Abstract
Study Design:
Retrospective study.
Objectives:
To evaluate the demographics, prevalence, etiology, severity, and outcomes of spinal cord injuries (SCIs) resulting from ischemic infarction.
Methods:
All patients with SCI and a diagnosis of cord infarct who were admitted to the inpatient rehabilitation unit at a level 1 trauma center from January 2003 to January 2014 were identified using an administrative billing database. Outcomes measures were evaluated.
Results:
Among 685 unique SCI patients who were identified, 30 (4.4%) had SCI due to spinal ischemic infarction. The mean age was 59 years (range 17-80 years). Fifty percent of patients had ASIA (American Spinal Injury Association) A and B severity. Most common causes were the following: 6 (20%) abdominal aortic aneurysm (AAA) repairs, 6 (20%) arteriovenous fistulas, and 6 (20%) with an unknown cause. Surgical complications led to 4 (13.3%) cord infarcts and was associated with a higher severity of injury (P = .02) compared with other etiologies. Other causes included systemic hypotension, AAA rupture, trauma, diabetic ketoacidosis, and after radiation therapy. At follow-up, 6 (20%) of patients were able to ambulate normally without assistance, 7 (23.3%) were ambulating with assistance, and 17 (56.7%) were still wheelchair bound. Clinical improvement in ambulatory status was noted in 6 (20%) patients and was associated with less severe initial injury (P = .02).
Conclusions:
While the existing literature associates spinal cord infarction with aortic pathologies and surgery, these caused less than 30% of cases, while nonaortic surgical complications were associated with the most severe injuries. Outcomes were worse than previously reported in the literature.
Keywords: spinal cord injury, epidemiology, etiology, spinal cord infarct, spinal cord ischemia, abdominal aortic aneurysm, ASIA grade, outcomes
Introduction
Patients with spinal cord injuries (SCI) have high lifelong health care expenses and a significantly diminished quality of life.1 Spinal cord ischemia and infarcts represent only 5% to 8% of all acute myelopathies and less than 2% of vascular neurologic pathologies. They are rare and often overlooked causes of cord injury that deserve further analysis.2,3
Spinal cord ischemia and infarction occur as a result of restricted blood flow to the spinal cord, leading to disrupted oxygen and glucose delivery, and subsequent metabolic failure of the cord tissue. The spinal cord is supplied by an anterior spinal artery and 2 posterior spinal arteries, which in turn receive their blood supply based on region: C1-T3 is supplied by the vertebral arteries, T3-T7 receives a branch from the intercostal arteries, and T8 to the medullary conus is derived from the Adamkiewicz artery as well as branches of the common or internal iliac arteries.4,5 Etiologies of spinal cord infarction vary greatly and include atherosclerosis, systemic hypotension, infection, emboli, vasculitis, aortic pathologies, coagulopathy, vascular surgery, and other surgical procedures, to name just a few. Conflicting risk factors for more severe cord infarction have been reported. Younger age, cardiac diseases, and higher blood glucose levels have been associated with increased severity.6 Some studies report male gender to be a risk factor whereas other studies have found female sex to be associated with worse severity and outcomes.2,6 Studies regarding the clinical outcomes of spinal cord infarction are limited and vary.7,8
One important factor that has been changing the health care landscape in the United States is the aging population. The geriatric population is growing rapidly, and the median age of initial SCI has been increasing at an even faster rate.9,10 Cord injuries of both traumatic and nontraumatic etiologies are anticipated to increase.11,12 Risk factors for spinal cord infarcts are undoubtedly changing as well, as the prevalence of abdominal aortic aneurysm (AAA) in patients older than 65 years and in particular those with a cigarette smoking history have increased.13 There has also been an increase in both elective and rupture repair in the elderly population.14
While many studies have been published on spinal cord infarction in relation to operations for aortic syndromes and other surgical procedures, aortic pathologies are just one of many risk factors that contribute to infarcts.15,16 In fact, the majority of cases are not a result of aortic aneurysms or surgical procedures.3,6,7 In up to 60% of cases the cause of spinal cord infarction is unknown.17,18 With the shifting healthcare landscape and a lack of recent literature encompassing all etiologies of cord infarct and clinical outcomes, an evaluation of spinal cord infarcts is necessary.
