Summary
Background
Hepatorenal syndrome and acute kidney injury are common complications of decompensated cirrhosis, and terlipressin is recommended as first‐line vasoconstrictor therapy. However, data on its use outside of clinical trials are lacking.
Aims
To assess practice patterns and outcomes around vasoconstrictor use for hepatorenal syndrome in UK hospitals.
Methods
This was a multicentre chart review study. Data were extracted from medical records of patients diagnosed with hepatorenal syndrome and treated by vasoconstrictor drugs between January 2013 and December 2017 at 26 hospitals in the United Kingdom. The primary outcome was improvement of kidney function, defined as complete response (serum creatinine improved to ≤1.5 mg/dL), partial response (serum creatinine reduction of ≥20% but >1.5 mg/dL) and overall response (complete or partial response). Other outcomes included need for dialysis, mortality, liver transplantation and adverse events.
Results
Of the 225 patients included in the analysis, 203 (90%) were treated with terlipressin (median duration, 6 days; range: 2‐24 days). Mean (±standard deviation) serum creatinine at vasopressor initiation was 3.25 ± 1.64 mg/dL. Terlipressin overall response rate was 73%. Overall response was higher in patients with mild acute kidney injury (baseline serum creatinine <2.25 mg/dL), compared to those with moderate (serum creatinine ≥2.25 mg/dL and <3.5 mg/dL) or severe (serum creatinine ≥3.5 mg/dL). Ninety‐day survival was 86% for all patients (93% for overall responders vs 66% for treatment nonresponders, P < 0.0001).
Conclusion
Terlipressin is the most commonly prescribed vasoconstrictor for patients with hepatorenal syndrome in the United Kingdom. Treatment with terlipressin in patients with less severe acute kidney injury (serum creatinine <2.25 mg/dL) was associated with higher treatment responses, and 90‐day survival.
1. INTRODUCTION
Acute kidney injury (AKI) is a complication of advanced liver disease, occurring in approximately 3.23% of hospitalised adult patients with chronic liver disease including cirrhosis, an orphan disease with a prevalence of 11.2 per 100 000 population. 1 Hepatorenal syndrome (HRS) is a type of functional AKI that results from portal hypertension leading to decreased effective circulating arterial volume, and renal vasoconstriction. 2 Diagnostic criteria for HRS‐AKI include cirrhosis with ascites; an acute elevation of serum creatinine (SCr) of at least 0.3 mg/dL above baseline, no improvement in SCr after at least 2 days of diuretic withdrawal and volume expansion with albumin, and the absence of shock, current or recent treatment with nephrotoxic drugs and parenchymal kidney disease. 3 , 4 Historically, the prognosis in patients with HRS is extremely poor, with multiple cohorts demonstrating approximately 50% survival at 1 month. 5 , 6
Clinical practice guidelines from the European Association of the Study of the Liver recommend terlipressin in combination with albumin as a first‐line intervention for HRS‐AKI, with the aim of decreasing SCr to <1.5 mg/dL. 3 While this approach is not curative of the underlying liver disease which drives the development of HRS‐AKI, in Europe it has become increasingly recognised that early treatment leads to reversal of AKI and that such patients may recover sufficiently to proceed to liver transplantation later. Thus, in many ways it is considered as a bridge to liver transplantation. 7 This is important in the United Kingdom, where there is a reluctance to undertake liver transplantation on patients receiving renal replacement therapy. Thus in 2018/2019, only 2% of all patients undergoing elective liver transplantation were on renal replacement therapy at the time of liver transplantation. 8 For patients who fail to respond to vasopressor therapy, renal replacement therapy (either intermittent haemodialysis or continuous venous hemofiltration) may be useful, 3 but is associated with a poor short term survival among those not listed for transplant. 9 Liver transplantation is ultimately the treatment of choice for patients who have developed HRS, but it has better outcomes if the AKI is reversed prior to liver transplantation. Prognosis is poor for patients who are not transplant candidates, and for whom renal replacement therapy has limited utility, unless there is a reversible element to their decline in liver function such as successful treatment of alcoholic hepatitis or of sepsis as a precipitating cause. 9
In clinical trials, HRS therapy with terlipressin and albumin have been shown to be significantly more effective than other vasopressors in reversing HRS‐AKI and are generally effective in approximately 40% of cases. 10 However, real‐world data on terlipressin's effectiveness and clinicians' treatment patterns are under‐represented in the literature, and in particular, whether early treatment of HRS‐AKI has an improved outcome compared to clinical trials, where patients are included with relatively advanced kidney injury. In medicine, it is generally recognised that early treatment is always better than rescue treatment. 11 In earlier years, there was a need for definitions of HRS to ensure that clinical studies were more homogeneous. However, with the advent of more straightforward AKI diagnostic guidelines, the International Ascites Club have recently re‐defined HRS‐AKI, and which is more in line with conventional medicine, and in effect recognises that earlier diagnosis and presumably treatment is better. 12
Therefore, we conducted a retrospective, multicentre chart review study to assess effectiveness, treatment patterns and other outcomes with terlipressin and other vasopressors use for patients hospitalised with HRS‐AKI in the United Kingdom.
