ABSTRACT
Over the past decade, a major debate has taken place on the underpinnings of cultural changes in human societies. A growing array of evidence in behavioural and evolutionary biology has revealed that social connectivity among populations and within them affects, and is affected by, culture. Yet the interplay between prehistoric hunter–gatherer social structure and cultural transmission has typically been overlooked. Interestingly, the archaeological record contains large data sets, allowing us to track cultural changes over thousands of years: they thus offer a unique opportunity to shed light on long‐term cultural transmission processes. In this review, we demonstrate how well‐developed methods for social structure analysis can increase our understanding of the selective pressures underlying cumulative culture. We propose a multilevel analytical framework that considers finer aspects of the complex social structure in which regional groups of prehistoric hunter–gatherers were embedded. We put forward predictions of cultural transmission based on local‐ and global‐level network metrics of small‐scale societies and their potential effects on cumulative culture. By bridging the gaps between network science, palaeodemography and cultural evolution, we draw attention to the use of the archaeological record to depict patterns of social interactions and transmission variability. We argue that this new framework will contribute to improving our understanding of social interaction patterns, as well as the contexts in which cultural changes occur. Ultimately, this may provide insights into the evolution of human behaviour.
Keywords: cultural evolutionary theory, cultural transmission, cultural complexity, social network analysis, archaeological networks, prehistoric hunter–gatherers, human social behaviour, computational archaeology, evolutionary archaeology
I. INTRODUCTION: AIMS AND SCOPE
This paper presents an evolutionary framework for studying human cultural evolution based on the interface between socio‐spatial structure and cultural transmission using the archaeological record. Scientists have been puzzling over cumulative changes in cultural traits for over a decade (Bar‐Yosef, 2002; Collard, Kemery, & Banks, 2005; Powell, Shennan, & Thomas, 2009), yet evidence from empirical and theoretical studies indicates that population size and social connectivity shape cultural evolution. A frequently explored assumption is that population size co‐varies with cultural complexity (Shennan, 2001; Henrich, 2004; Kline & Boyd, 2010; Derex et al., 2013; Kempe & Mesoudi, 2014). However, the interplay between social connectivity (hereafter social structure, i.e. patterns of social interactions or association among individuals, regional groups and populations; Hinde, 1976) and cultural transmission have been largely under‐researched. Interestingly, the archaeological record provides a data set allowing us to track cultural changes over thousands of years (Garvey, 2018). This represents a remarkable opportunity: it opens the way to exploring the manifold relationship between social structure and cultural complexity.
In this review, we aim to formalise social structure as a crucial component of the cultural evolution of prehistoric hunter–gatherer populations. More specifically, we suggest that such studies would benefit from considering the populations' socio‐spatial distribution, which encodes interaction patterns over geographic areas. Cultural transmission depends on connections between groups (Boyd & Richerson, 1985), and is likely to be affected by the degree and strength of intra‐ and inter‐regional group interactions as well as the spatial distribution of the prehistoric hunter–gatherer groups (Whallon, 2006). Consequently, the socio‐spatial structure of hunter–gatherers should clearly have a key impact on human cultural evolution.
We start by outlining the core concepts of cultural evolutionary theory before defining the terminology used herein. We then use a demographic hypothesis to explore the variation of cultural complexity while providing empirical and theoretical evidence of the role of social structure in cultural transmission. We thus follow an analytical approach to examine and interpret social connections based on material culture artefacts. In so doing, we present an evolutionary framework for studying cultural evolution considering the links between population size, social structure and culture. To finish, we advance that social structure mediates cultural transmission and is possibly one of its drivers.
II. CULTURAL EVOLUTIONARY THEORY
(1). Overview
In addition to the unified understanding of genes and their role in inheritance (sensu Huxley, 1942), there is a second form of evolution, recognised long ago (Darwin, 1859). Cultural evolutionary theory (CET) upholds social/cultural inheritance, in which individuals copy or learn from others. For a comprehensive approach to CET, it is important to distinguish its two underlying principles. First, culture is considered as a system of inheritance in which selective processes of evolution take place (e.g. individual variability in the population, differential survival and reproduction; Boyd & Richerson, 1985; Bettinger, 1991). Second, the mechanisms underlying the transmission of information differ from those governing genes. While in genetic transmission individuals inherit genes from their parents, in cultural transmission individuals can receive information from multiple sources, such as from unrelated peers (i.e. horizontal transmission) and/or across generations [i.e. from parents to their offspring (vertical transmission) or from one generation to another younger generation (oblique transmission); Boyd & Richerson, 1985)]. This process of learning from others (e.g. social learning) lies at the heart of research on culture, as it is widespread in the animal kingdom (Galef Jr & Laland, 2005). Cultural transmission rests on the creation of new traits (i.e. innovation) and their propagation through social contact (e.g. by imitation or active teaching), and evolutionary forces may introduce (e.g. errors during cultural transmission) or reduce (e.g. biased cultural transmission) variations in the diversity of cultural traits (Boyd & Richerson, 1985).
From this perspective, the concept of niche construction sheds light on how individual decisions may ultimately affect their social environment. The niche construction approach emphasises that individuals may have the capacity to alter the sources of natural selection in their environment and generate a new evolutionary outcome (Laland & Sterelny, 2006). One illustration is that animal and plant domestication, and the spread of agriculture, coincide with periods of demographic transition (Bocquet‐Appel, 2011). Culture has been identified as a significant amplification tool in human societies, and since cultural transmission generally operates faster than genetic transmission, it has been suggested that niche construction may play an important role in human evolution as well (Laland, Odling‐Smee, & Feldman, 2001). For example, culture is presumed to be maintained in population subgroups and to spread faster in more cohesive groups than in sub‐structured ones (Voelkl & Noë, 2010). These transmission variations can lead to variations in the spatial distribution of cultural attributes. Individuals might then cause variations in their social environment by learning and/or spreading new techniques in the socio‐spatial structure they are embedded in. This brings us to the conclusion that we cannot fully appreciate the significance of CET without a more complete understanding of our human ancestors' social structure.
The structural organisation of prehistoric hunter–gatherers is characterised by small clusters of bands forming regional groups nested in a multilevel social structure (Newell et al., 1990). These small groups are likely to have aggregated seasonally for social purposes (e.g. for information exchange, mate‐finding, alliance‐making, ritual/ceremonial events; Newell et al., 1990) and, under favourable conditions, viable long‐term maximum bands might have ranged between approximately 175 and over 300 individuals (Wobst, 1974). Prehistoric hunter–gatherer social structures can be studied at two different levels: at a microscale (intra‐band interaction) and at a macroscale (intra‐ and inter‐population interactions). Since data on individuals are scarce and studying prehistoric societies at a microscale is methodologically challenging, this review focuses on a macroscale perspective.
(2). Key concepts
In this subsection, we cover the terminology used in the literature on cultural evolutionary theory, reviewed below. We start with the term culture: following Laland & Hoppitt (2003, p. 151), culture can be defined as “group‐typical behaviour patterns shared by members of a community that relies on socially learned and transmitted information”.
Cultural complexity (i.e. technological complexity) is another key concept lying at the heart of much of the empirical and theoretical work of cultural evolutionists. Material culture (e.g. ostrich eggshells, pottery vessels, microlithic stone tools, ivory artefacts) carries information at archaeological timescales about a given society, its habitat, beliefs, diet and inter‐regional connections. As such, empirical archaeological data is well suited to model diffusions of innovations and long‐term cultural stability, to explore cultural extinctions and to investigate the effects of cultural transmission on cultural evolution, among others (Garvey, 2018). A fundamental idea in this approach is that artefact assemblages in the archaeological record are a proxy for cultural complexity. In this context, the term cultural complexity has been mostly used to discuss tool assemblage diversity related to subsistence [also called “food‐getting technology” (Collard et al., 2013) or “subsistants” (Oswalt, 1976)], the total number of technounits (i.e. “the different kinds of parts of a tool”; Collard et al., 2013), and the average number of technounits per tool (Oswalt, 1976; Torrence, 2001; Henrich, 2006; Read, 2008; Collard et al., 2011, 2013; Shott, 2016). The rationale is twofold: relative abundance and representativeness. Artefacts linked to the acquisition and management of food appear to have been produced since about 3.3 million years ago (McPherron et al., 2010) and have been well recorded. In addition, since 99% of hominin existence as a distinct lineage depended on hunting and gathering activities, hunter–gatherer subsistence technology is key to understanding cultural variation (Collard et al., 2011, 2013). Henceforth, for the purposes of this review, we use the term cultural complexity to refer to technological subsistence tools.
Cultural evolutionists also use the term cumulative culture (e.g. Cavalli‐Sforza & Feldman, 1981). The ability gradually to change cultural traits based on the knowledge of previous generations, exceeding what any single individual could invent (Boyd & Richerson, 1996) is considered to be the hallmark of human culture [but see Dean et al., 2014 and Mesoudi & Thornton, 2018 for a comprehensive discussion on this topic]. Humans have the capacity to accumulate large amounts of information, ratcheting up its complexity (Tomasello, 1994). A famous example is how humankind landed a manned spacecraft on the moon in the 1960s: this was the outcome of thousands of ‘small steps’, each leading to the accumulation of technical skills within a large team over the course of several years. Having established these definitions, we will now consider what is known about cultural variation in human prehistoric societies.
