Abstract
Study Design:
Retrospective cohort study.
Objectives:
Violation of the posterior soft tissues is believed to contribute to the development of proximal junctional kyphosis (PJK). Biomechanical and clinical studies suggest that augmentation of the posterior ligamentous structures (PLS) may help prevent PJK. The purpose of this study was to evaluate the effect of PLS augmentation on the rate of PJK at 1 year.
Methods:
A retrospective single-surgeon cohort study was performed of 108 adult spinal deformity patients who underwent 5 level fusions to the pelvis. Patients were divided into 2 groups: PLS+ patients had reconstruction of the PLS between upper instrumented vertebrae +1 (UIV+1) and UIV−1 with a surgical nylon tape while PLS− patients did not. Demographics, surgical data, and sagittal alignment parameters were compared between the cohorts. The primary outcome of interest was the development of PJK at final follow-up. A subgroup propensity match and logistic regression model were utilized to control for differences in the cohorts.
Results:
A total of 108 patients met final criteria, 31 patients (28.7%) were PLS+. There were no differences with regard to preoperative or final sagittal alignment parameters, number of levels fused, rates of 3-column osteotomies, and body mass index (P > .05), though the PLS+ cohort was older and had larger initial sagittal corrections (P < .05). The rates of PJK for PLS+ (27.3%) and PLS− (28.6%) were similar (P = .827). After controlling for sagittal correction via propensity matching, PLS+ had no impact on PJK (29% vs 38.7%, P = .367). In our multivariate analysis, only increased sagittal malalignment and failure to restore sagittal balance were retained as significant predictors of PJK.
Conclusion:
Even after controlling for extent of correction and preoperative sagittal alignment, PLS reinforcement at UIV+1 using a hand-tensioned nylon tape does not reduce the incidence of PJK at 1 year.
Keywords: posterior tether, ligamentous augmentation, proximal junctional kyphosis
Introduction
Proximal junctional kyphosis (PJK) is a common occurrence following surgery for adult spinal deformity with reported rates from 17% to 61.7%.1,2 Although a majority of patients with PJK are asymptomatic,3,4 PJK can have a significant impact on clinical outcomes5,6 and can result in neurologic injury and/or progressive sagittal plane deformities.7 PJK is among the most common causes for revision surgery in adult deformity.8
Despite the relatively high prevalence of PJK, there have been relatively few preventative strategies described in the literature. There is growing consensus that more judicious, age-appropriate sagittal plane deformity correction might help reduce the incidence of PJK.9 Other strategies to reduce PJK include cement augmentation at or above the upper instrumented vertebrae (UIV) and a “soft landing” at the top of the construct.10-17
None of these approaches, however, address the posterior ligamentous structures (PLS) at the UIV. The role of the PLS in the stability of the proximal motion segments has been well established in several biomechanical models.18,19 Damage to the PLS during posterior spinal dissection results in significant increases in the proximal junctional angle and proximal flexion forces.18,19 It follows, therefore, that repair and/or reinforcement of the PLS might represent an additional surgical strategy to help reduce the risk of PJK. A recent finite element analysis found that polyester tether reinforcement of the posterior elements was able to successfully reduce adjacent segment stress at levels above the upper instrumented vertebrae (UIV).20 Other authors have described a semitendinosus graft to augment the posterior spinous ligament at the UIV.21
In our practice, we utilize a surgical nylon tape (SNT) to reinforce the PLS at the UIV in select cases. This SNT is a fiber mesh tape that has a long history of use in general surgery22 and also as a novel technique in wrist ligament reconstruction.23-25 SNT has high mechanical stability and provokes minimal inflammatory reaction22; it allows for tissue ingrowth through the polyester mesh, making it particularly well suited to reconstructing the posterior soft tissues following surgery.26
We describe here the results of a single-surgeon study examining the impact of PLS reinforcement with SNT. We hypothesized that PLS augmentation at the UIV would reduce the incidence of PJK and prevent vertebral subluxation above the UIV.
Methods
Following approval by the institutional review board at our institution, we conducted a retrospective review of a single-surgeon series of procedures performed between 2014 and 2017. All adult spinal deformity patients who underwent greater than 5-level fusions to the pelvis with a minimum follow-up of 1 year were included (primary or revision cases). Patients with congenital scoliosis or neuromuscular disorders were excluded. Patients were divided into two groups: PLS+, patients in whom the posterior soft tissues were augmented with an SNT or PLS−, patients in whom no augmentation was performed.
Surgical Technique
All patients underwent a standard posterior approach to the spine. Patients were positioned prone on a Jackson table with the patients’ head suspended in Gardner-Wells traction in all cases. In both groups, an extensive posterior release was performed to gain exposure although care was taken to preserve as much of the PLS as possible, particularly from the UIV-2 and proximally. All patients had pedicle screws constructs including screws placed bilaterally at the UIV. Following correction of deformity and prior to closure, patients in the PLS+ group had 5-mm Mersilene Tape (Ethicon US, LLC) on a curved needle passed through the spinous process of the vertebra 1 level above the UIV (UIV+1) and secured below the pedicle screws at the UIV in a loop fashion. A drill was used to create a small hole in the spinous process if the needle could not be easily passed through the bone. The tape was then tensioned manually using a slip knot. The process was then repeated, placing the tape through the spinous process of the UIV and securing the tape below the pedicle screws at the UIV−1 (Figure 1). Crosslinks or cement augmentation at the UIV was not utilized. All patients were placed on a standard physical therapy regimen post-operatively which included postural training as well as encouraging the use of a walker for 6 weeks with an upright posture.
