Abstract
Global climate change is expected to further raise the frequency and severity of extreme events, such as droughts. The effects of extreme droughts on trees are difficult to disentangle given the inherent complexity of drought events (frequency, severity, duration, and timing during the growing season). Besides, drought effects might be modulated by trees’ phenotypic variability, which is, in turn, affected by long‐term local selective pressures and management legacies. Here we investigated the magnitude and the temporal changes of tree‐level resilience (i.e., resistance, recovery, and resilience) to extreme droughts. Moreover, we assessed the tree‐, site‐, and drought‐related factors and their interactions driving the tree‐level resilience to extreme droughts. We used a tree‐ring network of the widely distributed Scots pine (Pinus sylvestris) along a 2,800 km latitudinal gradient from southern Spain to northern Germany. We found that the resilience to extreme drought decreased in mid‐elevation and low productivity sites from 1980–1999 to 2000–2011 likely due to more frequent and severe droughts in the later period. Our study showed that the impact of drought on tree‐level resilience was not dependent on its latitudinal location, but rather on the type of sites trees were growing at and on their growth performances (i.e., magnitude and variability of growth) during the predrought period. We found significant interactive effects between drought duration and tree growth prior to drought, suggesting that Scots pine trees with higher magnitude and variability of growth in the long term are more vulnerable to long and severe droughts. Moreover, our results indicate that Scots pine trees that experienced more frequent droughts over the long‐term were less resistant to extreme droughts. We, therefore, conclude that the physiological resilience to extreme droughts might be constrained by their growth prior to drought, and that more frequent and longer drought periods may overstrain their potential for acclimation.
Keywords: acclimation, latitudinal gradient, Pinus sylvestris, predisposition, tree rings
We examined tree growth resilience of Scots pine along a 2,800 km latitudinal gradient from southern Spain to north‐eastern Germany using 615 adult trees from 30 different sites. We found that the resilience of Scots pine to extreme drought decreased in mid‐elevation and low productivity sites from 1980–1999 to 2000–2011 due to more frequent and severe droughts in the later period. We showed that the impact of drought on tree‐level resilience was not dependent on its latitudinal location, but rather on the type of sites trees were growing at and on their growth performances during the pre‐drought period.

1. INTRODUCTION
Climate change effects are broadly characterized by elevated temperature, changed precipitation regimes, and increased interannual variability, often resulting in more frequent and intense climate extremes such as severe droughts (Dai, 2012; Spinoni, Vogt, Naumann, Barbosa, & Dosio, 2018). The increased frequency and severity of droughts can significantly impact tree growth by reducing their photosynthetic activity (Flexas & Medrano, 2002; Reddy, Chaitanya, & Vivekanandan, 2004) and altering their cambial activity (Gruber, Strobl, Veit, & Oberhuber, 2010). In addition, severe drought events have been associated to forest decline either through direct abiotic effects leading to hydraulic failure and/or carbon starvation (Adams et al., 2017; Choat et al., 2018; McDowell et al., 2008) or mediated by biotic factors, such as insects (Rouault et al., 2006), fungi (Giordano, Gonthier, Varese, Miserere, & Nicolotti, 2009), and mistletoes (Rigling, Eilmann, Koechli, & Dobbertin, 2010). These effects may ultimately induce shifts in forest composition (Buras & Menzel, 2019; Walther et al., 2002) and reduction in forest productivity (Ciais et al., 2005).
Growing recognition of the impacts of extreme droughts on forest ecosystems has spurred on a number of long‐term experiments and observational studies (e.g., Breshears et al., 2005; Jentsch et al., 2011; Seidel, Matiu, & Menzel, 2019). The results of these studies revealed a large variability in pattern and magnitude of responses to extreme droughts (McDowell et al., 2008; Smith, 2011), because phenotypic acclimation to such extreme events may depend on a multitude of factors and their interactions, including drought characteristics (Anderegg et al., 2015; Gazol et al., 2018), drought history of the growing environment (Vicente‐Serrano et al., 2013), species‐specific functional traits and life‐history strategies (Anderegg et al., 2016; Greenwood et al., 2017; Lévesque et al., 2013), provenance (Sánchez‐Salguero et al., 2018; Seidel, Schunk, Matiu, & Menzel, 2016), tree size and age (Granda, Gazol, & Camarero, 2018; Magnani, Mencuccini, & Grace, 2000; Serra‐Maluquer, Mencuccini, & Martínez‐Vilalta, 2018), tree‐to‐tree competition (Linares, Camarero, & Carreira, 2010), nutrient imbalances (Hevia et al., 2019), nutrient availability (Gessler, Schaub, & McDowell, 2017), species composition and stocking of the forest stand (Bottero et al., 2017; Forrester et al., 2016; Grossiord et al., 2014), trees’ neighbourhood composition (Grossiord, 2019), microclimatic conditions related to forest edge and interior (Buras et al., 2018), and growth trends prior to drought (Zang, Hartl‐Meier, Dittmar, Rothe, & Menzel, 2014). In the longer term, acclimation is often complemented by evolutionary genotypic adaptation (Bose et al., 2020; Hamrick, 2004; Sánchez‐Salguero et al., 2018) leading to differentiation of populations and ecotypes with varying adaptive capacities to drought, often observed for marginal populations at dry species range margins (Bolte et al., 2016; Hampe & Petit, 2005).
Moreover, the effects of past drought and growing conditions (legacy effects), can remain for several years and modify the tree growth and physiological responses to the current drought (Anderegg et al., 2015; Kannenberg et al., 2019; Seidel et al., 2019). An important question in the debate on drought and acclimation is whether individuals will be able to acclimate fast enough to cope with increased frequency and severity of droughts (Dai, 2012; Szejner, Belmecheri, Ehleringer, & Monson, 2020). It is therefore important to understand how tree growth responses to extreme droughts vary across sites with different productivity (Valladares, Gianoli, & Gómez, 2007; Valladares et al., 2014), since site productivity can modify trees’ phenotypic strategies such as tree height, root to shoot ratio, and crown development for efficient conservation and utilization of water (Vanninen & Mäkelä, 2005). For example, tree height which is commonly used as an indicator of site productivity (e.g., Westoby, Falster, Moles, Vesk, & Wright, 2002) was reported to be the strongest predictor of tree mortality in southwestern United States where 1.8 million trees were studied (Stovall, Shugart, & Yang, 2019).
Several recent studies conducted in southern and central Europe have reported drought‐induced dieback of Scots pine (Buras et al., 2018; Camarero, Gazol, Sangüesa‐Barreda, Oliva, & Vicente‐Serrano, 2015; Etzold et al., 2019; Galiano, Martínez‐Vilalta, & Lloret, 2010; Hereş, Martínez‐Vilalta, & Claramunt López, 2012; Sánchez‐Salguero, Navarro‐Cerrillo, Camarero, & Fernández‐Cancio, 2012) causing a shift toward the dominance of oak (Quercus spp.) species (Carnicer et al., 2014; Galiano et al., 2010; Rigling et al., 2013). Although the impact of various tree‐ and site‐level factors on tree growth during drought has been studied from local to global scales (e.g., Anderegg et al., 2015; Buras et al., 2018; Gazol et al., 2018; Zang et al., 2014), their interactive effects are still not clearly understood (Maes et al., 2019). For example, some large‐scale studies found a low to moderate influence of drought severity on tree growth response (e.g., Gazol, Camarero, Anderegg, & Vicente‐Serrano, 2017; Sánchez‐Salguero et al., 2018), possibly because they did not consider interactive effects between drought characteristics and long‐term tree growth performances. In addition, large‐scale studies often characterize drought according to a predefined meteorological season (e.g., drought in spring–summer) irrespective of local site conditions, soil moisture content, and geographic location (e.g., Bottero et al., 2017; Gao et al., 2018; Gazol et al., 2018). As a consequence, site‐specific climate‐growth signals might be overlooked if a particular studied season is not the most relevant period for tree radial growth (Pasho, Camarero, de Luis, & Vicente‐Serrano, 2011; Sánchez‐Salguero et al., 2015).
Here we combined Scots pine tree‐ring width data from 30 sites into a network to determine how growth responses to extreme drought varied along a latitudinal gradient across Europe stretching from southern Spain to northern Germany. Tree growth response was assessed to retrospectively quantify short‐ and long‐term drought effects on growth for numerous individuals, sites, and species at annual resolution. Tree growth resilience was defined as the capacity of a tree to reach growth rates similar to those prior to a given drought event. Thus, resilience encompasses the capacity to buffer the impact of a disturbance (resistance), as well as the ability to return to predisturbance growth levels (recovery; Lloret, Keeling, & Sala, 2011). Specifically, we asked four research questions: (a) How does the impact of the climatic water balance (CWB; i.e., precipitation minus potential evapotranspiration) of different seasons on tree growth vary along a latitudinal gradient? (b) How do radial growth rates of Scots pine during drought and nondrought years vary across sites? (c) Has tree growth resilience to extreme drought changed over the past decades due to an increased frequency and severity of droughts (Serra‐Maluquer et al., 2018; Szejner et al., 2020)? (d) How do drought characteristics, site conditions, and tree growth‐related variables modulate the tree growth resilience to extreme drought events? For this last research question, we considered a list of biological hypotheses based on a literature review (see Table S1: e.g., Gazol et al., 2017, 2018; Sánchez‐Salguero et al., 2018; Vitali, Büntgen, & Bauhus, 2017; Zang et al., 2014).
2. MATERIALS AND METHODS
2.1. Study sites and tree‐ring data
We compiled tree‐ring width data of Scots pine from 30 sites (Table S2) along an approximately 2,800 km long latitudinal gradient from southern Spain (Baza; 37.2°N, 4.0°W) to north‐eastern Germany (Torgelow; 53.6°N, 14°E; Figure 1). To avoid age‐related growth effects only trees older than 30 years at the time of examined drought were selected, resulting in 615 adult Scots pine trees (6–60 trees per site). From each tree, two to four tree ring width series were included, measured from increment cores extracted at breast height (1.3 m height) and cross‐dated following standard dendrochronological procedures (Grissino‐Mayer, 2001).
FIGURE 1.
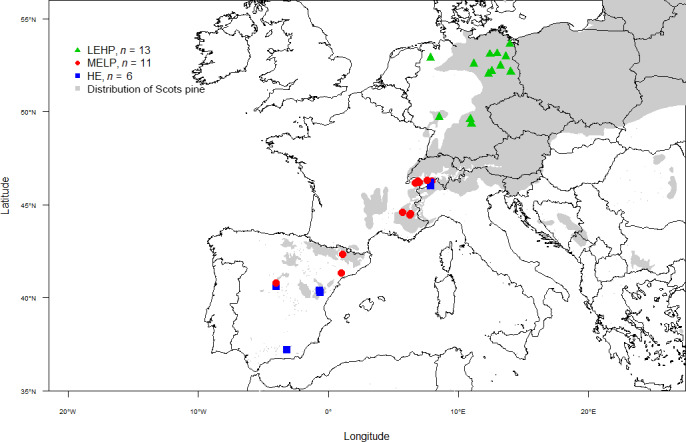
Location of the 30 Scots pine study sites distributed along a latitudinal gradient that ranged from southern Spain to northern Germany. HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity. The grey shade used as a background within the map represents the natural distribution of Scots pine adapted from Mátyás, Ackzell, & Samuel (2004)
Considering the large differences in productivity among study sites along this long gradient, the sites were grouped using a hierarchical cluster analysis (Kaufman & Rousseeuw, 1990). The classification was based on site productivity index (i.e., dominant tree height at 50 years of stand age) and site elevation. Dominant tree height has been commonly used as an indicator of site productivity (e.g., Bugmann, 1996; Diéguez‐Aranda, Burkhart, & Rodríguez‐Soalleiro, 2005; Westoby et al., 2002) including Scots pine sites (e.g., Diéguez‐Aranda, Álvarez González, Marcos Barrio, & Alberto Rojo, 2005; Hökkä & Ojansuu, 2004; Mäkinen, Yue, & Kohnle, 2017; Palahí, Tomé, Pukkala, Trasobares, & Montero, 2004). The hierarchical clustering was done using the hclust function and ward.D method in R (R Development Core Team, 2018). Based on the hierarchical cluster analysis (Figure S1), three groups (i.e., site types) were characterized as (a) high‐elevation sites (1,865–2,011 m a.s.l.) with low productivity (6.0–14.0 m in stand dominant tree height) referred as “HELP”, (b) mid‐elevation sites (600–1,450 m a.s.l.) with low productivity (7.5–11.0 m in stand dominant tree height) referred as “MELP,” and (c) low‐elevation sites (33–326 m a.s.l.) with high productivity (15–23.7 m in stand dominant tree height) referred as “LEHP” (Figure S1).
