Summary
Serum thymus and activation regulated chemokine (TARC) levels reflect classical Hodgkin lymphoma (cHL) disease activity and correspond with treatment response. We compared mid‐treatment interim TARC (iTARC) with interim 18F‐fluorodeoxyglucose positron‐emission tomography (iPET) imaging to predict modified progression‐free survival (mPFS) in a group of 95 patients with cHL. High iTARC levels were found in nine and positive iPET in 17 patients. The positive predictive value (PPV) of iTARC for a 5‐year mPFS event was 88% compared to 47% for iPET. The negative predictive value was comparable at 86% for iTARC and 85% for iPET. Serum iTARC levels more accurately reflect treatment response with a higher PPV compared to iPET.
Key points.
Interim TARC levels are highly predictive of modified progression‐free survival in classical Hodgkin lymphoma
Interim TARC levels have a better positive predictive value than interim 18F‐FDG‐PET imaging for predicting modified progression‐free survival
Early response to first‐line treatment determined by interim 18F‐fluorodeoxyglucose (FDG) positron‐emission tomography (iPET) after one or two cycles of chemotherapy is a strong predictor of progression‐free survival (PFS) in classical Hodgkin lymphoma (cHL) (Hutchings et al., 2014). iPET‐based treatment escalation or de‐escalation resulted in improved PFS and reduced treatment‐related toxicity, respectively (Radford et al., 2015; Johnson et al., 2016; Andre et al., 2017; Borchmann et al., 2018). Nevertheless, the iPET result does not accurately predict final outcome for all patients. In patients with a negative iPET after two cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazin) PFS event rates ranged from 10% up to 25% in early‐ and advanced‐stage patients, respectively (Johnson et al., 2016; Andre et al., 2017). On the other hand, 25% of patients with cHL with advanced‐stage disease and a positive iPET became PET negative after completion of ABVD treatment and experienced durable remissions (Biggi et al., 2013).
The CC‐chemokine, CCL17 (also known as TARC), is a very specific marker for cHL disease activity (van den Berg et al., 1999; Niens et al., 2008). TARC levels are elevated in pre‐treatment blood samples in >90% of patients with cHL and correlate with metabolic tumour volume. Serial TARC levels reflect treatment response even after one cycle of chemotherapy (Plattel et al., 2012; Plattel et al., 2016). In the present study, we compared interim TARC (iTARC) results with simultaneous performed iPET imaging to predict modified PFS (mPFS) in patients with cHL.
The primary end‐point of this study was the 5‐year mPFS rate for iTARC and iPET. Events for mPFS were defined as: progression, relapse, start of second‐line treatment for patients not achieving a complete response (CR) after completion of treatment including radiotherapy and death due to any cause. iTARC was considered elevated when the level was >1000 pg/ml as previously described (Plattel et al., 2012). Patients diagnosed with cHL from 2006 to 2017 in our centre (n = 106) were included, based on both the availability of iPET and iTARC. A total of 10 patients (9%) were excluded because pre‐treatment TARC was not elevated and one patient was excluded because of active atopic dermatitis, which interferes with accurate interpretation of the TARC measurements (Thijs et al., 2015). Data on pre‐ and post‐treatment TARC, but not iTARC and iPET were previously published for 75 patients (Plattel et al., 2016).