2. MATERIALS AND METHODS
2.1. Study design
In this medical chart review study, physicians specialising in gastroenterology (with or without subspecialty in hepatology), nephrology or critical care at 26 hospitals (11 in the Greater London and South East region; 7 in the Midlands and East region; 4 in North England; and 1 each in South West, South Central, Scotland and an unknown region) were recruited from a national database of centres treating HRS patients in the United Kingdom. Participating physicians (or their designated staff) were asked to review the medical charts of patients with a clinical diagnosis of HRS, as defined by their clinical International Classification of Disease, 10th revision (ICD10) coding (see below for details), from hospital admission to 90 days post‐discharge or until death, and extract data from these charts using an electronic case report form. The case report form was hosted on a secured website. Study sites and investigators remained anonymous to the sponsor and the sponsor was not disclosed to physicians; a decision tool by the United Kingdom Health Research Authority confirmed that this study did not need approval by a Research Ethics Committee. Data collection, processing, storage and usage followed data protection and privacy guidelines in the United Kingdom and the European Union General Data Protection Regulation. All results were reported in a de‐identified, aggregate manner. Physicians were identified and distinguished only by a randomly assigned identifier. Physician identifiers were stored for a time period that is consistent with the study sponsor's standard operating procedures and the General Data Protection Regulation.
2.2. Selection criteria
Eligible patients were identified by physicians using International Classification of Disease, 9th revision code of 572.4 (hepatorenal syndrome) or an ICD‐10 code of K76.7 (hepatorenal syndrome) or N17.9 (acute renal failure, unspecified) with K74.6 (other and unspecified cirrhosis of the liver), or based on chart documentation of HRS diagnosis. To obtain a representative sample of patients, physicians were prompted to select patients based on HRS‐AKI diagnosis date and first letter of the patient's last name. Patient inclusion criteria were as follows: ≥18 years of age at diagnosis; HRS‐AKI episode between 1 January 2013 and 31 December 2017 with a SCr >1.5 mg/dL; treated at participating physician's hospital with a vasopressor therapy; and the participating physician was able to report on the SCr on the day of diagnosis and on the last day or day 14 of vasopressor treatment (whichever date came first), and at the discharge date. Patients who had dialysis or a transjugular intrahepatic portosystemic shunt within 1 month prior to hospitalisation were excluded from the study to avoid confounding the data. Additional exclusions were patients who died within 24 hours of vasopressor initiation, prior liver transplantation and previous hospitalisation for HRS during the previous 6 months.
2.3. Study variables
Data were collected from hospital admission to 90 days post‐discharge, and included demographics, baseline clinical characteristics, treatment history, procedures and adverse events. Vasopressor therapy was grouped into (a) terlipressin monotherapy for at least 2 days; (b) monotherapy with other vasopressors (midodrine/octreotide, vasopressin, or noradrenaline) for at least 2 days; (c) miscellaneous (overlapping treatments groups or monotherapy for only 1 day). Hepatorenal syndrome treatment response was measured by change in SCr from 1 day prior to vasopressor initiation to last day of vasopressor treatment or day 14 (whichever came first). Treatment response was defined as complete response if SCr decreased to ≤1.5 mg/dL, as partial response if SCr decreased ≥20% but >1.5 mg/dL, and as no response if SCr decreased <20%. Overall response was defined as either complete or partial response. Patients were categorised as having mild AKI if the pre‐treatment SCr was <2.25 mg/dL, moderate AKI if the pre‐treatment SCr was ≥2.25 mg/dL but <3.5 mg/dL and severe if the pre‐treatment SCr was ≥3.5 mg/dL.