2.2. Analytical approaches
Addressing our four research questions, the analytical approach involved two steps: data preparation and data analysis. The data preparation step embodied four substeps, (a) quantification of tree‐ring width indices; (b) quantification of drought indices; (c) identification of drought, predrought, and postdrought periods (i.e., years); and (d) quantification of tree growth resilience indices. The data analysis step embodied four steps, that is, one for each research question.
2.3. Quantification of tree‐ring width indices
We aimed at quantifying growth responses to extreme drought events over the recent 50 years period roughly from year 1960 to year 2011. However, our studied trees largely differed in age across sites (Table S2). Hence, ring width data were transformed into dimensionless ring width indices (RWI) with both age‐related growth trends and lower frequency variation removed from the time series (Cook & Kairiukstis, 1990). For this, ring width data were detrended by fitting a negative exponential curve or using a 30 year cubic spline with a 50% frequency cutoff (Cook & Kairiukstis, 1990). In addition to these detrending methods, we also converted the raw ring width data into basal area increment (cm2 per year; Biondi & Quedan, 2008) using the dplR package in R (Bunn et al., 2018). We assessed the suitability of these approaches to disentangle the drought effects on tree growth by computing the correlation coefficient with the drought indices (cf. next section) and by characterizing the trend over a 50 year period (Table S3; Figure S2). The results showed that the negative exponential detrending method performed best among the used approaches in terms of the magnitude of correlation with the drought and of capturing the long‐term trends (Table S3; Figure S2). We thus used the detrended negative exponential RWI (hereafter referred to as RWI) for the analysis.
To build the site‐level tree‐ring chronology, we averaged the detrended individual RWI series with a Tukey's biweight robust mean (Cook & Kairiukstis, 1990; Fritts, 2001). The RWI and average tree‐level chronology were calculated using the detrend and chron functions, respectively, available from the dplR R package (Bunn et al., 2018; R Development Core Team, 2018).
2.4. Quantification of drought indices
Monthly mean temperature (°C) and total precipitation (mm) data were obtained for each site from different climate data sources (Table S4). To compute the correlation coefficient between drought indices and the RWI, we considered a 50 year period for all sites. However, the range of years for the 50 year period varied across sites due to differences in timing of data collection.
For drought index, we initially considered the De Martonne Index (De Martonne, 1926), the Standardized Precipitation Index (McKee, Doesken, & Kleist, 1993), and the Standardized Precipitation Evapotranspiration Index (SPEI; Vicente‐Serrano, Beguería, & López‐Moreno, 2010). The SPEI had a stronger correlation with RWI than the other indices examined for most of the sites (see Table S5). Hence, SPEI was used for defining the drought and nondrought years.
The SPEI is a unitless drought index, which takes into account both precipitation and potential evapotranspiration effects in the calculation of the CWB, and is commonly used in the literature for identifying and characterizing drought and nondrought years (e.g., Bottero et al., 2017; Gazol et al., 2018). The potential evapotranspiration was calculated using the Thornthwaite function of the R package SPEI (Begueria & Vicente‐Serrano, 2013). The SPEI was then calculated from CWB using the spei function of the R package SPEI (Begueria & Vicente‐Serrano, 2013). For each site, we calculated SPEI of various timescales that is, integrated over 1–15 months in order to represent different lengths of the growing season or at least different growth sensitive periods within the current and the previous growing season. We assessed the Pearson correlation between RWI and SPEIs (i.e. the different time intervals) for identifying the most relevant SPEI (i.e., most sensitive time interval) for each site to define the drought and nondrought years (see Table S6). The resulting SPEIs (i.e., those best correlated with RWI) are presented in the Table S7.
For identifying the extreme drought year of a site, we selected the year with the lowest SPEI value. For each site, we first selected the extreme drought years for the period of 1980–2011. We then selected the extreme drought year for the period of 1980–1999 and for the period of 2000–2011.
2.5. Identification of drought, predrought, and postdrought periods
We characterized drought periods by single or multiple years based on SPEI ≤ −1.00 and predrought or postdrought periods (i.e., without drought) based on SPEI ≥ −0.99. We limited the predrought and postdrought periods to a maximum of 3 years, but for drought periods we considered all consecutive years with SPEI ≤ −1.00 (see Table S6). We identified the most extreme droughts during 1980–1999, and during 2000–2011 for all study sites (see Table S6) for comparing the tree growth responses to extreme droughts during the recent decade (2000–2011) with the previous two decades (1980–1999). Since many sites had no drought during 1990–1999, we decided to enlarge the earlier period back until 1980.
2.6. Tree growth resilience indices
For tree growth resilience, we computed three resilience indices as suggested by Lloret et al. (2011): resistance, recovery, and resilience. The resistance quantifies the ratio between growth during a drought period and growth during the preceding nondrought period, representing thus the capacity of the trees to buffer the stress and maintain growth during drought. The recovery quantifies the growth reaction following the drought period and is defined by the ratio between growth during the postdrought period and growth during the drought period. The resilience quantifies the ratio between growth during the postdrought period and growth during the predrought period, which represents the capacity of trees to recover and regain the growth of the predrought period. We quantified resistance, recovery, and resilience for all trees of all sites during the most extreme droughts in 1980–1999 and in 2000–2011 (see Table S6).
2.7. Research question 1: Impact of seasonal drought (SPEI) on tree growth
Based on the results of preliminary analysis (i.e., correlation between RWI and different SPEIs), we identified the eight best correlated SPEIs for understanding the magnitude (i.e., degree of correlation) and pattern (i.e., type of correlation) of influences of drought on RWI, and how that magnitude and pattern of correlation varied across the latitudinal gradient examined in this study. The selected SPEI timescales were August 15 (i.e., from previous June to current August), May 12 (i.e., from previous June to current May), May 9 (i.e., from previous September to current May), May 6 (i.e., from previous December to current May), May 3 (i.e., spring, from current March to current May), August 6 (i.e., from current March to current August), August 3 (i.e., summer, from current June to current August), and November 6 (i.e., from current June to current November).
2.8. Research question 2: Tree growth rate in drought and nondrought years
For understanding the absolute tree radial growth performances during drought and nondrought years, we modeled absolute tree radial growth (non‐detrended tree ring width) as a function of site types (three levels: LEHP, MELP, and HELP), drought status (two levels: drought years and nondrought years), and the interaction between site types and drought status. For understanding the potential role of tree age on absolute tree radial growth, we considered tree age as a covariate in this analysis.
2.9. Research question 3: Temporal change in tree growth resilience to extreme droughts
We modeled resistance, recovery, and resilience as a function of time period (two levels: 1980–1999 and 2000–2011), site types (three levels: LEHP, MELP, and HELP), and the interaction between time period and site types.
2.10. Research question 4: Factors affecting tree growth resilience to extreme drought
For this research question, we selected the most extreme drought during the entire 1980–2011 study period and used the corresponding resistance, recovery, and resilience indices as response variables in a mixed‐effects model (cf. next section). We considered several tree‐, site‐, and drought‐level explanatory variables and various two‐way interaction terms (see Table S1). The variables included tree size (i.e., tree diameter at breast height [DBH] inside bark at the drought year), tree growth, and tree growth variability prior to drought representing the average and standard deviation of RWI, respectively of 10 consecutive years prior to the extreme drought excluding the years considered as predrought period for quantifying the three resilience indices, site types, elevation, latitude, drought severity (measured by the average SPEI during the drought period), drought duration (measured by the length of the drought period in years), and drought frequency (measured by the number of drought years (SPEI ≤ −1.00) within 10 years preceding the maximum drought period).
2.11. Statistical analyses
We used a linear mixed‐effect modeling approach for research question 2, 3, and 4 in which our variables of interest were considered as fixed effects and trees nested within sites were considered as random effects. The modeling was performed using the function lme of the R package nlme (Pinheiro & Bates, 2000; Pinheiro, Bates, DebRoy, & Sarkar, 2014). The response variables were log‐transformed to normalize residuals and homogenize variances and we checked the assumptions of normality of the residuals and homogeneity of the variances. Preliminary analysis indicated that an additional error structure to account for plot spatial autocorrelation did not improve model performance and thus was not incorporated into the final model. We also assessed potential multicollinearity among explanatory variables using the Variance Inflation Factor (VIF) and discarded variables when VIF > 2.0. The VIF was calculated using the function vif of the R package car (Fox & Weisberg, 2011). The post hoc Tukey multiple comparison test was performed to detect the statistical differences (Hothorn, Bretz, & Westfall, 2008).
For research question 4, we used the information‐theoretic approach (Burnham & Anderson, 2002; Johnson & Omland, 2004), which provides a measure of strength for each candidate model that represents a plausible hypothesis relative to the entire set of candidate models considered (Mazerolle, 2006). In the context of our research question (i.e., what are the factors driving the tree growth resilience to extreme drought?), we considered 16 hypotheses (i.e., candidate models; Table S1), which were developed based on the current understanding resulted from different studies that examined tree growth resilience to extreme droughts. Model selection was performed using the AICcmodavg package of R (Mazerolle, 2011). Candidate models were compared using Akaike's information criterion corrected for small sample sizes (AICc). Akaike weights were computed to assess the support for each model. We used multimodel inference to compute the model‐averaged estimates of the explanatory variables and their 95% confidence intervals (Burnham & Anderson, 2002). A confidence interval excluding 0 indicated that the corresponding explanatory variable had an effect on the response variable (Burnham & Anderson, 2002; Mazerolle, 2006). In addition to our candidate models we also considered a null model and a full model. The coefficient of variation (R 2) for fixed and random effects were calculated using the function r.squaredGLMM of the MuMIn package in R (Bartoń, 2013). The background map of Figure 2 was downloaded using the function map_data from ggmap package in R (Kahle & Wickham, 2013).
FIGURE 2.
