1.
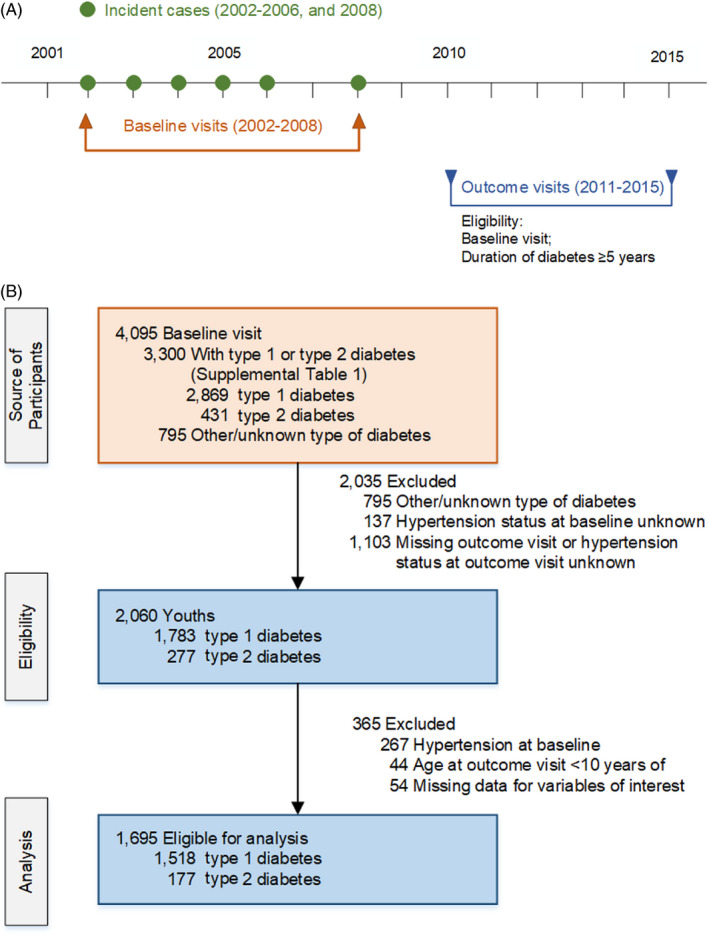
SEARCH for Diabetes in Youth Study design showing the years of baseline and outcome visits (1A) and flowchart (1B) of participants with youth‐onset type 1 and type 2 diabetes
For the current analysis, we included participants diagnosed with type 1 diabetes or type 2 diabetes. There were 4,095 participants with a baseline visit. We excluded participants with other or unknown diabetes type, hypertension or unknown hypertension status at baseline, and those with missing data for variables of interest (Figure 1B). After exclusions, 1,695 participants were included in the analysis.
2.2. Study procedures and variable definitions
Trained research staff conducted the in‐person baseline and outcome visits. Before each visit, participants were instructed to fast overnight for at least 8 hours and abstain from medications (including short‐acting insulin) on the morning of the visit. Participants (or parents, for participants <18 years of age) self‐reported their date of birth, sex, race, ethnicity, annual household income, type of health insurance, and highest level of parental education on their initial survey. Date of diabetes diagnosis was obtained from medical records and was used to calculate age at diagnosis and diabetes duration at each visit. 16
To define diabetes type, baseline blood samples were analyzed for glutamic acid decarboxylase‐65, insulinoma‐associated‐2, and zinc‐T8 autoantibodies. 17 Insulin sensitivity was estimated using a validated equation that includes waist circumference, glycated hemoglobin (HbA1c), and triglyceride concentrations measured at the visit. 17 Type 1 diabetes was defined as at least one positive antibody result (regardless of insulin sensitivity), or no positive antibody results and an insulin sensitivity score of at least 8.15. 17 Type 2 diabetes was defined as being negative for the three antibodies tested and insulin resistance (score <8.15). 17
Participant height and weight were measured in light indoor clothing without shoes and used to calculate BMI, and BMI z‐scores adjusted for age and sex. 18 Youths were categorized as under/normal weight (BMI‐for‐age <85th percentile or a BMI <25), overweight (BMI‐for‐age ≥85th to <95th percentile or a BMI ≥ 25 to <30 kg/m2), and obesity class 1 (BMI‐for age ≥95th to <97th percentile or a BMI ≥ 30 to <35 kg/m2), and obesity class 2 or higher (BMI‐for age ≥97th percentile or a BMI ≥ 35 kg/m2). Waist circumference was measured twice using the natural waist defined as midway between the lowest rib margin and the right iliac crest at the mid‐axillary line. A third measurement was made if the second measure differed from the first by >1.0 cm. We then calculated the ratio of waist‐to‐height (WHtR) to quantify central obesity. 19 , 20 , 21 If the rib margin could not be identified, the measurement was made at the point of natural bend (after asking the participant to lean to the side without swaying backward or forward). 21