Figure 1.
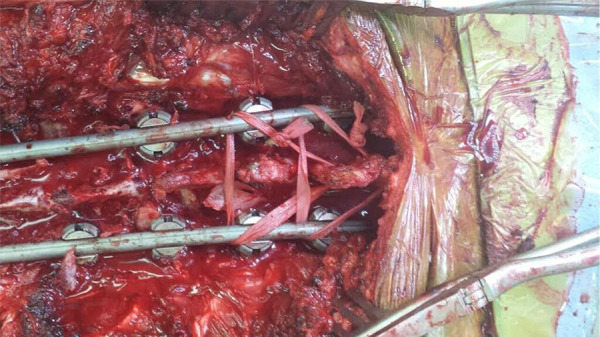
Clinical image depicting surgical nylon tape augmentation of the posterior soft tissues.
Radiographic Evaluation
All patients had anteroposterior (AP) and lateral standing radiographs performed prior to surgery, at 6 weeks and at final follow-up (minimum of 1 year postoperatively). The following sagittal radiographic variables were evaluated in the standard manner: sacral slope (SS), pelvic tilt (PT), pelvic incidence (PI), lumbar lordosis (LL), PI-LL mismatch, T10-T12 angle, T4-T12 kyphosis (TK), C2-C7 lordosis (CL), C7 sagittal vertical axis (C7 SVA), and the T1 pelvic angle (T1PA). Given that not all patients had standing radiographs before discharge, the 6-week postoperative changes in these variables were calculated as a measure of intraoperative correction. The proximal junctional angle (PJA) was defined as the sagittal Cobb angle between the UIV and UIV+2.23 PJK was defined as PJA ≥10° and increase in PJA ≥10° from the preoperative PJA at most recent follow-up (1 or 2 years).
Clinical and Surgical Factors
In addition to the radiographic data detailed above, relevant clinical and surgical variables were also gathered. These included: age, body mass index (BMI; kg/m2), gender, American Society of Anesthesiologists (ASA) class, operating time (skin incision to closure), estimated blood loss (EBL), upper instrumented level (UIV), lower instrumented level (LIV), presence of an interbody fusion (IBF), osteotomies and osteotomy type (Smith-Peterson [SPO] or 3-column osteotomies [3CO]).
Statistical Analysis
Continuous variables were examined for normality using Shapiro-Wilk’s test. Differences between continuous variables were compared using an independent Student’s t test or Wilcoxon rank sum as appropriate. Categorical variables were compared using the chi-square or Fisher’s exact test as appropriate. To control for baseline differences in the cohorts, two methods were employed. First, a backward, conditional multivariate regression model was constructed to determine if PLS reinforcement would be retained as a predictor of PJK. The initial model included the following variables: use of PLS, age, BMI, preoperative sagittal alignment parameters (SS, PT, PI, PI-LL, LL, T10-12 angle, TK, CL, C7 SVA, T1PA), UIV above T9, EBL (mL), presence of SPO, presence and location of 3CO, presence of IBF, and 6-week change in sagittal alignment parameters ((SS, PT, PI, PI-LL, LL, T10-12 angle, TK, CL, C7 SVA, T1PA). Hosmer-Lemeshow test and C-statistic were used to evaluate the goodness-of-fit and discrimination of the model. Second, a subgroup analysis was performed, where the PLS+ cohort was propensity-score matched to a PLS− cohort based on the 6-week sagittal correction parameters, and PJK rates were compared. A P value of .05 was used to determine the type I error. All statistics were performed using IBM SPSS Version 22.0 (Armonk, NY).
Results
A total of 126 patients meeting the inclusion criteria above were identified. Eighteen patients were excluded due to neuromuscular disorders or congenital scoliosis leaving 108 patients available for final analysis. Of these patients, 31 (28.7%) were PLS+ and the remainder were PLS−. Average follow-up was 17.6 ± 6.0 months. Follow-ups were nearly identical between the 2 cohorts (PLS+ 17.4 ± 6.0 vs PLS− 17.6 ± 6.1 months, P = .882). BMI, gender, and ASA class were similar between the 2 groups, though the PLS+ cohort was older (64.1 ± 10.4 vs 51.3 ± 21.4 years, P = .002) (Table 1). With regard to surgical factors, fusion levels, operating room time, EBL, and presence and location of 3CO were similar (P < .05) (Table 1). Of note, more patients in the PLS− cohort underwent 3CO and while more SPOs were performed in the PLS+ cohort, suggesting that correction was obtained through slightly different methods in each group. Bone mineral density data for the left femoral neck was the most common measurement available on DEXA (dual-energy X-ray absorptiometry) scans, available in 14 PLS+ patients (45%) and 29 PLS− patients (38%). Average T-score was −1.72 ± 0.89 PLS+ versus −1.30 ± 1.07 PLS−, P = .211.