The patients’ characteristics of the remaining 95 patients are listed in Table 1. The median (range) follow‐up for the entire cohort was 58 (7–130) months. In all, 54 (57%) patients had early‐ and 41 (43%) advanced‐stage disease. Most patients were treated according to European Organisation for Research and Treatment of Cancer (EORTC) protocols, active during the study period (Carde et al., 2016; Andre et al., 2017). Early‐stage patients were generally treated with three to four cycles of ABVD combined with involved‐node radiotherapy in 70% of patients. Advanced‐stage patients mainly received six–eight cycles of ABVD (59%) or (esc)BEACOPP (bleomycin, etoposide, adriamycin (doxorubicin), cyclophosphamide, vincristine, procarbazine, prednisone) (30%) combined with radiotherapy on remaining FDG‐PET‐positive lesions after completion of chemotherapy (7%). Response was re‐defined according to the Lugano classification (Cheson et al., 2014). Interim response evaluation with iTARC and iPET was performed at the same time‐point, that is, after two cycles of chemotherapy in early‐stage patients and after two or three cycles of chemotherapy in advanced‐stage patients. TARC was also measured after the first cycle of chemotherapy in most patients, but comparisons between iTARC and iPET were only performed on simultaneous evaluations after two or three cycles. No treatment adjustments were made based on iTARC or iPET results, except for omission of radiotherapy in 25% of early‐stage patients with a negative iPET, which is in accordance with the experimental arm of the EORTC trial H10 (Andre et al., 2017).
Table 1.
Patients’ characteristics according to iTARC.
| Characteristic |
Total (N = 95) |
iTARC <1000 pg/ml (n = 86) |
iTARC ≥1000 pg/ml (n = 9) |
|---|---|---|---|
| Age, years, median (range) | 32 (18–82) | 31 (18–82) | 49 (25–79) |
| Male, n (%) or n/N | 42 (44) | 37 (43) | 5/9 |
| Stage I/II, n (%) or n/N | 54 (57) | 51 (59) | 3/9 |
| Follow‐up, months, median (range) | 58 (7–130) | 62 (14–130) | 19 (7–82) |
| mPFS event, n (%) or n/N | 18 (19) | 10 (12) | 8/9 |
| iPET, n (%) or n/N | |||
| Negative (DS 1–3) | 78 (82) | 76 (88) | 2/9 |
| Positive (DS 4–5) | 17 (18) | 10 (12) | 7/9 |
| End of treatment TARC, n (%) or n/N | |||
| <1000 pg/ml | 84 (88) | 82 (95) | 2/9 |
| ≥1000 pg/ml | 11 (12) | 4 (5) | 7/9 |
| End of treatment FDG‐PET, n (%) or n/N | |||
| Negative | 82 (86) | 79 (92) | 3/9 |
| Positive | 13 (14) | 7 (8) | 6/9 |
| End of treatment response, n (%) or n/N | |||
| Complete response | 84 (88) | 81 (94) | 3/9 |
| Partial response | 6 (6) | 2 (2) | 4/9 |
| Progressive disease | 5 (5) | 3 (3) | 2/9 |
TARC, thymus and activation regulated chemokine; mPFS, modified progression‐free survival; FDG‐PET, fluorodeoxyglucose positron‐emission tomography; DS, Deauville Score.
At the mid‐treatment time‐point, iPET was positive (Deauville Score ≥4) in 17/95 (18%) and iTARC was elevated in nine of 95 (8%) patients (Figure S1). Concordance between iTARC and iPET was 87%. Both negative iPET and normal iTARC levels (double negative) were observed in 76 patients, both positive (double positive) in seven and discrepant results were found in 12 patients. Of the 76 double‐negative patients, 71 patients remained in remission, three patients were progressive at end of treatment (both TARC and PET became positive again at end‐treatment) and two experienced a relapse >1 year after completion of first‐line treatment with again elevated TARC and positive FDG‐PET at time of relapse. Six out of seven double‐positive patients were refractory to first‐line treatment and one patient with a massive pre‐treatment tumour load and an extremely high TARC level became both FDG‐PET and TARC negative at end of treatment and remained in remission. From the 12 patients with discrepant results at mid‐treatment, two patients had positive iTARC and negative iPET: one patient remained TARC positive at end of treatment, became FDG‐PET positive and was considered progressive and the other remained TARC positive and PET negative but experienced early relapse. The other 10 patients with discrepant results had a low iTARC and a positive iPET: seven of these 10 became FDG‐PET negative at end of treatment, remained TARC negative and did not experience relapse, one remained TARC negative and PET positive and was considered responsive based on a negative re‐biopsy, one became TARC positive at end of treatment and was considered refractory, and one remained TARC negative and proceeded to salvage treatment without re‐biopsy. In conclusion, eight out of nine iTARC‐positive patients were either primary refractory or had an early relapse. Of the 86 iTARC‐negative patients, 79 obtained a persistent complete remission. In contrast, nine of 17 iPET‐positive patients obtained a durable complete remission, seven patients were refractory, and one patient received second‐line treatment without re‐biopsy.