III. THE PUNCTUATED ACCUMULATION AND LOSS OF CULTURAL COMPLEXITY
The stone age archaeological record shows that long‐term evolutionary patterns of technological complexity were not linear – that is governed by slow and gradual changes towards more sophisticated toolkits – but punctuated and variable in space and time. Long periods of technological stability, such as the Middle Palaeolithic, were interrupted by phases of marked innovations such as the Chatelperronian technocomplex (Zilhão, 2011), the symbolic use of shells and pigments (Zilhão et al., 2010) or the first evidence of rock art among Neanderthals (Hoffmann et al., 2018). By contrast, the loss of technological capital, such as curated toolkits in stone, or bone and antler artefacts, have been documented across different regions of the world. For example, after Tasmania split from Australia, the resident Tasmanian hunter–gatherers reduced their toolkit to around 24 items whilst populations on the Australian mainland maintained hundreds of specialised tools [see Henrich, 2004 for details on the toolkit repertoire]. The Late Glacial and Early Holocene in Europe probably provide the best documented examples of abrupt changes in technological complexity entailing both the rapid spread of innovations (Marchand & Perrin, 2017) and the loss of technological capital (Wicks & Mithen, 2014; Riede, 2016), as clearly manifested in the Iberian archaeological record (Fig. 1). These kinds of changes in cultural traits have been linked to various causes (Bar‐Yosef, 2002), including the geographic expansion of modern humans (Klein, 2000), the risk of resource failure (Collard et al., 2011), environmental changes (Potts, 1996), and demography (Powell et al., 2009).
Figure 1.

Synthetic scheme illustrating the cultural patterns documented in Iberia during the Last Glacial–Early Holocene transition. We find that standardised and curated bone and antler toolkits vanished after the Late Magdalenian period. The blade debitage systems to produce standardised backed tips, points and microliths from the Late Magdalenian to the Epipaleolithic were abruptly replaced during the Early Mesolithic by much simpler flake debitage strategies to produce a reduced set of notches and denticulated tools. Finally, the Late Mesolithic period witnessed the reintroduction of blade debitage and the rapid spread of trapezoid microliths. The black curve at the bottom represents the palaeoclimatic framework (global temperature) according to the Greenland stratotype chronology (Rasmussen et al., 2014).
The primary theoretical basis of the demographic hypothesis was drawn up by Shennan (2001) and Henrich (2004) in essays that addressed the relationship between population size, cultural innovations and cumulative culture. Shennan (2001) simulated the effect of small population size on technological innovation rates. He demonstrated that once the population became small and isolated, imitation rate and levels of mean population fitness remained low, while larger populations had a greater probability of maintaining fitness‐enhancing innovations. Henrich (2004) modelled the loss of technological capital as a function of population size, density and interconnectedness. His model predicted that when one or several of these parameters dropped below a critical threshold, the number of interactions between social learners decreased, leading to stability or to a relative simplification of technological skills. A number of studies explored these theoretical predictions, but they presented mixed results: while some supported the positive relationship between population size and cultural complexity (Kline & Boyd, 2010; Derex et al., 2013; Kempe & Mesoudi, 2014), others found no effect (Collard et al., 2005; Read, 2012; Buchanan, O'Brien, & Collard, 2016b). These contradictory claims have led to heated debates on whether the linear and positive relationship between population size and cultural complexity can be generalised (Henrich et al., 2016; Vaesen et al., 2016). Integrating these perspectives may help to shape major insights into the processes underlying culture: (i) rather than population size, variation in cumulative culture may be caused by the interplay of several factors (e.g. environmental fluctuations, population size, social structure), and (ii) the relationship between population size and cultural complexity is not always linear because it depends, among other factors, on group connectivity.
Over the last few years, an increasing body of evidence has showed that the degree of social interactions, within and among populations, influences cultural complexity (Derex & Boyd, 2016; Kobayashi, Ohtsuki, & Wakano, 2016; Creanza, Kolodny, & Feldman, 2017). Powell et al. (2009), for example, demonstrated that subpopulation density and migratory activities resulted in the spatial structuring of knowledge. Parameters for estimating social structure have been so far developed for population density (e.g. Powell et al., 2009), the level of a population's fragmentation (e.g. Derex & Boyd, 2016) and population inter‐connectivity through migratory events (e.g. Creanza et al., 2017). Findings such as these highlight the need for further theoretical and empirical studies that examine how the complex social structure of prehistoric hunter–gatherers can have an impact on cultural transmission dynamics, which feed back into the heterogeneous spatial and temporal variations of cultural attributes. In the next section, we explore empirical and theoretical evidence that social structure affects cultural evolution.
IV. EVIDENCE OF THE EFFECTS OF SOCIAL STRUCTURE
The idea that social connectivity influences cumulative culture was introduced decades ago (e.g. Cavalli‐Sforza & Feldman, 1981). A main reason is that like social interactions, cultural transmission does not occur randomly. Instead, an individual's acquisition of a behavioural trait from another follows a matrix of interactions: individuals copy skills or learn from others by means of social contacts or spatial proximity. Based on this process, it is possible to track the transmission through the population, iterate it over generations and make predictions regarding the system's evolution (Cavalli‐Sforza & Feldman, 1981). For example, groups inhabiting the same region may show a higher probability of sharing a similar cultural trait among themselves than with groups living far away (Miller‐Atkins & Premo, 2018).
The field of human experimental biology has recently found evidence that social structure affects cultural evolution. A computer‐based experiment, in which individuals developed a communal task, showed that a propensity to learn from successful individuals reduced cultural diversity within fully connected groups, while partially connected groups showed higher rates of innovation and more diverse toolkits than the fully connected groups (Derex & Boyd, 2016). An agent‐based model – designed to explore the effects of population fragmentation on cumulative culture – showed that intermediate levels of fragmentation can maximise cultural complexity, depending on the extent to which innovation relies on a population's pre‐existing cultural richness (Derex, Perreault, & Boyd, 2018). Social connectivity and its relationship with cultural complexity underlies much of how archaeology studies societies and how they evolve through the analysis of material culture. Yet the discipline lacks an integrated framework that would enable studying the dynamics of cultural transmission based on social structures. In the following subsection we bring together what we have learned from other fields, especially from behavioural and evolutionary biology, to explore transmission processes in well‐studied systems.
(1). Insights from experiments on information transmission in modern societies
The aim of this subsection is to summarise current evidence regarding the diachronic patterns of information transmission through social structure. Cumulative cultural evolution may be unique to humankind [for comprehensive reviews, see Dean et al., 2014 and Mesoudi & Thornton, 2018], but insights into the behaviour of non‐human species shed light on how sociality influences transmission processes (Allen et al., 2013). Thus, the literature on animal behaviour can help us to make cultural transmission predictions while taking into account the observed distribution of cumulative culture. Our focus here is on non‐human primates and contemporary hunter–gatherers, although many other comparisons are potentially useful.
a. Non‐human primate societies
Many theoretical and empirical studies have focused on the mechanisms of information transmission in non‐human primates (e.g. Bonnie & de Waal, 2006; Huffman, Nahallage, & Leca, 2008; van de Waal, Borgeaud, & Whiten, 2013; Coelho et al., 2015). Most of these works have firmly established the importance of social structure [for comprehensive reviews on diffusion studies in humans and other animals see Duboscq et al., 2016 and Whiten, Caldwell, & Mesoudi, 2016]. It seems to be widespread that the position of individuals in a group (such as being more or less socially connected) as well the whole group structure (such as overall group connectivity) govern transmission dynamics, ultimately affecting individual fitness (e.g. Cheney, Silk, & Seyfarth, 2016). Key individuals, usually those with the most and/or strongest number of social connections within their groups, play a crucial role in information attaining and sharing, while more cohesive groups favour the faster transmission of social information (e.g. Voelkl & Noë, 2010; Claidière et al., 2013). Despite the highly diverse social repertoire of non‐human primate species, these results seem to be genus independent, demonstrating the overwhelming generality of social information transmission through social structure (Watson et al., 2018).
In particular, studies of great apes have shown that our closest living relatives present social learning mechanisms (Whiten et al., 1999; van Schaik, Fox, & Fechtman, 2003; Hobaiter et al., 2014) that are similar to ours (Whiten, 2017), ultimately helping to shed light on the evolutionary roots of human behaviour (e.g. Carvalho et al., 2012; Clay & Tennie, 2018). Chimpanzees (Pan troglodytes) for example, present 39 behaviours suspected of being socially acquired (most of them described as tool‐use; Whiten et al., 1999). This has been demonstrated recently through the social transmission of two novel tool‐use behaviours in the Sonso chimpanzee community (Hobaiter et al., 2014). In parallel, the so‐called ‘primate archaeology’ field outlines that some stone‐tool‐using primates share behavioural characteristics (such as tool selection and transport; Haslam et al., 2017), providing a fruitful means of investigating possible mechanisms of behavioural changes. Archaeological evidence, for example, has shown remains of nut‐cracking materials around 4300 years old in the Taï National Park, Côte d'Ivoire, where present‐day chimpanzees (> 200 generations later) still present the practice (Mercader et al., 2007).
b. Contemporary hunter–gatherers
Only a few modern societies are classified as hunter–gatherer, but a solid field of research complements our knowledge of social structure and the transmission of cultural attributes (Kelly, 2007). One study described a worldwide sample of 32 extant hunter–gatherer social structures as bilateral kin associations, brother–sister co‐residence and flexible dispersal patterns (Hill et al., 2011). This multilevel system includes frequent contacts between relatives (Kelly, 2007), but non‐kin relationships formed during childhood lead to a collaborative network that favours cultural exchange beyond households (Migliano et al., 2017). It is important to understand this patterning of interactions because it can help us to make predictions about the evolution of culture. For example, the intensity of inter‐band interaction (among the by‐products of which is the estimated number of observations of others making tools) was hypothesised to contribute to cumulative culture (Hill et al., 2014). Thus, the degree of social connectivity likely played a role in cumulative cultural change.