Blood pressure at baseline was measured using a standard mercury sphygmomanometer with one of five cuff sizes chosen based on the circumference of the participant's arm according to guidelines. 22 Follow‐up blood pressure was measured using a Welch Allyn Tycos 767‐series mobile aneroid manometer (Welch Allyn, Inc). Mean blood pressure was calculated based on the average of three readings, each taken 1 minute apart, after the participant had 5 minutes of seated rest. Using the American 2017 Academy of Pediatrics Clinical Practice Guidelines, prehypertension was defined for youths <13 years as a blood pressure between the 90th percentile to <95th percentile for age, sex, and height or 120/80 mm Hg to <95th percentile (whichever was lower); for youths ≥13 years and adults, it was defined as a blood pressure of 120‐129/<80 mm Hg. 22 Hypertension was defined for youths <13 years as a blood pressure ≥95th percentile for age, sex, and height or ≥130/80 mm Hg (whichever was lower); for youths ≥13 years and adults, it was defined as ≥130/80 mm Hg. Participants with self‐reported antihypertensive therapy were defined as having hypertension, regardless of age. 22 , 23
2.3. Statistical analysis
Descriptive data were presented as mean ± standard deviation (SD) for continuous and count (%) for categorical variables. Delta WHtR was defined as annualized change in WHtR measures from the baseline visit to the outcome visit (change in WHtR over time in years between baseline and outcome visit). The association between WHtR at baseline, WHtR change during follow‐up, and incident hypertension was evaluated. Because WHtR is often not measured as part of clinical care, we also evaluated the association between BMI z‐score at baseline, delta BMI during follow‐up, and incident hypertension in a similar manner.
The relative risk of WHtR, BMI, and incident hypertension was estimated using modified Poisson regression models with robust standard errors for confidence intervals (CI). Analyses included unadjusted and adjusted models including sex, race/ethnicity, age at diagnosis, duration of diabetes at baseline, parental education, and clinical site. Considering differences between youths with type 1 and type 2 diabetes with regard to sample size, demographics, and mechanism of diabetes (insulin deficiency versus insulin resistance), as well as observing statistically significant or trending to significant diabetes by WHtR interactions, the analyses were a priori stratified by diabetes type. The selection of adjustment variables was informed by development of a directed acyclic graph. 24 All analyses were conducted using SAS version 9.4 (SAS Institute).
3. RESULTS
The analytic sample was comprised of 1695 youths without hypertension at their baseline visit: n = 1,518 with type 1 diabetes and n = 177 with type 2 diabetes and was similar to those excluded from the analyses (see Table S1). Participants with type 1 diabetes excluded due to hypertension at baseline were less likely to be non‐Hispanic white, were more likely to be obese, and had a higher baseline BMI z‐score and WHtR than those excluded for other reasons such as incomplete information (P < .05 for each). Participants with type 2 diabetes excluded due to prevalent hypertension at baseline had a higher baseline BMI z‐score and WHtR than those excluded for other reasons (P < .05 for each).
Participants with type 2 diabetes were more likely to have class II obesity (67.6% vs 7.3%) and to have prehypertension (23.2% vs 8.0%) at their baseline visit compared to participants with type 1 diabetes (Table 1). Participants with type 1 diabetes and type 2 diabetes had a comparable baseline HbA1c and annual change in HbA1c during follow‐up. Participants with type 2 diabetes had a higher baseline WHtR than youths with type 1 diabetes.
Table 1.
