Abstract
Objectives
To investigate whether serial prostate magnetic resonance imaging (MRI) may guide the utility of repeat targeted (TBx) and systematic biopsy (SBx) when monitoring men with low‐risk prostate cancer (PCa) at 1‐year of active surveillance (AS).
Patients and Methods
We retrospectively included 111 consecutive men with low‐risk (International Society of Urological Pathology [ISUP] Grade 1) PCa, who received protocolled repeat MRI with or without TBx and repeat SBx at 1‐year of AS. TBx was performed in Prostate Imaging‐Reporting and Data System (PI‐RADS) score ≥3 lesions (MRI‐positive men). Upgrading defined as ISUP Grade ≥2 PCa (I), Grade ≥2 with cribriform growth/intraductal carcinoma PCa (II), and Grade ≥3 PCa (III) was investigated. Upgrading detected by TBx only (not by SBx) and SBx only (not by TBx) was investigated in MRI‐positive and ‐negative men, and related to radiological progression on MRI (Prostate Cancer Radiological Estimation of Change in Sequential Evaluation [PRECISE] score).
Results
Overall upgrading (I) was 32% (35/111). Upgrading in MRI‐positive and ‐negative men was 48% (30/63) and 10% (5/48) (P < 0.001), respectively. In MRI‐positive men, there was upgrading in 23% (seven of 30) by TBx only and in 33% (10/30) by SBx only. Radiological progression (PRECISE score 4–5) in MRI‐positive men was seen in 27% (17/63). Upgrading (I) occurred in 41% (seven of 17) of these MRI‐positive men, while this was 50% (23/46) in MRI‐positive men without radiological progression (PRECISE score 1–3) (P = 0.534). Overall upgrading (II) was 15% (17/111). Upgrading in MRI‐positive and ‐negative men was 22% (14/63) and 6% (three of 48) (P = 0.021), respectively. In MRI‐positive men, there was upgrading in three of 14 by TBx only and in seven of 14 by SBx only. Overall upgrading (III) occurred in 5% (five of 111). Upgrading in MRI‐positive and ‐negative men was 6% (four of 63) and 2% (one of 48) (P = 0.283), respectively. In MRI‐positive men, there was upgrading in one of four by TBx only and in two of four by SBx only.
Conclusion
Upgrading is significantly lower in MRI‐negative compared to MRI‐positive men with low‐risk PCa at 1‐year of AS. In serial MRI‐negative men, the added value of repeat SBx at 1‐year surveillance is limited and should be balanced individually against the harms. In serial MRI‐positive men, the added value of repeat SBx is substantial. Based on this cohort, SBx is recommended to be performed in combination with TBx in all MRI‐positive men at 1‐year of AS, also when there is no radiological progression.
Keywords: low‐risk prostate cancer, active surveillance, prostate MRI, PI‐RADS, PRECISE, upgrading, #ProstateCancer, #PCSM
Abbreviations
- AS
active surveillance
- CR
cribriform growth pattern
- csPCa
clinically significant prostate cancer
- EAU
European Association of Urology
- IDC
intraductal carcinoma
- IQR
interquartile range
- IRB
Institutional Review Board
- ISUP
International Society of Urological Pathology
- mpMRI
multiparametric MRI
- MRI ± TBx
MRI with or without TBx
- PCa
prostate cancer
- PI‐RADS
Prostate Imaging Reporting and Data System
- PRECISE
Prostate Cancer Radiological Estimation of Change in Sequential Evaluation
- PRIAS
Prostate cancer Research International Active Surveillance
- SBx
systematic TRUS‐guided prostate biopsy
- START
Standards of reporting for MRI‐targeted biopsy studies
- TBx
targeted biopsy
Introduction
Active surveillance (AS) is a widely used strategy for managing men with low‐risk prostate cancer (PCa) to reduce overtreatment and treatment‐related side‐effects, with confirmed oncological safety at long‐term follow‐up [1]. The fear of under grading at time of diagnostic biopsy has led to the development of AS protocols with strict criteria for inclusion and monitoring, like the Prostate cancer Research International Active Surveillance (PRIAS) study (www.prias‐project.org) [2].
Today, MRI and targeted biopsy (TBx) are increasingly used in the evaluation of patients with low‐risk PCa who initially opt for AS, based on systematic TRUS‐guided prostate biopsy (SBx) findings [3]. The additional use of a first pre‐biopsy MRI and subsequent TBx in these men can aid in the exclusion of higher risk men with International Society of Urological Pathology (ISUP) Grade ≥2 PCa, irrespective of the timing of the MRI during follow‐up (i.e., at baseline, confirmatory or surveillance biopsy) [4, 5, 6, 7, 8, 9]. A first pre‐biopsy MRI in the evaluation of men on AS for low‐risk PCa has therefore recently been adopted in the European Association of Urology (EAU) PCa guidelines [10].