Table 1.
Demographics and Surgical Characteristics.
| PLS+ (31) | PLS− (77) | ||||
|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | P | |
| Age, y | 64.1 | 10.4 | 51.3 | 21.4 | .002 |
| BMI, kg/m2 | 24.9 | 5.2 | 27 | 6.9 | .143 |
| Gender | .836 | ||||
| Male | 7 | 23% | 16 | 21% | |
| Female | 24 | 77% | 61 | 79% | |
| ASA | .224 | ||||
| 1 | 0 | 0% | 9 | 12% | |
| 2 | 17 | 55% | 36 | 47% | |
| 3 | 7 | 23% | 18 | 23% | |
| 4 | 0 | 0% | 1 | 1% | |
| No. of levels fused | 10.7 | 3.8 | 9.7 | 3.8 | .331 |
| Fusion above T9 | 14 | 45% | 40 | 52% | .444 |
| Average UIV | T8 | 3.6 | T8 | 4.2 | .892 |
| OR time, min | 318.2 | 63.6 | 313.8 | 103.8 | .842 |
| EBL, mL | 1650 | 846 | 1848 | 1535 | .503 |
| SPO | 24 | 77% | 33 | 43% | .001 |
| No. of SPOs | 2.6 | 2 | 1.4 | 2 | .001 |
| 3COa | 3 | 10% | 18 | 23% | .120 |
| Thoracic | 0 | 0% | 6 | 8% | |
| Lumbar | 3 | 10% | 12 | 16% | |
| IBF | 18 | 58% | 13 | 17% | .041 |
Abbreviations: PLS, posterior ligamentous structures; BMI, body mass index; ASA, American Society of Anesthesiologists; UIV, upper instrumented vertebrae; OR, operating room; EBL, estimated blood loss; SPO, Smith-Peterson osteotomy; 3CO, 3-column osteotomy; IBF, interbody fusion.
a One patient in the PLS− cohort had two 3CO.
There were no significant differences between the PLS+ and PLS− groups with regard to preoperative sagittal alignment (Table 2). At final follow-up, radiographic parameters were also comparable (Table 2). However, the PLS+ group underwent larger intraoperative corrections as measured by both the change in sagittal parameters baseline measurements to 6-week follow-up (Table 3). The average change in C7 SVA was −69.5 ± 63.7 mm in the PLS+ cohort compared with −28.1 ± 72.3 mm in the controls. Lumbar lordosis was also increased by a larger degree (24.3° ± 14.9° vs 10.1° ± 17.5°, P < .001).
Table 2.
Preoperative and Final Postoperative Radiographic Parameters.
| Preoperative | Final Postoperative | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLS+ (31) | PLS− (77) | PLS+ (31) | PLS− (77) | |||||||
| Mean | SD | Mean | SD | P | Mean | SD | Mean | SD | P | |
| Sacral slope | 27.2 | 12.0 | 31.8 | 14.8 | .134 | 29.2 | 10.2 | 32.6 | 12.7 | .380 |
| Pelvic tilt | 23.5 | 9.1 | 21.4 | 13.1 | .404 | 18.4 | 8.6 | 16.5 | 11.6 | .583 |
| Pelvic incidence (PI) | 50.8 | 12.1 | 53.2 | 14.4 | .420 | 47.7 | 12.6 | 49.1 | 14.2 | .753 |
| PI-LL | 18.8 | 17.8 | 13.5 | 21.5 | .228 | −2.1 | 14.0 | −1.3 | 15.7 | .870 |
| Lumbar lordosis (LL) | 32.0 | 19.1 | 39.7 | 23.6 | .111 | 49.9 | 17.7 | 50.4 | 17.8 | .917 |
| T10-L2 | −18.4 | 15.1 | −10.9 | 19.7 | .059 | −10.3 | 8.3 | −6.6 | 15.2 | .398 |
| Thoracic kyphosis | −29.7 | 15.3 | −34.2 | 18.5 | .241 | −39.9 | 12.2 | −40.1 | 16.2 | .981 |
| Cervical lordosis | 7.8 | 19.7 | 10.5 | 16.6 | .491 | 10.4 | 19.5 | 10.1 | 16.4 | .944 |
| C7 SVA | 72.5 | 80.1 | 58.9 | 77.7 | .420 | 2.6 | 36.7 | 9.8 | 42.7 | .584 |
| T1PA | 22.8 | 11.2 | 20.4 | 15.3 | .430 | 12.7 | 10.0 | 11.9 | 11.9 | .841 |
| PJA | −1.9 | 8.2 | −2.8 | 12.9 | .718 | −11.4 | 10.3 | −10.9 | 14.3 | .850 |
| PJK | — | — | — | — | — | 9.0 | 27.3% | 22.0 | 28.6% | .827 |
Abbreviations: PLS, posterior ligamentous structures; SVA, sagittal vertical axis; T1PA, T1 pelvic angle; PJA, proximal junctional angle; PJK, proximal junctional kyphosis.
Table 3.