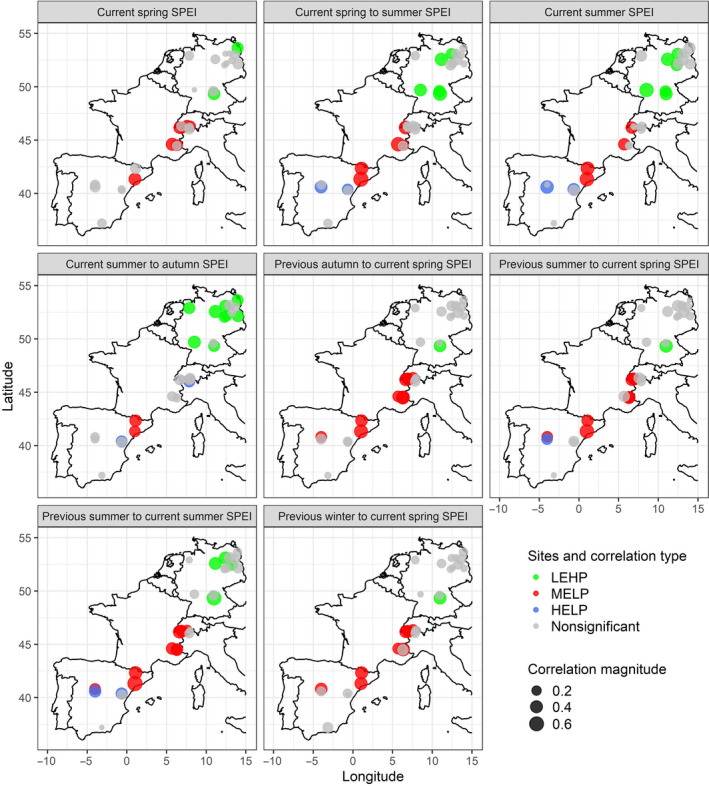
Seasonal correlations between Scots pine tree‐ring width indices and the Standardized Precipitation Evapotranspiration Index (SPEI) for the period of approximately 1960–2011 across the latitudinal gradient. Only the seasons that exhibited the strongest effect on tree‐ring width indices are plotted (see Section 2). Note. ‘previous’ refers to the year previous to tree ring formation, while ‘current’ refers to the current year of ring formation, summer: June, July, and August, spring: March, April, and May, autumn: September, October, and November, and winter: December, January, and February. HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity. Pearson's product‐moment correlation with a threshold <0.05 was used for statistical significance. Correlation magnitude: the larger the circles, the stronger the correlations. See Table S7 for correlation scores that are displayed in this figure
3. RESULTS
3.1. Impact of seasonal drought (SPEI) on tree growth
Our results showed significant differences in the response of tree growth to the different time periods of SPEI. The current year summer to autumn (June–November) SPEI significantly controlled tree growth at LEHP sites of northern Germany (Figure 2), while tree growth at MELP sites was driven by SPEI of spring (March–May), summer (June–August), and spring and summer combined. Tree growth in HELP sites was either nonrelated or negatively correlated with different time periods of the SPEI (Figure 2; Table S3). Overall, the magnitude of correlation between RWI and different SPEIs was higher for MELP than the two other site types (Table S3). Three sites of HELP site type had a negative correlation with SPEIs while one site of HELP site type was not significantly correlated with any SPEI considered in our analysis (Table S3).
3.2. Tree growth rate in drought and nondrought years
In drought and nondrought years, tree radial growth was higher at LEHP than at HELP and at MELP sites (Figure 3). The MELP sites had significantly lower tree radial growth in drought years than in nondrought years (Table S8; Figure 3). Contrary to MELP, tree growth was not significantly different between drought and nondrought years at LEHP and at HELP sites (Table S8; Figure 3). Tree age was negatively associated with the radial growth (p < .0001) irrespective of site types (Table S8).
FIGURE 3.
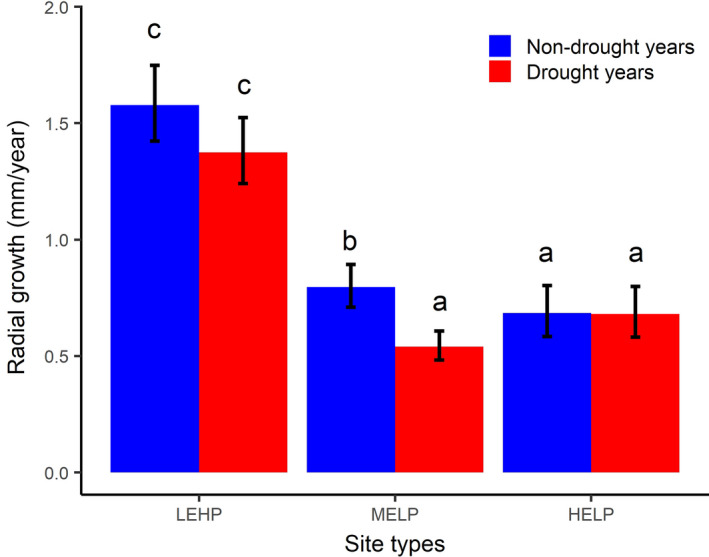
Mean annual radial growth in drought and non‐drought years for the period of approximately 1980–2011 across the three site types (i.e., LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity; HELP, high‐elevation sites with low productivity). Error bars represent the mean ± standard error (n = 615). Letters on top of the bars show the results (a < b < c) of the post hoc Tukey multiple comparison test with a threshold <0.05 for statistical significance indicating the differences among the three site types and between non‐drought years and drought years within each site type
3.3. Temporal change in tree growth resilience to extreme droughts
Tree growth resistance to extreme drought for all site types (i.e., HELP, LEHP, and MELP) did not change over the two periods (i.e., 1980–1999 and 2000–2011; Figure 4a). Nevertheless, resistance was higher at HELP than at LEHP, and higher in the latter compared to MELP, irrespective of the period (Table S9; Figure 4a).
FIGURE 4.
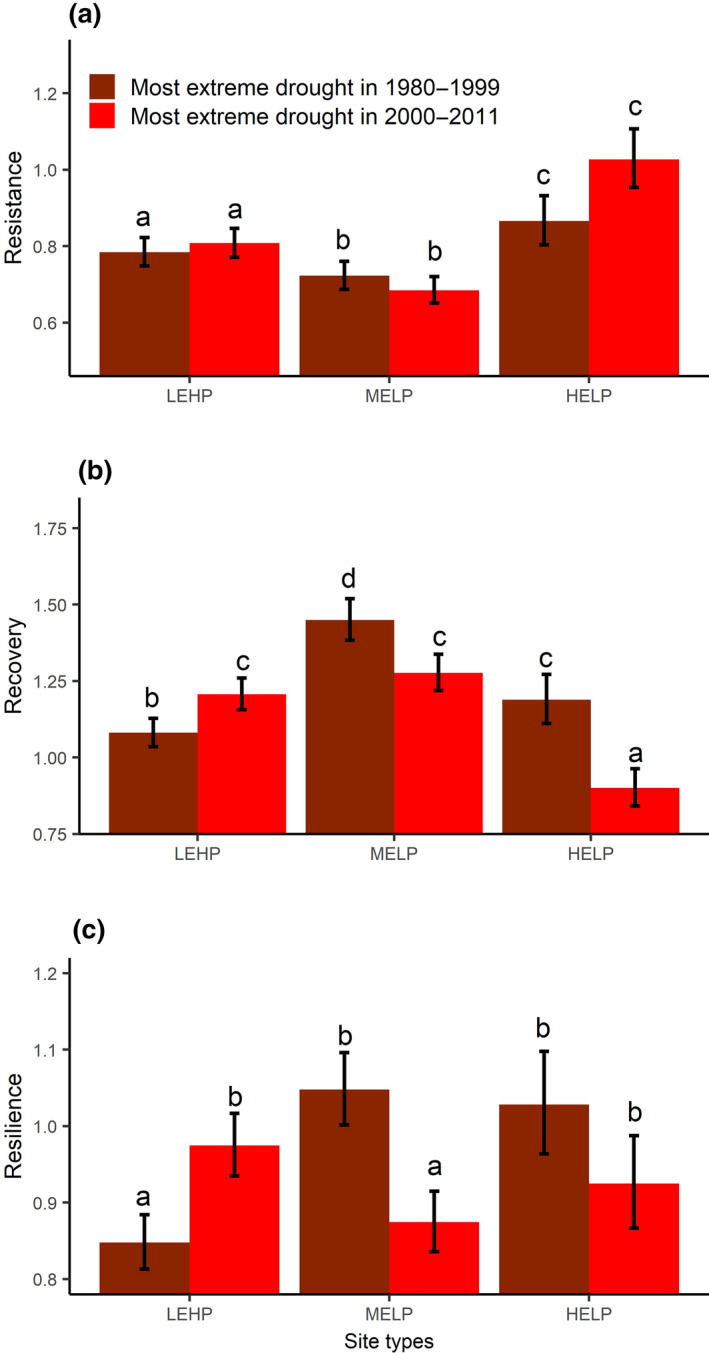
Tree‐level resistance (a), recovery (b), and resilience (c) to the most extreme drought during 1980–1999 and during 2000–2011 for three site types. Error bars represent the mean ± standard error (n = 615). Letters on top of the bars show the results (a < b < c) of the post hoc Tukey multiple comparison test with a threshold <0.05 for statistical significance indicating the differences among the three site types and between the two periods within each site type. HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity
Tree growth recovery changed significantly over the two periods for all site types, where recovery decreased from 1980–1999 to 2000–2011 at MELP and HELP sites, while increased from 1980–1999 to 2000–2011 at LEHP sites (Table S9; Figure 4b). In 1980–1999, recovery was significantly higher at MELP compared to the two other site types irrespective of period (Table S9; Figure 4b).
Tree growth resilience changed significantly over the two periods for LEHP and MELP sites, but not for HELP sites. Resilience decreased from 1980–1999 to 2000–2011 at MELP sites, while it increased from 1980–1999 to 2000–2011 at LEHP sites (Table S9; Figure 4c).
3.4. Factors affecting tree growth resilience to extreme drought
The model that included additive and interaction effects of all variables considered in the analysis had full support of Akaike weight for resistance (Table 1). A lower resistance was associated with higher predrought growth rate (Table 2). In addition, a lower resistance was associated with greater drought frequency, and with longer drought but depending upon predrought growth rate (Table 2). Resistance was higher at HELP and LEHP sites than at MELP sites (Table 2; Figure 5a).
TABLE 1.
Results of the best models explaining tree growth resistance, recovery, and resilience of Scots pine trees along the studied gradient. From the 16 tested models, only the three with the highest Akaike's information criterion (AICc) weight are presented
| Models | Hypotheses | References | AICc | ∆AICc | AICc weight | R 2 (fixed) | R 2 (fixed and random) |
|---|---|---|---|---|---|---|---|
| Resistance (RT) | Tree resistance to drought is affected by | ||||||
| RT~all variables | Full model | 117.9 | 0.0 | 1.00 | .33 | .49 | |
| RT~D_INT*PGR+D_INT*ST | The intensity of the drought, but depending upon the growth prior to drought and site types | Adapted from Gazol et al. (2018) | 130.4 | 12.5 | 0.00 | .22 | .46 |
| RT~D_FRE*PGR | The frequency of the drought, but depending upon the growth prior to drought | Adapted from Gao et al. (2018) | 130.5 | 12.6 | 0.00 | .14 | .47 |
| Recovery (RC) | Tree recovery after drought | ||||||
| RC~D_INT*ST | Is affected by the intensity of the drought, but depending upon the site types | Adapted from Gazol et al. (2018) | 301.9 | 0.0 | 0.74 | .16 | .36 |
| RC~ST | Decreased with site types | Sánchez‐Salguero et al. (2018) | 307.0 | 5.1 | 0.06 | .07 | .37 |
| RC~D_INT+D_FRE+D_DUR | Is affected combinedly by intensity of drought, duration of drought, and frequency of drought | Gao et al. (2018) | 308.4 | 6.5 | 0.03 | .08 | .37 |
| Resilience (RS) | Tree resilience to drought is | ||||||
| RS~D_DUR*PGR | Affected by the duration of the drought, but depending upon the growth prior to drought | Adapted from Taeger et al. (2013) | 342.4 | 0.0 | 0.45 | .03 | .35 |
| RS~PGR | Negatively associated with the growth prior to drought | Zang et al. (2014); Ruijven and Berendse (2010) | 344.3 | 1.9 | 0.17 | .02 | .35 |
| RS~D_FRE*PGR | Affected by the frequency of the drought, but depending upon the growth prior to drought | Adapted from Gao et al. (2018) | 344.6 | 2.2 | 0.15 | .02 | .36 |
PGR = average tree growth (ring width indices) prior to drought, ST = site type (LEHP (low‐elevation sites with high productivity), MELP (mid‐elevation sites with low productivity), and HELP (high‐elevation sites with low productivity)), D_FRE = drought frequency measured by the number of drought years within the past 10 years from the studied drought, D_INT = intensity of drought, and D_DUR = duration of drought, *indicates an interaction term and +indicates an additive term, PGR was quantified from tree growth during the 10 consecutive years prior to drought excluding the years considered as predrought period quantifying the three indices (i.e., resistance, recovery, and resilience).
