Abstract
Objectives
To review the literature to determine the sensitivity and specificity of gallium‐68 prostate‐specific membrane antigen (68Ga‐PSMA) positron‐emission tomography (PET) for detecting pelvic lymph node metastases in patients with primary prostate cancer (PCa), and the positive predictive value in patients with biochemical recurrence (BCR) after initial curative treatment, and, in addition, to determine the detection rate and management impact of 68Ga‐PSMA PET in patients with BCR after radical prostatectomy (RP).
Materials and Methods
We performed a comprehensive literature search. Search terms used in MEDLINE, EMBASE and Science Direct were ‘(PSMA, 68Ga‐PSMA, 68Gallium‐PSMA, Ga‐68‐PSMA or prostate‐specific membrane antigen)’ and ‘(histology, lymph node, staging, sensitivity, specificity, positive predictive value, recurrence, recurrent or detection)’. Relevant abstracts were reviewed and full‐text articles obtained where possible. References to and from obtained articles were searched to identify further relevant articles.
Results
Nine retrospective and two prospective studies described the sensitivity and specificity of 68Ga‐PSMA PET for detecting pelvic lymph node metastases before initial treatment, which ranged from 33.3% to 100% and 80% to 100%, respectively. In eight retrospective studies, the positive predictive value of 68Ga‐PSMA PET in patients with BCR before salvage lymph node dissection ranged from 70% to 100%. The detection rate of 68Ga‐PSMA PET in patients with BCR after RP in the PSA subgroups <0.2 ng/mL, 0.2–0.49 ng/mL and 0.5 to <1.0 ng/mL ranged from 11.3% to 50.0%, 20.0% to 72.7% and 25.0% to 87.5%, respectively.
Conclusion
The review results showed that 68Ga‐PSMA PET had a high specificity for the detection of pelvic lymph node metastases in primary PCa. Furthermore, 68Ga‐PSMA PET had a very high positive predictive value in detecting lymph node metastases in patients with BCR. By contrast, sensitivity was only moderate; therefore, based on the currently available literature, 68Ga‐PSMA PET cannot yet replace pelvic lymph node dissection to exclude lymph node metastases. In the salvage phase, 68Ga‐PSMA PET had both a high detection rate and impact on radiotherapy planning in early BCR after RP.
Keywords: 68Ga‐PSMA PET/CT, pelvic lymph node dissection, detection rate, pelvic lymph node metastases, #ProstateCancer, #PCSM
Introduction
Prostate cancer (PCa) has the second‐highest incidence of all cancers in men worldwide 1. Disease staging at primary diagnosis and at early biochemical recurrence (BCR) after initial curative treatment is important in determining optimal treatment strategy, and prognosis. Traditionally, CT or MRI is used for nodal staging, and bone scintigraphy (using 99mTc‐labelled bisphosphonates) is used to detect bone metastases; however, all techniques have limited sensitivity for detecting lymph node and/or bone metastases 2. In current practice, therefore, an extended pelvic lymph node dissection (PLND) is performed to accurately stage patients with a risk of > 5% of having nodal metastases 3.
The use of gallium‐68 (68Ga)‐prostate‐specific membrane antigen (PSMA) for positron‐emission tomography (PET) in patients with PCa was first described in 2012 4. PSMA is a type 2 transmembrane glycoprotein that is highly overexpressed in PCa tumours and PCa metastasis. PSMA represents a large extracellular domain which can be targeted by ligands for imaging and treatment purposes 5. Expression of PSMA is increased in more aggressive PCa 6. Since the first report of the use of 68Ga‐PSMA PET in 2012, there has been a growing body of evidence that this technique is superior to other imaging approaches, both in the primary as well as in the recurrence setting 7, 8. 68Ga‐PSMA PET has demonstrated a significantly higher detection rate than choline PET/CT and a high overall impact on management 9. The 2019 European Association of Urology guideline recommends performing a PSMA PET at early BCR (>0.2 ng/mL) after initial treatment if the result will influence subsequent treatment decisions (level of evidence 2b; weak recommendation) 3.
The primary aim of the prsent review was to obtain insight into the sensitivity and specificity of 68Ga‐PSMA PET for the detection of pelvic lymph node metastases in patients with primary PCa. In addition, we aimed to evaluate the positive predictive value of 68Ga‐PMSA PET for the detection of lymph node metastases in patients with BCR after initial curative treatment. The secondary aim was to assess the detection rate of 68Ga‐PSMA PET in patients with BCR after radical prostatectomy (RP), the location of PCa recurrence and the impact of 68Ga‐PSMA PET outcome on the planning of salvage treatment in these patients.
Methods
In May 2019, we performed a comprehensive literature search. Relevant manuscripts were found through searches of Medline, EMBASE and Science Direct databases. To review the sensitivity, specificity and positive predictive value of 68Ga‐PSMA PET, the following search terms were used: ‘(PSMA, 68Ga‐PSMA, 68Gallium‐PSMA, Ga‐68‐PSMA or prostate‐specific membrane antigen)’ in combination with ‘(histology, lymph node, staging, sensitivity, specificity or positive predictive value)’. To review the detection rate of 68Ga‐PSMA PET in patients with BCR after RP the following search terms were used: ‘(PSMA, 68Ga‐PSMA, 68Gallium‐PSMA, Ga‐68‐PSMA or prostate‐specific membrane antigen)’ in combination with ‘(recurrence, recurrent or detection)’. Studies were initially selected based on title, followed by screening on abstract. References to and from obtained articles were searched to identify further relevant articles. Only articles in the English language were included.
For the first section of the present review, we included all studies that determined the diagnostic ability of 68Ga‐PSMA PET for preoperative lymph node staging of PCa, using histopathology as the ‘gold standard’. The sensitivity, specificity and positive predictive value were retrieved from the studies included in our review. The studies were divided into two categories: (1) patients undergoing a 68Ga‐PSMA PET before initial treatment consisting of RP with extended PLND, i.e. the primary diagnosis and (2) patients with BCR after initial treatment undergoing 68Ga‐PSMA PET, followed by salvage PLND, i.e. salvage therapy. We excluded studies that included a mixed group of patients (primary diagnosis and salvage therapy) and did not present the results separately. Studies reporting only the per‐region sensitivity and specificity were also excluded.