Changes in Radiographic Parameters Between Preoperative and 6-Week Follow-up.
| PLS+ (31) | PLS− (77) | PLS− Matched (31)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | Mean | SD | P | |
| Sacral slope | 10.4 | 9.3 | 3.0 | 9.0 | .000 | 9.2 | 9.5 | .485 |
| Pelvic tilt | −10.8 | 9.2 | −3.5 | 8.9 | .000 | −9.5 | 10.1 | .285 |
| Pelvic incidence (PI) | −0.5 | 1.6 | −0.4 | 2.7 | .614 | −0.3 | 2.0 | .121 |
| PI-LL | −24.7 | 15.0 | −10.5 | 17.5 | .000 | −20.9 | 17.7 | .556 |
| Lumbar lordosis (LL) | 24.3 | 14.9 | 10.1 | 17.5 | .000 | 20.6 | 16.6 | .707 |
| T10-L2 | 13.1 | 17.4 | 3.6 | 14.7 | .003 | 6.8 | 16.8 | .274 |
| Thoracic kyphosis | −10.3 | 13.2 | −3.9 | 15.7 | .023 | −10.8 | 15.4 | .974 |
| Cervical lordosis | −4.5 | 9.3 | 0.4 | 15.3 | .087 | −1.9 | 15.2 | .308 |
| C7 SVA | −69.5 | 63.7 | −28.1 | 72.3 | .002 | −50.4 | 73.4 | .840 |
| T1PA | −14.5 | 9.3 | −5.6 | 11.4 | .000 | −11.9 | 11.9 | .427 |
Abbreviations: PLS, posterior ligamentous structures; SVA, sagittal vertical axis; T1PA, T1 pelvic angle.
The overall rate of PJK at final follow up was 28.7%. There was no difference in the rate of PJK between the PLS− (n = 22, 28.6%) and PLS+ (n = 9, 27.3%) groups at final follow-up. The 6-week rate of PJK was 5 (56%) PLS+ patients versus 16 (73%) PLS− patients (P = .353), while the 6-month rate of PJK was 7 (78%) PLS+ versus 17 (77%) PLS− patients (P = .976). Four revisions were performed for PJK during the follow-up period (1 at 6 weeks, 1 at 1 year, and 2 at 2 years), all in PLS− patients. In our multivariate analysis, only preoperative sagittal alignment parameters and degree of correction was associated with development of PJK (Table 4). Larger corrections of T1PA were found to have the largest effect on PJK (odds ratio [OR] 1.32, 95% confidence interval [CI] 1.07-1.64, P = .01), while decreasing the PI-LL mismatch was found to be most protective (OR 0.81, 95% CI 0.70-0.94, P = .005). PLS augmentation was not associated with PJK (OR 0.36, 95% CI 0.16-1.94, P = .561). The model had an appropriate goodness of fit (Hosmer-Lemeshow 0.136), decent discrimination (C-statistic 0.768), but could only account for 27% in the variability of PJK (R2 = 0.268).
Table 4.
Multivariate Analysis of Factors Associated With PJK.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Odds Ratio | Lower | Higher | P | |
| PLSa | 0.36 | 0.16 | 1.94 | .561 |
| Preoperative PI-LL | 0.87 | 0.79 | 0.97 | .008 |
| Preoperative cervical lordosis | 0.96 | 0.93 | 1.00 | .028 |
| Preoperative T1PA | 1.19 | 1.04 | 1.36 | .013 |
| UIV above T9 | 0.34 | 0.10 | 1.16 | .085 |
| BMI | 1.09 | 0.99 | 1.20 | .081 |
| Change in PI-LL | 0.81 | 0.70 | 0.94 | .005 |
| Change in T10-L2 | 1.04 | 1.00 | 1.09 | .063 |
| Change in thoracic kyphosis | 1.06 | 1.00 | 1.13 | .048 |
| Change in T1PA | 1.32 | 1.07 | 1.64 | .010 |
Abbreviations: PLS, posterior ligamentous structures; PI, pelvic incidence; LL, lumbar lordosis; T1PA, T1 pelvic angle; UIV, upper instrumented vertebrae; BMI, body mass index.
aExcluded from final regression model.
Given the importance of correction to PJK risk, a propensity score match was performed to match the PLS+ cohort to a PLS− cohort who underwent the same degree of sagittal correction (Table 4). In this propensity-matched cohort, PJK rates were 29% PLS+ versus 38.7% PLS−, P = .367. Age was also noted to be similar between these cohorts, average age 64.1 ± 10.4 years PLS+ vs 57.6 ± 15.3 years PLS−, P = .081.
Two subgroup analyses were performed to elucidate the differential effect of PLS based on UIV and degree of correction. In the 54 patients with UIV of T9 or below (17 [31.5%] PLS+), PJK rates were slightly higher in the PLS− cohort though this was not significant (29.4% PLS+ vs 37.8% PLS−, P = .547). In the 54 patients with a UIV above T9 (14 [25.9%] PLS+), PJK rates were 28.6% PLS+ versus 25.0% PLS−, P = .793.
