Abstract
Objective
To assess pharmacokinetics and safety of diazepam nasal spray (NRL‐1; VALTOCO®) in pediatric and adult patients with epilepsy in seizure and nonseizure states.
Methods
A single dose of diazepam nasal spray (5, 10, 15, or 20 mg based on weight) was administered during each of two conditions (ictal/peri‐ictal and interictal condition) to patients 6‐65 years old with partial or generalized epilepsy with motor seizures or seizures with clear alteration of awareness; a second dose was permitted if needed for persistent seizures. Dosing could be interictal or ictal/peri‐ictal first, with a washout of ≥14 days. Blood samples for pharmacokinetic analysis were taken at prespecified time points. Treatment‐emergent adverse events (TEAEs), sedation, nasal irritation, nasal mucosal pain, and olfactory changes were assessed.
Results
Of 57 patients in the study (mean age = 28.1 years [range = 6‐59], 54.4% female, 80.7% white), 49 were included in the primary pharmacokinetic analyses. Diazepam pharmacokinetic profiles were similar under both conditions, with approximately 2‐hour median time to mean (SD) maximum plasma concentrations of 164 (88) and 189 (110) ng/mL for ictal/peri‐ictal and interictal conditions, respectively; drug exposure during the first 6 hours postdosing was 532 (313) and 615 (368) h•ng/mL, respectively. Seventeen patients (29.8%) reported TEAEs, of whom eight (14%) had treatment‐related TEAEs, with those reported in ≥2 patients being dysgeusia (n = 3, 5.3%) and nasal discomfort (n = 2, 3.5%). One patient had serious TEAEs (recurrent seizures, metabolic encephalopathy), which were deemed unrelated to study treatment. No changes in respiratory rate were observed, nor were there clinically relevant changes in sedation, olfaction, nasal irritation, or acute nasal mucosal pain.
Significance
The epileptic conditions (ictal/peri‐ictal, interictal) had minimal impact on diazepam nasal spray pharmacokinetics in patients with epilepsy. Therefore, diazepam nasal spray can be administered ictally and interictally. Diazepam nasal spray safety was consistent with the profile of diazepam.
Keywords: diazepam, ictal, intranasal, pharmacokinetics, VALTOCO
Key Points.
Diazepam nasal spray administration in patients with epilepsy resulted in rapid diazepam absorption (reaching maximum plasma concentration at approximately 2 hours)
The epileptic condition (ictal/peri‐ictal, interictal) had minimal impact on diazepam nasal spray pharmacokinetics
The safety profile of diazepam nasal spray was consistent with expectations for diazepam
Sedation, nasal irritation, and olfactory changes after administration of diazepam nasal spray were mild, transient, and not clinically relevant
1. INTRODUCTION
Management of epilepsy relies on antiseizure drugs (ASDs) to prevent and control seizures. However, despite treatment with stable regimens of ASDs, some patients experience recognizable cluster patterns of increased seizure activity, often referred to as seizure clusters or acute repetitive seizures, 1 which require a readily available rescue medication that can be immediately and easily administered to manage these seizure emergencies in an outpatient setting.
Benzodiazepines have been the mainstay of rescue therapy, with rectal diazepam (Diastat), in particular, considered the standard of care in an outpatient setting, as it is appropriate for administration by non–health care professionals. However, rectal administration is associated with several limitations, including that it may be difficult to administer during active seizure particularly in larger patients, has wide pharmacokinetic variability 2 for reasons including that the gel can be expelled during seizure‐associated incontinence, and may result in embarrassment to patients and caregivers. Consequently, there remains a need for easier, more consistent, and more socially acceptable routes of administration.