Demographic and clinical characteristics at baseline and outcome visits of 1,695 participants with youth‐onset type 1 and type 2 diabetes and without baseline hypertension in the SEARCH for Diabetes in Youth Cohort Study
| Characteristic a |
Type 1 (N = 1518) |
Type 2 (N = 177) |
P value b |
|---|---|---|---|
| Demographics | |||
| Male sex, N (%) | 753 (49.6) | 51 (28.8) | <.001 |
| Age at diagnosis (y) | 10.0 (3.8) | 13.9 (2.7) | <.001 |
| Non‐Hispanic white race, N (%) | 1184 (78.0) | 51 (28.8) | <.001 |
| Parental education: some college or more, N (%) | 1242 (81.8) | 90 (50.8) | <.001 |
| Baseline visit measures | |||
| Duration of diabetes (mo) at visit | 9.1 (6.3) | 10.7 (7.2) | .0033 |
| Weight class, N (%) | <.001 | ||
| Under/normal weight | 1036 (68.4) | 14 (8.0) | |
| Overweight | 290 (19.1) | 21 (11.9) | |
| Obesity class 1 | 78 (5.1) | 22 (12.5) | |
| Obesity class ≥2 | 111 (7.3) | 119 (67.6) | |
| HbA1c(%), mean (SD) | 7.6 (1.4) | 7.5 (2.3) | .466 |
| HbA1c in mmol/mol, mean (SD) | 59.6 (15.8) | 58.1 (25.6) | .466 |
| Prehypertensive, N (%) | 122 (8.0) | 41 (23.2) | <.001 |
| Waist‐height ratio | 0.45 (0.05) | 0.60 (0.09) | <.001 |
| BMI z‐score | 0.5 (1.0) | 2.0 (0.6) | <.001 |
| Outcome Visit Measures | |||
| Interval (y) from baseline visit to follow‐up visit | 7.1 (1.9) | 6.8 (2.0) | .164 |
| Waist‐height ratio | 0.47 (0.06) | 0.61 (0.10) | <.001 |
| Annual change in waist‐height ratio c | 0.0017 (0.0079) | 0.0015 (0.0094) | .846 |
| BMI (kg/m2) | 24.1 (5.0) | 34.3 (8.2) | <.001 |
| Annual change in BMI c | 0.65 (0.51) | 0.18 (0.88) | <.001 |
| HbA1c (%) | 9.1 (1.8) | 9.2 (3.0) | .592 |
| HbA1c,(mmol/mol) | 76.0 (20.1) | 77.4 (33.1) | .592 |
| Annual change in HbA1c (%) c | 0.23 (0.32) | 0.28 (0.51) | .210 |
| Annual change in HbA1c (mmol/mol) c | 2.49 (3.53) | 3.03 (5.54) | .210 |
Data are shown as count (%), or mean (SD).
P‐value evaluating differences in characteristics across DM types: t tests (continuous) or chi‐square tests (categorical).
Annualized change from the baseline visit to follow‐up visit.
The average follow‐up was 7.1 ± 1.9 years for participants with type 1 and 6.8 ± 2.0 years for participants with type 2 diabetes (P = .164). During follow‐up, 225 participants with type 1 and 63 with type 2 diabetes developed hypertension. Participants with type 2 diabetes were more likely to develop hypertension during follow‐up than those with type 1 diabetes (35.6% vs 14.8%, P < .0001, Figure 2). Participants with prehypertension at baseline were more likely to develop hypertension when compared to those without prehypertension at baseline (P < .001 and P = .044 for type 1 or type 2 diabetes, respectively).
Figure 2.