Discussion
We report an overall rate of PJK of 28.7%, with no difference in the rate of PJK between the PLS− (n = 22, 28.6%) and PLS+ (n = 9, 27.3%) groups at final follow-up of at least 1 year. Even after accounting for potential confounders such as preoperative sagittal alignment, osteotomies, and degree of correction, PJK was still not associated with PLS. Our findings support that reinforcement of the PLS alone using the described technique may not reduce the incidence of PJK (Figure 2). Potential methods of failure include pullout through the spinous processes or under-tensioning of the SNT, or anterior column compression fracture. Given that PJK occurred at similar postoperative intervals in both cohorts (approximately 80% within 6 months), it is likely that failure of this particular construct occurs in the first few months postoperatively. While all revisions for PJK were performed in the PLS− cohort, the overall rate was very small and cannot be used to make conclusions.
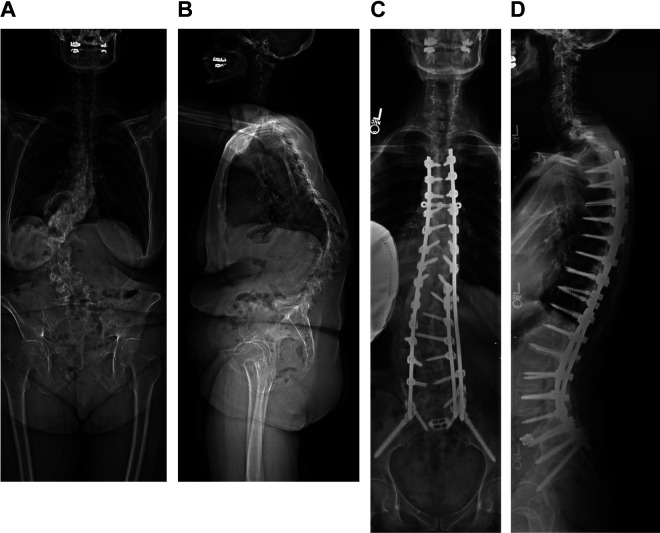
Figure 2.
67F presenting with several years of worsening back pain and deformity, refractory to conservative treatments. Preoperative bone density scans revealed osteoporosis; the patient underwent treatment with teriparatide in the months preceeding surgery. Preoperative standing radiographs (A, B), with baseline sagittal radiographic parameters included a sacral slope of 12°, pelvic tilt of 36°, pelvic incidence 47°, lumbar lordosis 16°, thoracolumbar junction (T10-L2) with 58° of kyphosis, and a C7 sagittal vertical axis (SVA) of 91 mm. The patient was instrumented from T3-pelvis, with Schwab grade 2 osteotomies from L1 to L4 and a transforaminal interbody fusion at L5/S1. The PLS was augmented at the top of the construct using the technique previously described. An increase in the proximal junctional angle from 2° of lordosis to 16° of kyphosis was first noted at the 6-week postoperative visit, which remained stable and asymptomatic through her 2-year postoperative radiographs (C, D).
Evidence in the literature examining techniques to prevent PJK is sparse but actively growing.11,17,21,27-29 Many investigators have examined the relationship between construct stiffness and PJK. Biomechanical studies have identified implants that reduce the stiffness of the proximal construct14 in hopes that one may reduce PJK in a clinical setting. Titanium rods, which are less stiff than cobalt chrome rods, have been associated with a lower incidence of PJK in retrospective studies (though also associated with a higher risk of rod breakage).30,31 Two groups of investigators have attempted to decrease the stiffness of the proximal construct and create a “soft landing” at the UIV. Hasanzadeh et al17 showed no instances of PJK in 20 patients treated with a hook at the UIV compared with a 29.6% (8/27 patients) rate of PJK in patients who were treated with a pedicle screw at the UIV (P = .01). Yanik et al27 studied the impact of leaving 2 screw threads out of the posterior cortex when placing pedicle screws at the UIV. In their cohort of Scheuermann’s kyphosis patients, they were able to show a reduced rate of PJK compared with a control group (0/31 cases vs 5/29, P = .02).27
Given that vertebral fractures represent a common etiology for PJK and proximal junctional failure, investigators have also studied the impact of prophylactic 1- and 2-level vertebroplasty above long fusions. Results reported include both biomechanical29 and clinical data.11,13 Ghobrial et al10 compared 47 controls to 38 patients who underwent cement augmentation at the UIV and UIV+1 at the time of adult spinal deformity correction, finding a 36.2% rate of PJF in controls compared to 23.7% in the cemented group. None of the cemented patients received revision surgery, while 12.6% of the control group underwent proximal extension of the fusion. More recent, longer-term data, however, suggests that while cement augmentation might reduce the risk of junctional failure in the early postoperative period, it does not reduce the incidence of PJK at 5 years.10 It should also be noted that vertebral augmentation is not without risk; the cost of augmentation as well as load transfer to adjacent vertebrae are factors that must be considered.32
Another surgical tactic to prevent PJK might be more judicious correction.9 Recent literature has shown that an increase in sagittal balance correction and an increase in lumbar lordosis correlate with the development of PJK.4,5,33,34 These theories are consistent with our data, as the only factors that were retained as potential predictors of PJK were preoperative sagittal alignment parameters and markers of overall sagittal correction (eg, change in PI-LL, change in T1PA).