Alternatives to the rectal route of diazepam administration include use of buccal and intranasal formulations. The intranasal route has advantages of accessibility during an ongoing seizure (ictal condition), a highly vascularized absorptive surface area, and the theoretical potential for direct nose to brain drug delivery that bypasses presystemic metabolism resulting from intestinal and hepatic first‐pass effects. 3 However, the nonaqueous solubility of benzodiazepines has presented a challenge for intranasal delivery of these drugs, 3 with the issues of solubility and absorption resulting in at least one prior failure for an experimental formulation to achieve a pharmacokinetic profile appropriate for this route of administration. 4
New approaches to intranasal delivery have resulted in two recent approvals from the US Food and Drug Administration. One, midazolam nasal spray (USL261; Nayzilam) is indicated for treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older. Approval was based on demonstrating that a significantly higher proportion of patients had “treatment success,” defined as seizure termination within 10 minutes and no seizure recurrence at 6 hours, compared with placebo (53.7% vs 34.3%; P < .05); 58.2% of the patients were seizure‐free at 6 hours relative to 37.3% with placebo, and these proportions were similar, 58.3% and 37.1%, respectively, at the 24‐hour time point. 5
Intranasal benzodiazepine formulations have relied on glycols as cosolvents. Diazepam nasal spray (NRL‐1; VALTOCO) is a unique intranasal preparation of diazepam in that it is formulated with Intravail A3 (n‐dodecyl beta‐D‐maltoside [DDM]) and vitamin E to enhance solubility and absorption. Three concentrations of diazepam, 5, 7.5, and 10 mg in 0.1 mL solution, facilitate weight‐based dosing. It is indicated for treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 6 years of age and older. Whereas vitamin E increases the nonaqueous solubility of diazepam, DDM is a nonionic surfactant that is used as an absorption enhancement agent to promote increased bioavailability of drugs across different types of mucosae, 6 and is a Generally Recognized as Safe excipient for oral administration. In early studies, diazepam nasal spray demonstrated 2‐ to 4‐fold less intrasubject pharmacokinetic variability than rectal diazepam, 7 and although the time from dosing to maximum plasma concentration (tmax) of intranasal diazepam is >1 hour, it has 97% bioavailability and a half‐life of ~49 hours. 8 However, because autonomic changes during a seizure could conceivably have an impact on mucosal drug absorption, the purpose of the current study was to assess the similarity of the pharmacokinetics and safety of diazepam nasal spray in patients with epilepsy during ictal/peri‐ictal and interictal periods.
2. MATERIALS AND METHODS
2.1. Study design
This phase 1, open‐label study was conducted on an inpatient basis at the study site. The study received approval from each site's institutional review board and was conducted in accordance with the Declaration of Helsinki; written informed consent was provided by all subjects or their parents/guardians prior to study participation.
Open‐label diazepam nasal spray was administered under two conditions, the ictal/peri‐ictal condition (defined as either during or immediately following a seizure; ideally within 5 minutes of seizure onset) and the interictal condition, with ≥14 days of washout between the two. Dosing could be done in either order (interictal first or ictal/peri‐ictal first); dosing for the interictal condition required that the subject be seizure‐free for at least the past 12 hours. In both conditions, a weight‐ and age‐ based single dose was administered. For patients 6–11 years of age, dosing was 5 mg (10–18 kg), 10 mg (19–37 kg), 15 mg (38–55 kg), or 20 mg (56–74 kg), and for patients 12 years of age and older, dosing was 5 mg (14–27 kg), 10 mg (28–50 kg), 15 mg (51–75 kg), or 20 mg (≥76 kg). [Corrections added on May 26, 2020, after first online publication: The last two sentences were revised.] A second dose permitted for subsequent seizures between 4 and 12 hours after the initial dose; the 5‐ and 10‐mg doses were administered into a single nostril, whereas the 15‐ and 20‐mg dose required an administration of half the dose into each nostril. Baseline for the ictal/peri‐ictal condition was considered the period within 7 days of admission to the clinical study site (ie, Epilepsy Monitoring Unit or Clinical Trial Research Center), and dosing was to occur at the time of a seizure considered suitable for administration of diazepam nasal spray.
2.2. Subjects
Eligible subjects were males and females between the ages of 6 and 65 years, inclusive, who had a clinical diagnosis of epilepsy, either focal (partial) or generalized, with motor seizures or seizures with clear alteration of awareness. Subjects were also required to have a body mass index ≤ 35 kg/m2 and no clinically significant abnormal findings in their medical history, or on physical examination, electrocardiogram (Fridericia correction factor for QT intervals [QTcF] < 450 milliseconds for males and QTcF < 470 milliseconds for females), or clinical laboratory results during screening. Female subjects of childbearing potential had to agree to use a medically acceptable method of contraception during the study duration, and those who were trying to conceive, were pregnant, or were lactating were excluded. Other key exclusion criteria were a history of major depression or a past suicide attempt or suicide ideation, a history of allergy or adverse response to diazepam, treatment with enzyme‐altering drugs in the past 14 days, over the counter oral and/or nasal decongestants in the past 14 days, or prescription medications, including benzodiazepines, in the past 14 days, excluding hormonal contraceptives.
2.3. Bioanalytical evaluation
Serial blood samples (3 mL) were collected into labeled K2‐EDTA tubes by direct venipuncture at prespecified time points including baseline (admission to the clinical site) and at 10, 20, 30, and 45 minutes, and 1, 1.25, 1.5, 1.75, 2, 3, 4, 5, and 6 hours after dosing. After collection, the plasma was separated by centrifugation (2000 g × 10 minutes at 0‐4°C), and equal aliquots were transferred to two labeled tubes and stored at approximately −20°C until analysis.