An MRI‐based monitoring strategy in men with low‐risk PCa on AS is attractive to health systems and patients, potentially avoiding a prostate biopsy procedure with its attendant morbidities as much as reasonably possible. The Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) criteria could help to qualify radiological risk of progression on serial prostate MRI [11, 12]. However, the role of MRI in monitoring and its potential to guide the indication for repeat biopsies (i.e., confirmatory and surveillance biopsies) during AS is still unclear. Unanswered issues in clinical practice are whether SBx could be omitted in cases of a negative follow‐up MRI, whether only TBx should be performed in cases of a positive follow‐up MRI, and whether biopsies should only be performed in cases of radiological disease progression on follow‐up MRI. Recent studies provide contradictory findings in men on AS for low‐risk PCa as to whether or not serial MRI could obviate the need for repeat biopsies [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. Hamoen et al. [18] showed an overall added value for repeat (confirmatory) SBx at 1‐year of AS of 42% as compared to 7% added value for serial MRI with or without TBx (MRI ± TBx). However, Thurtle et al. [19, 20] and Elkjaer et al. [19, 20] found much more added value for serial MRI ± TBx (30–50%), and less added value for repeat SBx (9–12%) in their cohorts. Substantial evidence on implementing prostate MRI as a monitoring tool in men on AS for low‐risk PCa is still lacking.
As virtually all AS protocols advise a repeat biopsy procedure after 1 year on AS, we aimed to determine the potential guidance of serial prostate MRI (i.e., positive or negative MRI, with or without radiological progression) in the utility of repeat TBx and SBx in men with low‐risk PCa at 1‐year of AS, using different definitions for clinically significant PCa (csPCa) as outcome measures.
Patients and Methods
Study Population
This retrospective study was approved by our Institutional Review Board (IRB; NL45884.078.13/A301321), and written informed consent with guarantee of confidentiality was obtained from all study participants. No additional data other than already collected as part of this IRB‐approved study was sought for the analyses done in this study. Men with low‐risk PCa (ISUP Grade 1) were prospectively enrolled in our in‐house clinical database as part of our AS protocol. All men were followed according to the MRI‐PRIAS study protocol (www.prias‐project.org). In summary, they underwent an MRI ± TBx at baseline (3 months after the detection of low‐risk PCa on diagnostic SBx), and during every repeat SBx scheduled at 1 year (confirmatory biopsy), and 4, 7 and 10 years (surveillance biopsy) after diagnosis (Fig. 1). The only upgrading or re‐classification criterion was the presence of ISUP Grade 2 (Gleason score 3 + 4) and higher PCa at biopsy.
Fig. 1.
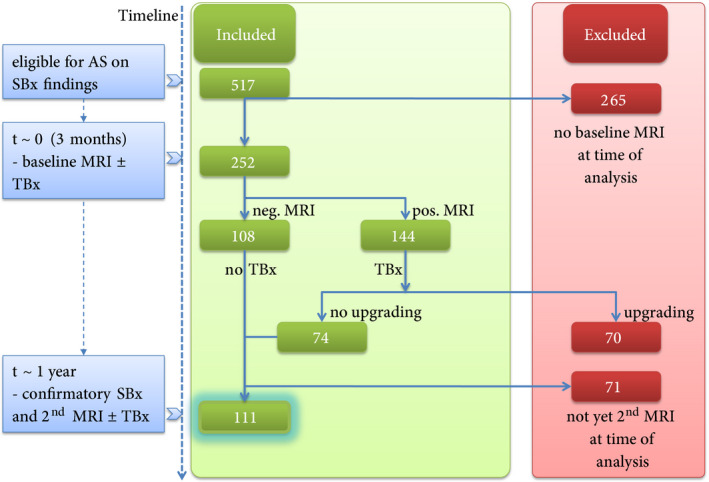
Flowchart of patients included in this study. AS, active surveillance; SBx, systematic biopsy; TBx, targeted biopsy.
From November 2013 to May 2019, 517 consecutive men on AS for ISUP Grade 1 PCa underwent at least one prostate MRI during follow‐up. At the time of analysis, 252 men had undergone an MRI ± TBx at baseline. Results of part of this cohort have been previously published [8, 9]. In all, 70/252 (28%) men had upgrading after their first MRI‐TBx and therefore ceased AS, and 71/252 (28%) men had not yet undergone a second MRI ± TBx at the time of analysis. In the present study, we included 111 men on AS for low‐risk PCa who had undergone both a MRI ± TBx at baseline and at the time of the scheduled confirmatory SBx 1 year after initiation of AS (Fig. 1).
Multiparametric MRI
Multiparametric MRI (mpMRI) at both time points was performed on a 3.0‐T MR scanner (Discovery MR750; General Electric Healthcare, Chicago, IL, USA) with a 32‐channel pelvic phased‐array coil. The institutional MRI protocol included T2‐weighted imaging, diffusion‐weighted imaging with apparent diffusion coefficient reconstructions, and dynamic contrast enhanced imaging, according to the Prostate Imaging‐Reporting and Data System (PI‐RADS) version 1 and 2 guidelines [25]. All MRIs were reviewed by one urogenital radiologist with >7 years’ experience of prostate MRI. Individual lesions were scored according to the PI‐RADS 5‐point likelihood scale for csPCa, and the index lesions were annotated and delineated [25]. Visible MRI lesions with a PI‐RADS score of 3–5 were defined as suspicious.