2.4. Statistical analysis
Categorical variables were summarised using the percentage and count in each category. Continuous variables were summarised using the summary statistics of mean and standard deviation or median and range, as appropriate. Group differences were tested using the Pearson's chi‐square test or Fisher's exact test for categorical variables and the Student's t test for continuous variables. Patient characteristics were compared in subgroups by SCr at treatment start (mild, moderate or severe) and treatment response (overall response vs no response). Missing data were reported as such and not imputed. All inferences were made assuming a two‐sided test with an alpha level of 0.05.
3. RESULTS
Study investigators collected data for 250 patients (range, 4‐15 patients per investigator). Twenty‐five patients had overlapping treatments or received monotherapy for only 1 day and were excluded from the final analysis. A total of 225 patients received vasopressor monotherapy for at least 2 days and were included in the final analyses. Out of these 225 patients, 68% were male, and the mean age was 53.6 years (Table 1). Mean (± standard deviation) SCr at vasopressor initiation was 3.25 ± 1.64 mg/dL for all patients. For most patients (83%), at least one precipitating event or treatment was reported in the week leading up to HRS diagnosis, with gastrointestinal bleeding being the most common (28%), followed by large‐volume paracentesis (19%) and diuretic treatment (14%). Alcoholic liver disease was the most common underlying cause of cirrhosis (68%), followed by non‐alcoholic steatohepatitis or non‐alcoholic fatty liver disease (17%) and hepatitis C (16%). History of ascites was reported in 75% of patients, encephalopathy in 33% of patients. Twenty‐four per cent of patients were reported to be eligible and/or listed for transplant.
TABLE 1.
Baseline and clinical characteristics patients with hepatorenal syndrome, by treatment group
|
Terlipressin (N = 203) |
Other vasopressors a (N = 22) |
P value b | ||
|---|---|---|---|---|
| Age (mean), y | 53.9 (11.6) | 50.7 (15.6) | 0.351 | |
| Male, N (%) | 136 (67) | 16 (73) | 0.585 | |
| Smoking status, N (%) | 0 (0) | 0.188 | ||
| Current smoker | 52 (26) | 8 (36) | ||
| Former smoker | 52 (26) | 6 (27) | ||
| Never smoked | 53 (26) | 7 (32) | ||
| Unknown | 46 (23) | 1 (5) | ||
| Eligible/listed for transplant, N (%) | 45 (22) | 8 (36) | 0.136 | |
| Precipitating event(s), N (%) c , d | ||||
| Diarrhoea | 18 (9) | 3 (14) | ||
| Gastrointestinal bleeding | 56 (28) | 6 (27) | ||
| Large‐volume paracentesis | 43 (21) | 0 (0) | ||
| Spontaneous bacterial peritonitis | 29 (14) | 2 (9) | ||
| Treatment with diuretics | 30 (15) | 2 (9) | ||
| Other infection | 79 (39) | 2 (9) | ||
| None of the above | 32 (16) | 7 (32) | 0.059 | |
| Underlying causes, N (%) c | ||||
| Alcoholic liver disease | 140 (69) | 12 (55) | ||
| Hepatitis C | 29 (14) | 7 (32) | ||
| Non‐alcoholic steatohepatitis/fatty liver disease | 36 (18) | 3 (14) | ||
| Other | 22 (11) | 2 (9) | ||
| Ascites at hospital admission | 160 (79) | 9 (41) | <0.001 | |
| Encephalopathy at hospital admission | 66 (33) | 9 (41) | 0.763 | |
Abbreviations: N, number; SD, standard deviation.
Vasopressin (N = 13), noradrenaline (N = 5), midodrine/octreotide (N = 4).
P values represent comparisons between terlipressin and other vasopressor groups, using Student's t test (continuous variables) or Fisher's exact test (categorical variables).
Precipitating events or treatments immediately (within 7 d) prior to the diagnosis of hepatorenal syndrome.
Responses are not mutually exclusive.
Of the 225 total patients, 203 patients (90%) received terlipressin monotherapy with a median duration of therapy of 6 days (range: 2‐24 days). Median time from hospital admission to terlipressin initiation was 4 days (range: 1‐57 days). Twenty‐two patients (10%) received other vasopressor treatment with a duration of 2 days or more, with vasopressin being the most common agent (N = 13). The median treatment duration of vasopressin monotherapy was 3 days (range: 2‐8 days), and the median time from admission to therapy initiation 3 days (range: 1‐11 days). Sixty‐eight per cent of all patients received concurrent treatment with albumin, including 72% of patients treated with terlipressin and 32% of patients treated with other vasopressors. In 27 patients (12%), use of albumin was recorded as unknown. Fifty‐two per cent of all patients received antibiotic treatment during their admission, of which penicillin‐class agents were the most common (75/225; 33%).