Analyses of all samples were performed at a central laboratory (Syneos Health, formerly inVentiv Health) using validated liquid chromatography–tandem mass spectrometry methods to determine sample concentrations of diazepam over the range of quantitation (1‐1000 ng/mL); 1.00 ng/mL represents the lower limit of quantitation for diazepam. Standard procedures were used. 9
2.4. Safety and tolerability
Safety and tolerability were evaluated by the reporting of treatment‐emergent adverse events (TEAEs) regardless of causality, with additional determination by the investigator as to whether the events were related to treatment. Evaluations also included clinical laboratory tests, physical examination, vital sign measurement, electrocardiography, and the Columbia Suicide Severity Rating Scale. 10 Nasal irritation was assessed by a trained observer using a 6‐point scale (Table S1), with results expressed as the number and percentage of subjects with scores indicative of severity. Sedation, also assessed on a 6‐point scale (Table S1) and expressed as the mean (SD) score among the patients, was reported by a trained observer. Changes in olfaction were assessed using the NIH Toolbox Odor Identification Test. 11 Acute nasal mucosal pain following nasal administration was assessed using a 10‐cm visual analogue scale (VAS) with the ends of the scale defined as 0 = no pain and 10 = extreme pain; severity thresholds are generally considered as mild (0‐3), moderate (4‐6), and severe (7‐10).
2.5. Statistical and pharmacokinetic analyses
The number of subjects was determined based on the results of a preliminary study in which maximum plasma concentration (Cmax) was the pharmacokinetic parameter with the greatest variability for diazepam, with a coefficient of variation for the natural log‐transformed Cmax of 38%. Assuming half of this value is a result of the intrasubject variability expressed as percentage coefficient of variation (CV%), approximately 27 subjects would be required per age group, and 45 subjects total were determined to obtain at least 30 evaluable subjects who complete both pharmacokinetic sampling periods of the study.
For analysis, the safety population was defined as subjects who received at least one dose of diazepam nasal spray, and subjects who completed the study through the 6‐hour pharmacokinetic sampling period on at least one of the dosing days comprised the pharmacokinetic population. Pharmacokinetic parameters for diazepam were calculated from the individual plasma concentrations by noncompartmental methods using Phoenix WinNonlin version 7.0 (Certara Company). The plasma pharmacokinetic parameters calculated were Cmax, tmax, and area under the plasma concentration‐time curve over the first 6 hours (AUC0‐6). Plasma pharmacokinetic parameters were summarized using descriptive statistics.
Although this study was not powered or intended to show bioequivalence, an exploratory analysis was conducted to provide a relative comparison of the ictal/peri‐ictal and interictal administration. Dose equivalence was considered to be met if the 90% confidence interval (CI) for the geometric mean ratios among the comparisons of the two parameters fell within the 80% to 125% region.
3. RESULTS
3.1. Population
Fifty‐seven subjects were enrolled, received study drug in at least one condition, and comprised the safety population. No subjects were assigned to the 5‐mg dose; 13 subjects received 10 mg, 19 subjects 15 mg, and 25 subjects 20 mg. Subjects were 54.4% female and 80.7% white (8.8% black/African American and 10.5% other), with a mean age of 28.1 ± 15.3 years and mean body weight of 65.3 ± 24.3 kg (Table 1). Fifty‐four subjects completed the study (94.7%), and none of the three discontinuations was related to TEAEs. The pharmacokinetic population consisted of 50 patients, and their demographic characteristics were similar to those of the safety population (Table 1). One subject was excluded from the pharmacokinetic analysis because samples were not stored per study specifications.
TABLE 1.
Demographic characteristics of the safety and pharmacokinetic populations
| Variable | Safety population | Pharmacokinetic population | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 mg, n = 13 | 15 mg, n = 19 | 20 mg, n = 25 | Total, N = 57 | 10 mg, n = 12 | 15 mg, n = 15 | 20 mg, n = 23 | Total, N = 49 a | |
| Age, y, mean (SD) | 11.4 (5.5) | 27.1 (12.9) | 37.6 (12.7) | 28.1 (15.3) | 11.8 (5.6) | 24.8 (12.1) | 37.3 (13.0) | 27.7 (15.3) |
| Age group, n (%) | ||||||||
| 6‐11 y | 8 (61.5) | 2 (10.5) | 1 (4.0) | 11 (19.3) | 7 (58.3) | 2 (13.3) | 1 (4.3) | 10 (20.4) |
| 12‐16 y | 2 (15.4) | 3 (15.8) | 1 (4.0) | 6 (10.5) | 2 (16.7) | 3 (20.0) | 1 (4.3) | 5 a (10.2) |
| >16 y | 3 (23.1) | 14 (73.7) | 23 (92.0) | 40 (70.2) | 3 (25.0) | 10 (66.7) | 21 (91.3) | 34 (69.4) |
| Sex, n (%) | ||||||||
| Male | 6 (46.2) | 4 (21.1) | 16 (64.0) | 26 (45.6) | 6 (50.0) | 3 (20.0) | 13 (56.5) | 22 (44.9) |
| Female | 7 (53.8) | 15 (78.9) | 9 (36.0) | 31 (54.4) | 6 (50.0) | 12 (80.0) | 10 (43.5) | 27 a (55.1) |
| Race | ||||||||
| White | 9 (69.2) | 16 (84.2) | 21 (84.0) | 46 (80.7) | 9 (75.0) | 12 (80.0) | 19 (82.6) | 39 a (79.6) |
| Black/African American | 2 (15.4) | 0 | 3 (12.0) | 5 (8.8) | 1 (8.3) | 0 | 3 (13.0) | 4 (8.2) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (5.3) | 0 | 1 (1.8) | 0 | 1 (6.7) | 0 | 1 (2.0) |
| Other | 2 (15.4) | 2 (10.5) | 1 (4.0) | 5 (8.8) | 2 (16.7) | 2 (13.3) | 1 (4.3) | 5 (10.2) |
Subject 16‐109 received a 15 mg in the seizure state and a 20 mg dose in the non‐seizure state.