In this way, understanding how cultural transmission varies across different social learning strategies may help us to understand how early human culture emerged. A meta‐ethnographic review provides details on how modern hunter–gatherers learn subsistence skills (Lew‐Levy et al., 2017). The authors showed that social learning begins in early infancy, and by the end of their childhood, hunter–gatherers are competent food collectors. It is not until adolescence, however, that they are actively taught more complex activities, such as hunting and some toolmaking (Lew‐Levy et al., 2017). In the Agta society for example, both vertical (i.e. between parents and their children) and oblique (i.e. between members of distinct generations) transmission was observed for hunting activities, but vertical and horizontal (i.e. between members of the same generation) transmission controlled knowledge of gathering activities (Hagen, van der Ploeg, & Minter, 2016). As regards gathering‐related skills, a mother is usually the first to take her daughter on a food procurement trip. Once the girl has sufficiently mastered some of the techniques, she joins her older sisters and cousins from whom she continues learning, through observation and imitation (Hagen et al., 2016). Altogether, these studies illustrate that observing and copying successful individuals frequently plays a major part in human cultural evolution. Thus, social structure is predicted to account for some variation in cumulative culture.
V. AN ANALYTICAL METHOD TO ESTIMATE THE SOCIAL STRUCTURE OF PREHISTORIC HUNTER–GATHERERS
A fine‐scale assessment of social connectivity and spatial distribution patterns of prehistoric hunter–gatherers can be obtained by applying social network analysis [SNA; see Barabási, 2016 and Scott, 2017 for an introduction to this series of methods and its multiple applications]. Social contacts within and among populations are mathematically quantified and graphically represented by nodes (individuals, regional groups, regional units, populations) connected by linkages (also called ties, edges or links) to other nodes in the network (Fig. 2). Each node can possess attributes, such as site functionality and geographical position. Linkages in the network provide quantitative information on the connections between nodes and/or the directionality of these connections. For example, binary networks (i.e. unweighted networks) consider only the presence and absence of social connections. Weighted networks account for variations in the frequency of the connections. Undirected networks do not take into account the difference in direction between social connections (i.e. either i started the interaction/association with j or j started the interaction/association with i) while directed networks do take them into account (Fig. 2).
Figure 2.
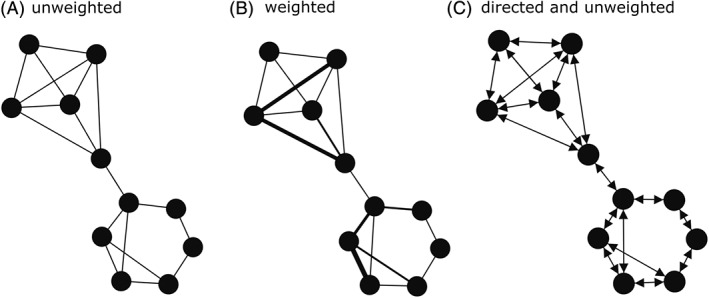
Theoretical networks of macro‐regional interactions. A node (i.e. circle) represents a regional group, and edges (i.e. lines) represent the interactions between nodes. Social interactions were defined by the occurrence (A) and/or frequency (B) of material culture sharing among regional groups. Directed and unweighted (C) networks consider the direction of interaction (i ➔ j or j ➔ i) but not the frequency of the relationships. Networks were built in Gephi 0.9.2 using Force Atlas Layout (Cherven, 2013).
SNA provides a set of specific metrics that can be used to characterise structural properties at the node level and at the level of the whole network. Centrality metrics are most commonly used to infer the relative importance of the network's nodes, generally referring to nodes that are more (and/or more strongly) connected than others. Other measures, such as network density, define global network metrics. Scott (2017) provides a detailed overview of these metrics, while Table 1 shows a subset known to facilitate or inhibit social transmission. Whether or not analytical models take these properties into account can change the predicted impact on cultural transmission (Table 1). We will examine the role of these metrics in more detail in Section VI. Here, we suggest that a hypothesis‐driven framework based on multilevel social network analysis will allow us to capture variations in the socio‐spatial structure, thus building a clearer understanding of the conditions of cultural changes. In this section, we revisit SNA applied to archaeology, together with its limitations and perspectives.
Table 1.
Definition of network properties and their biological meaning applied to the context of prehistoric hunter–gatherers. Network metrics are statistical measures used to characterise structural properties (Scott, 2017). Mathematical formulae describing each network metric can be found by consulting the respective references in the notes of this table. At the node level, a node's colour is directly related to its centrality: the stronger the colour, the higher its specific centrality. Here, we consider a node (a circle) as a regional group, but it may change according to the case study and research question. Networks were built in Gephi 0.9.2 using Force Atlas Layout (Cherven, 2013)
| Network metrics | Definition | Meaning in a prehistoric context | ||
|---|---|---|---|---|
| Node level | Degree |

|
The number of links connected to each node.a | Total number of interaction partners per regional group. |
| Strength |

|
The sum of the link weights connected to each node.b | The intensity of social relationships of a given regional group. | |
| Betweenness centrality |

|
The number of shortest paths passing through the considered node.a | The relative importance of regional groups in indirectly connecting other regional groups. Regional groups with high betweenness centrality are likely to connect independent units of the network. | |
| Eigenvector centrality |

|
The connectivity of a node within its network, also considering the connectivity of its neighbours.c | The connectivity of a regional group in terms of the connectivity of their interaction partners. Regional groups with high eigenvector centrality are connected to several other regional groups, which have a high connectivity themselves. | |
| Global level | Eigenvector centralisation |

|
Derived from individual eigenvector centrality, it estimates heterogeneity in connectedness across nodes in the networka. | The extent to which one or a few regional groups monopolise(s) the social connectivity in a network. A highly centralised network resembles a star, with an individual at the centre. |
| Modularity |

|
The extent to which a network is divided into differentiable modules (e.g. subgroups, subpopulations)d. | Regional groups which interact or are associated more frequently with each other within a subpopulation and that are loosely connectede to other subpopulations in the network. | |
| Density |

|
The ratio between the number of observed links and the number of possible links in the networkf. | The general level of connectivity between regional groups in a network. The network with the highest possible density is fully connected (i.e. all regional groups are connected to each other). | |
| Diameter |

|
The maximum length of the shortest paths between all node dyads in the networkf. | Regional groups in a population with a small diameter are connected to each other through few intermediaries. | |
| Average clustering coefficient |

|
The density of triads (trios of nodes)f. | How densely (or sparsely) the network is clustered around regional groups. | |
| Assortativity |

|
The level of homophily in the network. Assortativity is positive if similar nodes are more connected than expected and is negative otherwiseg. | Regional groups connected to those sharing similar characteristics, such as degree centrality (positive assortativity). | |
Over the past few years, SNA has become ever more prominent in archaeology (Brughmans, 2013; Östborn & Gerding, 2014; Collar et al., 2015; Mills, 2017). A chief motivation for applying a network perspective to study cultural evolution is the fact that human societies are part of socio‐spatial networks interconnected through direct or indirect social relationships. For example, Riede (2014), focusing on the features of the Late Glacial Bromme culture in Denmark, showed that a possible explanation for reduced technological complexity and absence of exotic materials was a rupture of the social networks. His study suggested that the decrease in cultural complexity was either a consequence of migratory events following the Laacher See eruption and/or due to the absence of long‐distance social exchanges (Riede, 2014). In another study, Mills et al. (2013) used a large‐scale database of material culture (i.e. decorated ceramic and obsidian) to research spatial and temporal variation in social connections during the late pre‐Hispanic period in the southwest of the USA. By combining SNA with geographic information system (GIS) techniques, and applying their methods to the archaeological record, the researchers showed that large variations in network structure (i.e. increase and collapse) corresponded to changes in migration patterns (Mills et al., 2013). Lastly, although the concept of ‘social network’ is well developed and is useful to depict social patterns and study cultural transmission (Cavalli‐Sforza & Feldman, 1981), few studies have implemented archaeological networks in the context of cultural evolutionary theory (e.g. Premo, 2012; Buchanan et al., 2016a).
This limited application of formal SNA to prehistoric hunter–gatherers may be partly due to archaeological taxonomies being widely interpreted as static blocks, and evolutionary theoretical frameworks being rarely applied (Riede, Hoggard, & Shennan, 2019). Traditional archaeological units consist of classifications of data into discrete subsystems (often based on assumptions about ‘ethnicity’, e.g. Barton, 1997). They disregard the dynamic processes that may have led to the spatio‐temporal distribution of identifiable material culture. Instead of considering that archaeological materials belong to preconceived categories, Riede et al. (2019) highlighted the urgent need to reconcile cultural taxonomies with evolutionary frameworks. The authors revisited the gene–culture co‐evolutionary theory and argued that a definition of cultural taxa, based on attributes that can be linked to cultural transmission, offers a robust theoretical grounding (Riede et al., 2019). The development and application of integrative approaches is expected to contribute to advances in the field (Kolodny, Feldman, & Creanza, 2018), and studies in archaeology will ultimately benefit from more robust analytical approaches.
SNA offers many advantages for studying cultural evolutionary theory. However, it requires careful consideration about the limitations of archaeological data, such as incomplete records, the ambiguities of trait similarities as a proxy for cultural links, poorly defined network boundaries, and the aggregation of data into varying time and spatial scales (Brughmans, 2010; Peeples et al., 2016; Prignano, Morer, & Diaz‐Guilera, 2017). More general considerations regarding the construction and analysis of social networks are explained elsewhere (Hanneman & Riddle, 2005; Scott, 2017), emphasising how important it is to assess the feasibility of the research question under study. For example, high‐quality archaeological data, such as well‐dated sites and artefacts, are required to evaluate spatio‐temporal patterns in social interactions and distinguish them from noise.