All patients with a CR after completion of treatment had a strong decrease in TARC levels, which was already evident after one cycle of chemotherapy (Fig 1A). Both at mid‐treatment and end of treatment high TARC levels were associated with a Deauville Score of 5 (Fig 1B,C). Concordance between TARC and FDG‐PET was 96% at end of treatment (Table 1). The 5‐year mPFS was 81% for the entire cohort, 84% for early‐stage and 74% for advanced‐stage patients. The iPET‐positive patients had significantly reduced mPFS at 5 years compared to the iPET‐negative patients (53% vs. 85% at 5‐years, P < 0.001; Fig 1D). In contrast, mPFS at 5 years for patients with elevated iTARC was 11% compared to 86% for patients with normal iTARC levels (P < 0.001, Fig 1E). In a combined model with iPET and iTARC, patients with normal iTARC levels generally had a favourable mPFS, whereas patients with elevated iTARC had very poor outcomes, irrespective of iPET results (Fig 1F). Consistent with this only iTARC remained predictive for mPFS in multivariate analysis, including both iPET and iTARC using the Cox proportional hazard method (hazard ratio for elevated iTARC 13·1, 95% confidence interval 3·5–49·4; P < 0.001).
Figure 1.
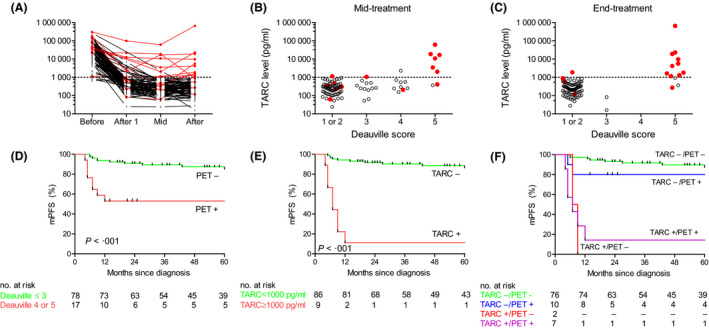
TARC and FDG‐PET results during and after treatment. (A) Dynamics of TARC before treatment, after one cycle of chemotherapy, at mid‐treatment and at end‐treatment. TARC levels were analysed using a sandwich enzyme‐linked immunosorbent assay (R&D systems). The cut‐off for TARC positivity at the mid‐treatment time point was 1000 pg/ml as previously defined (Plattel et al., 2012). Patients achieving a complete response without experiencing a relapse are displayed in black. Patients with refractory disease or patients experiencing a relapse are displayed in red. (B) TARC levels at mid‐treatment compared to mid‐treatment FDG‐PET Deauville score. FDG‐PET images were reconstructed according to the European Association of Nuclear Medicine criteria. All FDG‐PET scans were re‐analysed and visually re‐assessed according to the Lugano classification, which incorporates the Deauville 5‐point scale. A Deauville Score ≥4 was considered FDG‐PET positive. (C) TARC levels at end‐treatment compared to end‐treatment FDG‐PET Deauville Score. (D) Modified progression‐free survival (mPFS, see methods for definition) according to mid‐treatment FDG‐PET result. FDG‐PET negativity was defined as Deauville Score ≤3 and FDG‐PET positivity was defined as Deauville Score of 4 or 5. (E) mPFS according to mid‐treatment TARC result. TARC negativity was defined as TARC below the cut‐off of 1000 pg/ml. (F) mPFS according to combined FDG‐PET and TARC result. iTARC negativity generally correlated with favourable outcome, whereas iTARC positivity correlated with adverse outcome irrespective of the iPET result. Survival analyses were performed using the method of Kaplan and Meier and the log‐rank test was used to assess significance.