Using a large single institution SCI database that includes both traumatic and non-traumatic causes, our objective was to evaluate the demographics, prevalence, etiology, severity, and outcomes of SCIs resulting from infarction.
Materials and Methods
This study was approved by our institutional review board and is a retrospective review of all patients with SCI admitted to the inpatient rehabilitation unit of a level 1 trauma center from January 2003 to January 2014. Subjects were identified using an administrative billing database that generated a list of patients meeting our search criteria. These patients were managed at a single tertiary care academic center by multiple surgeons and providers. All patients that were enrolled demonstrated traumatic or nontraumatic SCI of the cervical, thoracic, and/or lumbar level. Some double entries due to readmissions were noted and excluded from the study, as were those with incomplete medical records. Finally, those with a diagnosis of spinal cord infarct (confirmed with magnetic resonance imaging) were evaluated in further detail.
Demographic and clinical data were analyzed, including age, sex, race, ethnicity, American Spinal Injury Association (ASIA) grade, infarct etiology, functional ambulatory outcomes at follow-up, and mortality. Patient outcomes (including mortality) were followed using electronic medical records up to June 1, 2018. Clinical improvement was defined in patients who had been completely dependent and wheelchair bound on admission but able to walk with or without assistance at follow-up, or in patients who had initially been walking with aids or assistance but were able to walk without aids or assistance at follow-up.
For statistical analysis, we used the chi-square test and Fisher’s exact test for correlations between categorical variables. Logistic regression analyses were utilized when there was a continuous independent variable (eg, age). Significance was set at P < .05.
Results
Demographics and Prevalence
A total of 757 initial SCI entries were identified and 685 unique patients met our inclusion criteria. Of those, 30 (4.4%) had SCI due to spinal ischemic infarction (Table 1). Twenty (66.7%) were males and 10 (33.3%) were females. Patients were predominantly white (25, 83.3%), followed by 2 (6.7%) black patients, and 1 (3.3%) each of Asian, other, and unknown. The mean age at admission was 59 years (range 17-80 years).
Table 1.
Demographics.
| n (%) of All SCI Infarct Patients (N = 30) | |
|---|---|
| Gender | |
| Males | 20 (66.7) |
| Females | 10 (33.3) |
| Race | |
| White | 25 (83.3) |
| Black | 2 (6.7) |
| Asian | 1 (3.3) |
| Other | 1 (3.3) |
| Unknown | 1 (3.3) |
| Age at admission (years) | |
| Mean (range) | 59 (17-80) |
Abbreviation: SCI, spinal cord injury.
Infarct Etiology and Injury Severity
Of the 30 patients, 12 (40%) had an ASIA grade A, 3 (10%) were noted to be ASIA B, 4 (13.3%) ASIA C, and 11 (36.7%) ASIA D (Table 2). The severity of cord injury was also noted for each etiology of cord infarct. Etiologies were led by 6 (20%) patients who had cord infarct as a result of AAA repair (4 open thoracoabdominal repairs, 1 thoracic endovascular repair, and 1 infrarenal endovascular repair), as well as 6 (20%) with arteriovenous (AV) fistula and 6 (20%) with an unknown cause of ischemic infarct. Half of the AAA repair patients had an ASIA grade of A and the other half ASIA D. AV Fistulas were predominantly associated with the lower ASIA C and D scores (5, 83.3%). Surgical complications led to 4 (13.3%) cord infarcts and was associated with a higher severity of injury (4 ASIA A) (P = .02) compared with other etiologies. These 4 surgeries were anterior corpectomy and fusion for an L1 burst fracture, appendectomy, video-assisted thoracoscopic surgery (VATS) for drainage and biopsies, and an aortic mesenteric bypass. Intraoperative neuromonitoring was not used in any of the surgeries.