Nevertheless, recent discussions have focused on developing methods to overcome such limitations (Peeples, 2019). Notwithstanding the technical difficulties of building networks, additional analytical techniques have become available allowing scientists to assess the robustness of their networks (e.g. Groenhuijzen & Verhagen, 2016), to manage missing node observations (e.g. Hoppitt & Farine, 2018), as well as to recreate a (quasi‐) realistic scenario by combining the data set used for SNA with other computational modelling approaches. Agent‐based modelling (ABM), for example, is a powerful tool for scientists facing the difficulties of interpreting the inherent complexity of biological systems. These computational models simulate the actions and interactions of autonomous agents and aim to provide a purposeful representation of real systems (Railsback & Grimm, 2012). Overall, network analysis is a useful approach for its capacity to depict complex systems (Kurvers et al., 2014). However, like any other analytical tool, it should be applied with caution – in terms of both defining valid research questions and recognising the data's potential limitations.
VI. A FRAMEWORK FOR STUDYING CULTURAL EVOLUTION IN PREHISTORIC HUNTER–GATHERER SOCIETIES
We reviewed above the importance of analysing social connectivity using archaeological data. In this section, we present an evolutionary framework to assess formally how different aspects of social structure can influence cultural transmission dynamics and ultimately, cumulative culture. A schematic framework outlines the dynamic relationship between environmental pressures, palaeodemography, socio‐spatial structure and cultural transmission in human societies (Fig. 3). As demographic variation is linked to social structure (e.g. David‐Barrett, 2019) and culture is fundamentally built on the transmission of information through social contacts (Whiten et al., 2016), demography, social structure and culture are linked, and can be considered as a whole (Fig. 3).
Figure 3.

A framework outlining the dynamic relationship between palaeodemography, socio‐spatial structure and cultural transmission in human societies. Environmental pressures, such as major climatic shifts, influence demographic patterns of prehistoric hunter–gatherer societies (i). Demographic factors, such as an increment in population size, cause variation in the socio‐spatial structure (ii). Social structures are represented using a network approach, in which node circles, (representing individuals at a microscale, and regional groups or populations at a macroscale) are connected by edges (links, with thickness representing the strength of social connections). These social interactions among nodes, reflected in the network topology, influence and are influenced by cultural transmission processes (iii).
Following the perspective adopted in this review, some factors affecting social structure at the individual level, such as age/sex attributes, are not considered in the diagram [but see Hinde, 1976 revisited by Whitehead, 2008, for an extensive list of these factors]. This was deliberate because the capacity to recreate social context characteristics at an individual level is generally limited to archaeological data relating to a prehistoric context [but see Hamilton, Buchanan, & Walker, 2018 for a reconstruction of prehistoric hunter–gatherer social organisation based on ethnoarchaeological data]. We explain our proposed framework in detail below.
(1). Environmental pressures on paleodemography
Climate–environmental changes, both abrupt and gradual, played a key role in the prehistory of human populations [Kelly et al., 2013; Fernández‐López de Pablo et al., 2019; (i) in Fig. 3]. There is evidence that major past climatic and environmental shifts, such as the Last Glacial Maximum and the Pleistocene–Holocene transition, affected the demographic dynamics of prehistoric hunter–gatherers (e.g. Gamble et al., 2005; Kelly et al., 2013; Tallavaara et al., 2015; Fernández‐López de Pablo et al., 2019). These severe changes in temperature caused widespread environmental stress and exerted, in some cases, a persistent influence on human demographic responses (e.g. Wyoming, USA; Kelly et al., 2013). In the Iberian Peninsula, for example, climate and palaeoenvironmental proxies (i.e. temperature, precipitation and temperate Mediterranean forests) predict a three‐phase demographic model that can be derived from empirical archaeological data during the Pleistocene–Holocene transition (Fernández‐López de Pablo et al., 2019). The first phase is characterised by exponential population increase during the Late Glacial warming period, followed by a phase of sustained population contraction and stagnation spanning the Younger Dryas and the first half of the Early Holocene. The third phase consists of density‐dependent logistic growth (Fernández‐López de Pablo et al., 2019). How prehistoric societies responded to climate changes is a core question in archaeology. Oscillations in average temperatures likely led to migration (Gamble et al., 2005), population decline and/or reorganisation of settlement patterning (Anderson et al., 2011). Some human populations, however, persisted throughout these climate events (Blockley et al., 2018). Together, these studies shed light on the demographic response to the pressures of climate and environmental change [(i) in Fig. 3].
(2). Demography and social structure
When studying the demographic dynamics of prehistoric hunter–gatherer societies, it is important to bear in mind two timescales. Demographic models are characterised by long‐term exponential growth (millennial scale) with short‐term fluctuations (centennial scale; e.g. Zahid, Robinson, & Kelly, 2016). These minor fluctuations are of interest: they allow examination of the influence of demographic variables (i.e. fertility/birth, mortality and migrations) on the social structure as they offer a fine‐grained view of the processes [(ii) in Fig. 3]. In this context, it is possible to make predictions about the system's behaviour in specific phases. For example, periods of demographic transitions in the Holocene are characterised by shifts in fertility and mortality (Bocquet‐Appel, 2011). During the Neolithic Demographic Transition, an abrupt increase in fertility and global increase in population size have been recorded (Bocquet‐Appel, 2011). This might have had an impact on the social structure. For example, a study under the scope of recent demographic transitions showed that falling fertility led to a decrease in the number of children who were related to each other, which in turn caused a drop in the local clustering coefficient and average network distance (i.e. individuals were less likely to be connected to each other but there were fewer steps from unconnected individuals; David‐Barrett, 2019). Demographic trends causing changes to a social structure's composition will then inevitably entail the loss of some social connectivity and the creation of new connectivity [(ii) in Fig. 3]. However, it is important to highlight that it is difficult to obtain levels of natality, mortality and population structure from the archaeological record when seeking to analyse long‐term patterns. In this way, most of what is inferred estimates relative changes in population size.
(3). Social structure influences cultural changes
In previous sections, we showed that social structure impacts cultural transmission (Section IV) and that a network‐based approach produces a refined evaluation of social connectivity (Section V). However, the extent to which social structure affected cultural changes in prehistoric hunter–gatherer societies is still subject to debate. We must therefore ask which role each group had in spreading innovations and how spatial configuration changes influence cultural transmission. To address some of these questions in more depth, we now present an interpretation of network metrics from a cultural transmission perspective.
a. The relative importance of nodes in the network
Several measures of node centrality exist (the most common are defined in Table 1), and each is expected to present a different relationship with cultural transmission. The number of direct connections (i.e. immediate contacts) provides the degree centrality, or the strength centrality if the intensity of connections is considered (Scott, 2017). These measures illustrate how well connected these nodes are in the local environment and can be used to assess a node's probability of cultural transmission to and from its immediate contacts (Barabási, 2016). To achieve a more refined vision of the transmission mechanisms, we can assess how individual centrality is dependent on the values of other individual connections; a measure known as eigenvector centrality. This measure extends the probability of cultural transmission to include the friendship of an individual's friend (Barabási, 2016).
Concepts were also developed to estimate whether some nodes behave as intermediaries of other nodes in the network (Scott, 2017). This idea is usually represented by the betweenness centrality (i.e. nodes that connect other nodes which are not otherwise connected; Freeman, 1979). It estimates the role of nodes in transmitting information to peripheral nodes, which favour transmission to the complete network (Barabási, 2016). The transmission rate is expected to be faster during any transmission between these central individuals (Barabási, 2016). However, the diversity of the centrality metrics plays an important part because they represent distinct facets of the transmission mechanisms. For example, a theoretical study simulating social transmission (a model applied to both information and pathogen transmission) in wild Japanese macaques (Macaca fuscata) suggested that direct connections, such as that estimated by degree centrality, are most predictive of an individual's probability of being informed. By contrast, indirect connections, such as that estimated by the betweenness centrality, are most predictive of the latency of transmission to the whole group (Romano et al., 2016).
b. The overall network structure
Evaluating the overall network structure is also important to understanding transmission dynamics. While some generalisations can be made (e.g. more cohesive networks tend to be highly efficient in their social transmission; Latora & Marchiori, 2001), the literature presents mixed evidence regarding the influence of some metrics on the transmission processes. Properties of the whole network might favour information transmission (e.g. density; Pasquaretta et al., 2014), reduce spreading processes (e.g. modularity; Nunn et al., 2015) or those same properties might even have dual roles regarding transmission (e.g. modularity; Romano et al., 2018). We must bear in mind that global network properties are the outcome of individual decisions about who to interact with and how frequently. Thus, emergent network properties may change under prevailing environmental and social pressures (Sueur et al., 2019). We will take modularity – the extent to which a network is divided into differentiable modules (Newman, 2006), such as subpopulations – as an illustration.
Among the global network properties, modularity has been regarded as a major contributor to the capacity of biological networks to evolve (e.g. bacterial metabolic systems; Wagner, Pavlicev, & Cheverud, 2007). One study showed that selective pressures to reduce connection costs lead to modular networks, which adapt faster to new environments than less‐modular networks (Clune, Mouret, & Lipson, 2013). Interestingly, the relationship between the degree of modularity and social transmission is seemingly not linear, with network efficiency (as a proxy for social transmission) peaking when modularity values are intermediate (Romano et al., 2018). This potential dual modularity role, with low values favouring transmission and high values inhibiting transmission (Nematzadeh et al., 2014; Romano et al., 2018) may help to explain the distinct interpretations in the literature (e.g. Lentz, Selhorst, & Sokolov, 2012; Nunn et al., 2015). A comparative study hypothesised that larger group sizes may be more subdivided structurally, and consequently, such group/population substructures (i.e. modularity) act as transmission bottlenecks [i.e. the so‐called “social bottleneck hypothesis” (Griffin & Nunn, 2012; Nunn et al., 2015)]. However, the diffusion delay may only occur over a determined threshold (Sah et al., 2017; Romano et al., 2018). As such, not one value, but a range of optimised modularity values predicting peaks of cultural diversity may exist.