This is the first study demonstrating that the blood‐based biomarker TARC can improve interim response evaluation in cHL. We and others already found a strong correlation between early TARC decrease and final favourable outcome (Plattel et al., 2012; Guidetti et al., 2017; Hsi et al., 2019). Guidetti et al. confirmed early TARC decrease as a predictor for iPET negativity. In their study, normalisation of TARC levels after one cycle of chemotherapy highly corresponded with a negative PET after two cycles of ABVD treatment (Guidetti et al., 2017). However, the positive predictive value (PPV) of TARC after one cycle for PET positivity after two cycles was rather limited. This might be due to the high rate of false positive iPET scans. Also, the PPV of elevated TARC for PFS was lower compared to our present study, likely due to the combination of a lower threshold for TARC positivity, different timing of TARC measurement and the uniform treatment escalation based on a positive FDG‐PET, which might have biased their study (Guidetti et al., 2017). The exclusion of patients with treatment escalation based on iPET allowed us to directly compare prognostic value of both iPET and iTARC. Very recently, Hsi et al. analysed among others serial TARC levels in the prospective Southwest Oncology Group (SWOG) S0816 trial and found that end of treatment TARC could aid in prognostication independent of PET imaging (Hsi et al., 2019). We found a high concordance between TARC and FDG‐PET, especially at the end of treatment time‐point. iTARC‐based response evaluation showed an improved PPV for 5‐year mPFS (from 47% to 89%) and similar negative predictive value (88%) as compared to iPET imaging, despite a possible bias in the use of FDG‐PET for final response assessment. Similar to the study by Hsi et al., end of treatment TARC elevation was highly predictive for mPFS: all 11 patients with elevated end of treatment TARC levels were either refractory or experienced early relapse. The higher PPV of TARC compared to PET can be explained by the high specificity of elevated TARC for tumour activity, as TARC is specifically produced and excreted by Hodgkin Reed–Sternberg cells. Serum TARC‐based response evaluation is non‐invasive and cheap, allowing response adapted therapy in cHL worldwide. A limitation of the use of TARC as a biomarker is that it is not applicable in the 10% of patients who do not have elevated pre‐treatment TARC. Although results of our present study are very promising, our modest cohort size warrants validation in a larger cohort.
In conclusion, elevated iTARC levels determined at mid‐treatment are highly predictive for inferior mPFS with a higher PPV compared to iPET. As TARC is elevated at baseline in about 90% of patients with cHL, iTARC measurements might serve as a substitute for iPET in these patients.
Authorship contributions
Wouter J. Plattel, Lydia Visser, Arjan Diepstra, Gustaaf W. van Imhoff and Anke van den Berg contributed to the design of the study. Wouter J. Plattel, Lydia Visser, Marcel Nijland, Tom van Meerten. Hanneke C. Kluin‐Nelemans, Andor W. J. M. Glaudemans, Gustaaf W. van Imhoff and Anke van den Berg collected the samples and/or clinical data. All authors significantly contributed to the manuscript writing.
Conflicts of interest
None.
Supporting information
Figure S1. Outcome of patients with interim PET and interim TARC results.
Acknowledgements
None.