Serial MRI scans were all compared to the initial imaging by the reporting radiologist according to the PRECISE criteria [11, 12]. The PRECISE recommendations use a 5‐point likelihood scale to qualify radiological progression on MRI in men on AS with serial prostate MRIs. PRECISE score 1–2 corresponds to resolution/regression of previous features suspicious on MRI (based on a decreased radiological size/stage/conspicuity/PI‐RADS score), PRECISE score 3 corresponds to radiological stable disease, and PRECISE score 4–5 to disease progression on MRI (based on an increased radiological size/stage/conspicuity/PI‐RADS score). In clinical practice, a positive serial MRI without radiological progression is defined as PRECISE score 1–3.
MRI‐Tbx and SBx
Biopsies were performed in a separate session. All men with a positive (serial) MRI underwent TBx. An MRI‐ultrasound fusion system (UroStation™, Koelis, France) was used to take TBx of all suspicious lesions identified on MRI. The suspicious MRI lesions, delineated on Digital Imaging and Communications in Medicine (DICOM) images, were targeted with 2–5 cores/lesion. An additional SBx (8–12 cores, depending on the prostate volume) was taken in all men at the time of confirmatory biopsy and was not blinded from MRI results. The biopsy procedures were performed by four experienced operators.
Pathological Review of Biopsy Specimens
One expert uropathologist reviewed all biopsy specimens according to the ISUP 2014 modified Gleason score/Grade Group system [26]. The presence of an invasive cribriform growth pattern (CR) and/or intraductal carcinoma (IDC) was routinely recorded. Upgrading, and thereby the recommendation to switch to active treatment, was defined in clinical practice as any ISUP Grade ≥2 PCa found by MRI ± TBx and/or SBx.
Study Endpoints
We compared the percentage of upgrading of ISUP Grade 1 PCa to csPCa between the results of MRI ± TBx and SBx in MRI‐positive and ‐negative men on AS at the time of confirmatory biopsy (1‐year surveillance). In addition, we assessed the percentage of upgrading related to the PRECISE score (i.e., regressive, stable and progressive features on prostate MRI). The percentage of upgrading was calculated using three different definitions of csPCa: definition I, ISUP Grade ≥2 PCa; definition II, ISUP Grade ≥2 with CR and/or IDC PCa; and definition III, ISUP Grade ≥3 PCa.
Primary outcomes are:
Upgrading (definition I) in MRI‐positive and ‐negative men.
Upgrading (definition I) related to the radiological changes between first and second MRI (PRECISE score), in MRI‐positive and ‐negative men.
Secondary outcome is:
Upgrading based on higher thresholds (definitions II and III) for csPCa.
Statistical Analysis
Descriptive statistics were used to report the clinical patient characteristics and percentages of upgrading. Statistically significant differences in continuous non‐parametric data were assessed with the Mann–Whitney U‐test and Wilcoxon signed‐rank test. The chi‐square test for trend, McNemar test and Wilcoxon signed‐rank test were used to test for differences in categorical data. In accordance with the Standards of reporting for MRI‐TBx studies (START) recommendations, cross‐tabulation of the confirmatory biopsy outcomes was performed to compare the percentage of upgrading detected by MRI ± TBx vs SBx [27]. Analyses were performed using the Statistical Package for the Social Sciences (SPSS®), version 24.0 (SPSS Inc., IBM Corp., Armonk, NY, USA), with a two‐tailed level of significance set at P < 0.05.
Results
Patients’ Characteristics
The clinical patients’ characteristics with subsequent low‐risk PCa profiles did not show significant differences (except for age) at baseline and at confirmatory biopsy at 1‐year of AS (Table 1).
Table 1.
Patients’ characteristics at baseline and at 1‐year confirmatory biopsy.
| Characteristic | Baseline | Confirmatory biopsy (1‐year surveillance) | P * |
|---|---|---|---|
| Total cohort (n = 111) | Total cohort (n = 111) | ||
| Median (IQR) | |||
| Age, years | 66 (60–70) | 67 (61–71) | <0.001 |
| PSA level, ng/mL | 6.8 (5.1–9.1) | 6.9 (5.2–9.4) | 0.352 |
| Prostate volume, mL | 42 (30–56) | 41 (31–55) | 0.695 |
| PSA density, ng/mL/mL | 0.17 (0.11–0.25) | 0.15 (0.12–0.27) | 0.864 |
| N (%) | |||
| Clinical stage | |||
| T1c | 85 (77) | 80 (72) | 0.665 |
| T2a | 22 (20) | 25 (23) | |
| T2b | 2 (2) | 4 (4) | |
| T2c | 1 (1) | 2 (2) | |
| T3a | 1 (1) | 0 (0) | |
| TRUS findings | |||
| Benign | 93 (84) | 91 (82) | 0.774 |
| Suspected | 18 (16) | 20 (18) | |
| Number of positive diagnostic cores | |||
| 1 | 46 (41) | N/A | N/A |
| 2 | 36 (32) | N/A | |
| 3 | 19 (17) | N/A | |
| 4 | 7 (6) | N/A | |
| 5 | 2 (2) | N/A | |
| 6 | 1 (1) | N/A | |
| PI‐RADS score of MRI | |||
| 1–2 | 52 (47) | 48 (43) | 0.303 |
| 3 | 15 (14) | 13 (12) | |
| 4 | 35 (32) | 40 (36) | |
| 5 | 9 (8) | 10 (9) | |
| PRECISE score of MRI | |||
| 1–2 | N/A | 14 (13) | N/A |
| 3 | N/A | 80 (72) | |
| 4–5 | N/A | 17 (15) | |
| Time between MRIs, months, median (IQR) | N/A | 10 (9–13) | N/A |
| Overall ISUP Grade at biopsy, n (%) | |||
| No PCa | N/A | 31 (28) | N/A |
| G 1 | 111 (100) | 45 (41) | |
| G 2 | N/A | 18 (16) | |
| G 2 with CR and/or IDC | N/A | 12 (11) | |
| G 3 | N/A | 5 (5) | |
| G 4–5 | N/A | 0 (0) | |
CR, cribriform growth pattern; G, grade; IDC, intraductal carcinoma; ISUP, International Society of Urological Pathology; IQR, interquartile range; N/A, not applicable; PCa, prostate cancer; PI‐RADS, Prostate Imaging‐Reporting and Data System; PRECISE, Prostate Cancer Radiological Estimation of Change in Sequential Evaluation.