Overall response rate was 73% among terlipressin‐treated patients and 59% among other vasopressor‐treated patients (Table 2). Complete response rates were 50% among 203 terlipressin‐treated patients and 23% among 22 other patients. Lower SCr at the time of terlipressin initiation was associated with higher complete response rates (79% for mild AKI vs 55% for moderate AKI vs 14% for severe AKI (P < 0.001; Figure 1). The median time to complete response was 7.0 days in patients with mild AKI, 8.0 days in moderate AKI and 9.0 days in severe AKI (Table 2). Change in SCr from baseline was −0.44, −0.90 and −1.37 mg/dL in patients with mild, moderate and severe AKI respectively (P < 0.001). Complete response rate was not significantly different in patients who received concomitant albumin (48%) compared to those who did not receive albumin (30%) and those in whom use was unknown (37%; P = 0.107).
TABLE 2.
Clinical outcomes of patients with hepatorenal syndrome by treatment group and acute kidney injury severity
|
By treatment group (N = 225) |
By AKI severity, terlipressin treated patients (N = 203) d |
||||||
|---|---|---|---|---|---|---|---|
|
Terlipressin (N = 203) |
Other vasopressor b (N = 22) |
P value c |
Mild (N = 67) |
Moderate (N = 73) |
Severe (N = 63) |
P value e | |
| Pre‐treatment SCr (mg/dL), mean (SD) | 3.23 (1.70) | 3.38 (1.11) | 0.071 | 1.84 (0.27) | 2.85 (0.41) | 5.17 (1.73) | <0.001 |
| SCr at end of therapy, mean (SD) a | 2.34 (1.87) | 2.17 (0.72) | 0.091 | 1.39 (0.84) | 1.96 (1.37) | 3.80 (2.27) | <0.001 |
| Change in SCr, mean (SD) | 0.89 (1.77) | 1.22 (1.24) | 0.569 | 0.44 (0.89) | 0.90 (1.40) | 1.37 (2.58) | <0.001 |
| Complete response, N (%) | 102 (50.2) | 5 (22.7) | 0.014 | 53 (79.1) | 40 (54.8) | 9 (14.3) | <0.001 |
| Overall response (complete or partial), N (%) | 148 (72.9) | 13 (59.1) | 0.172 | 53 (79.1) | 57 (78.1) | 38 (60.3) | 0.025 |
| Time to response in days, median (95% CI) | 8 (8, 11) | 11 (4, 11) | 0.230 | 7 (5, 8) | 8 (6, 8) | 9 (7, 11) | <0.001 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; N, number; SCr, serum creatinine; SD, standard deviation.
At day 14 of treatment or at time of treatment discontinuation, whichever event occurred first.
Vasopressin (N = 13), noradrenaline (N = 5), midodrine/Octreotide (N = 4).
P values represent comparisons between terlipressin and other vasopressor groups, using Wilcoxon rank sum tests for SCr, Fisher's exact test for overall response, and Kaplan‐Meier with log rank test for time to response. Terlipressin monotherapy vs other vasopressor monotherapy.
Mild AKI: SCr <2.25 mg/dL; moderate AKI: SCr ≥2.25 mg/dL and <3.5 mg/dL; severe AKI, SCr ≥3.5 mg/dL.
P values represent comparisons between mild, moderate and severe AKI groups, using Wilcoxon rank sum tests for SCr, Fisher's exact test for overall response, and Kaplan‐Meier with log rank test for time to response.
FIGURE 1.