[Corrections added on May 26, 2020, after first online publication: Some of the values in the table were changed and a footnote has been included.]
3.2. Pharmacokinetics
As shown in Figure 1, the log‐transformed mean plasma concentration‐time profiles had generally similar patterns of diazepam exposure after intranasal administration of diazepam nasal spray under ictal/peri‐ictal and interictal conditions. Diazepam was rapidly absorbed, with a Cmax of approximately 2 hours after a nominal lag time.
FIGURE 1.
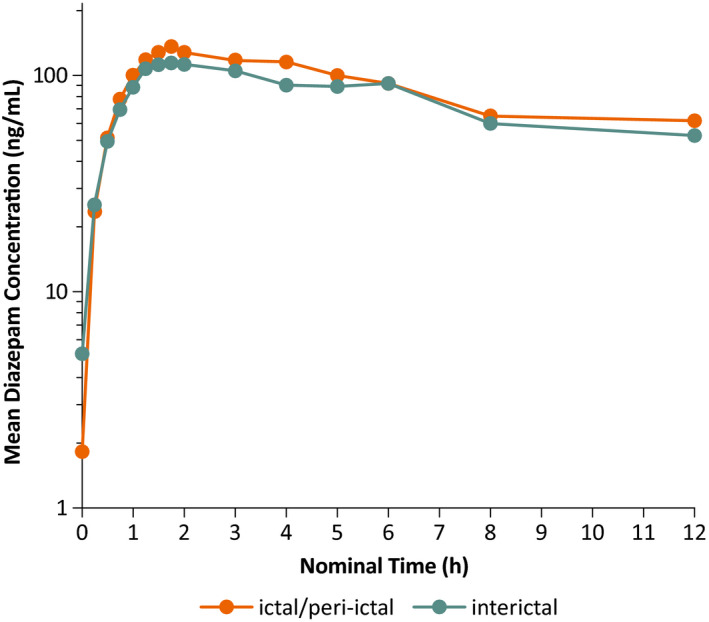
Log‐transformed mean plasma concentration versus time profile of diazepam after administration of diazepam nasal spray in the ictal/peri‐ictal and interictal conditions regardless of dose
Summary statistics of diazepam pharmacokinetic parameters were similar under ictal/peri‐ictal and interictal conditions (Table 2). The mean (SD) diazepam Cmax was 164 (87.7) ng/mL and 189 (110) ng/mL after administration under the ictal/peri‐ictal and interictal conditions, respectively, both with a median tmax of approximately 2 hours (Table 2). Extent of exposure over the first 6 hours after administration, expressed as the mean (SD) AUC0‐6, was 532 (313) h•ng/mL after ictal/peri‐ictal administration and 615 (368) h•ng/mL after interictal administration. Geometric means of the pharmacokinetic parameters were also similar between the two conditions (Table 2). The CV%, a measure of interpatient variability, ranged from 53.8% to 60.9% for arithmetic means of the pharmacokinetic parameters of Cmax and AUC0‐6, and 75.5% to 89.6% for the geometric means (Table 2).
TABLE 2.
Summary statistics of diazepam pharmacokinetic parameters regardless of dose
| Pharmacokinetic variable | Ictal/peri‐ictal, n = 46 a | Interictal, n = 47 a |
|---|---|---|
| Cmax, ng/mL | ||
| Mean ± SD (CV%) | 164 ± 88 (53.6) | 189 ± 110 (58.2) |
| Geometric mean (CV%) | 135 (89.6) | 153 (81.2) |
| t max, h, median (min, max) | 2.2 (0.5, 12.3) | 2.0 (0.5, 12.0) |
| AUC0‐6, h•ng/mL | ||
| Mean ± SD (CV%) | 532 ± 313 (58.8) | 615 ± 368 (59.7) |
| Geometric mean (CV%) | 435 (85.0) | 502 (78.1) |
| AUC0‐t, h•ng/mL | ||
| Mean ± SD (CV%) | 604 ± 351 (58.1) | 665 ± 386 (58.1) |
| Geometric mean (CV%) | 502 (75.5) | 546 (76.8) |
Abbreviations: AUC0‐6, area under the plasma concentration‐time curve over the first 6 hours; AUC0‐t, area under the plasma concentration‐time curve to the final sample with a measurable concentration; Cmax, maximum plasma concentration; CV%, percentage coefficient of variation; tmax, time to maximum plasma concentration.