Table 2.
Cord Infarct Etiology and SCI Severity.
| Severity of SCI, n (% of all SCI Infarct Patients) | |||||
|---|---|---|---|---|---|
| All Patients | ASIA A | ASIA B | ASIA C | ASIA D | |
| Overall | 30 | 12 (40) | 3 (10) | 4 (13.3) | 11 (36.7) |
| Etiology | |||||
| AAA repair | 6 (20) | 3 | 0 | 0 | 3 |
| AV fistula | 6 (20) | 0 | 1 | 2 | 3 |
| Other ischemia | 6 (20) | 1 | 2 | 1 | 2 |
| Surgical complication | 4 (13.3) | 4 | 0 | 0 | 0 |
| Systemic hypotension | 3 (10) | 1 | 0 | 1 | 1 |
| AAA rupture | 2 (6.7) | 1 | 0 | 0 | 1 |
| Trauma | 1 (3.3) | 0 | 0 | 0 | 1 |
| Diabetic ketoacidosis | 1 (3.3) | 1 | 0 | 0 | 0 |
| Radiation therapy | 1 (3.3) | 1 | 0 | 0 | 0 |
Abbreviations: SCI, spinal cord injury; ASIA, American Spinal Injury Association; AV, arteriovenous; AAA, abdominal aortic aneurysm.
Three (10%) cord infarct cases were due to systemic hypotension, and 2 (6.7%) were due to aortic rupture. Hypotension in the setting of trauma accounted for 1 (3.3%) case. Finally, there was 1 patient (3.3%) with infarct in the setting of diabetic ketoacidosis (DKA) (Figure 1) and 1 (3.3%) after radiation therapy for a C7 plasmacytoma lesion. Both of these were associated with ASIA A severity.
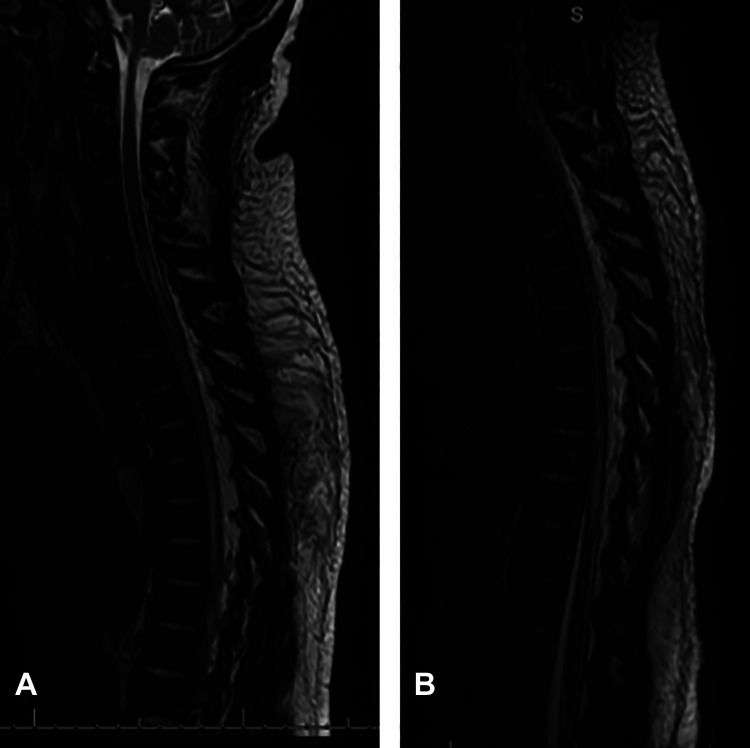
Figure 1.
A 44-year-old-male with ASIA (American Spinal Injury Association) A spinal cord infarction associated with diabetic ketoacidosis. (A) Sagittal T2-weighted magnetic resonance image of the thoracic spine demonstrating the increased spinal cord signal and infarction at the cervicothoracic junction and (B) thoracolumbar junction.