TABLE 2.
Log‐transformed estimates of predictor variables and 95% confidence intervals (CI) based on model averaging for the three response variables resistance, recovery, and resilience. Only predictor variables that had a strong effect (i.e., a 95% confidence interval excluding 0) are presented
| Parameters | Types of effect | Estimate (β) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Resistance | ||||
| Predrought growth | Additive | −0.13 | −0.22 | −0.04 |
| HELP versus MELP | Additive | 0.31 | 0.09 | 0.52 |
| LEHP versus MELP | Additive | 0.19 | 0.02 | 0.36 |
| Drought intensity*predrought growth | Interaction | 0.005 | 0.002 | 0.008 |
| Drought duration*predrought growth | Interaction | −0.22 | −0.35 | −0.09 |
| Drought frequency | Additive | −0.13 | −0.20 | −0.06 |
| Recovery | ||||
| HELP versus MELP | Additive | −0.23 | −0.45 | −0.02 |
| LEHP versus MELP | Additive | −0.16 | −0.34 | −0.01 |
| Drought frequency | Additive | 0.08 | 0.01 | 0.16 |
| Resilience | ||||
| Predrought growth | Additive | −0.18 | −0.28 | −0.07 |
| Predrought growth variability | Additive | −0.22 | −0.43 | −0.01 |
HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity.
FIGURE 5.
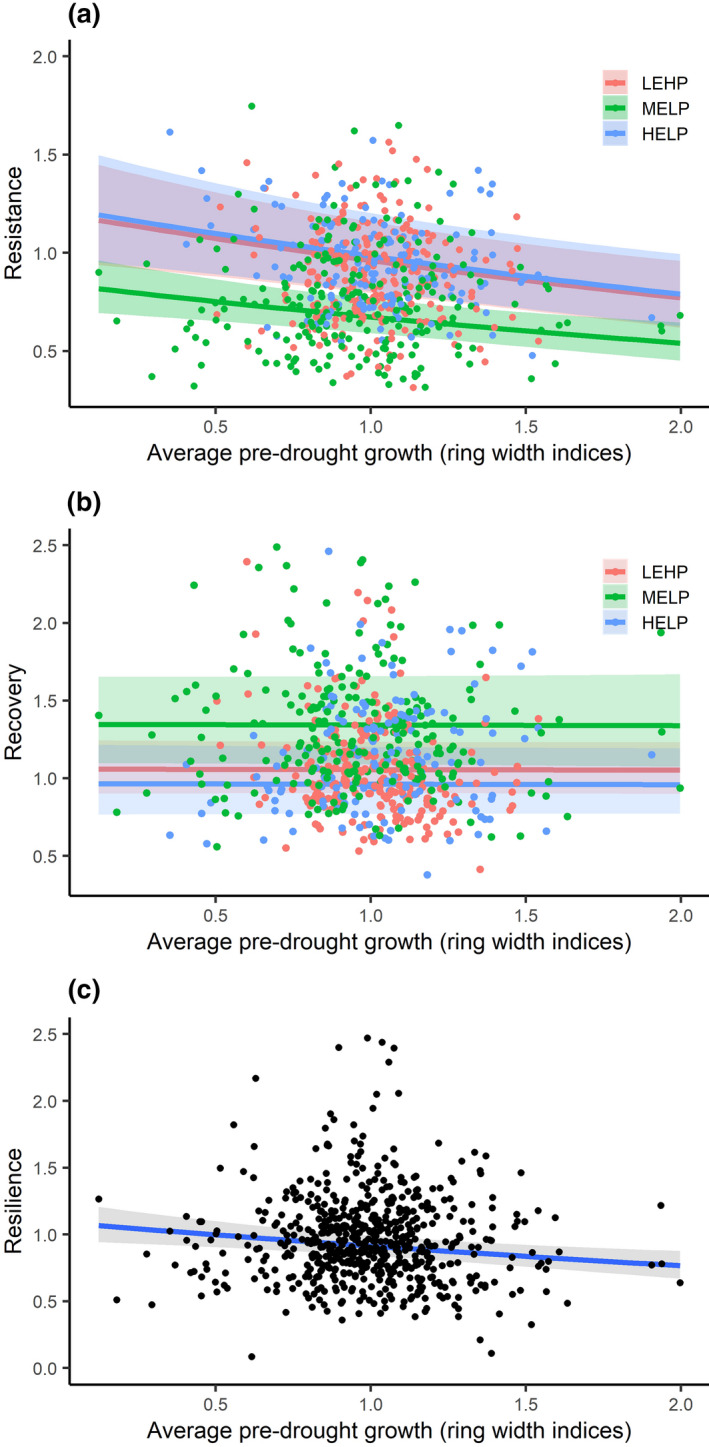
Tree growth resistance (a), recovery (b), and resilience (c) to the most extreme drought during 1980–2011 with 95% confidence intervals. Note. Average pre‐drought growth (ring width indices) was quantified from tree growth during the 10 consecutive years prior to drought excluding the years considered as pre‐drought period for quantifying the three indices (resistance, recovery, and resilience). HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity. See statistics for the fitted line in Tables 1 and 2
The model that included drought severity and site types, and the interaction between the two variables had the highest support of Akaike weight for recovery (0.74; Table 1). Recovery was lower at HELP and LEHP sites than at MELP sites (Table 2; Figure 5b). In addition, the recovery was higher where trees experienced a higher frequency of droughts (Table 2).
The model that included predrought growth rate and drought duration, and the interaction between the two variables had the highest support of Akaike weight for resilience (Table 1). Resilience was negatively associated with predrought growth rate and predrought growth variability and there was no difference across the three site types (Table 2; Figure 5c).
4. DISCUSSION
Using tree ring width data from 30 sites along a 2,800 km latitudinal gradient across Europe we analyzed whether tree growth resilience to extreme drought depended on the geographical location of the tree (Isaac‐Renton et al., 2018) and if resilience to extreme drought decreased over time due to more frequent drought events in recent years (Serra‐Maluquer et al., 2018; Spinoni, Naumann, Carrao, Barbosa, & Vogt, 2014). We examined these questions on Scots pine, one of the most widely distributed tree species in the world which is also considered vulnerable to extreme drought conditions (Camarero, Gazol, Sangüesa‐Barreda, et al., 2015; Galiano et al., 2010; Matías, Linares, Sánchez‐Miranda, & Jump, 2017; Rigling et al., 2013). Our study shows that tree‐level resilience to drought was not dependent on the latitudinal location, but rather on the type of site they were growing at and their growth performance (i.e., magnitude and variability of growth) during the predrought period. Our results indicate that trees with higher magnitude and variability in growth are more vulnerable to long and severe droughts. In addition, we found that tree growth resilience to extreme drought was lower during 2000–2011 than during 1980–1999 at mid‐elevation and lower productivity sites. These results may indicate that more frequent drought events that occurred in 2000–2011 than in earlier period make Scots pine trees more vulnerable to extreme droughts. However, we found high variability in tree‐level responses (Figure 5) as detected by previous studies (e.g., Gazol et al., 2018; Maes et al., 2019). This high tree‐level variability (Figure 5) and low marginal R 2 values (Table 2) may indicate that combining tree information from different sites without direct measurements of local site‐related factors (e.g., soil water content, nutrient availability, stand stocking, and rooting depth) compromises the predictive power of the models (DeSoto et al., 2020; Gazol et al., 2018; Grossiord et al., 2014).
4.1. Impact of seasonal drought on tree growth
Our results reveal important seasonal differences across lower and higher productivity sites in terms of SPEI‐RWI correlations. Scots pine trees growing at mid‐elevation lower productivity (MELP) sites showed a greater sensitivity to spring–summer SPEI while trees growing at low‐elevation higher productivity (LEHP) sites of northern Germany were more sensitive to summer–autumn SPEI (Figure 2). This is consistent with results from Pasho et al. (2011), who studied eight tree species including Scots pine for the period of 1950–2005 using the Standardized Precipitation Index at timescales from 1 to 48 months. Our results showed that Scots pine trees growing in MELP are more sensitive to drought (measured by SPEI) than trees growing in LEHP and HELP sites (Figure 2; Table S3), which is consistent with the findings of other studies on Scots pine (Lévesque, Rigling, Bugmann, Weber, & Brang, 2014; Pasho et al., 2011; Sánchez‐Salguero et al., 2015). Trees at MELP sites are likely to grow under water limitation during extended periods of the year, and changes in precipitation or evaporative demand during these periods will directly affect the water availability of the trees and thus their physiological responses, resulting in lower growth (Cabon, Peters, Fonti, Martínez‐Vilalta, & De Cáceres, 2020). A tree at a less dry site in contrast is growing mostly without water limitations. During such periods with plenty of water supply there will be no strong direct effect of precipitation (and at least within a certain range of evaporative demand) on tree physiology and on growth (Martínez‐Vilalta et al., 2009; Sterck, Zweifel, Sass‐Klaassen, & Chowdhury, 2008). Thus, it is reasonable to consider that the climatic control of tree growth is stronger at MELP which are relatively drier than LEHP sites (Table S2). However, we need to improve our understanding on local drought characteristics because the CWB and SPEI do not consider the soil water holding capacity and the depth of water table which are important parameters for estimating the soil water available for trees (Zang et al., 2020).
4.2. Tree growth rate in drought and nondrought years
We found higher annual growth among trees at lower‐elevation sites than trees at higher‐elevation sites (Figure 3). Trees at low‐elevation sites were relatively younger than trees at high‐elevation sites and thus more likely in their full growth ontogenetic stage. However, Scots pine trees at high‐elevation sites had no growth difference between our selected drought and nondrought years (Figure 3) irrespective of the fact that they experienced similar drought severity and frequency as the two other site types in terms of SPEI (Figure 6). Therefore, tree growth at high‐elevation Scots pine forests is probably controlled by other factors than changes in water availability during drought (Carrer, Nola, Eduard, Motta, & Urbinati, 2007; Cudlín et al., 2017). In our dataset, three out of six high‐elevation sites had negative correlation with SPEI (Table S3) indicating that tree radial growth at high‐elevation sites examined in our study was more related to other factors such as ontogeny and temperature (Camarero, Gazol, Galván, Sangüesa‐Barreda, & Gutiérrez, 2015; Körner, 2003) than to water availability (Hagedorn et al., 2014). Our high‐elevation site type has three sites with trees that were older than 150 years during our selected drought period and older than the trees at any other site examined in our analysis. Besides, the mean annual temperature at four out of six high‐elevation sites was <7.0°C which is almost 2°C cooler than at any other site examined in our analysis. Trees in our high‐elevation sites are perhaps well adapted to such low mean annual temperature and thus short growing season, confirming the findings of other studies conducted in southern Europe (Peñuelas, Hunt, Ogaya, & Jump, 2008) and in southwestern United States (Adams & Kolb, 2004). It is also important to mention that our drought intensity index may not fully capture the absolute water balance differences between drought and nondrought years because of the elevational differences between the sites and climate stations (Table S4), which may partly explain the lack of growth differences between drought and nondrought years for our high‐elevation sites.
FIGURE 6.