Concentration data were not available for three subjects who were not dosed during either the interictal or ictal/peri‐ictal state.
Box and whisker plots of dose‐ and weight‐normalized diazepam Cmax and AUC0–6 displayed substantial overlap between the ictal/peri‐ictal and the interictal conditions (Figure 2). Using the interictal condition as the reference, the geometric mean ratios were 87.3% (90% CI = 71.4%‐106.8%) for Cmax and 86.5% (90% CI = 71.7%‐104.4%) for AUC0–6.
FIGURE 2.

Box and whisker plots of dose‐ and weight‐normalized diazepam maximum plasma concentration (Cmax) and area under the plasma concentration‐time curve over the first 6 hours (AUC0‐6) after administration of diazepam nasal spray under interictal and ictal/peri‐ictal conditions. The dashed line represents the median, the solid line is the arithmetic mean, the ends of the “box” are the first and third quartiles, and the whiskers show the lowest and highest data values within 1.5 of the interquartile range of the lower and upper quartiles, respectively. Data values that do not fall between the whiskers are plotted as markers outside of the whiskers
3.3. Safety
Seventeen subjects (29.8%) reported TEAEs, of which none resulted in discontinuation (Table 3). One subject (1.8%), who had a history of mental retardation and seizure and type 1 diabetes, had two serious TEAEs (recurrent seizures and metabolic encephalopathy), and although both events required prolonged hospitalization (15 and 17 days, respectively, and ultimately resolved), neither was considered by the investigator to be related to study drug.
TABLE 3.
Treatment‐emergent adverse events
| Event | Incidence, n (%) | |||
|---|---|---|---|---|
| 10 mg, n = 13 | 15 mg, n = 19 | 20 mg, n = 25 | Total, N = 57 | |
| Any TEAE | 4 (30.8) | 9 (47.4) | 4 (16.0) | 17 (29.8) |
| Serious TEAEs | 0 | 0 | 1 (4.0) | 1 (1.8) |
| Discontinuation due to TEAE | 0 | 0 | 0 | 0 |
| Most common TEAEs, ≥2 subjects | ||||
| Dysgeusia | 0 | 3 (15.8) | 0 | 3 (5.3) |
| Seizure | 1 (7.7) | 0 | 1 (4.0) | 2 (3.5) |
| Nasopharyngitis | 2 (15.4) | 0 | 0 | 2 (3.5) |
| Nasal discomfort | 0 | 2 (10.5) | 0 | 2 (3.5) |
| Treatment‐related TEAEs | 1 (7.7) | 6 (31.6) | 1 (4.0) | 8 (14.0) |
| Dysgeusia | 0 | 3 (15.8) | 0 | 3 (5.3) |
| Nasal discomfort | 0 | 2 (10.5) | 0 | 2 (3.5) |
| Burning sensation | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Dizziness | 0 | 0 | 1 (4.0) | 1 (1.8) |
| Epistaxis | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Hot flush | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Nasal congestion | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Nasopharyngitis | 1 (7.7) | 0 | 0 | 1 (1.8) |
| Paresthesia | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Sneezing | 0 | 1 (5.3) | 0 | 1 (1.8) |
| Urine odor abnormal | 0 | 1 (5.3) | 0 | 1 (1.8) |
Abbreviation: TEAE, treatment‐emergent adverse event.
The most common TEAEs, defined as those occurring in ≥2 subjects, included dysgeusia (5.3%), seizure (3.5%), nasopharyngitis (3.5%), and nasal discomfort (3.5%; Table 3). Of the two patients with seizures reported as TEAEs, both recovered (one required rescue treatment with intravenous diazepam) and neither withdrew from the study; these TEAEs were deemed unlikely to be related to study treatment.
TEAEs deemed possibly or probably related to treatment occurred in eight patients (14.0%) and mainly consisted of events that may be expected with nasal administration, such as dysgeusia (5.3%), nasal discomfort (3.5%), and burning sensation (1.8%; Table 3). Nasal leakage was not spontaneously reported. There were no clinically meaningful abnormalities noted for vital signs, and more specifically, no changes in respiratory rate or heart rate were observed in either the ictal/peri‐ictal period or the interictal period with intranasal diazepam administration. No clinically significant changes were noted in clinical laboratory tests or electrocardiograms; the abnormal electrocardiograms observed in 27 subjects (47.4%) at baseline were considered by the investigator to not be of clinical significance. No subjects had suicidal ideation at any time postdosing.