Violation of the posterior soft tissues has always been viewed as a potential source of PJK due to biomechanical studies18,35 and intuition. Finite element analysis suggests that polyester tethers may improve the biomechanics thought to contribute to PJK.20 Zagahoul et al36 used SNT at the proximal junctional level in 18 patients, finding a 0% incidence of PJK at an average of 11.9 months of follow-up. Of note, this series included a wide variety of procedures, including isolated lumbar fusions, notably different from the population in this study. On the other hand, Buell et al37 performed a case-control study of adult spinal deformity patients with radiographic PJK at 1 year after posterior instrumented fusion to the lower thoracic spine (T9-T11). The authors compared the radiographic and surgical characteristics of 49 patients with PJK to 71 patients without, reporting a higher rate of posterior polyethylene tethers in the non PJK cohort (70% vs 47%, P = .010). PLS reinforcement was also found to be protective against PJK in their multivariate model (OR 0.063 95% CI 0.016-0.247). The contrasting findings are likely due to the difference between our patient samples and difference in surgical technique. While their sample was limited to T9 as the UIV, our average UIV was T8 and half of our sample had a UIV above T9. Many of our patients had coronal and sagittal plane deformity extending into the upper thoracic spine. The effect of tethers may be lessened when the UIV is at the mid-to-upper thoracic spine. Furthermore, Buell et al38 attached the tether to a crosslink at the UIV for some of their patients, which was associated with an even lower rate of PJK in a pilot study by the same authors. Thus, the efficacy of the surgical technique, with regard to level placement, use of crosslinks, and method of tensioning, will require continued investigation.
On the other hand, the literature on this topic is relatively scarce, and more studies will be needed to truly elucidate whether tethers have a meaningful effect on PJK rates. It is certainly possible that PLS augmentation does not fully restore the stiffness of the spine biomechanical segment to match the native PLS. Finally, while PLS reinforcement provides a static restraint at the UIV, it is possible that the violation of the posterior musculature (and dynamic stabilization) plays an equally important role in the etiology of PJK. Last, given that there were larger corrections obtained in the PLS+ cohort, it could be theorized that the PLS augmentation actually lowered the potential for PJK, bringing the rate to the level of the PLS− cohort. This theory was supported by our matched subgroup analysis, in which the PLS+ cohort that underwent similar corrections as a matched PLS− cohort trended toward a lower rate of PJK (29% PLS+ vs 38.7% PLS−, P = .367). Of note, definitive conclusions should not be made given the low power of this subgroup analysis.
The major limitation of this study is selection bias on the part of the surgeon, as PLS augmentation was utilized in patients who had a larger overall correction of sagittal parameters. While a matched subgroup analysis and multivariate regression model was utilized to control for these differences in the patient cohorts, such analyses are imperfect at approximating a randomized trial. A randomized trial of a single-surgeon cohort would be ideal to evaluate the impact of PLS on rates of PJK, as the surgical technique would be preserved among cases. Unfortunately, given the selectivity with which this surgical intervention is utilized, it would be difficult to enroll the required number of patients to detect these differences in a single-surgeon cohort, and a multisurgeon study would be subject to significant differences in surgical technique. A second limitation was the inability to capture bone mineral density data for all patients in the study. While those with bone mineral density data showed similar average T-scores, the PLS+ patients were older on average and more likely to be osteoporotic, which may have affected the risk of PJK and biased results toward the null given the use of PLS in this higher-risk population. A third limitation is related to the surgical technique itself, as the patients presented in this study represent the senior surgeon’s first use of PLS augmentation. Furthermore, biomechanical analyses have shown that augmentation to the level of the UIV+2 is superior at distributing forces through the upper levels of the construct and that crosslinks may enhance the efficacy of the tether at preventing PJK.20,38 The surgical technique utilized in this study was limited to PLS augmentation of the UIV+1 and crosslinks were not used. A fourth limitation was that our single-surgeon series was underpowered to allow significance in our subgroup analyses. However, we believe that the advantages conferred by having consistent surgical technique among cases forms a unique contribution to the body of literature on PLS augmentation and PJK. Finally, a limitation inherent to the technique of PLS augmentation is the degree of force applied to the SNT prior to “locking” it into the top of the construct. While our technique relied on the surgeon’s judgement to determine exactly how much tension to provide through the SNT, the physiologic forces experienced by the PLS may be higher or lower than this threshold. Further biomechanical and in vivo studies may allow surgeons to more effectively apply this technique.
Despite these limitations, we believe the current study is an important contribution to the literature. There are several methods by which the PLS may be reconstructed, and we show our results with an easily reproducible technique. This series once again highlights the multifactorial nature of PJK and raises further questions on how we may prevent this undesirable complication. Future research on the prevention of PJK must keep this fact in mind; it is unlikely that a single surgical modification will solve the problem of PJK. Optimal posterior ligamentous augmentation strategies should be pursued and refined.