Seventy percent of females had severe cord infarct injuries (ASIA A and B), compared with only 40% in males, though this was not statistically significant. Increased age was not associated with higher ASIA grade (P = .75).
Clinical Outcomes
Mean follow-up for clinical outcomes was 2.7 ± 3.0 years (Table 3). At follow-up, 6 (20%) of the patients were able to ambulate normally without assistance, 7 (23.3%) were ambulating with assistance, and 17 (56.7%) were still wheelchair bound. Those who were ambulating normally all had an initial ASIA grade of D, and the majority of those who were still wheelchair bound at follow-up had severe initial injury (ASIA A or B, n = 14, 82.4%, P = .0001). Clinical improvement in ambulatory status was noted in 6 (20%) patients, of whom 1 was initially ASIA B and 5 were ASIA D. Clinical improvement was significantly associated with less severe initial injury (P = .02).
Table 3.
Functional Outcomes at Follow-up.
| Outcomes by Initial ASIA Grade | |||||
|---|---|---|---|---|---|
| All (N = 30) | A (n = 12) | B (n = 3) | C (n = 4) | D (n = 11) | |
| Mean follow-up, years (SD) | 2.7 (3.0) | 2.0 (2.8) | 3.1 (2.4) | 1.9 (2.1) | 3.7 (3.7) |
| Ambulation at follow-up, n (%) | |||||
| Normal | 6 (20) | 0 | 0 | 0 | 6 (54.5) |
| Walk with assistance | 7 (23.3) | 0 | 1 (33.3) | 2 (50) | 4 (36.4) |
| Wheelchair | 17 (56.7) | 12 (100) | 2 (66.7) | 2 (50) | 1 (9.1) |
| Clinical improvement, n (%) | 6 (20) | 0 | 1 (33.3) | 0 | 5 (45.5) |
Abbreviation: ASIA, American Spinal Injury Association.
Mortality outcomes were also noted (Table 4). 1 (3.3%) died in the hospital prior to discharge, 25 (83.3%) were still alive at 1 year after discharge, and 21 (70.0%) were alive at 6 years postdischarge. Two (6.7%) patients were deceased with an unknown date of death, the status of 4 (13.3%) were unknown, and 15 (50%) were still alive as of June 1, 2018. The overall deceased rate was 36.7%, and this was not significantly associated with increased age (P = .24).
Table 4.
Mortality Outcomes.
| n (%) of all SCI Infarct Patients | |
|---|---|
| Years from hospital discharge, N (%) alive | |
| At discharge | 29 (96.7) |
| 1 | 25 (83.3) |
| 2 | 24 (80.0) |
| 3 | 23 (76.7) |
| 4 | 22 (73.3) |
| 5 | 22 (73.3) |
| 6 | 21 (70.0) |
| Overall deceased | 11 (36.7) |
| Unknown status | 4 (13.3) |
| Still alivea | 15 (50.0) |
Abbreviation: SCI, spinal cord injury.
a As of June 1, 2018.
Discussion
SCIs are associated with high health care costs (currently estimated to be $40 billion in the United States) and significantly reduced quality of life, and the epidemiology is shifting rapidly.11 Spinal cord infarcts are a commonly overlooked cause of these devastating injuries. The existing literature primarily focuses on spinal cord infarcts as a result of aortic pathologies, but etiologies of infarcts vary greatly.6,7 In this study, we evaluated all patients with SCI caused by infarcts and note their etiologies and outcomes.