There were no reported TEAEs of somnolence, and although sedation scores indicated small increases in sedation overall, there was variability among the patients (Table 4). These small increases generally appeared to be higher during ictal/peri‐ictal administration, were transient, and were not dose‐dependent.
TABLE 4.
Sedation scores
| Time point, mean ± SD; minimum, maximum | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ictal/peri‐ictal period | Interictal period | |||||||
| 10 mg, n = 13 | 15 mg, n = 19 | 20 mg, n = 25 | Total, N = 57 | 10 mg, n = 13 | 15 mg, n = 19 | 20 mg, n = 25 | Total, N = 57 | |
| Baseline | 0.27 ± 0.65; 0.0, 2.0 | 0.32 ± 0.75; 0.0, 3.0 | 0.19 ± 0.51; 0.0, 2.0 | 0.25 ± 0.63; 0.0, 3.0 | 0.0 ± 0.0; 0.0, 0.0 | 0.21 ± 0.54; 0.0, 2.0 | 0.08 ± 0.28; 0.0, 1.0 | 0.11 ± 0.38; 0.0, 2.0 |
| Postdose, 15 min, ±5 min | 0.82 ± 0.98; 0.0, 2.0 | 1.07 ± 1.39; 0.0, 4.0 | 0.50 ± 0.80; 0.0, 3.0 | 0.74 ± 1.05; 0.0, 4.0 | 0.50 ± 1.00; 0.0, 3.0 | 0.21 ± 0.42; 0.0, 1.0 | 0.38 ± 0.77; 0.0, 3.0 | 0.35 ± 0.73; 0.0, 3.0 |
| Postdose, 30 min, ±5 min | 1.45 ± 1.44; 0.0, 4.0 | 1.07 ± 1.28; 0.0, 4.0 | 0.59 ± 0.73; 0.0, 2.0 | 0.94 ± 1.14; 0.0, 4.0 | 1.17 ± 1.34; 0.0, 3.0 | 0.26 ± 0.56; 0.0, 2.0 | 0.38 ± 0.71; 0.0, 2.0 | 0.51 ± 0.90; 0.0, 3.0 |
| Postdose, 1 h, ±10 min | 0.82 ± 0.87; 0.0, 3.0 | 0.80 ± 1.21; 0.0, 3.0 | 0.82 ± 1.05; 0.0, 4.0 | 0.81 ± 1.05; 0.0, 4.0 | 0.75 ± 1.29; 0.0, 4.0 | 0.74 ± 0.93; 0.0, 3.0 | 0.63 ± 0.97; 0.0, 4.0 | 0.69 ± 1.02; 0.0, 4.0 |
| Postdose, 2 h, ±15 min | 0.73 ± 1.10; 0.0, 3.0 | 1.07 ± 1.58; 0.0, 5.0 | 0.68 ± 0.72; 0.0, 2.0 | 0.81 ± 1.12; 0.0, 5.0 | 1.00 ± 1.54; 0.0, 4.0 | 0.53 ± 0.77; 0.0, 2.0 | 1.04 ± 1.08; 0.0, 4.0 | 0.85 ± 1.11; 0.0, 4.0 |
| Postdose, 4 h, ±30 min | 0.73 ± 1.56; 0.0, 5.0 | 0.87 ± 1.36; 0.0, 4.0 | 1.14 ± 0.94; 0.0, 3.0 | 0.96 ± 1.22; 0.0, 5.0 | 1.17 ± 1.64; 0.0, 4.0 | 0.74 ± 0.90; 0.0, 3.0 | 0.63 ± 0.82; 0.0, 3.0 | 0.78 ± 1.08; 0.0, 4.0 |
| Postdose, 6 h, ±30 min | 0.45 ± 0.82; 0.0, 2.0 | 1.13 ± 1.60; 0.0, 5.0 | 0.55 ± 0.74; 0.0, 2.0 | 0.71 ± 1.11; 0.0, 5.0 | 1.08 ± 1.44; 0.0, 4.0 | 0.47 ± 0.77; 0.0, 2.0 | 0.33 ± 0.64; 0.0, 2.0 | 0.55 ± 0.94; 0.0, 4.0 |
| Discharge | 0.43 ± 0.79; 0.0, 2.0 | 0.18 ± 0.53; 0.0, 2.0 | 0.50 ± 0.71; 0.0, 2.0 | 0.36 ± 0.66; 0.0, 2.0 | 0.67 ± 1.21; 0.0, 3.0 | 0.29 ± 0.61; 0.0, 2.0 | 0.33 ± 0.58; 0.0, 2.0 | 0.37 ± 0.70; 0.0, 3.0 |
The few reports of nasal irritation were mild, did not exceed a score of 1A (focal nasal mucosal irritation or inflammation), were transient, and were more frequent under ictal/peri‐ictal administration (Table S2). Subjects reported negligible nasal mucosal pain, with all mean VAS scores in the range of 0‐1 (data not shown) except for a single time point (15 minutes postadministration in ictal/peri‐ictal condition) at the 20‐mg dose, for which the mean (SD) score was 1.1 (2.7). Smell tests showed no clinically relevant olfactory changes from baseline (data not shown).