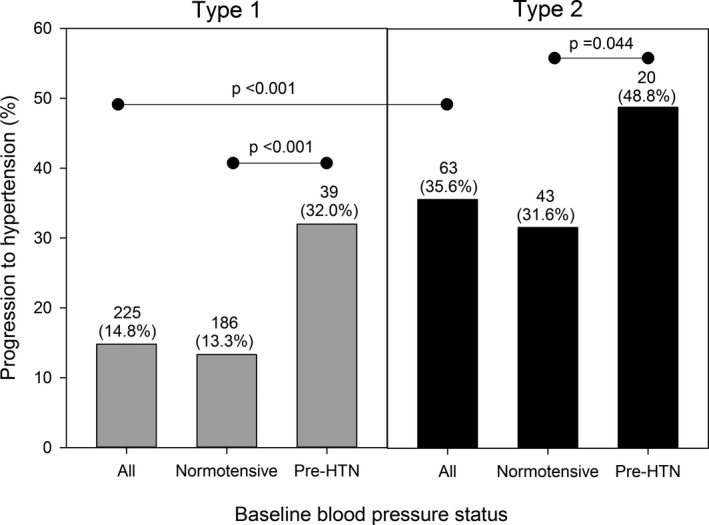
Progression to hypertension by baseline blood pressure status of 1695 participants with youth‐onset type 1 and type 2 diabetes in the SEARCH for Diabetes in Youth Cohort Study
For each 0.1 unit increase in WHtR at baseline, the unadjusted relative risk (95% CI) of progression to hypertension was 1.31 (1.08, 1.61) and 1.19 (0.97, 1.45) in participants with type 1 or type 2 diabetes, respectively (Figure 3A). After adjusting for sex, race/ethnicity, age at diagnosis, parental education, diabetes duration, and clinical site, the magnitude of association increased marginally to 1.36 (1.13, 1.63) and 1.22 (1.00, 1.49) for participants with type 1 or type 2 diabetes, respectively.
Figure 3.
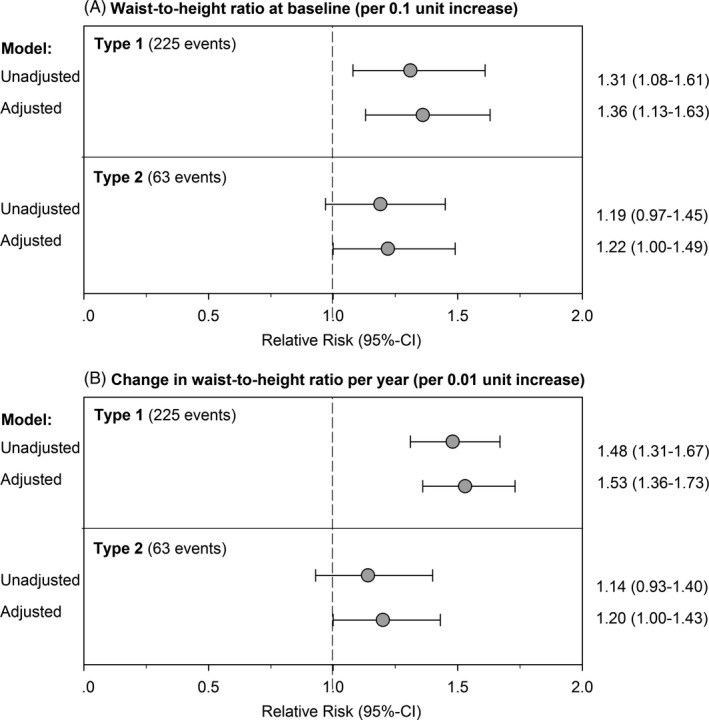
Relative risk of progression to hypertension for each 0.1 unit increase in waist‐to‐height ratio at baseline (3A) and for 0.01 unit increase in change per year (3B) by diabetes type in 1695 participants in the SEARCH for Diabetes in Youth Cohort Study
For each 0.01 unit increase in change in WHtR per year of follow‐up, the relative risk (95% CI) of progression to hypertension was 1.48 (1.31, 1.67) and 1.14 (0.93, 1.40) in participants with type 1 or type 2 diabetes, respectively (Figure 3B). After adjusting for sex, race/ethnicity, age at diagnosis, parental education, diabetes duration, clinical site, and baseline WHtR, the relative risk was 1.53 (1.36, 1.73) and 1.20 (1.00, 1.43) for participants with type 1 or type 2 diabetes, respectively.
The association between BMI z‐score at baseline, BMI change during follow‐up, and the risk of progression to hypertension was similar but slightly less pronounced with lower magnitude of association and wider confidence intervals than the association observed with WHtR (Figure S1).
4. DISCUSSION
In this cohort of youths and young adults with diabetes, we found a mild‐to‐moderate association between baseline WHtR as well as change in WHtR, and incident hypertension. This relationship was observed in both participants with type 1 and with type 2 diabetes. This relationship was less pronounced in participants with type 2 diabetes, in part due to the much smaller sample size among this group.