Of 685 unique SCI patients who met our inclusion criteria, 30 (4.4%) were due to spinal cord infarcts, which is in line with existing literature.2,3 Our cohort was 66.7% male and 33.3% female. Some studies have had primarily males while others have noted females to be risk factors, but the sample size of these studies is too small to make definitive conclusions regarding gender and cord infarcts.2,6,19 Age of these patients also vary greatly although our data aligns with prior studies.18,20 Half of the patients had ASIA A and ASIA B severity, followed by 13.3% ASIA C and 36.7% ASIA D, with the overall severity being greater than previously described.2 We found that the most common causes of infarct were AAA repair (20%), AV fistula (20%), and other (20%). AAA rupture caused 6.7% of cases. Some studies have shown aortic surgery (eg, AAA repair) to be a more common cause of infarct compared with nonsurgical aortic pathology (eg, AAA rupture) while others reveal the opposite, but our data is consistent with existing literature in that aortic pathologies and surgeries together account for less than 30% of cord infarcts.18,20 Among the aortic related infarcts, severity is split, with half having an SCI grade of ASIA A or B and the other half ASIA C or D. Other surgeries were associated with the highest severity of injury (P = 0.02), with all 4 cases leading to ASIA A injuries. In all cases, injury was confirmed postoperatively with magnetic resonance imaging. Given the lack of neuromonitoring during these surgeries, it is difficult to discern any specific intraoperative moments that may have caused the infarcts.
Two rare cases deserve special attention. One patient (a 44-year-old African American male) had spinal cord infarction that was attributed to DKA, leading to ASIA A SCI (Figure 1). There have been only 3 prior reported cases of this during our literature search.21-23 Because of the rarity of this clinical scenario, the pathophysiology of DKA leading to spinal cord edema and thus infarction is unclear. Theories have included: systemic hypotension and hypoxia, rapid changes in serum osmolarity, hyperviscosity, and electrolyte abnormalities.24 In the most recent published case described by Christodoulidou et al,23 cord edema and infarct was attributed to increased hyperosmolar state at baseline in addition to rapid fluid administration for the management of DKA. Because of the disease state of diabetes mellitus, there is chronic hyperosmolarity in the serum, and central nervous system (CNS) cells protect themselves by producing intracellular osmoles. When serum osmolarity decreases sharply, as can happen during rapid fluid resuscitation for the management of DKA, the relative hyperosmolarity in CNS cells results in edema and infarction of the spinal cord.24,25 Our second unique case of infarction (in a 77-year-old white female) related to radiation therapy is discussed in basic and translational research realms but still relatively rare in clinical literature. The mechanism here is more obvious: radiation leading to changes in permeability of the blood-brain barrier, altered blood flow, and ultimately ischemic necrosis of CNS tissue.26,27
Literature on the clinical outcomes of spinal cord infarct is sparse. The mean follow-up represented here was 2.7 years, though the range varied greatly from 3 days to 10.8 years. Owing to lack of consistency in follow-up data regarding other clinical measures (eg, bowel or bladder function), ambulation was used as the primary functional assessment of clinical outcomes. At last known follow-up, 20% of patients were ambulating normally, 23.3% were able to walk with assistance, and 56.7% were wheelchair bound. Those who were able to ambulate normally all had an initial ASIA grade of D, and those with severe initial injury (ASIA A or B) were almost all wheelchair bound at follow-up (P = 0.0001). Clinical improvement was seen in 6 (20%) patients and was associated with less severe initial injury (P = 0.02). Our outcomes are significantly worse than most prior studies. One study reported that only 20% were wheelchair bound at follow-up, and another reported 35.2% rate of nonambulation, compared with our findings of 56.7%.2,8 Novy et al7 noted a 70% clinical improvement rate, significantly higher than our 20%. Overall mortality rate at latest follow-up was 36.7%, which was also higher than previously reported.2,8 Unsurprisingly, mortality in the cord infarct patients is greater than that of the overall SCI population.11
The etiologies of spinal cord infarcts listed in this study align with existing literature in some realms but deviate in others. Consistent with other studies, we found that aortic pathologies and associated surgeries generally account for 25% to 30% of spinal infarcts.18,20 Thus, while most cord infarct literature is within the aortic pathology and surgical realm, more than 70% of cord infarcts are caused by other mechanisms, such as AV fistulas (20%), other, or unknown (20%), nonaortic surgeries (13.3%), or systemic hypotension (10%), to name just a few. We found clinical outcomes to be significantly worse than prior studies, which was likely due to multiple factors, including differences in mean age, etiology of injury, length of follow-up, and most notably our higher proportion of ASIA A patients, indicating the significant poor prognostic predictor of a severe initial cord injury. Our data reveals that clinical improvement and normal ambulatory status are seen primarily in those who had the least severe initial SCI (ASIA D). We hope the findings of our large single-center study of spinal cord infarcts will prompt providers and researchers to consider spinal cord infarcts with a more holistic approach, recognizing that these injuries occur not only with aortic pathologies but with many others, including rare ones such as diabetic ketoacidosis and radiation therapy. Providers and caretakers should be aware of the relatively poor prognosis of SCIs as a result of infarction (particularly in those with more severe initial presentation), and these cases should be managed at or referred to SCI rehabilitation centers.