For the second section of this review, we included studies that reported the detection rate of the 68Ga‐PSMA PET in patients with BCR after RP and a PSA value < 1.0 ng/mL. The detection rates were subdivided into three PSA categories: 0.01–0.19 ng/mL; 0.20–0.49 ng/mL; and 0.50 to <1.00 ng/mL. Finally, the sites of PCa recurrence were collated when reported in patients with BCR after RP without additional treatment after RP. Therefore, studies describing patients who received radiotherapy and/or androgen deprivation therapy (ADT) before 68Ga‐PSMA PET were excluded.
The results of the included studies are reported in the text as ranges; a meta‐analysis was not performed. The individual reported results are provided in Tables 1–4.
Results
Diagnostic Performance of 68Ga‐PSMA PET for Pelvic Lymph Node Metastases
Eleven studies were included in this review that presented data on the sensitivity and specificity of 68Ga‐PSMA PET for detection of pelvic lymph node metastases in men with newly diagnosed PCa (Table 1, Fig. 1) 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. Two prospective studies, including a total of 63 patients, showed a per‐patient sensitivity (range) and specificity (range) of 64–100% and 90–95%, respectively. The per‐node sensitivity and specificity were 50–58% and 96–100%, respectively 15, 18. Nine retrospective studies, including 696 patients, showed a per‐patient sensitivity and specificity of 33.3–100% and 80–100%. The per‐node sensitivity and specificity was 24.4–96.1% and 98.6–100%, respectively 8, 10, 11, 12, 13, 14, 16, 17, 20.
Table 1.
The per‐patient and per‐node sensitivity and specificity of gallium‐68 prostate‐specific membrane antigen positron‐emission tomography/CT in patients undergoing radical prostatectomy with extended lymph node dissection (primary diagnosis).
| Author (year) | Total number of patients (patients with LNM) |
Number of lymph nodes dissected Mean ± sd Median (IQR) |
Per‐patients sensitivity, % | Per‐patients specificity, % | Per‐node sensitivity | Per‐node specificity |
|---|---|---|---|---|---|---|
| Budaus (2016) 10 | 30 (12) |
20.3 18.5 (13.5–27.5) |
33.3 | 100 | – | – |
| Gupta (2017) 8 | 12 (7) |
20.25 20 |
100 | 80 | 66.67% | 98.61% |
| Maurer (2016) 11 | 130 (41) |
– 21 (12–30) |
65.9 | 98.9 | ||
| Obek (2017) 12 | 51 (15) |
20.2 ± 8.5 18.5 |
53.3 | 86.1 | – | – |
| Thalgott (2018) 13 | 73 (25) |
26.1 ± 16.9 23 (17–29) |
60 | 100 | ||
| Van Leeuwen (2018) 14 | 140 (51) |
– 16 (12–21) |
53 | 88 | – | – |
| Van Leeuwen (2017) 15 | 30 (11) |
17.8 ± 7 16 (12–20) |
64 | 95 | 58% | 100% |
| Yaxley (2019) 16 | 208 (55) |
– 13 |
38.2 | 93.5 | 24.4% | 99.5% |
| Zhang (2017) 17 | 42 (15) |
7.095 – |
93.3 | 96.3 | 96.08% | 99.65% |
| Park (2018)* 18 | 33 (3) |
11.6 – |
100 | 90 | 50% | 98.38% |
| Yilmaz (19) | 10 (2) |
– – |
100 | 100 | – | – |
IQR, interquartile range; LNM, lymph node metastases.
Gallium‐68 prostate‐specific membrane antigen positron‐emission tomography (68Ga‐PSMA PET)/MRI was used instead of 68Ga‐PSMA PET/CT.
Figure 1.
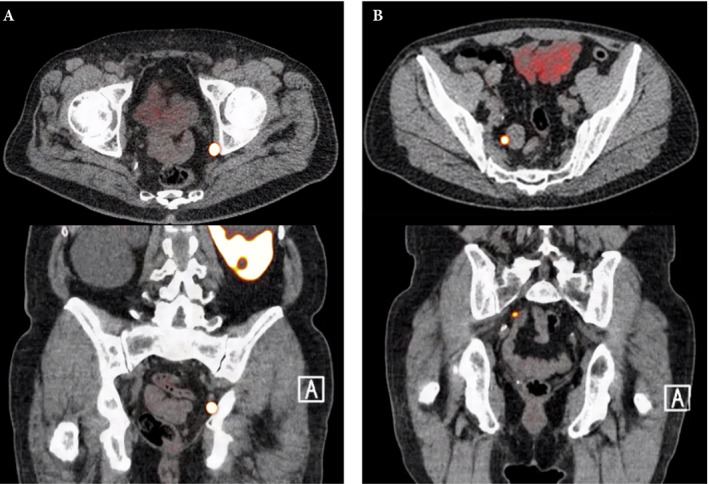
Axial and sagittal plane gallium‐68 prostate‐specific membrane antigen positron‐emission tomography /CT images of two patients with locoregional lymph node recurrence after initial curative treatment. The metastasis in patient A is located in the obturator area and the metastasis in patient B is located in the presacral area.
In this review, eight retrospective studies were included, reporting the positive predictive value of 68Ga‐PSMA PET in detecting pelvic lymph node metastases in patients with BCR after initial curative treatment 21, 22, 23, 24, 25, 26, 27, 28. All patients underwent salvage PLND, and histopathology was used as the gold standard reference. The positive predictive value, among the total of 290 patients included, ranged from 70% to 100% (Table 2) 21, 22, 23, 24, 25, 26, 27, 28.
Table 2.