Considering that culture is specific on a regional basis, understanding how human societies are spatially subdivided is central to the development and constraining of culture within and among populations. For example, a theoretical study investigating the link between social structure and cultural innovations showed that in situations where the separation between social modules increased, subdivided populations developed more independent strategies leading to higher cultural diversity (Whitehead & Lusseau, 2012). More recently, another theoretical study indicated that with intermediate population fragmentation values, the balance between cultural loss and innovation maximised cultural complexity (Derex et al., 2018). As such, social structure can strongly influence culture [(ii) in Fig. 3], and this phenomenon (which is not new in biology) has been observed, in particular, for networks that are sufficiently modular in their structure.
c. Interpreting network metrics
The interpretation of network metrics (at both a node level and globally) requires caution (Scott, 2017), as it will change according to how the nodes (e.g. regional groups or individuals) and linkages (e.g. travelling cost or cultural similarity) are defined, according to the network size, and the metrics used (e.g. degree or betweenness centrality, density, assortativity; Farine & Whitehead, 2015). For example, comparing degree centrality between two different‐sized networks will be misleading (Scott, 2017). As the estimation of degree is based – among other factors – on network size, the absolute number of connections can potentially be less representative (e.g. networks with five links between 10 nodes will have lower degree centrality when compared to a network with six links between 20 nodes). Therefore, it is important to consider different network interpretations when developing the research question and study design. Readers will find further information on this subject in Farine & Whitehead (2015) as well as Scott (2017).
(4). The other way around: does culture affect social structure?
While the social structure (i.e. social network) affects patterns of cultural transmission, culture can also feed back into the structure of prehistoric hunter–gatherer societies [(iii) in Fig. 3]. Based on the archaeological record, much research assumes that certain cultural traits can be used as population or cultural markers (Gamble et al., 2005). Conceivably, individuals from a given regional group would have interacted more frequently with individuals sharing similar characteristics (such as within the household and with nearby regional groups), and some cultural traits could have been maintained in the group through conformism (i.e. when individuals choose the most common behaviour when starting to learn). For most of the archaeological record, we have no means to evaluate empirically whether culture shaped prehistoric hunter–gatherer societies in this way or not. Yet, evidence from modern species shows that individuals may preferentially interact with conspecifics who share similar cultural behaviour (i.e. a mechanism called homophily), causing a feeding back into the social structure. For example, sympatric communities of cetaceans create social bonds based on the similarity of their vocal repertoire, which creates a biased cultural transmission (Cantor et al., 2015). Captive cowbirds (Molothrus ater) copulate preferentially with individuals sharing the same cultural background (Freeberg, 1996). Modern humans preferentially mate with individuals sharing similar phenotypes, such as education level (Domingue et al., 2014). Altogether, these examples illustrate feedback links between culture and social structure.
VII. PREDICTIONS AND OUTSTANDING QUESTIONS
The feedback loop diagram (Fig. 3) provides a theoretical basis for the study of human cultural evolution. As the population grows, the complexity of social interactions increases (i.e. diversity in social partners and frequency of social connections; but see Kappeler (2019) for a comprehensive discussion on the definition of social complexity), generating different social patterns. The accumulation of interactions gives rise to the social structure (Hinde, 1976), which can be translated into network properties. Using SNA, we can estimate the impact that each property (e.g. eigenvector centrality, assortativity; Table 1) has on cultural transmission dynamics and investigate how a given cultural attribute might have spread through the network and then accumulated, or been lost, over generations. We can now make several predictions based on our findings in the literature and the proposed multilevel and evolutionary framework (Table 2).
Table 2.
Summary of predictions proposed in our current framework translated into outstanding questions for future studies
| Network thinking | Outstanding research questions |
|---|---|
| Regional groups with many connections to other regional groups (e.g. degree centrality) are potentially super‐spreaders of information. In other words, if the transmission processes start with them, information is more likely to spread faster and to a higher number of regional groups. |
|
| Regional groups occupying intermediate positions in their network (i.e. high betweenness centrality) determine the spreading of material culture to peripheral groups. |
|
| A network's modularity has a direct influence on the extent and speed of information diffusion. |
|
VIII. OTHER FACETS OF THE STUDY
To understand cultural patterns better, it is also pertinent to consider the temporal variation of the socio‐spatial structure. Social relationships are dynamic: they occur discontinuously over time and potentially according to, among other factors, variations in the environmental conditions, group size, and the health status of individuals (Kelly, 2007). Consequently, the network's topology is altered: the node variations affect the linkages, and the linkages affect the transmission paths. To evaluate the temporal patterns of cultural shifts, a major archaeological consideration is the choice of time resolution. While cultural transmission mechanisms (e.g. vertical transmission) unfold over a short timescale (e.g. from one generation to the next), such a resolution is unlikely in the case of using material culture (Garvey, 2018). Since archaeological data are most frequently presented as a record of aggregated events over time (years, centuries, or millennia), researchers may keep in mind that their final outputs are snapshots of a superposition of social interactions for a given time. Thus, while including temporal dynamics in the analytical models may help to understand the formation and stabilisation of social structure (e.g. Hobson, Avery, & Wright, 2013) and to predict transmission processes, integrating temporal variation in social network analysis requires caution.
Finally, social structure should not be considered as an independent factor that drives cumulative culture. In any complex system, the combination of several conditions may influence the variation of cultural attributes. In the case of prehistoric hunter–gatherers, these conditions include, among others: type of habitat and prey abundance, landscape connectivity, and environmental conditions. Cumulative culture is a product of the relationships between social and ecological variables. Understanding the interplay between social structure and cultural transmission is a crucial step towards comprehending historical and current cultural dynamics. Herein we argued that the socio‐spatial structure of prehistoric hunter–gatherers is a fundamental component of cultural evolutionary theory. We thus hope this review encourages researchers to venture deeper into the interface between social structure, cultural transmission and cumulative cultural evolution.
IX. CONCLUSIONS
Cultural evolution is fundamentally a multidisciplinary field, connecting gaps between anthropology, archaeology, evolutionary biology, behavioural ecology and animal behaviour. Although considerable efforts have been made to introduce network science into the discipline of archaeology, few formal studies have applied a network perspective to cultural evolutionary theory. To understand the evolution of human behaviour, it is essential to study the ancestral patterns of social connections. The prehistoric hunter–gatherer model plays a key role in understanding the link between social structure and the evolution of cultural complexity.
Most cultural evolution models consider the production of new traits (i.e. innovation, imitation) and their final outcome (i.e. accumulation, loss or stability) rather than the mechanisms underlying the spread of these traits (such as the impact of hunter–gatherer socio‐spatial structure on cultural transmission). The fundamental assumption of the demographic hypothesis is that larger groups present toolkits that are more complex than those of smaller groups. However, the relationship between population size and cultural complexity is not always linear: it depends, among other factors, on social connectivity. We have presented evidence that social structure does affect transmission processes.
By integrating a multilevel analytical framework in the study of prehistoric hunter–gatherer society culture, we draw attention to the interplay between social structure and cultural evolution. Current evidence supports the view that network properties predict the path and efficiency of social transmission in myriad social species. We propose that a close evaluation of social network structure, in which regional groups are embedded, can greatly contribute to assessing how different aspects of a social structure could influence cultural transmission patterns, and ultimately cumulative culture. This has enabled us to present predictions based on our current framework, archaeological data, and the literature concerning animal behaviour.
Finally, there is a clear need for further research on two subjects: first, how social relationships between prehistoric hunter–gatherers cause variations in the extent to which cultural changes occur and the dynamics of cumulative culture; and second, whether and how culture might have shaped hunter–gatherer networks. By applying social network analyses, we can achieve greater accuracy regarding the selective mechanisms underlying cultural transmission. The latter will ultimately shed light on the process of human cultural evolution itself.
X. ACKNOWLEDGEMENTS
We are grateful to Rowan Mclaughlin and two anonymous referees for comments that greatly improved the manuscript. This work was funded by the European Research Council (ref. ERC‐2015 Co‐Grant 683018) awarded to J.F.‐L.d.P. – PALEODEM project: “Late Glacial and Postglacial Population History and Cultural Transmission in Iberia (C.15,000‐8,000cal BP)”. The authors were also supported by the Catalan Agency for Management of University and Research Grants (AGAUR), Grant 2017SGR836.