References
- Andre, M.P.E. , Girinsky, T. , Federico, M. , Reman, O. , Fortpied, C. , Gotti, M. , Casasnovas, O. , Brice, P. , van der Maazen, R. , Re, A. , Edeline, V. , Ferme, C. , van Imhoff, G. , Merli, F. , Bouabdallah, R. , Sebban, C. , Specht, L. , Stamatoullas, A. , Delarue, R. , Fiaccadori, V. , Bellei, M. , Raveloarivahy, T. , Versari, A. , Hutchings, M. , Meignan, M. & Raemaekers, J. (2017) Early positron emission tomography response‐adapted treatment in stage I and II hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology, 35, 1786–1794. [DOI] [PubMed] [Google Scholar]
- van den Berg, A. , Visser, L. & Poppema, S. (1999) High expression of the CC chemokine TARC in reed‐sternberg cells. A possible explanation for the characteristic T‐cell infiltratein hodgkin's lymphoma. American Journal of Pathology, 154, 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggi, A. , Gallamini, A. , Chauvie, S. , Hutchings, M. , Kostakoglu, L. , Gregianin, M. , Meignan, M. , Malkowski, B. , Hofman, M.S. & Barrington, S.F. (2013) International validation study for interim PET in ABVD‐treated, advanced‐stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. Journal of Nuclear Medicine, 54, 683–690. [DOI] [PubMed] [Google Scholar]
- Borchmann, P. , Goergen, H. , Kobe, C. , Lohri, A. , Greil, R. , Eichenauer, D.A. , Zijlstra, J.M. , Markova, J. , Meissner, J. , Feuring‐Buske, M. , Huttmann, A. , Dierlamm, J. , Soekler, M. , Beck, H.J. , Willenbacher, W. , Ludwig, W.D. , Pabst, T. , Topp, M.S. , Hitz, F. , Bentz, M. , Keller, U.B. , Kuhnhardt, D. , Ostermann, H. , Schmitz, N. , Hertenstein, B. , Aulitzky, W. , Maschmeyer, G. , Vieler, T. , Eich, H. , Baues, C. , Stein, H. , Fuchs, M. , Kuhnert, G. , Diehl, V. , Dietlein, M. & Engert, A. (2018) PET‐guided treatment in patients with advanced‐stage hodgkin's lymphoma (HD18): final results of an open‐label, international, randomised phase 3 trial by the german hodgkin study group. The Lancet, 390, 2790–2802. [DOI] [PubMed] [Google Scholar]
- Carde, P. , Karrasch, M. , Fortpied, C. , Brice, P. , Khaled, H. , Casasnovas, O. , Caillot, D. , Gaillard, I. , Bologna, S. , Ferme, C. , Lugtenburg, P.J. , Morschhauser, F. , Aurer, I. , Coiffier, B. , Meyer, R. , Seftel, M. , Wolf, M. , Glimelius, B. , Sureda, A. & Mounier, N. (2016) Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score ≥3, high‐risk hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. Journal of Clinical Oncology, 34, 2028–2036. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Fisher, R.I. , Barrington, S.F. , Cavalli, F. , Schwartz, L.H. , Zucca, E. & Lister, T.A. ; Alliance, Australasian Leukaemia and Lymphoma Group , Eastern Cooperative Oncology Group , European Mantle Cell Lymphoma Consortium , Italian Lymphoma Foundation , European Organisation for Research , Treatment of Cancer/Dutch Hemato‐Oncology Group , Grupo Espanol de Medula Osea , German High‐Grade Lymphoma Study Group , German Hodgkin's Study Group , Japanese Lymphorra Study Group , Lymphoma Study Association , NCIC Clinical Trials Group , Nordic Lymphoma Study Group , Southwest Oncology Group & United Kingdom National Cancer Research Institute . (2014) Recommendations for initial evaluation, staging, and response assessment of hodgkin and non‐hodgkin lymphoma: the lugano classification. Journal of Clinical Oncology, 32, 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti, A. , Mazzocchi, A. , Miceli, R. , Paterno', E. , Taverna, F. , Spina, F. , Crippa, F. , Farina, L. , Corradini, P. , Gianni, A.M. & Viviani, S. (2017) Early reduction of serum TARC levels may predict for success of ABVD as frontline treatment in patients with hodgkin lymphoma. Leukemia Research, 62, 91–97. [DOI] [PubMed] [Google Scholar]