P values calculated based on the comparison between the baseline and confirmatory characteristics for the total cohort.
Upgrading (Definition I) at 1‐year Surveillance, in MRI‐Positive and ‐Negative Men
At 1‐year surveillance, 57% (63/111) of men had a positive follow‐up MRI and 43% (48/111) had a negative follow‐up MRI. Overall, upgrading (definition I) occurred in 32% (35/111, 95% CI 23–41), as a result of TBx and/or SBx (Table 2). Upgrading in MRI‐positive and ‐negative men was 48% (30/63, 95% CI 35–61) and 10% (five of 48, 95% CI 4–23) (P < 0.001, 95% CI for the difference 21–51), respectively. In MRI‐positive men, upgrading was 23% (seven of 30) by TBx only, 33% (10/30) by SBx only, and 43% (13/30) by both TBx and SBx (Table S1 for cross‐tabulation of biopsy data).
Table 2.
Upgrading in MRI‐positive and ‐negative men per definition for csPCa at 1‐year surveillance.
| Definition I | Definition II | Definition III | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow‐up MRI at 1‐year surveillance | No upgrading | Upgrading | No upgrading | Upgrading | No upgrading | Upgrading | |||||||||||
| N (% of total) | Overall, n (%) | Overall, n (%) | Biopsy technique, n (% of overall) or n/N | Overall, n (%) | Overall, n (%) | Biopsy technique, n (% of overall) or n/N | Overall, n (%) | Overall, n (%) | Biopsy technique, n (% of overall) | ||||||||
| All biopsy | TBx + SBx | SBx only | TBx only | All biopsy | TBx + SBx | SBx only | TBx only | All biopsy | TBx + SBx | SBx only | TBx only | ||||||
| Total | 111 (100) | 76 (68) | 35 (32) | 13 (37) | 15 (43) | 7 (20) | 94 (85) | 17 (15) | 4 (24) | 10 (59) | 3 (18) | 106 (95) | 5 (5) | 1/5 | 3/5 | 1/5 | |
| Positive MRI | PI‐RADS 3–5 | 63 (57) | 33 (52) | 30 (48) | 13 (43) | 10 (33) | 7 (23) | 49 (78) | 14 (22) | 4/14 | 7/14 | 3/14 | 59 (94) | 4 (6) | 1/4 | 2/4 | 1/4 |
| Negative MRI | PI‐RADS 1–2 | 48 (43) | 43 (90) | 5 (10) | x | 5/5 | x | 45 (94) | 3 (6) | x | 3/3 | x | 47 (98) | 1 (2) | x | 1/1 | x |
PI‐RADS, Prostate Imaging‐Reporting and Data System; SBx, systematic biopsy; TBx, targeted biopsy.
In a total of 23 MRI‐positive men, SBx detected upgrading. 43% (10/23, 95% CI 23–66) of the detected upgrading by SBx in these men was (also) located on the contralateral side of the suspicious MRI lesion(s). The overall upgrading in MRI‐positive men increased with the PI‐RADS score from 38% (five of 13, 95% CI 14–68) in PI‐RADS score 3, 48% (19/40, 95% CI 32–64) in PI‐RADS score 4, 60% (six of 10, 95% CI 26–88) in PI‐RADS score 5 MRIs. Based on TBx results no correlation was found for upgrading related to higher PI‐RADS score.
Upgrading (Definition I) at 1‐year Surveillance, Related to Changes on MRI in MRI‐Positive and ‐Negative Men
Radiological progression (PRECISE score 4–5, i.e., from non‐suspicious to suspicious and suspicious to more suspicious) in MRI‐positive men was observed in 27% (17/63). Upgrading (definition I) in these men was 41% (seven of 17, 95% CI 18–67), as a result of TBx and/or SBx. Upgrading occurred in three of seven by TBx only and in three of seven by SBx only (Table 3). No radiological progression (PRECISE score 1–3) in MRI‐positive men occurred in 73% (46/63). Upgrading in these men was 50% (23/46, 95% CI 35–65), found by TBx and/or SBx. Upgrading was 17% (four of 23) by TBx only and 30% (seven of 23) by SBx only.
Table 3.