The positive predictive value of gallium‐68 prostate‐specific membrane antigen positron‐emission tomography/CT in patients undergoing salvage lymph node dissection (salvage phase).
| Author (year) | Total number of patients | Initial treatment (n) |
Number of lymph nodes dissected Mean ± sd Median (IQR) |
PPV per patient, % | PPV per region, % |
|---|---|---|---|---|---|
| Fendler (2019)* 21 | 87 | RP and/or RT | – | 84 | 84 |
| Linxweiler (2018) 22 | 25 | RP (25) |
– 9 (5–14) |
92 | – |
| Mandel (2018)* 23 | 23 | RP (23) |
– 15 |
70 | 87.5 |
| Pfister (2016) 24 | 28 |
RP (23) RT (3) HIFU (2) |
11 – |
82 | 75.7 (per node) |
| Rauscher (2016) 25 | 48 |
RP (45) RT (3) |
– – |
93 | 94.6 |
| Siriwardana (2017) 26 | 35 |
RP (28) Brachytherapy (5) ERBT (1) ERBT + brachytherapy (1) |
– 9 (3–14) |
91 | – |
| Jilg (2017) 27 | 30 |
RP (29) RT (1) |
– 33 (25.5–41.5) |
100% | 98.3 |
| Herlemann (2016) 28 | 14 | RP (14) | – | – | 86 |
EBRT, external beam radiation therapy; HIFU, high‐intensity focused ultrasonography; IQR, interquartile range; PPV, positive predictive value; RP, radical prostatectomy; RT, radiation therapy.
Patients underwent either gallium‐68 prostate‐specific membrane antigen positron‐emission tomography (PET)/CT or PET/MRI.
Detection Rate of 68Ga‐PSMA PET in Patients with Biochemical Recurrence after Radical Prostatectomy
The detection rates of 68Ga‐PSMA PET in men with BCR after RP are shown per PSA subgroup in Table 3. Eight studies reported the detection rate of 68Ga‐PSMA PET in patients with a PSA value <0.2 ng/mL 29, 30, 31, 32, 33, 34, 35, 36. This included 286 patients. Eighty‐six of the 286 patients (30.1%) had a positive 68Ga‐PSMA PET, with the detection rate ranging from 11.3% to 50.0% 29, 30, 31, 32, 33, 34, 35, 36.
Table 3.
Detection rate of gallium‐68 prostate‐specific membrane antigen (PSMA) positron‐emission tomography in different PSMA subgroups.
| Author (year) | Number of patients (percentage positive scans) | Number of patients (percentage positive scans) | Number of patients (percentage positive scans) |
|---|---|---|---|
| PSA 0.50 to <1.00 ng/mL | PSA 0.01–0.19 ng/mL (%) | PSA 0.20–0.49 ng/mL | |
| Kranzbühler (2018) 29 | 9 (44.4) | 11 (72.7) | 8 (87.5) |
| Van Leeuwen (2016) 30 | 35 (40.0) | 28 (71.4) | 7 (57.1) |
| Meredith (2016) 31 | 124 (11.3) | 79 (26.6) | 45 (53.3) |
| Gupta (2017) 32 | 11 (45.5) | 16 (43.8) | 17 (29.4) |
| Schmuck (2017) 33 | 18 (38.9) | 34 (55.9) | 28 (60.7) |
| Emmett (2017) 36 | 64 (50.0) | 81 (67.9) | 24 (79.2) |
| Boreta (2019) 34 | 9 (33.3) | 75 (52.0) | – |
| Yilmaz (2019) 35 | 16 (43.8) | 17 (52.9) | 14 (64.3) |
| Habl (2017)* 45 | 42 (50.0) | 21 (85.7) | |
| Fendler (2019) 21 | – | 136 (38.2) | 79 (57.0) |
| Berliner (2017) 37 | – | 33 (51.5) | 11 (54.5) |
| McCarthy (2019) 38 | – | 63 (50.8) | 21 (76.2) |
| Bashir (2019)* 39 | – | 28 (60.7) | – |
| Farolfi (2019) 40 | – | 119 (34.5) | – |
| Calais (2018) 41 | – | 153 (40.5) | 117 (59.8) |
| Sanli (2017) 42 | – | 10 (20) | 4 (25.0) |
| Eiber (2015) 43 | – | 19 (57.9) | 33 (72.7) |
| Rauscher (2018) 44 | – | 134 (55.2) | 138 (73.9) |
| Total | 286 (30.1) | 1078 (47.0) | 546 (63.9) |
PSA level between 0.01 and 0.5 ng/mL.
Eighteen studies reported the detection rate of 68Ga‐PSMA PET in patients with a PSA 0.20–0.49 ng/mL 21, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45. Combined, this involved 1078 patients. Of the 1078 patients, 507 (47.0%) had a positive 68Ga‐PSMA PET. At this PSA range, the detection rate varied from 20.0% to 72.7% 21, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45.
Fifteen studies described the detection rate of 68Ga‐PSMA PET in patients with a PSA level of 0.50 to <1.0 ng/mL 21, 29, 30, 31, 32, 33, 35, 36, 37, 38, 41, 42, 43, 44, 45. Collectively, these studies included 567 patients. Of these 567 patients, 367 (64.7%) had a positive 68Ga‐PSMA PET. The detection rate ranged from 25% to 87.5% 21, 29, 30, 31, 32, 33, 35, 36, 37, 38, 41, 42, 43, 44, 45.
Location of Prostate Cancer Recurrence Based on the 68Ga‐PSMA PET
In total, three studies reported the anatomical sites of 68Ga‐PSMA‐positive lesions in patients with BCR after RP in the absence of treatment between RP and 68Ga‐PSMA PET.
The location of PCa recurrence in the different PSA groups is shown in Table 4. Emmett et al. 36 and van Leeuwen et al. 30, combined, identified 254 patients with a PSA <1 ng/mL detecting a local recurrence only in 63 patients, pelvic lymph node metastases ± local recurrence in 55 patients, and distant metastases ± local recurrence in 39 patients. Boreta et al. 34 included 125 patients with a PSA <2.0 ng/mL. 68Ga‐PSMA PET detected a local recurrence only in eight patients, pelvic lymph node metastases ± local recurrence in 33 patients, and distant metastases ± local recurrence in 25 patients.