Several mechanisms link obesity and high blood pressure, including upregulation of pro‐inflammatory adipokines due to adipose tissue dysfunction, increased sympathetic nervous system, abnormal kidney function associated with increased tubular sodium reabsorption, and increasing arterial stiffness. 25 , 26 , 27 , 28 , 29 , 30 , 31 It has also been hypothesized that obesity‐related changes in the composition and function of gut microbiota might be associated with hypertension. 32 Central obesity, rather than BMI, has been shown to be a better predictor for hypertension and cardiovascular disease mortality in adults. 13 , 14 Visceral fat accumulation has been proposed as “dysfunctional adipose tissue,” resulting in an altered metabolic profile which may not be present in individuals with obesity and subcutaneous but low visceral fat deposition. 25 , 33 Results from the present study indicate that WHtR as a marker of central obesity may be an important factor for hypertension in youths and young adults with diabetes.
The SEARCH study has previously shown that the number of cardiovascular risk factors did not change significantly in participants with type 1 diabetes, but increased over time in participants with type 2 diabetes, a diabetic phenotype with a higher prevalence of obesity at diagnosis. 34 Early complications of diabetes co‐occurred in adolescents and young adults with diabetes, and included arterial stiffness and hypertension. 35 Youths and young adults with type 2 diabetes had a higher age‐adjusted prevalence of macrovascular outcomes such as arterial stiffness and hypertension than youths and young adults with type 1 diabetes. 6 Moreover, adjusting for WHtR attenuated the difference in risk for arterial stiffness and hypertension for youths and young adults with type 1 versus type 2 diabetes to non‐significance, indicating that central obesity explains, statistically, the higher burden of macrovascular outcomes in youths with type 2 versus those with type 1 diabetes. 6 While type 1 diabetes is characterized by insulin deficiency mostly due to autoimmune destruction of the pancreatic beta cells and type 2 diabetes by insulin resistance which may lead to beta‐cell failure, 36 it can be speculated that central obesity which is associated with insulin resistance, adds to the burden of disease in both type 1 and type 2 diabetes. Central obesity has been linked with metabolic outcomes, particularly through their impact on β‐cell function and insulin sensitivity. However, growing evidence suggests that they might also interact with the immune system to amplify the autoimmune response in type 1 diabetes. 37
The magnitude of the association between BMI and hypertension prevalence in pediatric populations has been estimated with odds ratios ranging from 3.5 to 4.4 for children with obesity compared to normal weight children. 38 , 39 , 40 , 41 , 42 Three cross‐sectional studies suggested that children with severe obesity are 2‐6 times more likely to have hypertension than those with moderate obesity. 42 , 43 However, these studies did not investigate central obesity or the effect among youths with diabetes. In the present study, youths with type 2 diabetes had a higher WHtR and BMI at baseline than youths with type 1 diabetes while the latter showed a more rapid increase in both markers of obesity than the former. Results from our study suggest that the prevention of central obesity may play an important role in the prevention of hypertension in adolescents and young adults with type 1 or type 2 diabetes. This may prevent obesity‐related metabolic changes involved in the development of hypertension. 25 , 26 , 27 , 28 , 29 , 30 , 31 However, previous analyses also showed that microvascular complications of diabetic kidney disease, retinopathy, and peripheral neuropathy were higher among youths and young adults with type 2 diabetes compared to those with type 1 even after adjustment for central obesity, glycemic control, and other factors over time. 6 This indicates that obesity alone is not responsible for these complications and potential pathways of disease progression need to be explored.
In absence of data to calculate WHtR, BMI, a marker of obesity, may be used as predictor of hypertension in youths and young adults with diabetes. Previous studies in children have shown that BMI correlated reasonably well with body fat 44 and is comparable with WHtR in performance as predictor of metabolic syndrome 45 and hypertension 15 in children. However, in youths with diabetes, central obesity may be a better indicator of risk for diabetes‐related complications than BMI. 6 , 11 In the present analysis, associations between BMI and progression to hypertension were slightly weaker with wider confidence intervals than associations between WHtR and progression to hypertension.