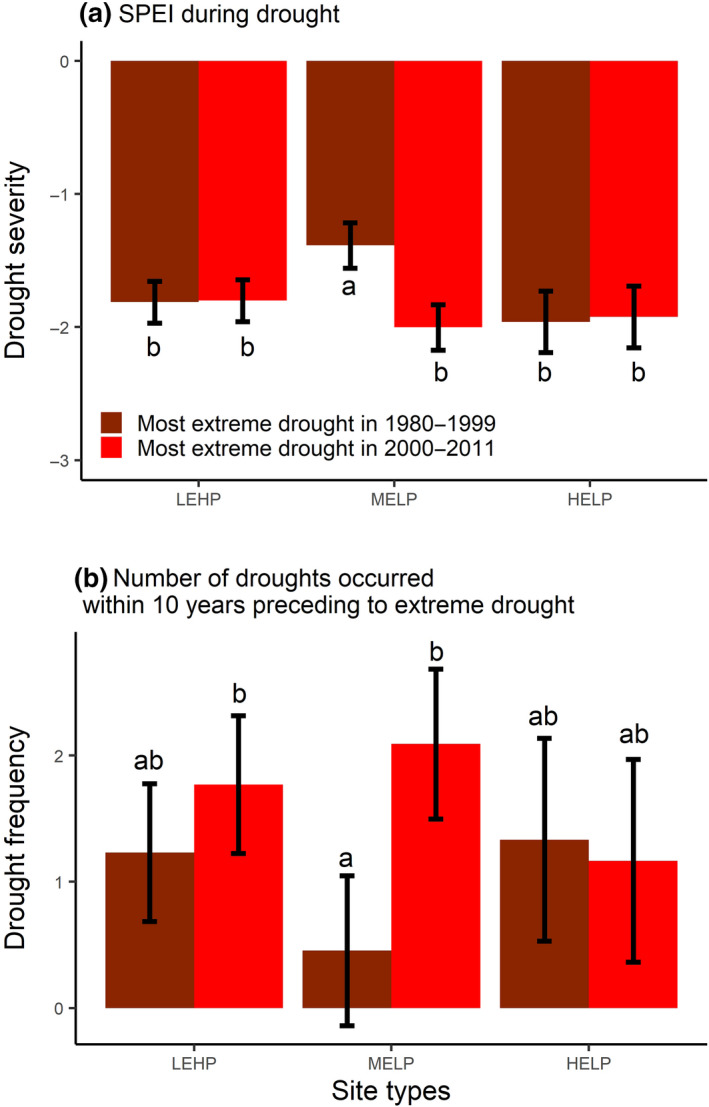
Drought severity (measure by the SPEI [Standardized Precipitation and Evapotranspiration Index]) (a) and drought frequency (measured by the number of droughts that occurred within 10 years preceding extreme drought) (b) during and prior to the examined extreme drought, respectively. Error bars represent the mean ± standard error (n = 30). Letters on top of the bars show the results (a < b < c) of the post hoc Tukey multiple comparison test with a threshold <0.05 for statistical significance. HELP, high‐elevation sites with low productivity; LEHP, low‐elevation sites with high productivity; MELP, mid‐elevation sites with low productivity
4.3. Temporal change in tree growth resilience to extreme droughts
Although Scots pine is a relatively drought‐tolerant species, our results showed a decreased growth recovery and resilience to extreme drought at mid‐elevation and lower productivity sites. This is largely consistent with the patterns reported by Serra‐Maluquer et al. (2018) in three pine species (including Scots pine) growing in NE Spain. In our case, this result likely reflects the higher frequency and severity of droughts events prior to the extreme drought occurring in 2000–2011 than prior to the extreme drought occurring in 1980–1999 (Figure 6). Moreover, trees became older and larger over time. A larger tree carries higher nonphotosynthetic biomass, which requires a greater investment for defense and maintenance (Ryan & Yoder, 1997; Scholz, Phillips, Bucci, & Goldstein, 2011) and increasing tree height may increase hydraulic constraints and xylem cavitation risks under drought (Olson et al., 2018). This reduced tree resilience over time may challenge the physiological potential for acclimation to more intensive and frequent drought events that we expect in the future (Dai, 2012). The decreased tree growth resilience at MELP sites may indicate that drought hardening (i.e., physiological processes by which a tree becomes more acclimate to drought conditions; Villar‐Salvador, Peñuelas, & Jacobs, 2013) is not very important and does not allow trees to acclimate to frequent and prolonged drought events irrespective of their drought experiences.
In contrast to MELP sites, tree growth recovery and resilience to extreme droughts increased at LEHP sites during the recent period (i.e., 2000–2011) compared to the previous period (i.e., 1980–1999). Although drought severity and drought frequency did not change significantly at LEHP sites over these two periods (Figure 6), the duration of the extreme drought was longer in 1980–1999 than in 2000–2011. For example, seven sites of the LEHP site type experienced a 2 year long drought during 1980–1999, while none of the droughts occurring during 2000–2011 was longer than 1 year at that site type (Table S6).
4.4. Factors affecting tree growth resilience to extreme drought
Several studies conducted at local to global scales provided understanding on tree, site, and drought‐related variables influencing tree‐level resilience to extreme drought (e.g., Gao et al., 2018; Gazol et al., 2017, 2018; Sánchez‐Salguero et al., 2018; Taeger, Zang, Liesebach, Schneck, & Menzel, 2013; Vitali et al., 2017; Zang et al., 2014). To our knowledge, however, none of these studies compared explicitly the model performances with and without interaction effects among all these variables.
Our results showed that the top‐ranked model (according to Akaike weights) for all the three resilience indices (resistance, recovery, and resilience) included interaction effects between tree and drought‐related variables (Table 1). This means that the impact of drought on tree‐level resilience is not independent, but rather dependent on how the trees were growing (i.e., magnitude and variability of growth) during the predrought period and on the type of site they were growing at. In addition to drought severity, which was usually found to significantly affect tree‐level resilience at various spatial scales variables (Gazol et al., 2017, 2018; Zang et al., 2014), we also considered drought duration and drought frequency as explanatory variables as suggested by Gao et al. (2018). We indeed found a significant negative effect of drought frequency on tree resistance (Table 2), suggesting that trees that experienced more frequent droughts were less resistant to extreme droughts. Therefore, trees that display higher variability in growth are not only sensitive to extreme drought but also to frequent drought occurrence as for example, shown by Seidel et al. (2016) for different Scots pine provenances. Higher aboveground biomass growth can be related to lower or at least nonproportional biomass allocation to roots (Gessler et al., 2017) and might consequently increase tree‐level sensitivity to upcoming drought periods (Martínez‐Vilalta, López, Loepfe, & Lloret, 2012). Long or repeated droughts may reduce the number of living branches and, because of needle multiyear life span, the leaf area per branch (Galiano, Martínez‐Vilalta, & Lloret, 2011; Vennetier et al., 2013). This holds back short‐term tree leaf area recovery and may drive tree growth to very low levels during and after drought. Although a reduced leaf area limits water stress during and after droughts and may favor resilience, it also hampers carbohydrate reserves buildup and may lead to carbon starvation. Accordingly, higher growth variability may be related to a higher vulnerability to the upcoming stresses (Cailleret et al., 2019; DeSoto et al., 2020; McDowell et al., 2008; Ogle, Whitham, & Cobb, 2000). For instance, Ogle et al., (2000) noticed a 1.5 times higher growth variability in dead pinyon pine trees relative to surviving ones in drought years preceding mortality across forests of southwestern United States.
Although the predrought growth of Scots pine had a strong relationship with resistance, it had no‐relationship with recovery. This lack of relationship between predrought growth and recovery resulted in a relatively weak relationship (although statistically significant) between predrought growth and resilience (Figure 5), because the resilience is mathematically related to both resistance and recovery (Lloret et al., 2011), and in the context of our study, recovery was more closely related to resilience than the relationship between resistance and resilience (Figure S3). Scots pine trees growing at MELP sites displayed lower resistance but higher recovery than trees growing at LEHP and HELP sites (Table 2; Figure 5), suggesting different growth strategies exercised by trees from different sites to cope with drought (Sánchez‐Salguero et al., 2018). However, resistance and recovery are relative indices and do not allow comparison across trees in terms of their absolute growth performances. Although resistance and recovery hold a negative mathematical relationship (Lloret et al., 2011), they provide useful insights on disentangling trees that tried to remain firm during drought years (i.e., higher resistance and lower recovery) from trees that tried to conform drought impact (i.e., lower resistance and higher recovery; Gazol et al., 2017). These two different strategies to extreme droughts by trees from high productivity and low productivity sites are also reported by other local (Vitali et al., 2017; Zang et al., 2014) and global‐scale studies (Gazol et al., 2017). For instance, a multispecies comparison across Spain found higher resistance for species dominating mesic sites than for species from more xeric sites, which presented a higher recovery (Gazol et al., 2018). At our LEHP, Scots pine trees were on average taller and larger than MELP sites (Table S2). These larger trees in mesic sites most likely developed larger crowns, which might be able to still provide sufficient photosynthates to sustain growth during the drought period. Alternatively, these larger trees had sufficient reserves to compensate for the drought‐induced reduction in photosynthesis. Both mechanisms would result in a higher resistance compared to trees in lower productivity sites (Martínez‐Vilalta et al., 2009). However, taller trees associated with larger crowns and concomitantly greater water demand can be more vulnerable to drought‐induced hydraulic failure (McDowell et al., 2008; Olson et al., 2018) and the required postdrought investment of assimilates for restoring their hydraulic system could slow down their recovery process (Brodribb, Bowman, Nichols, Delzon, & Burlett, 2010).
In this analysis, we considered tree, site, and drought level variables and their interactions, but available data did not allow to quantify the effects of forest stand‐level variables including tree‐to‐tree competition (Serra‐Maluquer et al., 2018), site conditions including soil and topography (Vennetier, Ripert, & Rathgeber, 2018; Zalloni, Battipaglia, Cherubini, Saurer, & De Micco, 2019), species mixtures (Pretzsch, Schütze, & Uhl, 2013), trees neighborhood composition (Grossiord, 2019), and stand stocking (Bottero et al., 2017; D'Amato, Bradford, Fraver, & Palik, 2013), which will be the subject of subsequent analyses. Moreover, we did not include possible subspecies variation due to local evolutionary adaptation induced by drought‐related selection (Hampe & Petit, 2005). We detected large tree‐level variability within and across sites, and a larger proportion of the variance of our models was explained by the random effects (i.e., trees nested within sites) than by the fixed effect variables (Table 1). Therefore, these points need to be considered when interpreting our results.
To conclude, we show that tree radial growth responses of Scots pine to extreme drought depend on site condition, tree growth prior to drought, and the number of droughts that a tree experienced within the 10 years before the selected drought. Our study identified a reduced tree growth resilience to extreme drought in Scots pine trees growing at mid‐elevation and low productivity sites likely driven by the more frequent and severe drought events that occurred at those sites in recent years. We show that the assessment of tree growth responses in terms of resistance, recovery, and resilience to extreme drought using radial growth data is challenging along large ecological and biogeographical gradients, since a multitude of location‐specific tree‐, site‐, and drought‐related factors and their interactions drive tree growth performances.