Table 4.
Detection rate and location of recurrence in patients without treatment between radical prostatectomy and gallium‐68 prostate‐specific membrane antigen positron‐emission tomography/CT at different PSA values.
| Emmett (2017) 36 | Van Leeuwen (2016) 30 | Boreta (2019) 34 | |
|---|---|---|---|
| PSA range | 0.01–0.19 | 0.01–0.19 | 0.01–0.19 |
| Total patients (total positive, percentage positive) | 77 (45, 58.4%) | 35 (11, 31.4%) | 9 (3, 33.3%) |
| Recurrence fossa only | 12 | 5 | 0 |
| Pelvic lymph nodes ± fossa | 16 | 4 | 2 |
| Distant metastases ± fossa | 17 | 2 | 1 |
| PSA range | 0.2–0.49 | 0.2–0.49 | 0.20–0.59 |
| Total patients (total positive; % positive) | 82 (56; 68.3) | 28 (18; 64.3) | 80 (39; 48.8) |
| Recurrence fossa only | 22 | 10 | 6 |
| Pelvic lymph nodes ± fossa | 23 | 5 | 22 |
| Distant metastases ± fossa | 12 | 3 | 11 |
| PSA range | 0.50 to <1.0 | 0.5 to <1.0 | 0.60–1.19 |
| Total patients (total positive; % positive) | 25 (19; 76.0) | 7 (4; 57.1) | 24 (13; 54.2) |
| Recurrence fossa only | 9 | 5 | 2 |
| Pelvic lymph nodes ± fossa | 6 | 1 | 4 |
| Distant metastases ± fossa | 4 | 1 | 7 |
Discussion
Recently, the ability of imaging to detect PCa metastases and PCa recurrence at very low PSA values has notably improved after the introduction of the PSMA PET. We conducted this review to determine the sensitivity, specificity and positive predictive value of 68Ga‐PSMA PET in detecting pelvic lymph node metastases. Furthermore, we assessed the detection rates of 68Ga‐PSMA PET and the location of PCa recurrence in patients with early BCR after RP. On the basis of the current literature, we conclude that 68Ga‐PSMA PET has an excellent specificity and positive predictive value for the detection of pelvic lymph node metastases in primary and recurrent PCa, that the detection rate achieved by 68Ga‐PSMA PET in patients with BCR after RP increases in parallel with rises in PSA values, and that the impact of PSMA in these men with recurrent PCa is considerable.
The results of this review highlight the high specificity and positive predictive value of 68Ga‐PSMA PET in detecting pelvic lymph node metastases. Most studies report a per‐patient specificity of 90% or higher 10, 11, 13, 15, 16, 17, 19. This high specificity is a result of the high expression of PSMA in PCa and lymph node metastases, resulting in a high tumour to background ratio 46, 47, 48, 49.
An extended PLND is the gold standard for lymph node staging in PCa, but lymph node metastasis can be missed during extended PLND or misclassified during histological examination. Increased sensitivity of extended PLND can further increase the specificity of 68Ga‐PSMA PET. This is demonstrated by Horn et al. 50, who detected metastatic tissue in 120 of 121 patients with a positive 68Ga‐PSMA PET using PSMA‐targeted radioguided surgery. Owing to the high specificity and positive predictive value of 68Ga‐PSMA PET in detecting pelvic lymph node metastases, it is not always necessary to obtain histology to confirm lymph node metastasis in case of a positive 68Ga‐PSMA PET result.
The specificity of 68Ga‐PSMA PET for detecting local recurrence or bone metastases is unknown because histopathological confirmation is often not obtained; however, the specificity of 68Ga‐PSMA PET in detecting a local recurrence is thought to be high because of the high efficacy of salvage radiotherapy with regard to local recurrence detected by 68Ga‐PSMA PET 36. By increased PSMA expression, fractures and Paget’s disease can mimic bone metastases on 68Ga‐PSMA PET, with high expression in the vasculature of neo‐angiogenesis. The specificity of 68Ga‐PSMA PET for early bone metastases is almost certainly lower because of these benign conditions that also express low levels of PSMA 51, 52, 53, 54.
The specificity and sensitivity of 68Ga‐PSMA PET for detecting intraprostatic lesions at diagnosis is calculated on the basis of whole prostate gland histology. The reported specificity and sensitivity range from 92% to 100% and 60.1% to 67%, respectively 55, 56. A high lesion to normal prostate tissue ratio is seen in lesions with Gleason score ≥7 55. The use of 68Ga‐PSMA PET in the diagnostic pathway of PCa is yet to be determined.
The sensitivity of 68Ga‐PSMA PET for detecting lymph node metastases, in contrast to the specificity, is only moderate. The reported per‐patient sensitivity ranges from 33.3% to 100%. The fact that the 68Ga‐PSMA PET misses lymph node metastases is highlighted by a lower per‐node sensitivity, which is in the range of 24.4–66.67%. Lymph node metastases can be missed because of the lack of PSMA expression in 0–9% of primary prostate tumours and lymph node metastases 57, 58. Moreover, 68Ga‐PSMA PET misses small lymph node metastases 10, 12, 15, 25, 27. In the studies by van Leeuwen et al. 15, Budaus et al. 10 and Obek et al. 12 the 68Ga‐PSMA PET missed all lymph node metastases smaller than 2, 4 and 5 mm, respectively therefore, 68Ga‐PSMA PET cannot yet replace extended PLND to exclude pelvic lymph node metastases.
As extended PLND is used as the gold standard reference, the number of nodes removed influences the sensitivity of 68Ga‐PSMA PET. 68Ga‐PSMA PET results can wrongly be classified as true‐negative if lymph node metastasis is missed during extended PLND. Although there is no consensus regarding the minimum number of lymph nodes that should be removed during extended PLND, studies in which a low number of lymph nodes are removed should be interpreted with caution.