Upgrading related to radiological changes on MRI in MRI‐positive and ‐negative men per definition for csPCa at 1‐year surveillance.
| Definition I | Definition II | Definition III | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow‐up MRI at 1‐year surveillance | No upgrading | Upgrading | No upgrading | Upgrading | No upgrading | Upgrading | |||||||||||
| N (% of total) | Overall, n (%) or n/N | Overall, n (%) or n/N | Biopsy technique, n (% of overall) or n/N | Overall, n (%) or n/N | Overall, n (%) or n/N | Biopsy technique, n/N | Overall, n (%) or n/N | Overall, n (%) or n/N | Biopsy technique, n/N | ||||||||
| All biopsy | TBx + SBx | SBx only | TBx only | All biopsy | TBx + SBx | SBx only | TBx only | All biopsy | TBx + SBx | SBx only | TBx only | ||||||
| Total | PRECISE 4–5 | 17 (15) | 10 (59) | 7 (41) | 1 (14) | 3 (43) | 3 (43) | 14 (82) | 3 (18) | 0/3 | 3/3 | 0/3 | 16 (94) | 1 (6) | 0/1 | 1/1 | 0/1 |
| PRECISE 1–3 | 94 (85) | 66 (70) | 28 (30) | 12 (43) | 12 (43) | 4 (14) | 80 (85) | 14 (15) | 4/14 | 7/14 | 3/14 | 90 (96) | 4 (4) | 1/4 | 2/4 | 1/4 | |
| Positive MRI | PRECISE 4–5 | 17 (27) | 10 (59) | 7 (41) | 1/7 | 3/7 | 3/7 | 14 (82) | 3 (18) | 0/3 | 3/3 | 0/3 | 16 (94) | 1 (6) | 0/1 | 1/1 | 0/1 |
| PRECISE 1–3 | 46 (73) | 23 (50) | 23 (50) | 12 (52) | 7 (30) | 4 (17) | 35 (76) | 11 (24) | 4/11 | 4/11 | 3/11 | 43 (93) | 3 (7) | 1/3 | 1/3 | 1/3 | |
| Negative MRI | PRECISE 4–5 | N/A | |||||||||||||||
| PRECISE 1–3 | 48 (100) | 43 (90) | 5 (10) | x | 5/5 | x | 45 (94) | 3 (6) | x | 3/3 | x | 47 (98) | 1 (2) | x | 1/1 | x | |
| PRECISE 3 | 42 (88) | 38 (90) | 4 (10) | x | 4/4 | x | 40 (95) | 2 (5) | x | 2/2 | x | 41 (98) | 1 (2) | x | 1/1 | x | |
| PRECISE 1–2 | 6 (13) | 5/6 | 1/6 | x | 1/1 | x | 5/6 | 1/6 | x | 1/1 | x | 6/6 | 0/6 | x | 0/0 | x | |
PRECISE, Prostate Cancer Radiological Estimation of Change in Sequential Evaluation; SBx, systematic biopsy; TBx, targeted biopsy.
PRECISE score 3 (i.e., stable radiological features) in MRI‐negative men was observed in 88% (42/48), in whom upgrading occurred in 10% (four of 42, 95% CI 3–23) (Table 3). Radiological regression from suspicious to non‐suspicious findings (PRECISE score 1–2) in follow‐up MRI‐negative men was observed in 13% (six of 48), in whom upgrading occurred in 17% (one of six, 95% CI 1–64).
Upgrading at 1‐year Surveillance, Based on Higher Thresholds (Definitions II and III) for csPCa
Overall upgrading (definition II) was 15% (17/111, 95% CI 9–23). Upgrading in MRI‐positive and ‐negative men was 22% (14/63, 95% CI 13–35) and 6% (three of 48, 95% CI 1–17) (P = 0.021, 95% CI for the difference 2–28), respectively. In MRI‐positive men, there was upgrading in three of 14 by TBx only, and in four of 14 by SBx only (Table 2). Related to PRECISE, upgrading was 18% (three of 17, 95% CI 4–43) for PRECISE score 4–5 and 15% (14/94, 95% CI 8–24) for PRECISE score 1–3 (Table 3).
Overall upgrading (definition III) was 5% (five of 111, 95% CI 2–10). Upgrading in MRI‐positive and ‐negative men was 6% (four of 63, 95% CI 2–16) and 2% (one of 48, 95% CI 1–11) (P = 0.283, 95% CI for the difference −5 to 13), respectively. In MRI‐positive men, there was upgrading in one of four by TBx only, and in two of four by SBx only (Table 2). Related to PRECISE, upgrading was 6% (one of 17, 95% CI 1–29) for PRECISE score 4–5 and 4% (four of 94, 95% CI 1–11) for PRECISE score 1–3 (Table 3).