The SEARCH for Diabetes in Youth Study includes a large, racially, and ethnically diverse cohort. 6 The longitudinal measurements of blood pressure, body weight, height, and waist circumference are another strength of the present study. However, the use of blood pressure measured at study visits is a proxy for hypertension because it was not possible to confirm the presence of hypertension at other clinical visits as recommended by current guidelines. 22 Finally, the number of participants in the sample with type 2 diabetes was relatively small, resulting in less precise relative risk estimates.
In conclusion, we found that increasing central obesity was a major risk factor for incident hypertension in youths and young adults with diabetes. The development and implementation of effective strategies to reduce the risk of central obesity and the prevention of weight gain may lead to a reduction in the risk for incident hypertension among youths and young adults with diabetes.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
C Koebnick and JM Stafford had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. C Koebnick, JM Lawrence, G Imperatore, ET Jensen, JM Stafford, AS Shah, AK Mottl, RA Bell, D Dabelea, AD Liese, SM Marcovina, RB D’Agostino, Jr., and EM Urbina contributed to concept and design; acquisition, analysis, or interpretation of data. C Koebnick, JM Lawrence, ET Jensen, JM Stafford, AK Mottl, and RB D’Agostino, Jr. involved in drafting the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. C Koebnick, ET Jensen, JM Stafford, and RB D’Agostino, Jr. performed statistical analysis. D Dabelea, RB D’Agostino, Jr., and JM Lawrence obtained funding.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The SEARCH for Diabetes in Youth Study is indebted to the many youths and their families, and their health care providers, whose participation made this study possible. The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California's Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group); the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062, UL1 Tr001450; Seattle Children's Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077, UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Koebnick C, Imperatore G, Jensen ET, et al. Progression to hypertension in youth and young adults with type 1 or type 2 diabetes: The SEARCH for Diabetes in Youth Study. J Clin Hypertens. 2020;22:888–896. 10.1111/jch.13849
Funding information
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP‐05‐069, and DP‐10‐001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The Population Based Registry of Diabetes in Youth Study (1U18DP006131, U18DP006133, U18DP006134, U18DP006136, U18DP006138, U18DP006139) is funded by the Centers for Disease Control and Prevention and supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Sites: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241‐3, U01 DP000247, and U18DP000247‐06A1), Cincinnati's Children's Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children's Hospital (U18DP006136, U58/CCU019235‐4, U01 DP000244, and U18DP002710‐01), Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200‐2010‐35171).
REFERENCES
- 1. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376:1419‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abduljabbar MA, Aljubeh JM, Amalraj A, et al. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J. 2010;31:413‐418. [PubMed] [Google Scholar]
- 3. Fox DA, Islam N, Sutherland J, et al. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19:501‐505. [DOI] [PubMed] [Google Scholar]
- 4. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62:408‐417. [DOI] [PubMed] [Google Scholar]
- 5. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 Diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dabelea D, Stafford JM, Mayer‐Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Constantino MI, Molyneaux L, Limacher‐Gisler F, et al. Long‐term complications and mortality in young‐onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863‐3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luk AO, Lau ES, So WY, et al. Prospective study on the incidences of cardiovascular‐renal complications in Chinese patients with young‐onset type 1 and type 2 diabetes. Diabetes Care. 2014;37:149‐157. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds K, Saydah SH, Isom S, et al. Mortality in youth‐onset type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Diabetes Complications. 2018;32:545‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez BL, Dabelea D, Liese AD, et al. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157(2):245–251.e1. [DOI] [PubMed] [Google Scholar]
- 11. Wadwa RP, Urbina EM, Anderson AM, et al. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2010;33:881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2010;11:4‐11. [DOI] [PubMed] [Google Scholar]
- 13. Obesity in Asia Collaboration . Is central obesity a better discriminator of the risk of hypertension than body mass index in ethnically diverse populations? J Hypertens. 2008;26:169‐177. [DOI] [PubMed] [Google Scholar]
- 14. Czernichow S, Kengne AP, Stamatakis E, et al. Body mass index, waist circumference and waist‐hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual‐participant meta‐analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12:680‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui Z, Truesdale KP, Cai J, et al. Anthropometric indices as measures of body fat assessed by DXA in relation to cardiovascular risk factors in children and adolescents: NHANES 1999–2004. Int J Body Compos Res. 2013;11:85‐96. [PMC free article] [PubMed] [Google Scholar]
- 16. Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;(135):1‐55. [PubMed] [Google Scholar]
- 17. Dabelea D, Pihoker C, Talton JW, et al. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;11:1‐190. [PubMed] [Google Scholar]
- 19. World Health Organization . Waist circumference and waist–hip ratio. Report of a WHO expert consultation, Geneva, 8–11 December, 2008. 2011. http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf. Published Last Modified Date|. Accessed Dated Accessed|.