In the salvage phase, the detection rate of 68Ga‐PSMA PET is strongly correlated to the PSA value at the time of the PET 59. The high efficacy of blind salvage radiotherapy on the prostatic fossa at PSA values < 0.5 ng/mL demonstrates that most patients with BCR after RP at this PSA level actually experience a local recurrence 60. In the present review, local recurrence was detected only in 22.1% (49/222) of all men at a PSA < 0.5 ng/mL, suggesting that 68Ga‐PSMA PET misses local recurrences (Table 4). This is confirmed by the high efficacy of salvage radiotherapy (SRT) to the prostatic fossa in patients with a negative 68Ga‐PSMA PET result 36. Failure to detect local recurrence is probably attributable to the inability to distinguish tumour activity from intense activity in the nearby bladder owing to urinary radiotracer excretion and to the fact that less aggressive PCa more often returns locally and less aggressive disease expresses less PSMA 46, 47, 48, 49.
In patients with BCR after RP 68Ga‐PSMA PET has been found to have a high management impact and detection rate 61; however, the recommendation to perform 68Ga‐PSMA PET after RP to guide salvage treatment is only weak 3. Although all patients in the different studies included in the present review underwent RP as primary therapy, not all treatments between RP and 68Ga‐PSMA PET were similar. Additional treatments after RP influence the outcome and detection rate of the 68Ga‐PSMA PET. First, ADT at the time of the PET influences the chance of having a positive 68Ga‐PSMA PET. In the nomogram presented by Rauscher et al. 44 for patients with BCR after RP, all patients with a PSA value >0.2 ng/mL and on ADT at time of the PET have a ~90% chance of having a positive PET. The study by Emmett et al. 62 showed that the effect of androgen blockade depends on the patient’s hormonal sensitivity, and impacts the scan results within 9 days of commencing treatment. In patients with hormone‐sensitive metastatic PCa, androgen blockage reduces the PSMA intensity. In contrast, androgen blockage in patients with castrate‐resistant PCa significantly increases PSMA intensity and the number of lesions detected 62. There is a clear effect, therefore, of ADT on the performance of 68Ga‐PMSA PET, which needs further evaluation. Second, patients who underwent SRT after RP, but before 68Ga‐PSMA PET, have a decreased risk of having local recurrence detected on 68Ga‐PSMA PET. In most papers reporting the detection rate of 68Ga‐PSMA PET that are included in this review, both patients with or without ADT at time of or prior to the PSMA PET were included. Moreover, a selection of patients underwent radiotherapy to the prostatic fossa between RP and 68Ga‐PSMA PET. This should be considered when interpreting the results.
Salvage radiotherapy is the last curative treatment possibility for patients with BCR after RP, and is most effective at low PSA values 60; however, SRT is unsuccessful if PCa has recurred outside the prostatic fossa. Only three studies describe a patient cohort without additional treatment between RP and 68Ga‐PSMA PET to determine the effect of 68Ga‐PSMA PET outcome on SRT planning 30, 34, 36. By detecting PCa recurrence outside the prostatic fossa, the outcome of 68Ga‐PSMA PET changed SRT planning in 34.7% (42/121) and 39.1% (43/110) patients at PSA values <0.2 and 0.2–0.49 ng/mL, respectively. In the study by Boreta et al. 34 68Ga‐PSMA PET changed SRT planning in 41.3% (33/80) of all patients with a PSA value of 0.2–0.59 ng/mL. Therefore, the use of 68Ga‐PSMA PET to prevent unsuccessful SRT seems indicated in patients considered for SRT, even at PSA values < 0.2 ng/mL.
The optimal time to perform 68Ga‐PSMA PET is unclear, but one conclusion can be drawn from the included studies, namely, 68Ga‐PSMA PET has poor sensitivity for local recurrences, especially at low PSA values. 68Ga‐PSMA PET should therefore be used to exclude metastases rather than to detect a local recurrence. In patients considered for SRT, the 68Ga PSMA PET should be obtained at a PSA value where the detection rate is considered high enough to exclude metastases and a negative 68Ga PSMA PET/CT triggers SRT.
The present review has some limitations. The first is inherent in its design. We did not perform a systematic review and meta‐analysis as not all data were available; therefore, we were only able to show the reported results of the different studies. The second limitation is the lack of prospective and randomized controlled trials included. Most studies included were retrospective in design and therefore have a possible selection bias.
A strength of this review is the strict inclusion criteria of articles included. This enabled adequate assessment of the specificity and sensitivity of 68Ga‐PSMA PET in detecting lymph node metastases. Moreover, by excluding papers describing the location of PCa recurrence after RP and additional treatments, we presented a well‐defined selected group of patients considered for SRT.
Future studies should focus on strategies to improve the sensitivity of 68Ga‐PSMA PET. To do so, different strategies could be considered. First, the stimulation of upregulation of PSMA in PCa cells might improve the sensitivity. In vitro models showed an upregulation of the PSMA surface levels in PCa cells by enzalutamide and dutasteride, although the effect of androgen blockage on the sensitivity of 68Ga‐PSMA PET seems to depend on the phenotype of the PCa 62, 63. More research is needed to evaluate the exact effect of androgen blockade on the 68Ga‐PSMA PET imaging. Second, next to the expression of the PSMA receptor, the detection of small lesions by PET scanners depends both on technical and tracer characteristics. Improvement of spatial resolution of PET systems and usage of tracers with higher positron yield and shorter positron range, such as 18F, might improve the detection of small lesions 64, 65. In eight patients who underwent 18F‐PSMA‐1007 PET/CT and subsequent RP with extended PLND, the sensitivity and specificity were 94.7% and 100%, respectively 66. Moreover, decreased urinary clearance of 18F‐PSMA‐1007 might reduce activity of radiotracer in the bladder and hence improve detection of local recurrence 66. In patients with BCR after RP, the detection rate of 18F‐PSMA‐1007 PET/CT was 61.5% (40/65) and 74.5% (35/47) for patients with PSA values of 0.2–0.49 ng/mL and 0.5–0.99 ng/mL, respectively 67. The reported detection rates of 18F‐DCFPyL PET/CT in the PSA range of 0.2–0.49 ng/mL and 0.5–0.99 are 59% (17/29) and 69% (20/29), respectively 68. In a recent matched‐pair comparison, the accuracy of 68Ga‐PSMA PET and 18F‐PSMA‐1007 PET was determined in patients with BCR after RP. Diagnostic performance was similar, however, 18F‐PSMA‐1007 PET showed positive lesions that were benign in origin (e.g. ganglia) five times more often. Readers of 18F‐PSMA PET should be aware of these potential pitfalls. In addition, future studies should focus on determining the optimal time to perform PSMA PET and the effectiveness of SRT after negative PSMA PET at different PSA values. Lastly, studies with long‐term follow‐up are needed to determine the role of PSMA‐guided salvage therapies in clinical practice.