CONFLICT OF INTEREST
None is declared.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The study was supported by a Marie Skłodowska‐Curie Individual Fellowship (PROJECT ID: 749051‐REFOREST) to A.K.B. R.S‐.S. was supported by Postdoctoral grant (IJCI‐2015‐25845, FEDER funds), J.J.C., J.C.L., and R.S‐.S. were supported by the RTI2018‐096884‐B‐C31 and RTI2018‐096884‐B‐C33 projects (Ministry of Science, Innovation and Universities, Spain) and VULBOS project (UPO‐1263216, FEDER Funds, Andalusia Regional Government, Consejería de Economía, Conocimiento, Empresas y Universidad 2014‐2020), A.H. by PinCaR project (UHU‐1266324, FEDER Funds, Andalusia Regional Government, Consejería de Economía, Conocimiento, Empresas y Universidad 2014‐2020), and A.M. by the Bavarian Ministry of Science via the Bavarian Climate Research Network (bayklif). Part of the sampling was funded within the project DENDROKLIMA by the German Waldklimafond (FKZ 28W‐C‐4‐077‐01) and ST327 by the Bavarian State Ministry for Food, Agriculture, and Forestry. We thank Kerstin Treydte, Richard Peters, and Stefan Klesse for providing valuable supports on tree ring width detrending methods, David Forrester for providing information on site productivity index, Tanja Sanders for providing valuable comments on earlier version of the manuscript, and Landesforst Mecklenburg‐Vorpommern, Landeskompetenzzentrum Forst Eberswalde, and Nordwestdeutsche Forstliche Versuchsanstalt for maintaining the intensive forest monitoring sites (Level II) used in this study, Javier Donés (Director of Montes de Valsaín), National Parks Autonomous Agency (OAPN), Junta de Castilla y León forest guards and all the participants involved in the “International Tree‐Ring Summer School” celebrated in 2012 in Valsaín (Segovia‐Spain), and Ramzi Touchan and Dave Meko. We also thank all colleagues who were involved in field sampling and laboratory measurements for the other sites.
Bose AK, Gessler A, Bolte A, et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob Change Biol. 2020;26:4521–4537. 10.1111/gcb.15153
DATA AVAILABILITY STATEMENT
The data of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams, H. D. , & Kolb, T. E. (2004). Drought responses of conifers in ecotone forests of northern Arizona: Tree ring growth and leaf δ13C. Oecologia, 140(2), 217–225. 10.1007/s00442-004-1585-4 [DOI] [PubMed] [Google Scholar]
- Adams, H. D. , Zeppel, M. J. B. , Anderegg, W. R. L. , Hartmann, H. , Landhäusser, S. M. , Tissue, D. T. , … McDowell, N. G. (2017). A multi‐species synthesis of physiological mechanisms in drought‐induced tree mortality. Nature Ecology & Evolution, 1(9), 1285–1291. 10.1038/s41559-017-0248-x [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. L. , Klein, T. , Bartlett, M. , Sack, L. , Pellegrini, A. F. A. , Choat, B. , & Jansen, S. (2016). Meta‐analysis reveals that hydraulic traits explain cross‐species patterns of drought‐induced tree mortality across the globe. Proceedings of the National Academy of Sciences of the United States of America, 113(18), 5024–5029. 10.1073/pnas.1525678113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg, W. R. L. , Schwalm, C. , Biondi, F. , Camarero, J. J. , Koch, G. , Litvak, M. , … Pacala, S. (2015). Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science, 349(6247), 528–532. [DOI] [PubMed] [Google Scholar]
- Bartoń, K. (2013). MuMIn: multi‐model inference. R package version, 1(5). Retrieved from https://cran.r-project.org/web/packages/MuMIn/index.html [Google Scholar]
- Begueria, S. , & Vicente‐Serrano, S. M. (2013). SPEI: Calculation of the standardised precipitation‐evapotranspiration index. R package version 1.6. Vienna, Austria: CRAN. [Google Scholar]
- Biondi, F. , & Quedan, F. (2008). A theory‐driven approach to tree‐ring standardization: Defining the biological trend from expected basal area increment. Tree Ring Research, 64, 81–96. 10.3959/2008-6.1 [DOI] [Google Scholar]
- Bolte, A. , Czajkowski, T. , Cocozza, C. , Tognetti, R. , de Miguel, M. , Pšidová, E. , … Müller, J. (2016). Desiccation and mortality dynamics in seedlings of different European beech (Fagus sylvatica L.) populations under extreme drought conditions. Frontiers in Plant Science, 7, 751 10.3389/fpls.2016.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose, A. K. , Moser, B. , Rigling, A. , Lehmann, M. M. , Milcu, A. , Peter, M. , … Gessler, A. (2020). Memory of environmental conditions across generations affects the acclimation potential of scots pine. Plant, Cell & Environment, 43(5), 1288–1299. 10.1111/pce.13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero, A. , D'Amato, A. W. , Palik, B. J. , Bradford, J. B. , Fraver, S. , Battaglia, M. A. , & Asherin, L. A. (2017). Density‐dependent vulnerability of forest ecosystems to drought. Journal of Applied Ecology, 54(6), 1605–1614. 10.1111/1365-2664.12847 [DOI] [Google Scholar]
- Breshears, D. D. , Cobb, N. S. , Rich, P. M. , Price, K. P. , Allen, C. D. , Balice, R. G. , … Meyer, C. W. (2005). Regional vegetation die‐off in response to global‐change‐type drought. Proceedings of the National Academy of Sciences of the United States of America, 102(42), 15144–15148. 10.1073/pnas.0505734102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb, T. J. , Bowman, D. J. M. S. , Nichols, S. , Delzon, S. , & Burlett, R. (2010). Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist, 188(2), 533–542. 10.1111/j.1469-8137.2010.03393.x [DOI] [PubMed] [Google Scholar]
- Bugmann, H. (1996). Functional types of trees in temperate and boreal forests: Classification and testing. Journal of Vegetation Science, 7(3), 359–370. 10.2307/3236279 [DOI] [Google Scholar]
- Bunn, A. , Korpela, M. , Biondi, F. , Campelo, F. , Merian, P. , Qeadan, F. , & Zang, C. (2018). dplR: Dendrochronology program library in R. R package version 1.6.4. Retrieved from https://CRAN.R-project.org/package=dplR
- Buras, A. , & Menzel, A. (2019). Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios. Frontiers in Plant Science, 9, 1986 10.3389/fpls.2018.01986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras, A. , Schunk, C. , Zeiträg, C. , Herrmann, C. , Kaiser, L. , Lemme, H. , … Menzel, A. (2018). Are Scots pine forest edges particularly prone to drought‐induced mortality? Environmental Research Letters, 13, 025001 10.1088/1748-9326/aaa0b4 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). New York: Springer‐Verlag. [Google Scholar]
- Cabon, A. , Peters, R. L. , Fonti, P. , Martínez‐Vilalta, J. , & De Cáceres, M. (2020). Temperature and water potential co‐limit stem cambial activity along a steep elevational gradient. New Phytologist, 226(5), 1325–1340. 10.1111/nph.16456 [DOI] [PubMed] [Google Scholar]
- Cailleret, M. , Dakos, V. , Jansen, S. , Robert, E. M. R. , Aakala, T. , Amoroso, M. M. , … Martínez‐Vilalta, J. (2019). Early‐warning signals of individual tree mortality based on annual radial growth. Frontiers in Plant Science, 9, 1964 10.3389/fpls.2018.01964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero, J. J. , Gazol, A. , Galván, J. D. , Sangüesa‐Barreda, G. , & Gutiérrez, E. (2015). Disparate effects of global‐change drivers on mountain conifer forests: Warming‐induced growth enhancement in young trees vs. CO2 fertilization in old trees from wet sites. Global Change Biology, 21(2), 738–749. 10.1111/gcb.12787 [DOI] [PubMed] [Google Scholar]
- Camarero, J. J. , Gazol, A. , Sangüesa‐Barreda, G. , Oliva, J. , & Vicente‐Serrano, S. M. (2015). To die or not to die: Early warnings of tree dieback in response to a severe drought. Journal of Ecology, 103(1), 44–57. 10.1111/1365-2745.12295 [DOI] [Google Scholar]
- Carnicer, J. , Coll, M. , Pons, X. , Ninyerola, M. , Vayreda, J. , & Peñuelas, J. (2014). Large‐scale recruitment limitation in Mediterranean pines: The role of Quercus ilex and forest successional advance as key regional drivers. Global Ecology and Biogeography, 23(3), 371–384. [Google Scholar]
- Carrer, M. , Nola, P. , Eduard, J. L. , Motta, R. , & Urbinati, C. (2007). Regional variability of climate–growth relationships in Pinus cembra high elevation forests in the Alps. Journal of Ecology, 95(5), 1072–1083. 10.1111/j.1365-2745.2007.01281.x [DOI] [Google Scholar]
- Choat, B. , Brodribb, T. J. , Brodersen, C. R. , Duursma, R. A. , López, R. , & Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature, 558(7711), 531–539. [DOI] [PubMed] [Google Scholar]
- Ciais, P. H. , Reichstein, M. , Viovy, N. , Granier, A. , Ogée, J. , Allard, V. , … Valentini, R. (2005). Europe‐wide reduction in primary productivity caused by the heat and drought in 2003. Nature, 437, 529–533. 10.1038/nature03972 [DOI] [PubMed] [Google Scholar]
- Cook, E. R. , & Kairiukstis, L. A. (1990). Methods of dendrochronology: Applications in the environmental sciences. Dordrecht, The Netherlands: Kluwer; ISBN‐13: 978‐0‐7923‐0586‐6. 10.1007/978-94-015-7879-0 [DOI] [Google Scholar]
- Cudlín, P. , Cudlín, P. , Cudlín, P. , Tognetti, R. , Malis, F. , Alados, C. L. , … Wielgolaski, F. E. (2017). Drivers of treeline shift in different European mountains. Climate Research, 73(1–2), 135–150. 10.3354/cr01465 [DOI] [Google Scholar]
- Dai, A. (2012). Increasing drought under global warming in observations and models. Nature Climate Change, 3, 52. [Google Scholar]
- D'Amato, A. W. , Bradford, J. B. , Fraver, S. , & Palik, B. J. (2013). Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecological Applications, 23(8), 1735–1742. 10.1890/13-0677.1 [DOI] [PubMed] [Google Scholar]
- De Martonne, E. (1926). Une nouvelle fanction climatologique. L'indice D'aridite La Meteriologie, 449–458. [Google Scholar]
- DeSoto, L. , Cailleret, M. , Sterck, F. , Jansen, S. , Kramer, K. , Robert, E. M. R. , … Martínez‐Vilalta, J. (2020). Low growth resilience to drought is related to future mortality risk in trees. Nature Communications, 11(1), 545 10.1038/s41467-020-14300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diéguez‐Aranda, U. , Álvarez González, J. G. , Marcos Barrio, A. , & Alberto Rojo, A. (2005). Site quality equations for Pinus sylvestris L. plantations in Galicia (northwestern Spain). Annals of Forest Science, 62(2), 143–152. 10.1051/forest:2005006 [DOI] [Google Scholar]
- Diéguez‐Aranda, U. , Burkhart, H. E. , & Rodríguez‐Soalleiro, R. (2005). Modeling dominant height growth of radiata pine (Pinus radiata D. Don) plantations in north‐western Spain. Forest Ecology and Management, 215(1), 271–284. 10.1016/j.foreco.2005.05.015 [DOI] [Google Scholar]