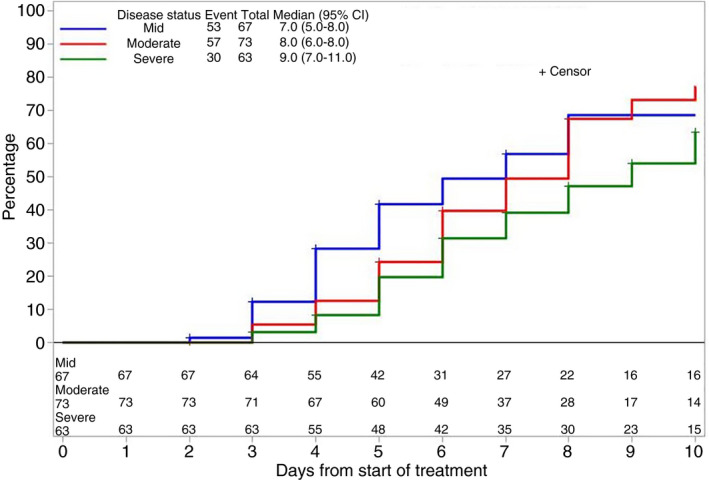
Cumulative response to terlipressin (decrease in serum creatinine to ≤1.5 mg/dL), according to kidney function at the time of initiation of therapy. AKI, acute kidney injury; CI, confidence interval. Groups were defined as mild AKI (serum creatinine <2.25 mg/dL within 24 h of treatment initiation), moderate AKI (serum creatinine 2.25‐3.5 mg/dL within 24 h of treatment initiation), and severe AKI (serum creatinine ≥3.5 mg/dL within 24 h of treatment initiation)
Change in mean arterial pressure was available for 187 out of 203 patients (92%) treated with terlipressin. In these patients, the mean (± standard deviation) change in mean arterial pressure from baseline to day 7 or the day of treatment discontinuation, whichever occurred first, was 3.6 ± 13.3 mm Hg. Change in mean arterial pressure was similar between mild, moderate and severe AKI subgroups (5.4, 2.5, and 3.0 mm Hg respectively, P = 0.541). Of the 203 patients who received terlipressin, 99 (49%) developed infections during hospitalisation. AKI severity was not associated with presence of infection (mild AKI, 48%; moderate AKI, 48%; severe AKI; P = 0.952).
For all patients, the overall survival was 92% at 1 month, 88% at 2 months and 86% at 3 months. Among terlipressin‐treated patients, survival was 92%, 88% and 85% at 1, 2 and 3 months respectively). Overall survival at 1, 2 and 3 months post‐vasopressor initiation was significantly higher in patients with partial or complete response compared to patients with no response (Figure 2).
FIGURE 2.
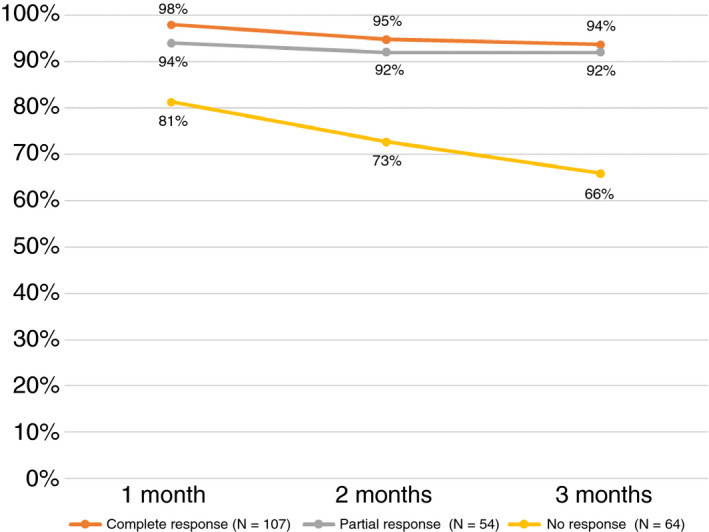
Overall survival by treatment response, all patients. Definitions of groups: Complete response (decrease in serum creatinine from the day before treatment initiation to a level of ≤1.5 mg/dL by end of therapy; Partial response (decrease in serum creatinine of ≥20% from the day before treatment initiation to a level >1.5 mg/dL by end of therapy); No response (decrease in serum creatinine of <20% from the day before treatment initiation by end of therapy)
Of the patients treated with terlipressin, 88 (43%) underwent a total paracentesis during their hospitalisation. Terlipressin responders were more likely to have received a paracentesis during admission compared to nonresponders (35% vs 15%, P = 0.004). Twelve per cent of patients required renal replacement therapy during their admission, and 3% of patients received a transjugular intrahepatic portosystemic shunt. No liver transplants were reported during the initial hospitalisation. Four of the 203 (2%) patients treated with terlipressin received a liver transplant within 90 days of treatment.