4. DISCUSSION
The current study shows that diazepam nasal spray, which incorporates DDM and vitamin E as excipients to resolve issues of solubility and absorption associated with benzodiazepines, resulted in rapid diazepam absorption after intranasal administration, with drug exposure that was similar between epileptic conditions (ictal/peri‐ictal, interictal). Under both conditions, plasma concentrations over the first 6 hours after dosing remained higher than the minimal diazepam concentration (~70 ng/mL) shown to elevate the seizure threshold in a rat model of epilepsy. 12 Regardless of ictal/peri‐ictal or interictal condition, the Cmax was comparable with concentrations reported when diazepam was administered using other routes, including intramuscularly (95‐125 ng/mL) and orally (148‐255 ng/mL). 13 The overall pharmacokinetic profile was also consistent with what has been reported in healthy subjects after intranasal administration of diazepam nasal spray 9 as well as after rectal administration of diazepam. 7 , 14 , 15 , 16 , 17
As indicated by the CV% for the pharmacokinetic parameters of Cmax and drug exposure (AUC), interpatient variability was relatively low, similar to previous comparisons of diazepam nasal spray with rectal diazepam. 7 This low interpatient variability suggests a robust pharmacokinetic profile that is more reliable than that obtained with rectal administration. These favorable pharmacokinetic characteristics of diazepam nasal spray are likely a result of the increased solubility and drug absorption associated with DDM. Because DDM has been shown to modify the pharmacokinetic profile for intranasal administration of other drugs (eg, sumatriptan for migraine and naloxone for opioid overdose), 18 , 19 based on its putative mechanism of relaxation of tight junctions combined with enabling greater penetration through cell membranes, the potential for direct nose to brain drug delivery exists. 6
The pharmacokinetic profiles were similar between the ictal/peri‐ictal and interictal conditions, as indicated by overlap of the dose‐normalized values, demonstrating that diazepam nasal spray can be given during the ictal/peri‐ictal and interictal states.
Diazepam nasal spray demonstrated a good safety profile that was similar to what has previously been reported in healthy subjects. 9 TEAEs did not appear to be dose‐dependent, and the low incidence of treatment‐related TEAEs was consistent with expectations for intranasal administration of diazepam; dysgeusia was the most common adverse event. Of specific relevance to clinicians and patients, somnolence was not reported as a TEAE, and only low levels of transient sedation were reported using a sedation scale. As previously reported in healthy subjects, 9 we observed no evidence for respiratory depression. Nasal irritation and olfactory changes were mild and transient. Importantly, in comparison with the previous study in healthy subjects, which was limited to adults, the population of the current study included pediatric patients. Therefore, the results presented here suggest that the safety and tolerability profiles of diazepam nasal spray extend to children age 6 years and above and adolescents.
In summary, intranasal administration of diazepam nasal spray in patients with epilepsy results in good diazepam absorption and exposure in both the ictal/peri‐ictal and interictal conditions, with pharmacokinetic profiles that were comparable to rectal diazepam administration, but with a lower interpatient variability (compared with previous studies 16 , 17 ). The safety profile is consistent with previous studies of diazepam, with no clinically relevant changes in sedation, olfaction, nasal irritation, or acute nasal pain. These results add to the body of evidence supporting diazepam nasal spray as a useful and socially acceptable rescue medication, which is indicated for managing seizure clusters in patients with epilepsy 6 years of age and older.
CONFLICT OF INTEREST
R.E.H. has received research support from UCB Pharmaceuticals, Neurelis, Biogen, and Engage Therapeutics. D.T. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Marinus and Avexis. M.R.S. has received personal compensation for speaking from NeurologyLive and Eisai, and consulting with payments to Thomas Jefferson University from Medtronic. M.R.S. has received research support from Eisai, Medtronic, Neurelis, SK Life Science, Takeda, Sunovion, UCB Pharma, Xenon, and Engage Pharmaceuticals. P.K. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Abbott, Alliance, Acquestive, Eisai, Lundbeck, SK Life Sciences, Sunovion Pharmaceuticals, and UCB Pharma. P.K. has received research support from Lundbeck. He is a member of the scientific advisory board of OB Pharmaceuticals. I.M. has served as a consultant/advisor to GW Pharmaceuticals, Insys Therapeutics, Visualase, and NeuroPace, and as a study investigator for GW Pharmaceuticals. E.B.S. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Eisai, Lundbeck, Nutricia, Novartis, Greenwich, Epitel, Encoded Therapeutics, and Q Biomed. A.L.R. is an employee of and has received stock options from Neurelis. E.C. has received personal compensation for consulting with Neurelis, Alexza, Marinus, and Zogenix, and holds stock in Neurelis. E.C. has received compensation for serving on the Board of Directors of Marinus Pharmaceuticals and Hawaii‐Biotech. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DATA SHARING STATEMENT
Neurelis will not be sharing individual deidentified participant data or other relevant study documents.