Discussion
The guidance of serial prostate MRI in the utility of repeat biopsies, when monitoring low‐risk PCa men on AS, has not been clearly established. In our clinical practice of men with low‐risk PCa on AS with subsequent MRI at baseline and at 1‐year follow‐up, overall upgrading from low‐ to intermediate/high‐risk PCa (definition I) at 1‐year surveillance was 32%. Upgrading was significantly lower in MRI‐negative men (10%) compared to MRI‐positive men (48%). In MRI‐positive men, SBx detected a substantial additional proportion of upgrading not detected by TBx; almost half detected on the contralateral side of the suspicious MRI lesion(s). Upgrading was similar in MRI‐positive men with radiological progression and without radiological progression. In these two groups the additional value of SBx in upgrading to ISUP Grade ≥2 PCa was 43% and 30%, respectively. This argues for additional repeat SBx in men with and without radiological progression on positive MRI. The other studied thresholds for upgrading ([definition II] ISUP Grade ≥2 with CR and/or IDC PCa, and [definition III] ISUP Grade ≥3 PCa) resulted in a lower overall upgrading (15% and 5%, respectively). At these thresholds, similar results were found with only limited upgrading in MRI‐negative men and substantial added value of SBx in MRI‐positive men. These results suggest that in serial MRI‐negative men with low‐risk PCa, repeat SBx at 1‐year surveillance should be balanced against the harms on an individual basis. The risk of missing a timely diagnosis of high‐risk PCa is low. However, in serial MRI‐positive men repeat SBx combined with TBx should be performed in all MRI‐positive low‐risk PCa men at 1‐year surveillance to gain maximal diagnostic precision. This strategy could save a repeat biopsy procedure at 1‐year follow‐up in 43% of men at the cost of missing 2–10% of csPCa (depending on the threshold used) in our population.
Two important clinical implications from our present results are: to consider omitting SBx in serial MRI‐negative men at 1‐year AS, and to perform both SBx and TBx in serial MRI‐positive men. Previous studies have also investigated the value of serial MRI and TBx in monitoring men on AS for low‐risk PCa. With respect to the applied AS protocol (i.e., the time interval between follow‐up testing), the studies of Thurtle et al. [19], Elkjaer et al. [20] and Hamoen et al. [18] are similar to our present study. Our present results of overall upgrading (32%) and added value of repeat SBx in MRI‐positive (33%) and MRI‐negative men (10%) at 1‐year surveillance of low‐risk PCa are mostly in line with the results of Hamoen et al. [18] (25% overall upgrading and an added value of repeat SBx in serial MRI‐positive men of 36%, while in MRI‐negative men of 50%). The difference in added value of SBx in MRI‐negative men is probably caused by the fact that in our present study 48 (43%) men had a negative MRI and repeat SBx, while only eight (11%) men in the Hamoen et al. [18] cohort had a negative MRI and SBx. Our present overall percentage of upgrading is also consistent with the stable 25% re‐classification found at each repeat SBx in the entire PRIAS study (without the use of MRI) [2]. This finding confirms the high value of re‐sampling the prostate with SBx in men at 1‐year AS, which after upfront risk stratification with MRI appears to have the most added value in MRI‐positive men.
Thurtle et al. [19] and Elkjaer et al. [20] showed, however, a lower overall upgrading (14–16%) at confirmatory biopsy in their cohorts and less added value of repeat SBx (12–18% added value of repeat SBx in serial MRI‐positive men and 5–7% in MRI‐negative men). These differences to our present study could be explained by our daily clinical practice AS cohort of men with low‐risk PCa as opposed to the men with very low‐risk PCa included in their studies. Furthermore, in the Hamoen et al. [18] study and in our present study, the repeat SBx was not taken blinded from the MRI results, which could beneficially influence the SBx outcomes.
Consistent with the upgrading results in other studies, most men, if upgraded, were upgraded from ISUP Grade 1 to Grade 2 PCa in our present cohort. This is probably (partially) caused by previous sampling error, as low‐risk PCa profiles remained equal. This confirms the finding that most men following an AS programme rarely have high‐risk disease (ISUP Grade ≥3 PCa) during follow‐up and therefore have a good cancer‐specific survival [1, 28].
Our present results indicate performing SBx in combination with TBx in all MRI‐positive men at 1‐year of AS, and also when there is no radiological progression, which is in line with the recommendations from Hsiang et al. [23] and Chesnut et al. [24]. In the total cohort, we detected more upgrading in men with a PRECISE score 4–5 (41%) compared to men with a PRECISE score 1–3 (30%) on follow‐up MRI. This finding is consistent with Dieffenbacher et al. [21], who studied the impact of serial MRIs in AS using the PRECISE score at 4‐years follow‐up. However, they showed a much better discrimination of the PRECISE scoring system for AS disqualification, with only 10% upgrading detected in men with a PRECISE score 1–3. Differences might be explained by the fact that we analysed a cohort at 1‐year of AS with substantial added value of repeat prostate sampling with SBx, while they analysed a cohort at the time of the third follow‐up SBx (4‐years after initial diagnosis). This has probably resulted in an improved patient selection for AS, with only limited added value of repeat sampling of the whole prostate at the time of their analysis. In addition, the fact remains that the assessment of serial MRIs in men on AS is challenging, as upgrading still occurs with some regularity in men with an apparent stable low‐risk disease on positive MRI due to the high value of repeat prostate sampling. Therefore, serial MRIs and the PRECISE criteria need to be investigated more often in clinical AS cohorts of men with low‐risk PCa to help with the creation of a robust dataset to define proper radiological thresholds of csPCa in men on AS.