Among terlipressin‐treated patients, mean (standard deviation) length of hospitalisation was 26 ± 16 days. Length of stay was not significantly different when comparing those who achieved a complete response (23.2 ± 15.2 days) to those patients with no response (27.1 ± 16.6 days; P = 0.150).
Adverse events were attributed to terlipressin in 50/203 (25%) patients and 9/22 (41%) in those treated with other vasopressors (Table 3). Fluid overload/pulmonary oedema and multiorgan failure were the most commonly reported events. Severe AKI at the time of treatment initiation was associated with higher rates of adverse events (mild AKI, 9%; moderate AKI, 25%; severe AKI, 41; P < 0.001).
TABLE 3.
Adverse events attributed to vasoconstrictor treatment
|
Terlipressin (N = 203) |
Other vasopressors (N = 22) |
|
|---|---|---|
| Any, N (%) | 50 (25) | 9 (41) |
| Fluid overload or pulmonary oedema | 32 (16) | 7 (32) |
| Mesenteric ischemia | 3 (2) | 0 (0) |
| Multi‐organ failure | 18 (9) | 2 (9) |
| Myocardial infarction | 2 (1) | 0 (0) |
Adverse events were attributed to therapy by the treating clinician.
Abbreviation: N, number.
4. DISCUSSION
Results from this large real‐world chart review study show that vast majority of patients with a clinical diagnosis of HRS‐AKI were treated with terlipressin in the United Kingdom, consistent with European Association for the Study of the Liver guidelines. Approximately 50% of patients treated with terlipressin achieved a complete response, with an additional 23% experiencing partial response. Patients with mild HRS‐AKI were significantly more likely to achieve complete response compared to those with moderate or severe HRS‐AKI.
Our patient cohort cannot be compared directly with clinical trial patients diagnosed with HRS, since our cohort of patients were identified directly by clinicians through the ICD10 Code on discharge or death. Thus, they did not necessarily fulfil guideline definitions of HRS‐AKI. However, the results of this study complement those of randomised controlled trial data of terlipressin plus albumin. Presented in November 2019 at the American Association for the Study of Liver Disease Liver Meeting, the CONFIRM trial randomised patients to receive terlipressin plus albumin or albumin alone, and found that 36.2% of patients who received terlipressin plus albumin achieved HRS reversal, defined as a SCr ≤1.5 mg/dL. 13 The higher response rate in this study compared to CONFIRM could be explained by differences in baseline characteristics, including a lower SCr at the time of treatment initiation. Lower SCr has been linked to higher rates of treatment response in various clinical trials, 10 , 14 , 15 , 16 , 17 as well as in our current study. Alternatively, because this population was drawn from real clinical practice settings, patients may have been selected for terlipressin use and managed differently compared to those patients enrolled in clinical trials. For example, only 68% of patients were reported as having received intravenous albumin and only 75% were noted to have a history of ascites on presentation. Both of these features are part of the 2015 Ascites Club diagnostic criteria for HRS, and would have been required as part of any clinical trial examining the effect of vasopressors such as terlipressin. 4 This paper represents the largest real world population experience of terlipressin for HRS, and thus holds great value in bridging clinical trial with real‐world data and making it available to clinicians.
It is well known that clinical practice evolves from clinical trial data. Furthermore, clinicians treating patients with oliguric HRS‐AKI often witness a dramatic response in some patients, with rapidly improving urine output and renal function. These anecdotal cases serve to reinforce the belief and confidence of individual clinicians that earlier treatment in patients with developing HRS‐AKI may be more effective. This is borne out both in the clinical trial data and these real‐world data. Thus, many clinicians in the United Kingdom will commence treatment with terlipressin before such patients fulfil the diagnostic criteria originally set out by the International Ascites Club. It is striking to note that terlipressin was by far the most commonly prescribed vasoconstrictor. While this limited our ability to compare efficacy across treatment groups, it does suggest that an approach using terlipressin with or without albumin translates well into real clinical practice.
The reported adverse events rates in patients treated with terlipressin was lower in our study (25%) compared to a recent systematic review of randomised trials (56%). 18 One other observational study of 21 patients treated with terlipressin reported lower adverse event rates as well. 19 While data are conflicting whether adverse event rates are reported at higher rates in randomised trials compared to observational studies, 20 it may be that adverse events were under‐reported in our population.