There are several limitations to this study. First, this was a single center study, the demographics of which cannot be extrapolated to other regions of the United States or globally. Second, follow-up length varied greatly and the loss of follow-up in some patients may have biased the outcomes data. For example, the patients who had clinical improvement may have decided that they no longer needed to follow-up, and this positive functional outcome would have been missed and not represented in our data. In addition, patient mortalities prior to rehabilitation center admission (the inclusion event for this study) lead to an underestimation of the scope of acute SCI at our facility. It should also be noted that this study was limited in sample size and lacked a control group. Finally, we did not have standardized patient-reported outcomes because these patients were treated by multiple providers from many different specialties.
In conclusion, spinal cord ischemic infarct is a rare and often overlooked cause of SCI. While the majority of existing literature associates spinal cord infarction with aortic pathologies and surgery, this study demonstrates that the etiologies vary greatly and include rare ones such as diabetic ketoacidosis and radiation therapy. Infarcts cause severe SCI, with half of our patients having an ASIA A or B grade. Nonaortic surgical complications were associated with the most severe injuries. Ambulatory functional outcomes and clinical improvement were worse than previously reported. We hope that this study will direct future evaluation and assessment of ischemic cord infarction and lead to effective care measures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Addisu Mesfin, MD  https://orcid.org/0000-0003-0076-4185
https://orcid.org/0000-0003-0076-4185
References
- 1. National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202018.pdf. Accessed September 5, 2019.
- 2. Nedeltchev K, Loher TJ, Stepper F, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35:560–565. doi:10.1161/01.STR.0000111598.78198.EC [DOI] [PubMed] [Google Scholar]
- 3. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68:282–292. [PubMed] [Google Scholar]
- 4. Vuong SM, Jeong WJ, Morales H, Abruzzo TA. Vascular diseases of the spinal cord: infarction, hemorrhage, and venous congestive myelopathy. Semin Ultrasound CT MR. 2016;37:466–481. doi:10.1053/J.SULT.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 5. Sliwa JA, Maclean IC. Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes. Arch Phys Med Rehabil. 1992;73:365–372. [DOI] [PubMed] [Google Scholar]
- 6. Romi F, Naess H. Characteristics of spinal cord stroke in clinical neurology. Eur Neurol. 2011;66:305–309. doi:10.1159/000332616 [DOI] [PubMed] [Google Scholar]
- 7. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113–1120. doi:10.1001/archneur.63.8.1113 [DOI] [PubMed] [Google Scholar]
- 8. Robertson CE, Brown RD, Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78:114–121. doi:10.1212/WNL.0b013e31823efc93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–177. doi:10.1097/01.SLA.0000081085.98792.3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikpeze TC, Mesfin A. Spinal cord injury in the geriatric population: risk factors, treatment options, and long-term management. Geriatr Orthop Surg Rehabil. 2017;8:115–118. doi:10.1177/2151458517696680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ge L, Arul K, Ikpeze T, Baldwin A, Nickels JL, Mesfin A. Traumatic and nontraumatic spinal cord injuries. World Neurosurgery. 2018;111:e142–e148. doi:10.1016/J.WNEU.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 12. New PW, Sundararajan V. Incidence of non-traumatic spinal cord injury in Victoria, Australia: a population-based study and literature review. Spinal Cord. 2008;46:406–411. doi:10.1038/sj.sc.3102152 [DOI] [PubMed] [Google Scholar]