The present study is the first to investigate the role of serial prostate MRIs in a daily clinical practice of AS (following a strict protocol) related to the presence of CR and IDC in biopsy specimens. CR and IDC are prognostic drivers in survival, even more than other Gleason 4 subpatterns [29]. Kweldam et al. [30] showed that men with ISUP Grade 2 PCa with the presence of CR/IDC were associated with a worse disease‐specific survival (67%) at 15‐years follow‐up, compared to men with ISUP Grade 2 PCa without the presence of CR/IDC (94%) and men with Grade 1 PCa (99%). Identifying these Gleason 4 patterns in men on AS may therefore be of high clinical relevance, with subsequently a large population staying on AS without CR/IDC. In our present population, overall upgrading defined by ISUP Grade ≥2 with CR and/or IDC PCa (definition II) decreased from 32% to 15%, potentially saving even more biopsy procedures (e.g., in PI‐RADS score 3 men) and keeping more men on AS. Incorporation of this tumour‐specific information into risk stratification could further improve selection of men who will benefit from active treatment. We may argue that the threshold for upgrading in men on AS should be changed to ISUP Grade ≥2 with CR and/or IDC PCa, to (falsely) exclude less men from AS and thereby to reduce the rate of overtreatment and treatment‐related side‐effects.
Some limitations of our present study should be highlighted. First, our study has a retrospective design and could thereby introduce a selection bias. However, our study represents a prospective cohort of consecutive men on AS with strict monitoring. Second, clinicians involved were not blinded to clinical data and MRI results. Hence, this process is daily clinical practice and therefore can be extrapolated to other hospitals. Third, the sample size of our study is relatively small, which could reduce generalisability. However, in comparison to similar studies in the recent literature, it is the second largest sample size available in a study on the use of serial MRI in men on AS at 1‐year surveillance for low‐risk PCa. Lastly, the median follow‐up time was limited to 33 months. We acknowledge that the outcome measurement of our analysis was upgrading at 1‐year surveillance. The cancer‐specific survival rate in a long‐term follow‐up would have been more appropriate to make hard inferences about the need for and frequencies of surveillance testing. This outcome may, however, be debatable in a cohort of men with low‐risk disease who exhibit excellent long‐term cancer‐specific survival and who furthermore experience most shifts from AS to active treatment during the first 2 years of follow‐up [31].
In conclusion, at 1‐year surveillance the performance of repeat SBx in serial MRI‐negative men should be discussed per individual based on one’s balance between benefits (e.g. not missing any intermediate/high‐risk disease) and harms (e.g. unnecessary biopsy, biopsy complications). In serial MRI‐positive men, repeat SBx should be performed together with MRI‐TBx in all MRI‐positive men, and also when there is no radiological progression. These findings are irrespective of upgrading threshold. Future large‐scale studies should confirm this and focus on other surveillance issues in the current MRI era, such as the need for, the intervals, and frequencies of surveillance testing from 2 years after the diagnosis of low‐risk PCa.
Conflicts of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. Cross‐tabulation of serial MRI with or without targeted biopsy results vs repeat systematic biopsy results, at 1‐year surveillance. MRI, magnetic resonance imaging; TBx, targeted biopsy; ISUP, International Society of Urological Pathology; PI‐RADS, Prostate Imaging Reporting and Data System; TBx, targeted biopsy; SBx, systematic biopsy; PCa, prostate cancer; G, grade; CR, cribriform growth pattern; IDC, intraductal carcinoma.
References
- 1. Klotz L, Vesprini D, Sethukavalan P et al. Long‐term follow‐up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272–7 [DOI] [PubMed] [Google Scholar]
- 2. Bokhorst LP, Valdagni R, Rannikko A et al A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol 2016; 70: 954–60 [DOI] [PubMed] [Google Scholar]
- 3. Schoots IG, Moore CM, Rouviere O. Role of MRI in low‐risk prostate cancer: finding the wolf in sheep's clothing or the sheep in wolf's clothing? Curr Opin Urol 2017; 27: 238–45 [DOI] [PubMed] [Google Scholar]
- 4. Marliere F, Puech P, Benkirane A et al. The role of MRI‐targeted and confirmatory biopsies for cancer upstaging at selection in patients considered for active surveillance for clinically low‐risk prostate cancer. World J Urol 2014; 32: 951–8 [DOI] [PubMed] [Google Scholar]
- 5. Ouzzane A, Renard‐Penna R, Marliere F et al. Magnetic resonance imaging targeted biopsy improves selection of patients considered for active surveillance for clinically low risk prostate cancer based on systematic biopsies. J Urol 2015; 194: 350–6 [DOI] [PubMed] [Google Scholar]
- 6. Abdi H, Pourmalek F, Zargar H et al. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology 2015; 85: 423–8 [DOI] [PubMed] [Google Scholar]
- 7. Recabal P, Ehdaie B. The role of MRI in active surveillance for men with localized prostate cancer. Curr Opin Urol 2015; 25: 504–9 [DOI] [PubMed] [Google Scholar]
- 8. Alberts AR, Roobol MJ, Drost FH et al. Risk‐stratification based on magnetic resonance imaging and prostate‐specific antigen density may reduce unnecessary follow‐up biopsy procedures in men on active surveillance for low‐risk prostate cancer. BJU Int 2017; 120: 511–9 [DOI] [PubMed] [Google Scholar]