Footnotes
Authors’ Note: The initial 6-week results of this series were presented at the International Meeting for Advanced Spine Techniques in Cape Town, South Africa in 2017.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Francis Lovecchio, MD  https://orcid.org/0000-0001-5236-1420
https://orcid.org/0000-0001-5236-1420
Han Jo Kim, MD  https://orcid.org/0000-0003-2170-3592
https://orcid.org/0000-0003-2170-3592
References
- 1. Kim HJ, Lenke LG, Shaffrey CI, Van Alstyne EM, Skelly AC. Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: a systematic review. Spine (Phila Pa 1976). 2012;37(22 suppl):S144–S164. [DOI] [PubMed] [Google Scholar]
- 2. Lee JH, Kim JU, Jang JS, Lee SH. Analysis of the incidence and risk factors for the progression of proximal junctional kyphosis following surgical treatment for lumbar degenerative kyphosis: minimum 2-year follow-up. Br J Neurosurg. 2014;28:252–258. [DOI] [PubMed] [Google Scholar]
- 3. Hart R, McCarthy I, O'Brien M. et al. International Spine Study Group. Identification of decision criteria for revision surgery among patients with proximal junctional failure after surgical treatment of spinal deformity. Spine (Phila Pa 1976). 2013;38:E1223–E1227. [DOI] [PubMed] [Google Scholar]
- 4. Yagi M, King AB, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine (Phila Pa 1976). 2012;37:1479–1489. [DOI] [PubMed] [Google Scholar]
- 5. Kim HJ, Bridwell KH, Lenke LG. et al. Patients with proximal junctional kyphosis requiring revision surgery have higher postoperative lumbar lordosis and larger sagittal balance corrections. Spine (Phila Pa 1976). 2014;39:E576–E580. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Bridwell KH, Lenke LG. et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine (Phila Pa 1976). 2013;38:896–901. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine (Phila Pa 1976). 2010;35:138–145. [DOI] [PubMed] [Google Scholar]
- 8. Reames DL, Kasliwal MK, Smith JS, Hamilton DK, Arlet V, Shaffrey C. Time to development, clinical and radiographic characteristics, and management of proximal junctional kyphosis following adult thoracolumbar instrumented fusion for spinal deformity. J Spinal Disord Tech. 2015;28:E106–sE114. [DOI] [PubMed] [Google Scholar]
- 9. Lafage R, Schwab F, Glassman S. et al. International Spine Study Group. Age-adjusted alignment goals have the potential to reduce PJK. Spine (Phila Pa 1976). 2017;42:1275–1282. [DOI] [PubMed] [Google Scholar]
- 10. Raman T, Miller E, Martin CT, Kebaish KM. The effect of prophylactic vertebroplasty on the incidence of proximal junctional kyphosis and proximal junctional failure following posterior spinal fusion in adult spinal deformity: a 5-year follow-up study. Spine J. 2017;17:1489–1498. [DOI] [PubMed] [Google Scholar]
- 11. Ghobrial GM, Eichberg DG, Kolcun JPG. et al. Prophylactic vertebral cement augmentation at the uppermost instrumented vertebra and rostral adjacent vertebra for the prevention of proximal junctional kyphosis and failure following long segment fusion for adult spinal deformity. Spine J. 2017;17:1499–1505. [DOI] [PubMed] [Google Scholar]
- 12. Theologis AA, Burch S. Prevention of acute proximal junctional fractures after long thoracolumbar posterior fusions for adult spinal deformity using 2-level cement augmentation at the upper instrumented vertebra and the vertebra 1 level proximal to the upper instrumented vertebra. Spine (Phila Pa 1976). 2015;40:1516–1526. [DOI] [PubMed] [Google Scholar]
- 13. Martin CT, Skolasky RL, Mohamed AS, Kebaish KM. Preliminary results of the effect of prophylactic vertebroplasty on the incidence of proximal junctional complications after posterior spinal fusion to the low thoracic spine. Spine Deform. 2013;1:132–138. [DOI] [PubMed] [Google Scholar]
- 14. Lange T, Schmoelz W, Gosheger G. et al. Is a gradual reduction of stiffness on top of posterior instrumentation possible with a suitable proximal implant? A biomechanical study. Spine J. 2017;17:1148–1155. [DOI] [PubMed] [Google Scholar]
- 15. Metzger MF, Robinson ST, Svet MT, Liu JC, Acosta FL. Biomechanical analysis of the proximal adjacent segment after multilevel instrumentation of the thoracic spine: do hooks ease the transition? Global Spine J. 2016;6:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thawrani DP, Glos DL, Coombs MT, Bylski-Austrow DI, Sturm PF. Transverse process hooks at upper instrumented vertebra provide more gradual motion transition than pedicle screws. Spine (Phila Pa 1976). 2014;39:E826–E832. [DOI] [PubMed] [Google Scholar]
- 17. Hassanzadeh H, Gupta S, Jain A, El Dafrawy MH, Skolasky RL, Kebaish KM. Type of anchor at the proximal fusion level has a significant effect on the incidence of proximal junctional kyphosis and outcome in adults after long posterior spinal fusion. Spine Deform. 2013;1:299–305. [DOI] [PubMed] [Google Scholar]
- 18. Anderson AL, McIff TE, Asher MA, Burton DC, Glattes RC. The effect of posterior thoracic spine anatomical structures on motion segment flexion stiffness. Spine (Phila Pa 1976). 2009;34:441–446. [DOI] [PubMed] [Google Scholar]
- 19. Heuer F, Schmidt H, Klezl Z, Claes L, Wilke HJ. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J Biomech. 2014;40:271–280. [DOI] [PubMed] [Google Scholar]
- 20. Bess S, Harri JE, Turner A. et al. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: a finite element analysis. J Neurosurg Spine. 2017;26:125–133. [DOI] [PubMed] [Google Scholar]
- 21. Pham MH, Tuchman A, Smith L. et al. Semitendinosus graft for interspinous ligament reinforcement in adult spinal deformity. Orthopedics. 2017;40:e206–e210. [DOI] [PubMed] [Google Scholar]
- 22. Schumpelick V, Klinge U, Rosch R, Junge K. Light weight meshes in incisional hernia repair. J Minim Access Surg. 2006;2:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin JA, Jr, Wehbé MA. A carpal ligament substitute part 1. polyester suture. Hand Clin. 2013;29:143–148. [DOI] [PubMed] [Google Scholar]
- 24. Chen CY, Yang SW, Lin KY. et al. Comparison of single coracoclavicular suture fixation and hook plate for the treatment of acute unstable distal clavicle fractures. J Orthop Surg Res. 2014;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wehbé MA, Whitaker ML. A carpal ligament substitute part II. polyester suture for scapho-lunate and triqueto-lunate ligament reconstruction. Hand Clin. 2013;29:149–154. [DOI] [PubMed] [Google Scholar]
- 26. Ethicon. Wound closure platform. https://www.ethicon.com/na/epc/search/platform/wound%20closure?lang=en-default. Accessed April 10, 2019.