REFERENCES
- Allen, J. , Weinrich, M. , Hoppitt, W. & Rendell, L. (2013). Network‐based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488. [DOI] [PubMed] [Google Scholar]
- Anderson, D. G. , Goodyear, A. C. , Kennett, J. & West, A. (2011). Multiple lines of evidence for possible human population decline/settlement reorganization during the early younger dryas. Quaternary International 242, 570–583. [Google Scholar]
- Barabási, A. L. (2016). Network Science. Cambridge University Press, Cambridge. [Google Scholar]
- Barrat, A. , Barthélemy, M. , Pastor‐Satorras, R. & Vespignani, A. (2004). The architecture of complex weighted networks. Proceedings of the National Academy of Sciences of the United States of America 101, 3747–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, C. M. (1997). Stone tools, style, and social identity: an evolutionary perspective on the archaeological record. Archeological Papers of the American Anthropological Association 7, 141–156. [Google Scholar]
- Bar‐Yosef, O. (2002). The upper paleolithic revolution. Annual Review of Anthropology 31, 363–393. [Google Scholar]
- Bettinger, R. (1991). Hunter‐gatherers and neo‐darwinian cultural transmission In Hunter‐Gatherers: Archaeological and Evolutionary Theory, pp. 181–208. Plenun Press, New York. [Google Scholar]
- Blockley, S. , Candy, I. , Matthews, I. , Langdon, P. , Langdon, C. , Palmer, A. , Lincoln, P. , Abrook, A. , Taylor, B. , Conneller, C. , Bayliss, A. , MacLeod, A. , Deeprose, L. , Darvill, C. , Kearney, R. , Beavan, N. , Staff, R. , Bamforth, M. , Taylor, M. & Milner, N. (2018). The resilience of postglacial hunter‐gatherers to abrupt climate change. Nature Ecology & Evolution 2, 810–818. [DOI] [PubMed] [Google Scholar]
- Bocquet‐Appel, J.‐P. (2011). The agricultural demographic transition during and after the agriculture inventions. Current Anthropology 52, 497–510. [Google Scholar]
- Bonacich, P. (1987). Power and centrality: a family of measures. American Journal of Sociology 92, 1170–1182. [Google Scholar]
- Bonnie, K. E. & de Waal, F. B. M. (2006). Affiliation promotes the transmission of a social custom: handclasp grooming among captive chimpanzees. Primates 47, 27–34. [DOI] [PubMed] [Google Scholar]
- Boyd, R. & Richerson, P. J. (1985). Culture and the Evolutionary Process. The University of Chicago Press, Chicago. [Google Scholar]
- Boyd, R. & Richerson, P. J. (1996). Why culture is common, but cultural evolution is rare In Evolution of Social Behaviour Patterns in Primates and Man (eds Runciman W. G., Smith J. M. and Dunbar R. I. M.), pp. 77–93. Oxford University Press, New York. [Google Scholar]
- Brughmans, T. (2010). Connecting the dots: towards archaeological network analysis. Oxford Journal of Archaeology 29, 277–303. [Google Scholar]
- Brughmans, T. (2013). Thinking through networks: a review of formal network methods in archaeology. Journal of Archaeological Method and Theory 20, 623–662. [Google Scholar]
- Buchanan, B. , Hamilton, M. J. , Kilby, J. D. & Gingerich, J. A. M. (2016a). Lithic networks reveal early regionalization in Late Pleistocene North America. Journal of Archaeological Science 65, 114–121. [Google Scholar]
- Buchanan, B. , O'Brien, M. J. & Collard, M. (2016b). Drivers of technological richness in prehistoric Texas: an archaeological test of the population size and environmental risk hypotheses. Archaeological and Anthropological Sciences 8, 625–634. [Google Scholar]
- Cantor, M. , Shoemaker, L. G. , Cabral, R. B. , Flores, C. O. , Varga, M. & Whitehead, H. (2015). Multilevel animal societies can emerge from cultural transmission. Nature Communications 6, 8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, S. , Biro, D. , Cunha, E. , Hockings, K. , McGrew, W. C. , Richmond, B. G. & Matsuzawa, T. (2012). Chimpanzee carrying behaviour and the origins of human bipedality. Current Biology 22, 180–181. [DOI] [PubMed] [Google Scholar]
- Cavalli‐Sforza, L. L. & Feldman, M. W. (1981). Cultural Transmission and Evolution: A Quantitative Approach. Princeton University Press, Princeton. [PubMed] [Google Scholar]
- Cheney, D. L. , Silk, J. B. & Seyfarth, R. M. (2016). Network connections, dyadic bonds and fitness in wild female baboons. Royal Society Open Science 3, 160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherven, K. (2013). Network Graph Analysis and Visualization with Gephi. Packt Publishing Limited, Birmingham. [Google Scholar]
- Claidière, N. , Messer, E. J. E. , Hoppitt, W. & Whiten, A. (2013). Diffusion dynamics of socially learned foraging techniques in squirrel monkeys. Current Biology 23, 1251–1255. [DOI] [PubMed] [Google Scholar]
- Clay, Z. & Tennie, C. (2018). Is overimitation a uniquely human phenomenon? Insights from human children as compared to bonobos. Child Development 89, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Clune, J. , Mouret, J. & Lipson, H. (2013). The evolutionary origins of modularity. Proceedings of the Royal Society B: Biological Sciences 280, 20122863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, C. G. , Falótico, T. , Izar, P. , Mannu, M. , Resende, B. D. , Siqueira, J. O. & Ottoni, E. B. (2015). Social learning strategies for nut‐cracking by tufted capuchin monkeys (Sapajus spp.). Animal Cognition 18, 911–919. [DOI] [PubMed] [Google Scholar]
- Collar, A. , Coward, F. , Brughmans, T. & Mills, B. J. (2015). Networks in archaeology: phenomena, abstraction, representation. Journal of Archaeological Method and Theory 22, 1–32. [Google Scholar]
- Collard, M. , Kemery, M. & Banks, S. (2005). Causes of toolkit variation among hunter‐gatherers: a test of four competing hypotheses. Canadian Journal of Archaeology 29, 1–19. [Google Scholar]
- Collard, M. , Buchanan, B. , Morin, J. & Costopoulos, A. (2011). What drives the evolution of hunter‐gatherer subsistence technology? A reanalysis of the risk hypothesis with data from the Pacific northwest. Philosophical Transactions of the Royal Society B: Biological Sciences 366, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard, M. , Buchanan, B. , O'Brien, M. J. & Scholnick, J. (2013). Risk, mobility or population size? Drivers of technological richness among contact‐period western North American hunter‐gatherers. Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanza, N. , Kolodny, O. & Feldman, M. W. (2017). Greater than the sum of its parts? Modelling population contact and interaction of cultural repertoires. Journal of the Royal Society Interface 14, 20170171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C. (1859). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. John Murray, London. [PMC free article] [PubMed] [Google Scholar]
- David‐Barrett, T. (2019). Network effects of demographic transition. Scientific Reports 9, 2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, L. G. , Vale, G. L. , Laland, K. N. , Flynn, E. & Kendal, R. L. (2014). Human cumulative culture: a comparative perspective. Biological Reviews 89, 284–301. [DOI] [PubMed] [Google Scholar]
- Derex, M. & Boyd, R. (2016). Partial connectivity increases cultural accumulation within groups. Proceedings of the National Academy of Sciences of the United States of America 113, 2982–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derex, M. , Beugin, M.‐P. , Godelle, B. & Raymond, M. (2013). Experimental evidence for the influence of group size on cultural complexity. Nature 503, 389–391. [DOI] [PubMed] [Google Scholar]
- Derex, M. , Perreault, C. & Boyd, R. (2018). Divide and conquer: intermediate levels of population fragmentation maximize cultural accumulation. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue, B. W. , Fletcher, J. , Conley, D. & Boardman, J. D. (2014). Genetic and educational assortative mating among US adults. Proceedings of the National Academy of Sciences of the United States of America 111, 7996–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboscq, J. , Romano, V. , MacIntosh, A. & Sueur, C. (2016). Social information transmission in animals: lessons from studies of diffusion. Frontiers in Psychology 7, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine, D. R. & Whitehead, H. (2015). Constructing, conducting and interpreting animal social network analysis. Journal of Animal Ecology 84, 1144–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐López de Pablo, J. , Gutiérrez‐Roig, M. , Gómez‐Puche, M. , McLaughlin, R. , Silva, F. & Lozano, S. (2019). Palaeodemographic modelling supports a population bottleneck during the Pleistocene‐Holocene transition in Iberia. Nature Communications 10, 1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeberg, T. M. (1996). Assortative mating in captive cowbirds is predicted by social experience. Animal Behaviour 52, 1129–1142. [Google Scholar]
- Freeman, L. C. (1979). Centrality in social networks conceptual clarification. Social Networks 1, 215–239. [Google Scholar]
- Galef, B. G. Jr. & Laland, K. N. (2005). Social learning in animals: empirical studies and theoretical models. Bioscience 55, 489–499. [Google Scholar]
- Gamble, C. , Davies, W. , Pettitt, P. , Hazelwood, L. & Richards, M. (2005). The archaeological and genetic foundations of the European population during the late glacial: implications for ‘agricultural thinking’. Cambridge Archaeological Journal 15, 193–223. [Google Scholar]
- Garvey, R. (2018). Current and potential roles of archaeology in the development of cultural evolutionary theory. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvan, M. & Newman, M. E. J. (2002). Community structure in social and biological networks. Proceedings of the National Academy of Sciences of the United States of America 99, 7821–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, R. H. & Nunn, C. L. (2012). Community structure and the spread of infectious disease in primate social networks. Evolutionary Ecology 26, 779–800. [Google Scholar]