- Etzold, S. , Ziemińska, K. , Rohner, B. , Bottero, A. , Bose, A. K. , Ruehr, N. K. , … Rigling, A. (2019). One century of forest monitoring data in Switzerland reveals species‐ and site‐specific trends of climate‐induced tree mortality. Frontiers in Plant Science, 10, 307 10.3389/fpls.2019.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas, J. , & Medrano, H. (2002). Drought‐inhibition of photosynthesis in C3 plants: Stomatal and non‐stomatal limitations revisited. Annals of Botany, 89(2), 183–189. 10.1093/aob/mcf027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, D. I. , Bonal, D. , Dawud, S. , Gessler, A. , Granier, A. , Pollastrini, M. , & Grossiord, C. (2016). Drought responses by individual tree species are not often correlated with tree species diversity in European forests. Journal of Applied Ecology, 53(6), 1725–1734. 10.1111/1365-2664.12745 [DOI] [Google Scholar]
- Fox, J. , & Weisberg, S. (2011). An {R} companion to applied regression (2nd edn). Thousand Oaks, CA: SAGE Publications Inc. [Google Scholar]
- Fritts, H. C. (2001). Tree rings and climate. Blackburn, UK. ISBN‐13: 978‐1‐930665‐39‐2. [Google Scholar]
- Galiano, L. , Martínez‐Vilalta, J. , & Lloret, F. (2010). Drought‐induced multifactor decline of Scots pine in the Pyrenees and potential vegetation change by the expansion of co‐occurring oak species. Ecosystems, 13(7), 978–991. 10.1007/s10021-010-9368-8 [DOI] [Google Scholar]
- Galiano, L. , Martínez‐Vilalta, J. , & Lloret, F. (2011). Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytologist, 190(3), 750–759. [DOI] [PubMed] [Google Scholar]
- Gao, S. , Liu, R. , Zhou, T. , Fang, W. , Yi, C. , Lu, R. , … Luo, H. (2018). Dynamic responses of tree‐ring growth to multiple dimensions of drought. Global Change Biology, 24(11), 5380–5390. 10.1111/gcb.14367 [DOI] [PubMed] [Google Scholar]
- Gazol, A. , Camarero, J. J. , Anderegg, W. R. L. , & Vicente‐Serrano, S. M. (2017). Impacts of droughts on the growth resilience of Northern Hemisphere forests. Global Ecology and Biogeography, 26(2), 166–176. 10.1111/geb.12526 [DOI] [Google Scholar]
- Gazol, A. , Camarero, J. J. , Vicente‐Serrano, S. M. , Sánchez‐Salguero, R. , Gutiérrez, E. , de Luis, M. , … Galván, J. D. (2018). Forest resilience to drought varies across biomes. Global Change Biology, 24(5), 2143–2158. 10.1111/gcb.14082 [DOI] [PubMed] [Google Scholar]
- Gessler, A. , Schaub, M. , & McDowell, N. G. (2017). The role of nutrients in drought‐induced tree mortality and recovery. New Phytologist, 214(2), 513–520. 10.1111/nph.14340 [DOI] [PubMed] [Google Scholar]
- Giordano, L. , Gonthier, P. , Varese, G. C. , Miserere, L. , & Nicolotti, G. (2009). Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Diversity, 38, 69–83. [Google Scholar]
- Granda, E. , Gazol, A. , & Camarero, J. J. (2018). Functional diversity differently shapes growth resilience to drought for co‐existing pine species. Journal of Vegetation Science, 29(2), 265–275. 10.1111/jvs.12617 [DOI] [Google Scholar]
- Greenwood, S. , Ruiz‐Benito, P. , Martínez‐Vilalta, J. , Lloret, F. , Kitzberger, T. , Allen, C. D. , … Jump, A. S. (2017). Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecology Letters, 20(4), 539–553. 10.1111/ele.12748 [DOI] [PubMed] [Google Scholar]
- Grissino‐Mayer, H. D. (2001). Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree‐Ring Research, 57, 205–221. [Google Scholar]
- Grossiord, C. (2019). Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytologist, in press. 10.1111/nph.15667 [DOI] [PubMed] [Google Scholar]
- Grossiord, C. , Granier, A. , Ratcliffe, S. , Bouriaud, O. , Bruelheide, H. , Checko, E. , … Gessler, A. (2014). Tree diversity does not always improve resistance of forest ecosystems to drought. Proceedings of the National Academy of Sciences of the United States of America, 111(41), 14812–14815. 10.1073/pnas.1411970111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, A. , Strobl, S. , Veit, B. , & Oberhuber, W. (2010). Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris . Tree Physiology, 30(4), 490–501. 10.1093/treephys/tpq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn, F. , Shiyatov, S. G. , Mazepa, V. S. , Devi, N. M. , Grigor'ev, A. A. , Bartysh, A. A. , … Moiseev, P. A. (2014). Treeline advances along the Urals mountain range – Driven by improved winter conditions? Global Change Biology, 20(11), 3530–3543. 10.1111/gcb.12613 [DOI] [PubMed] [Google Scholar]
- Hampe, A. , & Petit, R. J. (2005). Conserving biodiversity under climate change: The rear edge matters. Ecology Letters, 8(5), 461–467. 10.1111/j.1461-0248.2005.00739.x [DOI] [PubMed] [Google Scholar]
- Hamrick, J. L. (2004). Response of forest trees to global environmental changes. Forest Ecology and Management, 197(1), 323–335. 10.1016/j.foreco.2004.05.023 [DOI] [Google Scholar]
- Hereş, A.‐M. , Martínez‐Vilalta, J. , & Claramunt López, B. (2012). Growth patterns in relation to drought‐induced mortality at two Scots pine (Pinus sylvestris L.) sites in NE Iberian Peninsula. Trees, 26(2), 621–630. [Google Scholar]
- Hevia, A. , Sánchez‐Salguero, R. , Camarero, J. J. , Querejeta, J. I. , Sangüesa‐Barreda, G. , & Gazol, A. (2019). Long‐term nutrient imbalances linked to drought‐triggered forest dieback. Science of the Total Environment, 690, 1254–1267. 10.1016/j.scitotenv.2019.06.515 [DOI] [PubMed] [Google Scholar]
- Hökkä, H. , & Ojansuu, R. (2004). Height development of Scots pine on peatlands: Describing change in site productivity with a site index model. Canadian Journal of Forest Research, 34(5), 1081–1092. 10.1139/x03-275 [DOI] [Google Scholar]
- Hothorn, T. , Bretz, F. , & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal, 50(3), 346–363. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- Isaac‐Renton, M. , Montwé, D. , Hamann, A. , Spiecker, H. , Cherubini, P. , & Treydte, K. (2018). Northern forest tree populations are physiologically maladapted to drought. Nature Communications, 9(1), 5254 10.1038/s41467-018-07701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch, A. , Kreyling, J. , Elmer, M. , Gellesch, E. , Glaser, B. , Grant, K. , … Beierkuhnlein, C. (2011). Climate extremes initiate ecosystem‐regulating functions while maintaining productivity. Journal of Ecology, 99(3), 689–702. 10.1111/j.1365-2745.2011.01817.x [DOI] [Google Scholar]
- Johnson, J. B. , & Omland, K. S. (2004). Model selection in ecology and evolution. Trends in Ecology & Evolution, 19(2), 101–108. 10.1016/j.tree.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Kahle, D. , & Wickham, H. (2013). ggmap: Spatial visualization with ggplot2. The R Journal., 5, 144–161. 10.32614/RJ-2013-014 [DOI] [Google Scholar]
- Kannenberg, S. A. , Maxwell, J. T. , Pederson, N. , D'Orangeville, L. , Ficklin, D. L. , & Phillips, R. P. (2019). Drought legacies are dependent on water table depth, wood anatomy and drought timing across the eastern US. Ecology Letters, 22(1), 119–127. 10.1111/ele.13173 [DOI] [PubMed] [Google Scholar]
- Kaufman, L. , & Rousseeuw, P. (1990). Finding groups in data: An introduction to cluster analysis. New York: John Wiley & Sons Inc. [Google Scholar]
- Körner, C. (2003). Carbon limitation in trees. Journal of Ecology, 91(1), 4–17. 10.1046/j.1365-2745.2003.00742.x [DOI] [Google Scholar]
- Lévesque, M. , Rigling, A. , Bugmann, H. , Weber, P. , & Brang, P. (2014). Growth response of five co‐occurring conifers to drought across a wide climatic gradient in Central Europe. Agricultural and Forest Meteorology, 197, 1–12. 10.1016/j.agrformet.2014.06.001 [DOI] [Google Scholar]
- Lévesque, M. , Saurer, M. , Siegwolf, R. , Eilmann, B. , Brang, P. , Bugmann, H. , & Rigling, A. (2013). Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Global Change Biology, 19(10), 3184–3199. 10.1111/gcb.12268 [DOI] [PubMed] [Google Scholar]
- Linares, J. C. , Camarero, J. J. , & Carreira, J. A. (2010). Competition modulates the adaptation capacity of forests to climatic stress: Insights from recent growth decline and death in relict stands of the Mediterranean fir Abies pinsapo. Journal of Ecology, 98(3), 592–603. [Google Scholar]
- Lloret, F. , Keeling, E. G. , & Sala, A. (2011). Components of tree resilience: Effects of successive low‐growth episodes in old ponderosa pine forests. Oikos, 120(12), 1909–1920. 10.1111/j.1600-0706.2011.19372.x [DOI] [Google Scholar]
- Maes, S. L. , Perring, M. P. , Vanhellemont, M. , Depauw, L. , Van den Bulcke, J. , Brūmelis, G. , … Verheyen, K. (2019). Environmental drivers interactively affect individual tree growth across temperate European forests. Global Change Biology, 25(1), 201–217. 10.1111/gcb.14493 [DOI] [PubMed] [Google Scholar]
- Magnani, F. , Mencuccini, M. , & Grace, J. (2000). Age‐related decline in stand productivity: The role of structural acclimation under hydraulic constraints. Plant, Cell & Environment, 23(3), 251–263. 10.1046/j.1365-3040.2000.00537.x [DOI] [Google Scholar]
- Mäkinen, H. , Yue, C. , & Kohnle, U. (2017). Site index changes of Scots pine, Norway spruce and larch stands in southern and central Finland. Agricultural and Forest Meteorology, 237–238, 95–104. 10.1016/j.agrformet.2017.01.017 [DOI] [Google Scholar]
- Martínez‐Vilalta, J. , Cochard, H. , Mencuccini, M. , Sterck, F. , Herrero, A. , Korhonen, J. F. J. , … Zweifel, R. (2009). Hydraulic adjustment of Scots pine across Europe. New Phytologist, 184(2), 353–364. 10.1111/j.1469-8137.2009.02954.x [DOI] [PubMed] [Google Scholar]
- Martínez‐Vilalta, J. , López, B. C. , Loepfe, L. , & Lloret, F. (2012). Stand‐ and tree‐level determinants of the drought response of Scots pine radial growth. Oecologia, 168(3), 877–888. 10.1007/s00442-011-2132-8 [DOI] [PubMed] [Google Scholar]
- Matías, L. , Linares, J. C. , Sánchez‐Miranda, Á. , & Jump, A. S. (2017). Contrasting growth forecasts across the geographical range of Scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Global Change Biology, 23(10), 4106–4116. 10.1111/gcb.13627 [DOI] [PubMed] [Google Scholar]
- Mátyás, C. , Ackzell, L. , & Samuel, C. J. A. (2004). EUFORGEN technical guidelines for genetic conservation and use for Scots pine (Pinus sylvestris). International Plant Genetic Resources Institute, Rome, Italy, 6 [Google Scholar]
- Mazerolle, M. J. (2006). Improving data analysis in herpetology: Using Akaike's Information Criterion (AIC) to assess the strength of biological hypotheses. Amphibia‐Reptilia, 27, 169–180. 10.1163/156853806777239922 [DOI] [Google Scholar]
- Mazerolle, M. J. (2011). AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 1.17. Retrieved from http://cran.r-project.org/web/packages/AICcmodavg/index.html
- McDowell, N. , Pockman, W. T. , Allen, C. D. , Breshears, D. D. , Cobb, N. , Kolb, T. , … Yepez, E. A. (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178(4), 719–739. 10.1111/j.1469-8137.2008.02436.x [DOI] [PubMed] [Google Scholar]