- 27. Yanik HS, Ketenci IE, Polat A. et al. Prevention of proximal junctional kyphosis after posterior surgery of Scheuermann kyphosis: an operative technique. J Spinal Disord Tech. 2015;28:E101–E105. [DOI] [PubMed] [Google Scholar]
- 28. Denis F, Sun EC, Winter RB. Incidence and risk factors for proximal and distal junctional kyphosis following surgical treatment for Scheuermann kyphosis: minimum five-year follow-up. Spine (Phila Pa 1976). 2009;34:E729–E734. [DOI] [PubMed] [Google Scholar]
- 29. Kebaish KM, Martin CT, O’Brien JR, LaMotta IE, Voros GD, Belkoff SM. Use of vertebroplasty to prevent proximal junctional fractures in adult deformity surgery: a biomechanical cadaveric study. Spine J. 2013;13:1897–1903. [DOI] [PubMed] [Google Scholar]
- 30. Han S, Hyun SJ, Kim KJ, Jahng TA, Lee S, Rhim SC. Rod stiffness as a risk factor of proximal junctional kyphosis after adult spinal deformity surgery: comparative study between cobalt chrome multiple-rod constructs and titanium alloy two-rod constructs. Spine J. 2017;17:962–968. [DOI] [PubMed] [Google Scholar]
- 31. Han S, Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Comparative study between cobalt chrome and titanium alloy rods for multilevel spinal fusion: proximal junctional kyphosis more frequently occurred in patients having cobalt chrome rods. World Neurosurg. 2017;103:404–409. [DOI] [PubMed] [Google Scholar]
- 32. Lau D, Clark AJ, Scheer JK. et al. SRS Adult Spinal Deformity Committee. Proximal junctional kyphosis and failure following spinal deformity surgery: a systematic review of the literature as a background to classification development. Spine (Phila Pa 1976). 2014;39:2093–2102. [DOI] [PubMed] [Google Scholar]
- 33. Maruo K, Ha Y, Inoue S. et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine (Phila Pa 1976). 2013;38:E1469–E1476. [DOI] [PubMed] [Google Scholar]
- 34. Mendoza-Lattes S, Ries Z, Gao Y, Weinstein SL. Proximal junctional kyphosis in adult reconstructive spine surgery results from incomplete restoration of the lumbar lordosis relative to the magnitude of the thoracic kyphosis. Iowa Orthop J. 2011;31:199–206. [PMC free article] [PubMed] [Google Scholar]
- 35. Cammarata M, Aubin CE, Wang X, Mac-Thiong JM. Biomechamical risk factors for proximal junctional kyphosis: a detailed numerical analysis of surgical instrumentation variables. Spine (Phila Pa 1976). 2014;39:E500–E507. [DOI] [PubMed] [Google Scholar]
- 36. Zaghloul KM, Matoian BJ, Denardin NB, Patel VV. Preventing proximal adjacent level kyphosis with strap stabilization. Orthopedics. 2016;39:e794–e799. [DOI] [PubMed] [Google Scholar]
- 37. Buell TJ, Chen CJ, Quinn JC. et al. Alignment risk factors for proximal junctional kyphosis and the effect of lower thoracic junctional tethers for adult spinal deformity. World Neurosurg. 2018;121:e96–e103. [DOI] [PubMed] [Google Scholar]
- 38. Buell TJ, Buchholz AL, Quinn JC. et al. A pilot study on posterior polyethylene tethers to prevent proximal junctional kyphosis after multilevel spinal instrumentation for adult spinal deformity. Oper Neurosurg (Hagerstown). 2019;16:256–266. [DOI] [PubMed] [Google Scholar]