- Groenhuijzen, M. R. & Verhagen, P. (2016). Testing the robustness of local network metrics in research on archeological local transport networks. Frontiers in Digital Humanities 3, 6. [Google Scholar]
- Hagen, R. , van der Ploeg, J. & Minter, T. (2016). How do hunter‐gatherers learn? The transmission of indigenous knowledge among the Agta of The Philippines. Hunter Gatherer Research 3, 389–413. [Google Scholar]
- Hamilton, M. J. , Buchanan, B. & Walker, R. S. (2018). Scaling the size, structure, and dynamics of residentially mobile hunter‐gatherer camps. American Antiquity 83, 701–720. [Google Scholar]
- Hanneman, R. & Riddle, M. (2005). Introduction to Social Network Methods. University of California, Riverside. [Google Scholar]
- Haslam, M. , Hernandez‐Aguilar, R. A. , Proffitt, T. , Arroyo, A. , Falótico, T. , Fragaszy, D. , Gumert, M. , Harris, J. W. K. , Huffman, M. A. , Kalan, A. K. , Malaivijitnond, S. , Matsuzawa, T. , McGrew, W. , Ottoni, E. B. , Pascual‐Garrido, A. , Piel, A. , Pruetz, J. , Schuppli, C. , Stewart, F. , Tan, A. , Visalberghi, E. & Luncz, L. V. (2017). Primate archaeology evolves. Nature Ecology and Evolution 1, 1431–1437. [DOI] [PubMed] [Google Scholar]
- Henrich, J. (2004). Demography and cultural evolution: how adaptive cultural processes can produce maladaptive losses: the Tasmanian case. American Antiquity 69, 197–214. [Google Scholar]
- Henrich, J. (2006). Understanding cultural evolutionary models: a reply to Read's critique. American Antiquity 71, 771–782. [Google Scholar]
- Henrich, J. , Boyd, R. , Derex, M. , Kline, M. A. , Mesoudi, A. , Muthukrishna, M. , Powell, A. T. , Shennan, S. J. & Thomas, M. G. (2016). Understanding cumulative cultural evolution. Proceedings of the National Academy of Sciences of the United States of America 113, E6724–E6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K. R. , Walker, R. S. , Božičević, M. , Eder, J. , Headland, T. , Hewlett, B. , Hurtado, A. M. , Marlowe, F. , Wiessner, P. & Wood, B. (2011). Co‐residence patterns in hunter‐gatherer societies show unique human social structure. Science 331, 1286–1289. [DOI] [PubMed] [Google Scholar]
- Hill, K. R. , Wood, B. M. , Baggio, J. , Hurtado, A. M. & Boyd, R. T. (2014). Hunter‐gatherer inter‐band interaction rates: implications for cumulative culture. PLoS One 9, e102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde, R. A. (1976). Interactions, relationships and social structure. Man 11, 1–17. [Google Scholar]
- Hobaiter, C. , Poisot, T. , Zuberbühler, K. , Hoppitt, W. & Gruber, T. (2014). Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biology 12, e1001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson, E. , Avery, M. & Wright, T. (2013). An analytical framework for quantifying and testing patterns of temporal dynamics in social networks. Animal Behaviour 85, 83–96. [Google Scholar]
- Hoffmann, D. L. , Standish, C. D. , García‐Diez, M. , Pettitt, P. B. , Milton, J. A. , Zilhão, J. , Alcolea‐González, J. J. , Cantalejo‐Duarte, P. , Collado, H. , de Balbín, R. , Lorblanchet, M. , Ramos‐Muñoz, J. , Weniger, G.‐C. & Pike, A. W. G. (2018). U‐Th dating of carbonate crusts reveals Neandertal origin of Iberian cave art. Science 915, 912–915. [DOI] [PubMed] [Google Scholar]
- Hoppitt, W. J. E. & Farine, D. R. (2018). Association indices for quantifying social relationships: how to deal with missing observations of individuals or groups. Animal Behaviour 136, 227–238. [Google Scholar]
- Huffman, M. A. , Nahallage, C. A. D. & Leca, J. B. (2008). Cultured monkeys: social learning cast in stones. Current Directions in Psychological Science 17, 410–414. [Google Scholar]
- Huxley, J. (1942). Evolution: The Modern Synthesis. George Allen & Unwin Ltd., London. [Google Scholar]
- Kappeler, P. M. (2019). A framework for studying social complexity. Behavioral Ecology and Sociobiology 73, 13. [Google Scholar]
- Kelly, R. L. (2007). The Foraging Spectrum: Diversity in Hunter‐Gatherer Lifeways. Percheron Press, New York. [Google Scholar]
- Kelly, R. L. , Surovell, T. A. , Shuman, B. N. & Smith, G. M. (2013). A continuous climatic impact on Holocene human population in the Rocky Mountains. Proceedings of the National Academy of Sciences of the United States of America 110, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe, M. & Mesoudi, A. (2014). An experimental demonstration of the effect of group size on cultural accumulation. Evolution and Human Behavior 35, 285–290. [Google Scholar]
- Klein, R. G. (2000). Archeology and the evolution of human behavior. Evolutionary Anthropology 9, 17–36. [Google Scholar]
- Kline, M. A. & Boyd, R. (2010). Population size predicts technological complexity in Oceania. Proceedings of the Royal Society B: Biological Sciences 277, 2559–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y. , Ohtsuki, H. & Wakano, J. Y. (2016). Population size vs. social connectedness — a gene‐culture coevolutionary approach to cumulative cultural evolution. Theoretical Population Biology 111, 87–95. [DOI] [PubMed] [Google Scholar]
- Kolodny, O. , Feldman, M. W. & Creanza, N. (2018). Integrative studies of cultural evolution: crossing disciplinary boundaries to produce new insights. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvers, R. H. J. M. , Krause, J. , Croft, D. P. , Wilson, A. D. M. & Wolf, M. (2014). The evolutionary and ecological consequences of animal social networks: emerging issues. Trends in Ecology & Evolution 29, 326–335. [DOI] [PubMed] [Google Scholar]
- Laland, K. N. & Hoppitt, W. (2003). Do animals have culture? Evolutionary Anthropology 12, 150–159. [Google Scholar]
- Laland, K. N. & Sterelny, K. (2006). Perspective: seven reasons (not) to neglect niche construction. Evolution: International Journal of Organic Evolution 60, 1751–1762. [PubMed] [Google Scholar]
- Laland, K. N. , Odling‐Smee, J. & Feldman, M. W. (2001). Cultural niche construction and human evolution. Journal of Evolutionary Biology 14, 22–33. [DOI] [PubMed] [Google Scholar]
- Latora, V. & Marchiori, M. (2001). Efficient behavior of small‐world networks. Physical Review Letters 87, 198701. [DOI] [PubMed] [Google Scholar]
- Lentz, H. H. K. , Selhorst, T. & Sokolov, I. M. (2012). Spread of infectious diseases in directed and modular metapopulation networks. Physical Review E 85, 066111. [DOI] [PubMed] [Google Scholar]
- Lew‐Levy, S. , Reckin, R. , Lavi, N. , Cristóbal‐Azkarate, J. & Ellis‐Davies, K. (2017). How do hunter‐gatherer children learn subsistence skills?: a meta‐ethnographic review. Human Nature 28, 367–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand, G. & Perrin, T. (2017). Why this revolution? Explaining the major technical shift in southwestern Europe during the 7th millennium cal. BC. Quaternary International 428, 73–85. [Google Scholar]
- McPherron, S. P. , Alemseged, Z. , Marean, C. W. , Wynn, J. G. , Reed, D. , Geraads, D. , Bobe, R. & Béarat, H. A. (2010). Evidence for stone‐tool‐assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860. [DOI] [PubMed] [Google Scholar]
- Mercader, J. , Barton, H. , Gillespie, J. , Harris, J. , Kuhn, S. , Tyler, R. & Boesch, C. (2007). 4,300‐year‐old chimpanzee sites and the origins of percussive stone technology. Proceedings of the National Academy of Sciences of the United States of America 104, 3043–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesoudi, A. & Thornton, A. (2018). What is cumulative cultural evolution? Proceedings of the Royal Society B: Biological Sciences 285, 20180712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliano, A. B. , Page, A. E. , Gómez‐Gardeñes, J. , Salali, G. D. , Viguier, S. , Dyble, M. , Thompson, J. , Chaudhary, N. , Smith, D. , Strods, J. , MacE, R. , Thomas, M. G. , Latora, V. & Vinicius, L. (2017). Characterization of hunter‐gatherer networks and implications for cumulative culture. Nature Human Behaviour 1, 0043. [Google Scholar]
- Miller‐Atkins, G. & Premo, L. S. (2018). Time‐averaging and the spatial scale of regional cultural differentiation in archaeological assemblages. STAR: Science & Technology of Archaeological Research 4, 12–27. [Google Scholar]
- Mills, B. J. (2017). Social network analysis in archaeology. Annual Review of Anthropology 46, 309–334. [Google Scholar]
- Mills, B. J. , Shackley, M. S. , Roberts, J. M. , Clauset, A. , Huntley, D. L. , Breiger, R. L. , Clark, J. J. , Borck, L. , Haas, W. R. , Peeples, M. A. & Hill, J. B. (2013). Transformation of social networks in the late pre‐hispanic US southwest. Proceedings of the National Academy of Sciences of the United States of America 110, 5785–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematzadeh, A. , Ferrara, E. , Flammini, A. & Ahn, Y. Y. (2014). Optimal network modularity for information diffusion. Physical Review Letters 113, 088701. [DOI] [PubMed] [Google Scholar]
- Newell, R. R. , Kielman, D. , Constandse‐Westermann, T. S. , Van der Sanden, W. A. & Van Gijn, A. (1990). An Inquiry into the Ethnic Resolution of Mesolithic Regional Groups: The Study of their Decorative Ornaments in Time and Space. Brill, New York. [Google Scholar]
- Newman, M. E. J. (2002). Assortative mixing in networks. Physical Review Letters 89, 208701. [DOI] [PubMed] [Google Scholar]
- Newman, M. E. J. (2006). Finding community structure in networks using the eigenvectors of matrices. Physical Review E 74, 036104. [DOI] [PubMed] [Google Scholar]
- Nunn, C. L. , Mccabe, C. M. , Verdolin, J. L. , Jorda, F. , Fewell, J. H. & Nunn, C. L. (2015). Infectious disease and group size: more than just a numbers game. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 20140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östborn, P. & Gerding, H. (2014). Network analysis of archaeological data: a systematic approach. Journal of Archaeological Science 46, 75–88. [Google Scholar]