In conclusion, 68Ga‐PSMA PET has a very high specificity and positive predictive value in detecting pelvic lymph node metastases, therefore, histological confirmation is not always necessary if 68Ga‐PSMA PET detects pelvic lymph node metastasis. In contrast, the sensitivity of the 68Ga‐PSMA PET in detecting pelvic lymph node metastases is only moderate and small lymph node metastases can be missed. 68Ga‐PSMA PET, therefore, cannot yet replace extended PLND to exclude pelvic lymph node metastases. Based on the available data, 68Ga‐PSMA PET seems to have a high management impact in patients considered for SRT at (early) BCR after RP. Future studies should focus on improving the sensitivity of 68Ga‐PSMA PET, and prospective studies focusing on long‐term outcomes of PSMA‐guided therapies are required.
Conflict of Interest
The authors declare to have no conflict of interest.
Abbreviations
- 68Ga‐PSMA
gallium‐68 prostate‐specific membrane antigen
- PSMA
prostate‐specific membrane antigen
- PET
positron‐emission tomography
- PCa
prostate cancer
- BCR
biochemical recurrence
- RP
radical prostatectomy
- PLND
pelvic lymph node dissection
- ADT
androgen deprivation therapy
- SRT
salvage radiotherapy
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424 [DOI] [PubMed] [Google Scholar]
- 2. Hövels AM, Heesakkers RA, Adang EM et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta‐analysis. Clin Radiol 2008; 63: 387–95 [DOI] [PubMed] [Google Scholar]
- 3. EAU Guidelines . Edn. Presented at the EAU Annual Congress Barcelona 2019. ISBN 978‐94‐92671‐04‐2.
- 4. Afshar‐Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium‐labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F‐FECH. Eur J Nucl Med Mol Imaging 2012; 39: 1085–6 [DOI] [PubMed] [Google Scholar]
- 5. Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate‐specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52: 637–40 [DOI] [PubMed] [Google Scholar]
- 6. Schwarzenboeck SM, Rauscher I, Bluemel C et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med 2017; 58: 1545–52 [DOI] [PubMed] [Google Scholar]
- 7. De Visschere PJL, Standaert C, Futterer JJ et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol 2019; 2: 47–76 [DOI] [PubMed] [Google Scholar]
- 8. Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of (68)Gallium‐prostate specific membrane antigen positron emission tomography‐computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med 2017; 16: 186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morigi JJ, Stricker PD, van Leeuwen PJ et al. Prospective comparison of 18F‐fluoromethylcholine versus 68Ga‐PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015; 56: 1185–90 [DOI] [PubMed] [Google Scholar]
- 10. Budaus L, Leyh‐Bannurah SR, Salomon G et al. Initial experience of (68)Ga‐PSMA PET/CT imaging in high‐risk prostate cancer patients prior to radical prostatectomy. Eur Urol 2016; 69: 393–6 [DOI] [PubMed] [Google Scholar]
- 11. Maurer T, Gschwend JE, Rauscher I et al. Diagnostic efficacy of (68)Gallium‐PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436–43 [DOI] [PubMed] [Google Scholar]
- 12. Obek C, Doganca T, Demirci E et al. The accuracy of (68)Ga‐PSMA PET/CT in primary lymph node staging in high‐risk prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 1806–12 [DOI] [PubMed] [Google Scholar]
- 13. Thalgott M, Duwel C, Rauscher I et al. One‐stop‐shop whole‐body (68)Ga‐PSMA‐11 PET/MRI compared with clinical nomograms for preoperative T and N staging of high‐risk prostate cancer. J Nucl Med 2018; 59: 1850–6 [DOI] [PubMed] [Google Scholar]
- 14. van Leeuwen PJ, Donswijk M, Nandurkar R et al. Gallium‐68‐prostate‐specific membrane antigen (68Ga‐PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate‐ and high‐risk prostate cancer. BJU Int 2019; 24: 62–8 [DOI] [PubMed] [Google Scholar]
- 15. van Leeuwen PJ, Emmett L, Ho B et al. Prospective evaluation of 68Gallium‐prostate‐specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int 2017; 119: 209–15 [DOI] [PubMed] [Google Scholar]
- 16. Yaxley JW, Raveenthiran S, Nouhaud FX et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga‐PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology: can preoperative 68Ga‐PSMA positron emission tomography/computerized tomography replace pelvic lymph node dissection for prostate cancer staging? J Urol 2019; 201: 815–20 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Q, Zang S, Zhang C et al. Comparison of (68)Ga‐PSMA‐11 PET‐CT with mpMRI for preoperative lymph node staging in patients with intermediate to high‐risk prostate cancer. J Transl Med 2017; 15: 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park SY, Zacharias C, Harrison C et al. Gallium 68 PSMA‐11 PET/MR imaging in patients with intermediate‐ or high‐risk prostate cancer. Radiology 2018; 288: 495–505 [DOI] [PubMed] [Google Scholar]
- 19. Yilmaz B, Turkay R, Colakoglu Y et al. Comparison of preoperative locoregional Ga‐68 PSMA‐11 PET‐CT and mp‐MRI results with postoperative histopathology of prostate cancer. Prostate 2019; 79: 1007–17 [DOI] [PubMed] [Google Scholar]
- 20. Tulsyan S, Das CJ, Tripathi M, Seth A, Kumar R, Bal C. Comparison of 68Ga‐PSMA PET/CT and multiparametric MRI for staging of high‐risk prostate cancer68Ga‐PSMA PET and MRI in prostate cancer. Nucl Med Commun 2017; 38: 1094–102 [DOI] [PubMed] [Google Scholar]