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
Medical writing support was provided at the direction of the authors by E. Jay Bienen, PhD, of the Curry Rockefeller Group, which also provided additional editorial assistance, including formatting and proofreading. This support was funded by Neurelis.
Hogan RE, Tarquinio D, Sperling MR, et al. Pharmacokinetics and safety of VALTOCO (NRL‐1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri‐ictal) and nonseizure (interictal) conditions: A phase 1, open‐label study. Epilepsia. 2020;61:935–943. 10.1111/epi.16506
Funding information
This study was supported by Neurelis.
REFERENCES
- 1. Jafarpour S, Hirsch LJ, Gainza‐Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. [DOI] [PubMed] [Google Scholar]
- 2. Garnett WR, Barr WH, Edinboro LE, Karnes HT, Mesa M, Wannarka GL. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: a pharmacokinetic comparison. Epilepsy Res. 2011;93:11–6. [DOI] [PubMed] [Google Scholar]
- 3. Kapoor M, Cloyd JC, Siegel RA. A review of intranasal formulations for the treatment of seizure emergencies. J Control Release. 2016;237:147–59. [DOI] [PubMed] [Google Scholar]
- 4. Acorda Therapeutics . PLUMIAZ™ (diazepam) nasal spray [press release]. [cited 2020 April 17]. Available at: http://ir.acorda.com/investors/investor‐news/investor‐news‐details/2016/Acorda‐to‐Discontinue‐Development‐of‐PLUMIAZ‐for‐Treatment‐of‐Epilepsy‐Seizure‐Clusters/default.aspx
- 5. Detyniecki K, Van Ess PJ, Sequeira DJ, Wheless JW, Meng TC, Pullman WE. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—a randomized, double‐blind, placebo‐controlled trial. Epilepsia. 2019;60:1797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maggio ET, Pillion DJ. High efficiency intranasal drug delivery using Intravail(R) alkylsaccharide absorption enhancers. Drug Deliv Transl Res. 2013;3:16–25. [DOI] [PubMed] [Google Scholar]
- 7. Hogan RE, Gidal BE, Koplowitz B, Koplowitz LP, Lowenthal RE, Carrazana E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105:362–7. [DOI] [PubMed] [Google Scholar]
- 9. Tanimoto S, Koplowitz LP, Lowenthal RE, Koplowitz B, Rabinowicz AL, Carrazana E. Evaluation of the pharmacokinetics and dose proportionality of diazepam after intranasal administration of NRL‐1 to healthy volunteers. Clin Pharmacol Drug Dev. 2020. 10.1002/cpdd.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Posner K, Brown GK, Stanley B, et al. The Columbia‐Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalton P, Doty RL, Murphy C, et al. Olfactory assessment using the NIH Toolbox. Neurology. 2013;80:S32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhir A, Rogawski MA. Determination of minimal steady‐state plasma level of diazepam causing seizure threshold elevation in rats. Epilepsia. 2018;59:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Divoll M, Greenblatt DJ, Ochs HR, Shader RI. Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983;62:1–8. [PubMed] [Google Scholar]
- 14. Cloyd JC, Lalonde RL, Beniak TE, Novack GD. A single‐blind, crossover comparison of the pharmacokinetics and cognitive effects of a new diazepam rectal gel with intravenous diazepam. Epilepsia. 1998;39:520–6. [DOI] [PubMed] [Google Scholar]
- 15. Henney HR III, Sperling MR, Rabinowicz AL, Bream G, Carrazana EJ. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014;108:1204–11. [DOI] [PubMed] [Google Scholar]
- 16. Lamson MJ, Sitki‐Green D, Wannarka GL, Mesa M, Andrews P, Pellock J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open‐label, single‐dose, crossover, single‐centre study. Clin Drug Investig. 2011;31:585–97. [DOI] [PubMed] [Google Scholar]
- 17. Ivaturi V, Kriel R, Brundage R, Loewen G, Mansbach H, Cloyd J. Bioavailability of intranasal vs. rectal diazepam. Epilepsy Res. 2013;103:254–61. [DOI] [PubMed] [Google Scholar]
- 18. Munjal S, Gautam A, Offman E, Brand‐Schieber E, Allenby K, Fisher DM. A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN‐02, an intranasal Sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous Sumatriptan in healthy adults. Headache. 2016;56:1455–65. [DOI] [PubMed] [Google Scholar]
- 19. Krieter P, Gyaw S, Chiang CN, Crystal R, Skolnick P. Enhanced intranasal absorption of naltrexone by dodecyl maltopyranoside: implications for the treatment of opioid overdose. J Clin Pharmacol. 2019;59(7):947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