We were surprised at the high survival rates in this population. The absence of a definitive, objective test for HRS precludes the ability to extract outcome data retrospectively from consecutive patients. As this was therefore a convenience sample, there may have been omission of more severe cases or of cases where complete data were not available. Thus, survival was higher than expected, which was likely due to a selection bias. For example, if the notes of patients deceased were not readily available, our participants could have simply selected more patients who were alive. That said, the data are invaluable in that they show the real‐world outcomes based on the patient demographics presented.
In our study, the incidence of terlipressin‐attributable respiratory events and multi‐organ failure was significantly higher in patients with severe AKI (24% and 21%) compared to mild (both 5%) or moderate AKI (19% and 3%). While it is possible that patients with severe AKI represent a sicker population (and thus are more prone to adverse events), this higher event rate is noteworthy and highlights that this subgroup warrants close monitoring and judicious use of vasopressors and volume expansion. We are also aware that patients treated with terlipressin in the CONFIRM trial also showed a greater number of respiratory complications compared to placebo, which is consistent with our results.
In line with our expectations, the use of transjugular intrahepatic portosystemic shunt in patients with terlipressin monotherapy in our study was low (3% during initial hospitalisation). The use of renal replacement therapy in patients treated with terlipressin was more common (11%). The utilisation of both of these procedures is in consistent with a study conducted in the United States, showing that 1.1% and 16.3% underwent TIPS and haemodialysis respectively. 21 Despite 24% of study patients reportedly eligible or listed for transplant, only 4 patients (2%) received a transplant during the 90‐day follow‐up period. These results are consistent with an observational study using electronic health records in the United States, in which 2.1% of patients underwent liver or combined kidney/liver transplant during a hospital admission for HRS. 21 However, overall rates of transplant vary from study to study, 5 , 6 , 9 and depend heavily on organ availability in different regions, the illness severity of the included population, and may be influenced by drug availability (for example, terlipressin is not an approved therapy in the United States).
As with any study, a discussion of its limitations will help contextualise its results. Similarly to randomised controlled trials, 22 observational studies in HRS‐AKI are inherently challenging, since sampling efforts need to balance achieving broad patient representation against the feasibility of data collection and accuracy of HRS‐AKI diagnosis. In our study, we aimed for a wide inclusion that represented ‘real‐world’ use of vasopressors for HRS‐AKI. Patients in this study likely represent a more heterogeneous population than is found in RCTs of HRS‐AKI. Identification of HRS relies on clinical judgement, guided by consensus criteria, rather than an objective diagnostic test, and as a result, all patients may not have met the full criteria for HRS. Furthermore, changes to the 2015 International Ascites Club HRS guidelines, 4 which occurred during the study period, may have altered clinicians' judgement when determining when to initiate vasoconstrictors. Our sampling strategy may have led to higher survival and treatment response rates than are seen in other settings, resulting in a selection bias towards surviving patients. Still, given that all patients were diagnosed with HRS by qualified physicians, this large population offers invaluable insights into effects of terlipressin in the real‐world clinical setting. Unfortunately, quantitative measures of liver disease severity, such as MELD score or Child‐Pugh score, 23 , 24 were not available, since the prothrombin time or international normalised ratio, were not collected at the time of study. This would have greatly helped generalise these findings to other settings. We believe that the multiple centres included (n = 26) and other details around renal function and complications of liver disease help mitigate this issue. Given the retrospective nature of the study design, patient outcomes were already known to providers, which may have resulted in reporting bias towards better outcomes. Our study included only 22 patients treated with non‐terlipressin vasopressors, and thus was under‐powered to compare the two treatment groups. Adverse events were recorded retrospectively from clinical documentation. Thus, less severe events were likely to have been underreported. Finally, as with any retrospective study, these results should be interpreted as associations, rather than a causal relationship.
5. CONCLUSIONS
Terlipressin was the most commonly prescribed vasoconstrictor for hospitalised patients HRS‐AKI in the United Kingdom, consistent with current guidelines. Initiation of terlipressin at lower serum creatinine was associated with higher rates of treatment response. Complete or partial response to terlipressin is associated with higher rate of 90‐day survival. These data demonstrate that treatment with terlipressin is an effective treatment for HRS in the real‐world.
FUNDING INFORMATION
This study was funded in full by Mallinckrodt Pharmaceuticals.
AUTHORSHIP
Guarantor of the article: Shelby Corman.
Author contributions: KM, XH, KJ, KV, SC and MH designed the study. CM and RL participated in data collection. LL, NK, KV and RB analysed the data. KM, XH, KJ, KV, NS, AA and SC interpreted the study results and drafted the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge Martina Sluga‐O'Callaghan's involvement in the management of the data collection process as an employee of AplusA.