- 21. Fendler WP, Calais J, Eiber M et al. Assessment of 68Ga‐PSMA‐11 PET accuracy in localizing recurrent prostate cancer: a prospective single‐arm clinical trial. JAMA Oncol 2019; 5: 856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linxweiler J, Saar M, Al‐Kailani Z et al. Robotic salvage lymph node dissection for nodal‐only recurrences after radical prostatectomy: perioperative and early oncological outcomes. Surg Oncol 2018; 27: 138–45 [DOI] [PubMed] [Google Scholar]
- 23. Mandel P, Tilki D, Chun FK et al. Accuracy of (68)Ga‐prostate‐specific membrane antigen positron emission tomography for the detection of lymph node metastases before salvage lymphadenectomy. Eur Urol Focus 2018; 10.1016/j.euf.2018.07.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. Pfister D, Porres D, Heidenreich A et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga‐PSMA‐HBED‐CC than with (18)F‐Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging 2016; 43: 1410–7 [DOI] [PubMed] [Google Scholar]
- 25. Rauscher I, Maurer T, Beer AJ et al. Value of 68Ga‐PSMA HBED‐CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med 2016; 57: 1713–9 [DOI] [PubMed] [Google Scholar]
- 26. Siriwardana A, Thompson J, van Leeuwen PJ et al. Initial multicentre experience of (68) gallium‐PSMA PET/CT guided robot‐assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int 2017; 120: 673–81 [DOI] [PubMed] [Google Scholar]
- 27. Jilg CA, Drendel V, Rischke HC et al. Diagnostic accuracy of Ga‐68‐HBED‐CC‐PSMA‐ligand‐PET/CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics 2017; 7: 1770–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herlemann A, Wenter V, Kretschmer A et al. (68)Ga‐PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol 2016; 70: 553–7 [DOI] [PubMed] [Google Scholar]
- 29. Kranzbühler B, Nagel H, Becker AS et al. Clinical performance of (68)Ga‐PSMA‐11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging 2018; 45: 20–30 [DOI] [PubMed] [Google Scholar]
- 30. van Leeuwen PJ, Stricker P, Hruby G et al. (68) Ga‐PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int 2016; 117: 732–9 [DOI] [PubMed] [Google Scholar]
- 31. Meredith G, Wong D, Yaxley J et al. The use of (68) Ga‐PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int 2016; 118 (Suppl. 3): 49–55 [DOI] [PubMed] [Google Scholar]
- 32. Gupta SK, Watson T, Denham J et al. Prostate‐specific membrane antigen positron emission tomography‐computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys 2017; 99: 701–9 [DOI] [PubMed] [Google Scholar]
- 33. Schmuck S, Nordlohne S, von Klot CA et al. Comparison of standard and delayed imaging to improve the detection rate of [(68)Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate‐specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 960–8 [DOI] [PubMed] [Google Scholar]
- 34. Boreta L, Gadzinski AJ, Wu SY et al. Location of recurrence by Gallium‐68 PSMA‐11 PET scan in prostate cancer patients eligible for salvage radiotherapy. Urology 2019; 129: 165–71 [DOI] [PubMed] [Google Scholar]
- 35. Yilmaz U, Komek H, Can C, Altindag S. The role of ((68)Ga)PSMA I&T in biochemical recurrence after radical prostatectomy: detection rate and the correlation between the level of PSA, Gleason score, and the SUVmax. Ann Nucl Med 2019; 33: 545–53 [DOI] [PubMed] [Google Scholar]
- 36. Emmett L, van Leeuwen PJ, Nandurkar R et al. Treatment outcomes from (68)Ga‐PSMA PET/CT‐informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med 2017; 58: 1972–6 [DOI] [PubMed] [Google Scholar]
- 37. Berliner C, Tienken M, Frenzel T et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [(68)Ga]PSMA I&T and comparison with published data of [(68)Ga]PSMA HBED‐CC. Eur J Nucl Med Mol Imaging 2017; 44: 670–7 [DOI] [PubMed] [Google Scholar]
- 38. McCarthy M, Francis R, Tang C, Watts J, Campbell A. A multicentre prospective clinical trial of (68)Gallium PSMA HBED‐CC PET‐CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution, compared to standard imaging. Int J Radiat Oncol Biol Phys 2019; 104: 801–08 [DOI] [PubMed] [Google Scholar]
- 39. Bashir U, Tree A, Mayer E et al. Impact of Ga‐68‐PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur J Nucl Med Mol Imaging 2019; 46: 901–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farolfi A, Ceci F, Castellucci P et al. (68)Ga‐PSMA‐11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging 2019; 46: 11–9 [DOI] [PubMed] [Google Scholar]
- 41. Calais J, Czernin J, Cao M et al. (68)Ga‐PSMA‐11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med 2018; 59: 230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanli Y, Kuyumcu S, Sanli O et al. Relationships between serum PSA levels, Gleason scores and results of 68Ga‐PSMAPET/CT in patients with recurrent prostate cancer. Ann Nucl Med 2017; 31: 709–17 [DOI] [PubMed] [Google Scholar]
- 43. Eiber M, Maurer T, Souvatzoglou M et al. Evaluation of hybrid 68Ga‐PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015; 56: 668–74 [DOI] [PubMed] [Google Scholar]
- 44. Rauscher I, Duwel C, Haller B et al. Efficacy, predictive factors, and prediction nomograms for (68)Ga‐labeled prostate‐specific membrane antigen‐ligand positron‐emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol 2018; 73: 656–61 [DOI] [PubMed] [Google Scholar]