- 9. Schoots IG, Osses DF, Drost FH et al. Reduction of MRI‐targeted biopsies in men with low‐risk prostate cancer on active surveillance by stratifying to PI‐RADS and PSA‐density, with different thresholds for significant disease. Transl Androl Urol 2018; 7: 132–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mottet N, Bellmunt J, Bolla M et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2019; 71: 618–29 [DOI] [PubMed] [Google Scholar]
- 11. Moore CM, Giganti F, Albertsen P et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE Recommendations‐A Report of a European School of Oncology Task Force. Eur Urol 2017; 71: 648–55 [DOI] [PubMed] [Google Scholar]
- 12. Giganti F, Allen C, Piper JW et al. Sequential prostate MRI reporting in men on active surveillance: initial experience of a dedicated PRECISE software program. Magn Reson Imaging 2019; 57: 34–9 [DOI] [PubMed] [Google Scholar]
- 13. Walton Diaz A, Shakir NA, George AK et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015; 33: 202.e1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felker ER, Wu J, Natarajan S et al. Serial magnetic resonance imaging in active surveillance of prostate cancer: incremental value. J Urol 2016; 195: 1421–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eineluoto JT, Jarvinen P, Kenttamies A et al. Repeat multiparametric MRI in prostate cancer patients on active surveillance. PLoS ONE 2017; 12: e0189272. DOI: 10.1371/journal.pone.0189272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olivier J, Kasivisvanathan V, Drumez E et al. Low‐risk prostate cancer selected for active surveillance with negative MRI at entry: can repeat biopsies at 1 year be avoided? A pilot study. World J Urol 2018; 37: 253–9 [DOI] [PubMed] [Google Scholar]
- 17. Gallagher KM, Christopher E, Cameron AJ et al. Four‐year outcomes from a multiparametric magnetic resonance imaging (MRI)‐based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies. BJU Int 2018; 123: 429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamoen EH, Hoeks CM, Somford DM et al. Value of serial multiparametric magnetic resonance imaging and magnetic resonance imaging‐guided biopsies in men with low‐risk prostate cancer on active surveillance after 1 yr follow‐up. Eur Urol Focus 2019; 5: 407–15. [DOI] [PubMed] [Google Scholar]
- 19. Thurtle D, Barrett T, Thankappan‐Nair V et al. Progression and treatment rates using an active surveillance protocol incorporating image‐guided baseline biopsies and multiparametric magnetic resonance imaging monitoring for men with favourable‐risk prostate cancer. BJU Int 2018; 122: 59–65 [DOI] [PubMed] [Google Scholar]
- 20. Elkjaer MC, Andersen MH, Hoyer S, Pedersen BG, Borre M. Multi‐parametric magnetic resonance imaging monitoring patients in active surveillance for prostate cancer: a prospective cohort study. Scand J Urol. 2018; 52: 8–13 [DOI] [PubMed] [Google Scholar]
- 21. Dieffenbacher S, Nyarangi‐Dix J, Giganti F et al. Standardized magnetic resonance imaging reporting using the prostate cancer radiological estimation of change in sequential evaluation criteria and magnetic resonance imaging/transrectal ultrasound fusion with transperineal saturation biopsy to select men on active surveillance. Eur Urol Focus 2019. [Epub ahead of print]. 10.1016/j.euf.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 22. Klotz L, Pond G, Loblaw A et al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2‐year postbiopsy follow‐up. Eur Urol 2020; 77: 311–7 [DOI] [PubMed] [Google Scholar]
- 23. Hsiang W, Ghabili K, Syed JS et al. Outcomes of serial multiparametric magnetic resonance imaging and subsequent biopsy in men with low‐risk prostate cancer managed with active surveillance. Eur Urol Focus 2019. [Epub ahead of print]. 10.1016/j.euf.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 24. Chesnut GT, Vertosick EA, Benfante N et al. Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for men with prostate cancer on active surveillance. Eur Urol 2020; 77: 501–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinreb JC, Barentsz JO, Choyke PL et al. PI‐RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urol 2016; 69: 16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Epstein JI, Egevad L, Amin MB et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016; 40: 244–52 [DOI] [PubMed] [Google Scholar]
- 27. Moore CM, Kasivisvanathan V, Eggener S et al. Standards of reporting for MRI‐targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 2013; 64: 544–52 [DOI] [PubMed] [Google Scholar]
- 28. Hamdy FC, Donovan JL, Lane JA et al. 10‐year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24 [DOI] [PubMed] [Google Scholar]
- 29. Zlotta AR, Egawa S, Pushkar D et al. Prevalence of prostate cancer on autopsy: cross‐sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013; 105: 1050–8 [DOI] [PubMed] [Google Scholar]
- 30. Kweldam CF, Kümmerlin IP, Nieboer D et al. Disease‐specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Modern Pathol 2016; 29: 630–6 [DOI] [PubMed] [Google Scholar]
- 31. Duffield AS, Lee TK, Miyamoto H, Carter HB, Epstein JI. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J Urol 2009; 182: 2274–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cross‐tabulation of serial MRI with or without targeted biopsy results vs repeat systematic biopsy results, at 1‐year surveillance. MRI, magnetic resonance imaging; TBx, targeted biopsy; ISUP, International Society of Urological Pathology; PI‐RADS, Prostate Imaging Reporting and Data System; TBx, targeted biopsy; SBx, systematic biopsy; PCa, prostate cancer; G, grade; CR, cribriform growth pattern; IDC, intraductal carcinoma.