Declaration of personal interest: Khurram Jamil, Xingyue Huang and Nisreen Shamseddine are employees of Mallinckrodt Pharmaceuticals. Katharina Verleger, Marieke Heisen, Rachel Bakker, Shelby Corman, Linlin Luo and Nehemiah Kebede are employees of Pharmerit International, which received consulting fees related to this study. Andrew Allegretti has served on advisory boards for Mallinckrodt Pharmaceuticals.
Moore K, Jamil K, Verleger K, et al. Real‐world treatment patterns and outcomes using terlipressin in 203 patients with the hepatorenal syndrome. Aliment Pharmacol Ther. 2020;52:351–358. 10.1111/apt.15836
The Handling Editor for this article was Dr Stephen Ryder, and it was accepted for publication after full peer‐review.
Funding information
This study was funded by Mallinckrodt Pharmaceuticals.
REFERENCES
- 1. Pant C, Jani BS, Desai M, et al. Hepatorenal syndrome in hospitalized patients with chronic liver disease: results from the Nationwide Inpatient Sample 2002–2012. J Investig Med. 2016;64:33‐38. [DOI] [PubMed] [Google Scholar]
- 2. Garcia‐Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064‐2077. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406‐460. [DOI] [PubMed] [Google Scholar]
- 4. Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968‐974. [DOI] [PubMed] [Google Scholar]
- 5. Martín–Llahí M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488‐496.e4. [DOI] [PubMed] [Google Scholar]
- 6. Allegretti AS, Ortiz G, Wenger J, et al. Prognosis of acute kidney injury and hepatorenal syndrome in patients with cirrhosis: a prospective cohort study. Int J Nephrol. 2015;2015:108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acevedo JG, Cramp ME. Hepatorenal syndrome: update on diagnosis and therapy. World J Hepatol. 2017;9:293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Health Service Blood and Transplant . Annual Report on Liver Transplantation. Report for. 2018/2019. https://nhsbtdbe.blob.core.windows.net/umbraco‐assets‐corp/16782/nhsbt‐liver‐transplantation‐annual‐report‐2018‐19.pdf. Accessed January 21, 2020.
- 9. Allegretti AS, Parada XV, Eneanya ND, et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol. 2018;13:16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567‐574. [DOI] [PubMed] [Google Scholar]
- 11. Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579‐1589.e2. [DOI] [PubMed] [Google Scholar]
- 12. Huelin P, Piano S, Solà E, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute‐on‐chronic liver failure. Clin Gastroenterol Hepatol. 2017;15:438‐445.e5. [DOI] [PubMed] [Google Scholar]
- 13. Wong F, Curry MP, Reddy KR, et al. The CONFIRM Study: a North American randomized controlled trial (RCT) of terlipressin plus albumin for the treatment of hepatorenal syndrome type 1 (HRS‐1). American Association for the Study of Liver Diseases: The Liver Meeting; November 8–12, 2019; Boston, MA, USA. [Google Scholar]
- 14. Boyer TD, Sanyal AJ, Garcia‐Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219‐226. [DOI] [PubMed] [Google Scholar]
- 16. Piano S, Schmidt HH, Ariza X, et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol. 2018;16:1792‐1800.e3. [DOI] [PubMed] [Google Scholar]
- 17. Sanyal AJ, Boyer TD, Frederick RT, et al. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT‐0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017;45:1390‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Liu A, Bo W, et al. Terlipressin in the treatment of hepatorenal syndrome: a systematic review and meta‐analysis. Medicine (Baltimore). 2018;97:e0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortega R, Gines P, Uriz J, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941‐948. [DOI] [PubMed] [Google Scholar]
- 20. Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8:e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamil K, Huang X, Lovelace B, et al. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ. 2019;22:421‐429. [DOI] [PubMed] [Google Scholar]
- 22. Gifford FJ, Fallowfield JA. Editorial: tackling hepatorenal syndrome‐terlipressin for all, or time for a stratified approach? Aliment Pharmacol Ther. 2017;46:193‐194. [DOI] [PubMed] [Google Scholar]
- 23. Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864‐871. [DOI] [PubMed] [Google Scholar]
- 24. Pugh RNH, Murray‐Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646‐649. [DOI] [PubMed] [Google Scholar]