- 20. Liu LL, Kahn HS, Pettitt DJ, et al. Comparing two waist‐to‐height ratio measurements with cardiometabolic risk factors among youth with diabetes. Int J Child Health Nutr. 2016;5:87‐94. [PMC free article] [PubMed] [Google Scholar]
- 21. Pettitt DJ, Talton JW, Liese AD, et al. Comparison of two waist circumference measurement protocols: the SEARCH for Diabetes in Youth Study. Pediatr Obes. 2012;7:e81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flynn JT, Kaelber DC, Baker‐Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 23. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 24. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37‐48. [PubMed] [Google Scholar]
- 25. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881‐887. [DOI] [PubMed] [Google Scholar]
- 26. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1‐7. [DOI] [PubMed] [Google Scholar]
- 27. Chrysant SG. Pathophysiology and treatment of obesity‐related hypertension. J Clin Hypertens (Greenwich). 2019;21:555‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brady TM. Obesity‐related hypertension in children. Front Pediatr. 2017;5:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14‐22. [DOI] [PubMed] [Google Scholar]
- 30. Urbina EM. Abnormalities of vascular structure and function in pediatric hypertension. Pediatr Nephrol. 2016;31:1061‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15(6):367‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathieu P, Boulanger MC, Despres JP. Ectopic visceral fat: a clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord. 2014;15:289‐298. [DOI] [PubMed] [Google Scholar]
- 34. Kim G, Divers J, Fino NF, et al. Trends in prevalence of cardiovascular risk factors from 2002‐2012 among youth early in the course of type 1 and type 2 diabetes. The SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2019;20(6):693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sauder KA, Stafford JM, Mayer‐Davis EJ, et al. Co‐occurrence of early diabetes‐related complications in adolescents and young adults with type 1 diabetes: an observational cohort study. Lancet Child Adolesc Health. 2018;3(1):35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S13‐S28. [DOI] [PubMed] [Google Scholar]
- 37. Redondo MJ, Evans‐Molina C, Steck AK, et al. The influence of type 2 diabetes‐associated factors on type 1 diabetes. Diabetes Care. 2019;42:1357‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McNiece KL, Poffenbarger TS, Turner JL, et al. Prevalence of hypertension and pre‐hypertension among adolescents. J Pediatr. 2007;150:640‐644. [DOI] [PubMed] [Google Scholar]
- 39. Chiolero A, Cachat F, Burnier M, et al. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25:2209‐2217. [DOI] [PubMed] [Google Scholar]
- 40. Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school‐aged children. Pediatrics. 2004;113:475‐482. [DOI] [PubMed] [Google Scholar]
- 41. Lo JC, Sinaiko A, Chandra M, et al. Prehypertension and hypertension in community‐based pediatric practice. Pediatrics. 2013;131:e415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koebnick C, Black MH, Wu J, et al. High blood pressure in overweight and obese youth: implications for screening. J Clin Hypertens (Greenwich). 2013;15:793‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lo JC, Chandra M, Sinaiko A, et al. Severe obesity in children: prevalence, persistence and relation to hypertension. Int J Pediatr Endocrinol. 2014;2014:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin‐Calvo N, Moreno‐Galarraga L, Martinez‐Gonzalez MA. Association between body mass index, waist‐to‐height ratio and adiposity in children: a systematic review and meta‐analysis. Nutrients. 2016;8(8):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Radetti G, Fanolla A, Grugni G, et al. Indexes of adiposity and body composition in the prediction of metabolic syndrome in obese children and adolescents: which is the best? Nutr Metab Cardiovasc Dis. 2019;29(11):1189‐1196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