- Oswalt, W. H. (1976). An Anthropological Analysis of Food‐Getting Technology. Wiley, Hoboken. [Google Scholar]
- Pasquaretta, C. , Levé, M. , Claidiere, N. , Van De Waal, E. , Whiten, A. , MacIntosh, A. J. J. , Pelé, M. , Bergstrom, M. L. , Borgeaud, C. , Brosnan, S. F. , Crofoot, M. C. , Fedigan, L. M. , Fichtel, C. , Hopper, L. M. , Mareno, M. C. , et al. (2014). Social networks in primates: smart and tolerant species have more efficient networks. Scientific Reports 4, 7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples, M. A. (2019). Finding a place for networks in archaeology. Journal of Archaeological Research 27, 451–499. [Google Scholar]
- Peeples, M. A. , Mills, B. J. , Randall Haas, W. , Clark, J. J. & Roberts, J. M. (2016). Analytical challenges for the application of social network analysis in archaeology In The Connected Past: Challenges to Network Studies in Archaeology and History (eds Brughmans T., Collar A. and Coward F.), pp. 59–84. Oxford University Press, Oxford. [Google Scholar]
- Potts, R. (1996). Humanity's Descent: The Consequences of Ecological Instability. William Morrow & Company, New York. [Google Scholar]
- Powell, A. , Shennan, S. & Thomas, M. G. (2009). Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301. [DOI] [PubMed] [Google Scholar]
- Premo, L. S. (2012). Local extinctions, connectedness, and cultural evolution in structured populations. Advances in Complex Systems 15, 1150002. [Google Scholar]
- Prignano, L. , Morer, I. & Diaz‐Guilera, A. (2017). Wiring the past: a network science perspective on the challenge of archeological similarity networks. Frontiers in Digital Humanities 4, 13. [Google Scholar]
- Railsback, S. F. & Grimm, V. (2012). Agent‐Based and Individual‐Based Modelling. Princeton University Press, Princeton. [Google Scholar]
- Rasmussen, S. O. , Bigler, M. , Blockley, S. P. , Blunier, T. , Buchardt, S. L. , Clausen, H. B. , Cvijanovic, I. , Dahl‐Jensen, D. , Johnsen, S. J. , Fischer, H. , Gkinis, V. , Guillevic, M. , Hoek, W. Z. , Lowe, J. J. , Pedro, J. B. , Popp, T. , Seierstad, I. K. , Steffensen, J. P. , Svensson, A. M. , Vallelonga, P. , Vinther, B. M. , Walker, M. J. C. , Wheatley, J. J. & Winstrup, M. (2014). A stratigraphic framework for abrupt climatic changes during the last glacial period based on three synchronized Greenland ice‐core records: refining and extending the INTIMATE event stratigraphy. Quaternary Science Reviews 106, 14–28. [Google Scholar]
- Read, D. (2008). An interaction model for resource implement complexity based on risk and number of annual moves. American Antiquity 73, 599–625. [Google Scholar]
- Read, D. (2012). Population Size Does Not Predict Artifact Complexity: Analysis of Data from Tasmania, Arctic Hunter‐Gatherers, and Oceania Fishing Groups by Dwight. UCLA Center for Human Complex Systems, Los Angeles. [Google Scholar]
- Riede, F. (2014). Eruptions and ruptures ‐ a social network perspective on vulnerability and impact of the Laacher see eruption (c. 13,000 BP) on late glacial hunter‐gatherers in northern Europe. Archaeological Review from Cambridge 29, 67–102. [Google Scholar]
- Riede, F. (2016). The loss and re‐introduction of bow‐and‐arrow technology: a case study from the northern European late Paleolithic. Lithic Technology 34, 27–45. [Google Scholar]
- Riede, F. , Hoggard, C. & Shennan, S. (2019). Reconciling material cultures in archaeology with genetic data requires robust cultural evolutionary taxonomies. Palgrave Communications 5, 55. [Google Scholar]
- Romano, V. , Duboscq, J. , Sarabian, C. , Thomas, E. , Sueur, C. & MacIntosh, A. J. J. (2016). Modeling infection transmission in primate networks to predict centrality‐based risk. American Journal of Primatology 78, 767–779. [DOI] [PubMed] [Google Scholar]
- Romano, V. , Shen, M. , Pansanel, J. , MacIntosh, A. J. J. & Sueur, C. (2018). Social transmission in networks: global efficiency peaks with intermediate levels of modularity. Behavioral Ecology and Sociobiology 72, 154. [Google Scholar]
- Sah, P. , Leu, S. T. , Cross, P. C. , Hudson, P. J. & Bansal, S. (2017). Unraveling the disease consequences and mechanisms of modular structure in animal social networks. Proceedings of the National Academy of Sciences of the United States of America 114, 4165–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik, C. P. , Fox, E. B. A. & Fechtman, L. T. (2003). Individual variation in the rate of use of tree‐hole tools among wild orang‐utans: implications for hominin evolution. Journal of Human Evolution 44, 11–23. [DOI] [PubMed] [Google Scholar]
- Scott, J. (2017). Social Network Analysis. SAGE Publications Ltd, London. [Google Scholar]
- Shennan, S. (2001). Demography and cultural innovation: a model and its implications for the emergence of modern human culture. Cambridge Archaeological Journal 11, 5–16. [Google Scholar]
- Shott, M. (2016). Technological organization and settlement mobility: an ethnographic examination. Journal of Anthropological Research 42, 15–51. [Google Scholar]
- Sueur, C. , Romano, V. , Sosa, S. & Puga‐Gonzalez, I. (2019). Mechanisms of network evolution: a focus on socioecological factors, intermediary mechanisms, and selection pressures. Primates 60, 167–181. [DOI] [PubMed] [Google Scholar]
- Tallavaara, M. , Luoto, M. , Korhonen, N. , Järvinen, H. & Seppä, H. (2015). Human population dynamics in Europe over the last glacial maximum. Proceedings of the National Academy of Sciences of the United States of America 112, 8232–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello, M. (1994). The question of chimpanzee culture In Chimpanzee Cultures (eds Wrangham R. W., McGrew W. C., de Waal F. B. M. and Heltne P. G.), pp. 301–317. Harvard University Press, Cambridge. [Google Scholar]
- Torrence, R. (2001). Hunter‐gatherer technology: macro‐ and microscale approaches In Hunter‐Gatherers: An Interdisciplinary Perspective (eds Panter‐Brick C., Layton R. H. and Rowley‐Conwy P.), pp. 73–98. New York: Cambridge University Press. [Google Scholar]
- Vaesen, K. , Collard, M. , Cosgrove, R. & Roebroeks, W. (2016). Population size does not explain past changes in cultural complexity. Proceedings of the National Academy of Sciences of the United States of America 113, E2241–E2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl, B. & Noë, R. (2010). Simulation of information propagation in real‐life primate networks: longevity, fecundity, fidelity. Behavioral Ecology and Sociobiology 64, 1449–1459. [Google Scholar]
- van de Waal, E. , Borgeaud, C. & Whiten, A. (2013). Potent social learning and conformity shape a wild primate's foraging decisions. Science 340, 483–485. [DOI] [PubMed] [Google Scholar]
- Wagner, G. P. , Pavlicev, M. & Cheverud, J. M. (2007). The road to modularity. Nature Reviews Genetics 8, 921–931. [DOI] [PubMed] [Google Scholar]
- Watson, S. K. , Botting, J. , Whiten, A. & van de Waal, E. (2018). Culture and selective social learning in wild and captive primates In Evolution of Primate Social Cognition (eds di Paolo L., di Vincenzo F. and de Petrillo F.), pp. 211–230. Springer International Publishing AG, Cham. [Google Scholar]
- Whallon, R. (2006). Social networks and information: non‐"utilitarian" mobility among hunter‐gatherers. Journal of Anthropological Archaeology 25, 259–270. [Google Scholar]
- Whitehead, H. (2008). Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. The University of Chicago Press, Chicago. [Google Scholar]
- Whitehead, H. & Lusseau, D. (2012). Animal social networks as substrate for cultural behavioural diversity. Journal of Theoretical Biology 294, 19–28. [DOI] [PubMed] [Google Scholar]
- Whiten, A. (2017). Social learning and culture in child and chimpanzee. Annual Review of Psychology 68, 129–154. [DOI] [PubMed] [Google Scholar]
- Whiten, A. , Goodall, J. , McGrew, W. C. , Nishida, T. , Reynolds, V. , Sugiyama, Y. , Tutin, C. E. G. , Wrangham, R. W. & Boesch, C. (1999). Cultures in chimpanzees. Nature 399, 682–685. [DOI] [PubMed] [Google Scholar]
- Whiten, A. , Caldwell, C. A. & Mesoudi, A. (2016). Cultural diffusion in humans and other animals. Current Opinion in Psychology 8, 15–21. [DOI] [PubMed] [Google Scholar]
- Wicks, K. & Mithen, S. (2014). The impact of the abrupt 8.2ka cold event on the Mesolithic population of western Scotland: a bayesian chronological analysis using ‘activity events’ as a population proxy. Journal of Archaeological Science 45, 240–269. [Google Scholar]
- Wobst, H. M. (1974). Boundary conditions for Paleolithic social systems: a simulation approach. American Antiquity 39, 147–178. [Google Scholar]
- Zahid, H. J. , Robinson, E. & Kelly, R. L. (2016). Agriculture, population growth, and statistical analysis of the radiocarbon record. Proceedings of the National Academy of Sciences of the United States of America 113, 931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhão, J. (2011). Aliens from outer time? Why the “human revolution” is wrong, and where do we go from here? In Continuity and Discontinuity in the Peopling of Europe One Hundred Fifty Years of Neanderthal Study (eds Condemi S. and Weniger G.‐C.), pp. 331–366. Springer, New York. [Google Scholar]
- Zilhão, J. , Angelucci, D. E. , Badal‐Garcia, E. , D'Errico, F. , Daniel, F. , Dayet, L. , Douka, K. , Higham, T. F. G. , Martinez‐Sanchez, M. J. , Montes‐Bernardez, R. , Murcia‐Mascaros, S. , Perez‐Sirvent, C. , Roldan‐Garcia, C. , Vanhaeren, M. , Villaverde, V. , et al. (2010). Symbolic use of marine shells and mineral pigments by Iberian Neandertals. Proceedings of the National Academy of Sciences of the United States of America 107, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]