- McKee, T. B. , Doesken, N. J. , & Kleist, J. (1993). The relationship of drought frequency and duration to time scales (pp. 179–184). Preprints, Eight Conference on Applied Climatology. Anaheim, CA: American Meteor Society. [Google Scholar]
- Ogle, K. , Whitham, T. G. , & Cobb, N. S. (2000). Tree‐ring variation in pinyon predicts likelihood of death following severe drought. Ecology, 81(11), 3237–3243. 10.1890/0012-9658(2000)081[3237:TRVIPP]2.0.CO;2 [DOI] [Google Scholar]
- Olson, M. E. , Soriano, D. , Rosell, J. A. , Anfodillo, T. , Donoghue, M. J. , Edwards, E. J. , … Méndez‐Alonzo, R. (2018). Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences of the United States of America, 115(29), 7551 10.1073/pnas.1721728115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palahí, M. , Tomé, M. , Pukkala, T. , Trasobares, A. , & Montero, G. (2004). Site index model for Pinus sylvestris in north‐east Spain. Forest Ecology and Management, 187(1), 35–47. 10.1016/S0378-1127(03)00312-8 [DOI] [Google Scholar]
- Pasho, E. , Camarero, J. J. , de Luis, M. , & Vicente‐Serrano, S. M. (2011). Impacts of drought at different time scales on forest growth across a wide climatic gradient in north‐eastern Spain. Agricultural and Forest Meteorology, 151(12), 1800–1811. 10.1016/j.agrformet.2011.07.018 [DOI] [Google Scholar]
- Peñuelas, J. , Hunt, J. M. , Ogaya, R. , & Jump, A. S. (2008). Twentieth century changes of tree‐ring δ13C at the southern range‐edge of Fagus sylvatica: Increasing water‐use efficiency does not avoid the growth decline induced by warming at low altitudes. Global Change Biology, 14(5), 1076–1088. [Google Scholar]
- Pinheiro, J. C. , & Bates, D. M. (2000). Mixed effects models in S and S‐PLUS. New York, NY: Springer Verlag. [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , & Sarkar, D. (2014). nlme: Linear and nonlinear mixed effects models. R package version 3.1‐117. Retrieved from https://cran.rproject.org/web/packages/nlme/index.html
- Pretzsch, H. , Schütze, G. , & Uhl, E. (2013). Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter‐specific facilitation. Plant Biology, 15(3), 483–495. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from www.r-project.org [Google Scholar]
- Reddy, A. R. , Chaitanya, K. V. , & Vivekanandan, M. (2004). Drought‐induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology, 161(11), 1189–1202. 10.1016/j.jplph.2004.01.013 [DOI] [PubMed] [Google Scholar]
- Rigling, A. , Bigler, C. , Eilmann, B. , Feldmeyer‐Christe, E. , Gimmi, U. , Ginzler, C. , … Dobbertin, M. (2013). Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Global Change Biology, 19(1), 229–240. 10.1111/gcb.12038 [DOI] [PubMed] [Google Scholar]
- Rigling, A. , Eilmann, B. , Koechli, R. , & Dobbertin, M. (2010). Mistletoe‐induced crown degradation in Scots pine in a xeric environment. Tree Physiology, 30, 845–852. 10.1093/treephys/tpq038 [DOI] [PubMed] [Google Scholar]
- Rouault, G. , Candau, J.‐N. , Lieutier, F. , Nageleisen, L.‐M. , Martin, J.‐C. , & Warzée, N. (2006). Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Annals of Forest Science, 63(6), 613–624. 10.1051/forest:2006044 [DOI] [Google Scholar]
- Ruijven, J. , & Berendse, F. (2010). Diversity enhances community recovery, but not resistance, after drought. Journal of Ecology, 98(1), 81–86. [Google Scholar]
- Ryan, M. G. , & Yoder, B. J. (1997). Hydraulic limits to tree height and tree growth. BioScience, 47, 235–242. 10.2307/1313077 [DOI] [Google Scholar]
- Sánchez‐Salguero, R. , Camarero, J. J. , Hevia, A. , Madrigal‐González, J. , Linares, J. C. , Ballesteros‐Canovas, J. A. , … Rigling, A. (2015). What drives growth of Scots pine in continental Mediterranean climates: Drought, low temperatures or both? Agricultural and Forest Meteorology, 206, 151–162. 10.1016/j.agrformet.2015.03.004 [DOI] [Google Scholar]
- Sánchez‐Salguero, R. , Camarero, J. J. , Rozas, V. , Génova, M. , Olano, J. M. , Arzac, A. , … Linares, J. C. (2018). Resist, recover or both? Growth plasticity in response to drought is geographically structured and linked to intraspecific variability in Pinus pinaster . Journal of Biogeography, 45(5), 1126–1139. 10.1111/jbi.13202 [DOI] [Google Scholar]
- Sánchez‐Salguero, R. , Navarro‐Cerrillo, R. M. , Camarero, J. J. , & Fernández‐Cancio, Á. (2012). Selective drought‐induced decline of pine species in southeastern Spain. Climatic Change, 113(3), 767–785. 10.1007/s10584-011-0372-6 [DOI] [Google Scholar]
- Scholz, F. , Phillips, N. , Bucci, S. , & Goldstein, G. (2011). Hydraulic capacitance: Biophysics and functional significance of internal water sources in relation to tree size In Meinzer F. C., Lachenbruch B. & Dawson T. E. (Eds.), Size‐ and age‐related changes in tree structure and function (Vol. 4, pp. 341–361). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Seidel, H. , Matiu, M. , & Menzel, A. (2019). Compensatory growth of Scots pine seedlings mitigates impacts of multiple droughts within and across years. Frontiers in Plant Science, 10, 519–519. 10.3389/fpls.2019.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel, H. , Schunk, C. , Matiu, M. , & Menzel, A. (2016). Diverging drought resistance of Scots pine provenances revealed by infrared thermography. Frontiers in Plant Science, 7, 1247 10.3389/fpls.2016.01247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra‐Maluquer, X. , Mencuccini, M. , & Martínez‐Vilalta, J. (2018). Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia, 187(1), 343–354. 10.1007/s00442-018-4118-2 [DOI] [PubMed] [Google Scholar]
- Smith, M. D. (2011). An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. Journal of Ecology, 99(3), 656–663. 10.1111/j.1365-2745.2011.01798.x [DOI] [Google Scholar]
- Spinoni, J. , Naumann, G. , Carrao, H. , Barbosa, P. , & Vogt, J. (2014). World drought frequency, duration, and severity for 1951–2010. International Journal of Climatology, 34(8), 2792–2804. 10.1002/joc.3875 [DOI] [Google Scholar]
- Spinoni, J. , Vogt, J. V. , Naumann, G. , Barbosa, P. , & Dosio, A. (2018). Will drought events become more frequent and severe in Europe? International Journal of Climatology, 38(4), 1718–1736. 10.1002/joc.5291 [DOI] [Google Scholar]
- Sterck, F. J. , Zweifel, R. , Sass‐Klaassen, U. , & Chowdhury, Q. (2008). Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiology, 28(4), 529–536. 10.1093/treephys/28.4.529 [DOI] [PubMed] [Google Scholar]
- Stovall, A. E. L. , Shugart, H. , & Yang, X. (2019). Tree height explains mortality risk during an intense drought. Nature Communications, 10(1), 4385 10.1038/s41467-019-12380-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szejner, P. , Belmecheri, S. , Ehleringer, J. R. , & Monson, R. K. (2020). Recent increases in drought frequency cause observed multi‐year drought legacies in the tree rings of semi‐arid forests. Oecologia, 192, 241–259. [DOI] [PubMed] [Google Scholar]
- Taeger, S. , Zang, C. , Liesebach, M. , Schneck, V. , & Menzel, A. (2013). Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. Forest Ecology and Management, 307, 30–42. 10.1016/j.foreco.2013.06.053 [DOI] [Google Scholar]
- Valladares, F. , Gianoli, E. , & Gómez, J. (2007). Ecological limits to plant phenotypic plasticity. New Phytologist, 176(4), 749–763. 10.1111/j.1469-8137.2007.02275.x [DOI] [PubMed] [Google Scholar]
- Valladares, F. , Matesanz, S. , Guilhaumon, F. , Araújo, M. B. , Balaguer, L. , Benito‐Garzón, M. , … Zavala, M. A. (2014). The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters, 17(11), 1351–1364. 10.1111/ele.12348 [DOI] [PubMed] [Google Scholar]
- Vanninen, P. , & Mäkelä, A. (2005). Carbon budget for Scots pine trees: Effects of size, competition and site fertility on growth allocation and production. Tree Physiology, 25(1), 17–30. 10.1093/treephys/25.1.17 [DOI] [PubMed] [Google Scholar]
- Vennetier, M. , Ripert, C. , & Rathgeber, C. (2018). Autecology and growth of Aleppo pine (Pinus halepensis Mill.): A comprehensive study in France. Forest Ecology and Management, 413, 32–47. 10.1016/j.foreco.2018.01.028 [DOI] [Google Scholar]
- Vennetier, M. , Thabeet, A. , Didier, C. , Girard, F. , Cailleret, M. , Taugourdeau, O. , … Caraglio, Y. (2013). Climate change impact on tree architectural development and leaf area In Singh B. R. (Eds.), Climate change—realities, impacts over ice cap, sea level and risks. INTECH Open Access Publisher; 10.5772/51510 [DOI] [Google Scholar]
- Vicente‐Serrano, S. M. , Beguería, S. , & López‐Moreno, J. I. (2010). A multiscalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index. Journal of Climate, 23(7), 1696–1718. 10.1175/2009JCLI2909.1 [DOI] [Google Scholar]
- Vicente‐Serrano, S. M. , Gouveia, C. , Camarero, J. J. , Begueria, S. , Trigo, R. , Lopez‐Moreno, J. I. , … Sanchez‐Lorenzo, A. (2013). Response of vegetation to drought time‐scales across global land biomes. Proceedings of the National Academy of Sciences of the United States of America, 110(1), 52–57. 10.1073/pnas.1207068110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar‐Salvador, P. , Peñuelas, J. L. , & Jacobs, D. F. (2013). Nitrogen nutrition and drought hardening exert opposite effects on the stress tolerance of Pinus pinea L. seedlings. Tree Physiology, 33(2), 221–232. 10.1093/treephys/tps133 [DOI] [PubMed] [Google Scholar]
- Vitali, V. , Büntgen, U. , & Bauhus, J. (2017). Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south‐western Germany. Global Change Biology, 23(12), 5108–5119. 10.1111/gcb.13774 [DOI] [PubMed] [Google Scholar]
- Walther, G.‐R. , Post, E. , Convey, P. , Menzel, A. , Parmesan, C. , Beebee, T. J. , … Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416(6879), 389–395. [DOI] [PubMed] [Google Scholar]
- Westoby, M. , Falster, D. S. , Moles, A. T. , Vesk, P. A. , & Wright, I. J. (2002). Plant ecological strategies: Some leading dimensions of variation between species. Annual Review of Ecology and Systematics, 33(1), 125–159. 10.1146/annurev.ecolsys.33.010802.150452 [DOI] [Google Scholar]
- Zalloni, E. , Battipaglia, G. , Cherubini, P. , Saurer, M. , & De Micco, V. (2019). Wood growth in pure and mixed Quercus ilex L. forests: Drought influence depends on site conditions. Frontiers Plant Science, 10, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, C. , Buras, A. , Esquivel‐Muelbert, A. , Jump, A. S. , Rigling, A. , & Rammig, A. (2020). Standardized drought indices in ecological research: Why one size does not fit all. Global Change Biology, 26, 322–324. 10.1111/gcb.14809 [DOI] [PubMed] [Google Scholar]
- Zang, C. , Hartl‐Meier, C. , Dittmar, C. , Rothe, A. , & Menzel, A. (2014). Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Global Change Biology, 20(12), 3767–3779. 10.1111/gcb.12637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.