- 13. Saucy F, Déglise S, Holzer T, et al. Abdominal aortic aneurysm: what about screening? Curr Pharm Des. 2015;21:4084–4087. [DOI] [PubMed] [Google Scholar]
- 14. Lilja F, Wanhainen A, Mani K. Changes in abdominal aortic aneurysm epidemiology. J Cardiovasc Surg (Torino). 2017;58:848–853. doi:10.23736/S0021-9509.17.10064-9 [DOI] [PubMed] [Google Scholar]
- 15. Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat. 2011;33:3–9. doi:10.1007/s00276-010-0654-0 [DOI] [PubMed] [Google Scholar]
- 16. Fehrenbacher JW, Siderys H, Terry C, Kuhn J, Corvera JS. Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg. 2010;140(6 suppl):S154–S160. doi:10.1016/j.jtcvs.2010.08.054 [DOI] [PubMed] [Google Scholar]
- 17. Naess H, Romi F. Comparing patients with spinal cord infarction and cerebral infarction: clinical characteristics, and short-term outcome. Vasc Health Risk Manage. 2011;7:497–502. doi:10.2147/VHRM.S22950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masson C, Pruvo JP, Meder JF, et al. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry. 2004;75:1431–1435. doi:10.1136/jnnp.2003.031724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadav N, Pendharkar H, Kulkarni GB. Spinal cord infarction: clinical and radiological features. J Stroke Cerebrovasc Dis. 2018;27:2810–2821. doi:10.1016/j.jstrokecerebrovasdis.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 20. Salvador de la Barrera S, Barca-Buyo A, Montoto-Marqués A, Ferreiro-Velasco M, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord. 2001;39:520–525. doi:10.1038/sj.sc.3101201 [DOI] [PubMed] [Google Scholar]
- 21. Dixon AN, Jude EB, Banerjee AK, Bain SC. Simultaneous pulmonary and cerebral oedema, and multiple CNS infarctions as complications of diabetic ketoacidosis: a case report. Diabet Med. 2006;23:571–573. doi:10.1111/j.1464-5491.2006.01822.x [DOI] [PubMed] [Google Scholar]
- 22. Eisenhut M. Interleukin-1 and the constellation of pulmonary oedema, and cerebral infarctions and oedema in diabetic ketoacidosis. Diabet Med. 2006;23:1386 doi:10.1111/j.1464-5491.2006.02002.x [DOI] [PubMed] [Google Scholar]
- 23. Christodoulidou M, Selmi F. Severe diabetic ketoacidosis leading to cardiac failure, pulmonary oedema and spinal cord oedema resulting in tetraplegia. BMJ Case Rep. 2012;2012:bcr2012006769 doi:10.1136/bcr-2012-006769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levin DL. Cerebral edema in diabetic ketoacidosis. Pediatr Crit Care Med. 2008;9:320–329. doi:10.1097/PCC.0b013e31816c7082 [DOI] [PubMed] [Google Scholar]
- 25. Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164–171. doi:10.1016/j.jpeds.2004.03.045 [DOI] [PubMed] [Google Scholar]
- 26. Okada S, Okeda R. Pathology of radiation myelopathy. Neuropathology. 2001;21:247–265. [DOI] [PubMed] [Google Scholar]
- 27. Hornsey S, Myers R, Jenkinson T. The reduction of radiation damage to the spinal cord by post-irradiation administration of vasoactive drugs. Int J Radiat Oncol Biol Phys. 1990;18:1437–1442. [DOI] [PubMed] [Google Scholar]