- Hsi, E.D. , Li, H. , Nixon, A.B. , Schoder, H. , Bartlett, N.L. , LeBlanc, M. , Smith, S. , Kahl, B.S. , Leonard, J.P. , Evens, A.M. , Scott, D.W. , Rimsza, L.M. & Friedberg, J.W. (2019) Serum levels of TARC, MDC, IL‐10, and soluble CD163 in hodgkin lymphoma: a SWOG S0816 correlative study. Blood, 133, 1762–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, M. , Kostakoglu, L. , Zaucha, J.M. , Malkowski, B. , Biggi, A. , Danielewicz, I. , Loft, A. , Specht, L. , Lamonica, D. , Czuczman, M.S. , Nanni, C. , Zinzani, P.L. , Diehl, L. , Stern, R. & Coleman, M. (2014) In vivo treatment sensitivity testing with positron emission tomography/computed tomography after one cycle of chemotherapy for Hodgkin lymphoma. Journal of Clinical Oncology, 32, 2705–2711. [DOI] [PubMed] [Google Scholar]
- Johnson, P. , Federico, M. , Kirkwood, A. , Fossa, A. , Berkahn, L. , Carella, A. , d'Amore, F. , Enblad, G. , Franceschetto, A. , Fulham, M. , Luminari, S. , O'Doherty, M. , Patrick, P. , Roberts, T. , Sidra, G. , Stevens, L. , Smith, P. , Trotman, J. , Viney, Z. , Radford, J. & Barrington, S. (2016) Adapted treatment guided by interim PET‐CT scan in advanced hodgkin's lymphoma. The New England Journal of Medicine, 374, 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niens, M. , Visser, L. , Nolte, I.M. , van der Steege, G. , Diepstra, A. , Cordano, P. , Jarrett, R.F. , Te Meerman, G.J. , Poppema, S. & van den Berg, A. (2008) Serum chemokine levels in hodgkin lymphoma patients: Highly increased levels of CCL17 and CCL22. British Journal of Haematology, 140, 527–536. [DOI] [PubMed] [Google Scholar]
- Plattel, W.J. , van den Berg, A. , Visser, L. , van der Graaf, A.M. , Pruim, J. , Vos, H. , Hepkema, B. , Diepstra, A. & van Imhoff, G.W. (2012) Plasma thymus and activation‐regulated chemokine as an early response marker in classical hodgkin's lymphoma. Haematologica, 97, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattel, W.J. , Alsada, Z.N. , van Imhoff, G.W. , Diepstra, A. , van den Berg, A. & Visser, L. (2016) Biomarkers for evaluation of treatment response in classical hodgkin lymphoma: Comparison of sGalectin‐1, sCD163 and sCD30 with TARC. British Journal of Haematology, 175, 868–875. [DOI] [PubMed] [Google Scholar]
- Radford, J. , Illidge, T. , Counsell, N. , Hancock, B. , Pettengell, R. , Johnson, P. , Wimperis, J. , Culligan, D. , Popova, B. , Smith, P. , McMillan, A. , Brownell, A. , Kruger, A. , Lister, A. , Hoskin, P. , O'Doherty, M. & Barrington, S. (2015) Results of a trial of PET‐directed therapy for early‐stage hodgkin's lymphoma. The New England Journal of Medicine, 372, 1598–1607. [DOI] [PubMed] [Google Scholar]
- Thijs, J. , Krastev, T. , Weidinger, S. , Buckens, C.F. , de Bruin‐Weller, M. , Bruijnzeel‐Koomen, C. , Flohr, C. & Hijnen, D. (2015) Biomarkers for atopic dermatitis: a systematic review and meta‐analysis. Current Opinion in Allergy and Clinical Immunology, 15, 453–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Outcome of patients with interim PET and interim TARC results.