- 45. Habl G, Sauter K, Schiller K et al. (68) Ga‐PSMA‐PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017; 77: 920–7 [DOI] [PubMed] [Google Scholar]
- 46. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 1998; 82: 2256–61 [DOI] [PubMed] [Google Scholar]
- 47. Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of [(68)Ga]PSMA‐HBED‐CC in patients with prostate cancer: characterization of uptake in normal organs and tumour lesions. Mol Imaging Biol 2016; 18: 428–36 [DOI] [PubMed] [Google Scholar]
- 48. Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin‐releasing peptide receptor, the prostate stem cell antigen and the prostate‐specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate 2009; 69: 1101–8 [DOI] [PubMed] [Google Scholar]
- 49. Woythal N, Arsenic R, Kempkensteffen C et al. Immunohistochemical validation of PSMA expression measured by (68)Ga‐PSMA PET/CT in primary prostate cancer. J Nucl Med 2018; 59: 238–43 [DOI] [PubMed] [Google Scholar]
- 50. Horn T, Kronke M, Rauscher I et al. Single lesion on prostate‐specific membrane antigen‐ligand positron emission tomography and low prostate‐specific antigen are prognostic factors for a favorable biochemical response to prostate‐specific membrane antigen‐targeted radioguided surgery in recurrent prostate cancer. Eur Urol 2019; 76: 517–23 [DOI] [PubMed] [Google Scholar]
- 51. Hoberuck S, Michler E, Kaiser D, Rohnert A, Zophel K, Kotzerke J. Prostate‐specific membrane antigen expression in distal radius fracture. Clin Nucl Med 2018; 43: 611–3 [DOI] [PubMed] [Google Scholar]
- 52. Jochumsen MR, Dias AH, Bouchelouche K. Benign traumatic rib fracture: a potential pitfall on 68Ga‐prostate‐specific membrane antigen PET/CT for prostate cancer. Clin Nucl Med 2018; 43: 38–40 [DOI] [PubMed] [Google Scholar]
- 53. Panagiotidis E, Paschali A, Giannoula E, Chatzipavlidou V. Rib fractures mimicking bone metastases in 18F‐PSMA‐1007 PET/CT for prostate cancer. Clin Nucl Med 2019; 44: e46–8 [DOI] [PubMed] [Google Scholar]
- 54. Rowe SP, Deville C, Paller C et al. Uptake of (18)F‐DCFPyL in Paget's disease of bone, an important potential pitfall in clinical interpretation of PSMA PET studies. Tomography 2015; 1: 81–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fendler WP, Schmidt DF, Wenter V et al. 68Ga‐PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med 2016; 57: 1720–5 [DOI] [PubMed] [Google Scholar]
- 56. Donato P, Roberts MJ, Morton A et al. Improved specificity with (68)Ga PSMA PET/CT to detect clinically significant lesions "invisible" on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging 2019; 46: 20–30 [DOI] [PubMed] [Google Scholar]
- 57. Chakraborty PS, Tripathi M, Agarwal KK, Kumar R, Vijay MK, Bal C. Metastatic poorly differentiated prostatic carcinoma with neuroendocrine differentiation: negative on 68Ga‐PSMA PET/CT. Clin Nucl Med 2015; 40: e163–6 [DOI] [PubMed] [Google Scholar]
- 58. Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ. 68Ga‐PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging 2017; 10.1111/cpf.12480. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59. Perera M, Papa N, Roberts M et al. Gallium‐68 prostate‐specific membrane antigen positron emission tomography in advanced prostate cancer‐updated diagnostic utility, sensitivity, specificity, and distribution of prostate‐specific membrane antigen‐avid lesions: a systematic review and meta‐analysis. Eur Urol 2019; 10.1016/j.eururo.2019.01.049. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60. Tendulkar RD, Agrawal S, Gao T et al. Contemporary update of a multi‐institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 2016; 34: 3648–54 [DOI] [PubMed] [Google Scholar]
- 61. Han S, Woo S, Kim YJ, Suh CH. Impact of (68)Ga‐PSMA PET on the management of patients with prostate cancer: a systematic review and meta‐analysis. Eur Urol 2018; 74: 179–90 [DOI] [PubMed] [Google Scholar]
- 62. Emmett LM, Yin C, Crumbaker M et al. Rapid modulation of PSMA expression by androgen deprivation: serial (68)Ga PSMA‐11 PET in men with hormone sensitive and castrate resistant prostate cancer commencing androgen blockade. J Nucl Med 2019; 60: 950–4 [DOI] [PubMed] [Google Scholar]
- 63. Kranzbühler B, Salemi S, Umbricht CA et al. Pharmacological upregulation of prostate‐specific membrane antigen (PSMA) expression in prostate cancer cells. Prostate 2018; 78: 758–65 [DOI] [PubMed] [Google Scholar]
- 64. Omami G, Tamimi D, Branstetter BF. Basic principles and applications of (18)F‐FDG‐PET/CT in oral and maxillofacial imaging: a pictorial essay. Imaging Sci Dent 2014; 44: 325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sanchez‐Crespo A. Comparison of Gallium‐68 and Fluorine‐18 imaging characteristics in positron emission tomography. Appl Radiat Isot 2013; 76: 55–62 [DOI] [PubMed] [Google Scholar]
- 66. Giesel FL, Hadaschik B, Cardinale J et al. F‐18 labelled PSMA‐1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging 2017; 44: 678–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giesel FL, Knorr K, Spohn F et al. Detection efficacy of (18)F‐PSMA‐1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med 2019; 60: 362–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wondergem M, Jansen BHE, van der Zant FM et al. Early lesion detection with (18)F‐DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2019; 46: 1911–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


