Abstract
Background and Aims
The enhanced liver fibrosis (ELF) score comprises serum markers of fibrogenesis and matrix remodelling and was developed to detect liver fibrosis, however, it may also be useful for the non‐invasive detection of portal hypertension (PHT).
Methods
ELF score and its single components (TIMP1/PIIINP/HA) were analysed in 201 patients with advanced chronic liver disease (ACLD; ie hepatic venous pressure gradient (HVPG) ≥6 mm Hg). Patients with pre‐/post‐hepatic PHT, hepatocellular carcinoma beyond Milan criteria, and history of TIPS implantation or liver transplantation were excluded.
Results
ELF and its single components correlated with HVPG in the overall cohort: ELF: r = .443, TIMP1: r = .368, PIIINP:r = .332, and HA:r = .419 (all P < .001). The strength of the correlation between ELF and HVPG decreased in higher HVPG strata: 6‐9 mm Hg:r = .569(P = .004), 10‐19 mm Hg:r = .304 (P = .001) and ≥20 mm Hg:r = −.023(P = .853).
Area under the receiver operating characteristics (AUROC) of ELF score to detect clinically significant PHT (CSPH; HVPG ≥ 10 mm Hg) was 0.833. Importantly, HA alone yielded an AUROC of 0.828. Detection of CSPH in strictly compensated ACLD (cACLD) patients was less accurate: AUROC: 0.759 (P < .001). CSPH was ruled‐in by ELF ≥ 11.1 with a PPV of 98% (sensitivity: 61%/specificity: 92%/NPV:24%), but CSPH could not be ruled‐out.
ELF score had a low AUROC of 0.677 (0.60‐0.75; P < .001) for the diagnosis of high‐risk PHT (HRPH; HVPG ≥ 20mm Hg) and, thus, HRPH could not be ruled‐in by ELF. However, ELF < 10.1 ruled‐out HRPH with a NPV of 95% (sensitivity: 97%/specificity: 26%/PPV: 39%).
Conclusion
The ELF score correlates with HVPG at values <20 mm Hg. An ELF ≥ 11.1 identifies patients with a high probability of CSPH, while an ELF < 10.1 may be used to rule‐out HRPH.
Keywords: ACLD, advanced chronic liver disease, cirrhosis, clinically significant portal hypertension, hepatic venous pressure gradient, non‐invasive, portal hypertension, prediction
Abbreviations
- ACLD
advanced chronic liver disease
- ALD
alcohol‐related liver disease
- AUROC
area under the receiver operating characteristics
- AVB
acute variceal bleeding
- cACLD
compensated ACLD
- CSPH
clinically significant portal hypertension
- CTP
Child‐Turcotte‐Pugh
- dACLD
decompensated ACLD
- ELF
enhanced liver fibrosis score
- HA
hyaluronic acid
- HCV
viral hepatitis C
- HRPH
high‐risk portal hypertension
- HVPG
hepatic venous pressure gradient
- INR
international normalized ratio
- IVD
in vitro diagnostic
- MELD
model for end‐stage liver disease
- NAFLD
non‐alcoholic fatty liver disease
- NASH
non‐alcoholic steatohepatitis
- NLR
negative likelihood ratio
- NPV
negative predictive value
- NSBB
non‐selective beta‐blockers
- PHT
portal hypertension
- PIIINP
Amino‐terminal propeptide of type III procollagen
- PLR
positive likelihood ratio
- PLT
platelet
- PPV
positive predictive value
- TIMP‐1
tissue inhibitor matrix metalloproteinase‐1
- TIPS
transjugular intrahepatic portosystemic shunt
- VCTE
vibration‐controlled transient elastography
Key points.
Patients with liver disease may develop increased pressure in the blood vessels that drain into the liver. This condition is termed portal hypertension (PHT). Invasive catheter measurements must be performed to diagnose PHT. Our study shows that a simple blood test named “enhanced liver fibrosis (ELF) score” can identify patients with a high versus a low likelihood of suffering from PHT.
1. INTRODUCTION
Chronic liver injury causes liver damage that is characterized by necroinflammation and fibrosis, which can ultimately progress towards advanced chronic liver disease (ACLD; ie advanced liver fibrosis and cirrhosis). Perpetual increase in intrahepatic resistance finally results in development of portal hypertension (PHT). 1 Hepatic venous pressure gradient (HVPG) above or equal to 10 mm Hg defines the presence of clinically significant portal hypertension (CSPH) and is assessed by hepatic vein catheterization. 2 CSPH is an important prerequisite for the development of complications in patients with ACLD. Most importantly, patients with CSPH are at risk of hepatic decompensation and increased mortality. 2 , 3
The enhanced liver fibrosis (ELF) score was firstly described to non‐invasively detect fibrosis in patients with non‐alcoholic fatty liver disease (NAFLD), 4 and subsequently validated for viral hepatitis C (HCV) 5 and is based on combining serum markers of matrix remodelling and fibrosis. 6 The ELF score includes three single components, ie tissue inhibitor of matrix metalloproteinases (TIMP1), aminoterminal peptide of procollagen type III (PIIINP) and hyaluronic acid (HA). 5 Ultimately, ELF score cut‐offs were proposed for the discrimination of patients with fibrosis from healthy individuals and for staging fibrosis in patients with HCV, 5 , 7 non‐alcoholic steatohepatitis (NASH), 8 alcohol‐related liver disease (ALD) 9 and paediatric NAFLD 10 patients. Importantly, multiple studies reported that ELF was a predictor of disease progression and mortality. 11 , 12 , 13 , 14
Previous studies assessing the ability of ELF score to detect CSPH have produced controversial results. 15 , 16 , 17 In one of these studies, Sandahl et al computed a prediction model for CSPH by combining ELF with serum concentrations of soluble CD163, a serum marker of activated macrophages, which yielded in a higher accuracy in discriminating CSPH from subclinical PHT, 16 as compared to ELF alone. Importantly, both ELF score and histological fibrosis stage as well as HVPG significantly decreased after cure from HCV in patients with PHT suggesting a correlation between the decline in HVPG and fibrosis that may be mirrored by changes in ELF score. 17 The varying accuracy of ELF score to predict CSPH in previous studies may derive from considerable differences in the respective study cohorts such as liver disease aetiology, as well as severity of liver disease and of PHT. Finally, previous studies included both compensated and decompensated patients. However, the non‐invasive detection of CSPH is particularly relevant in patients with compensated ACLD (cACLD), 18 who have not been analysed separately yet.
The aim of this study was to investigate the performance of ELF score to predict CSPH and high‐risk PHT (HRPH; ie HVPG ≥20 mm Hg) in a large prospective cohort of patients undergoing HVPG measurements and simultaneous assessment of ELF score.
2. PATIENTS AND METHODS
2.1. Study design
A total of 201 patients with clinically stable ACLD undergoing hepatic vein catheterization between January 2017 and August 2019 at the Vienna Hepatic Hemodynamic Lab of the Medical University of Vienna were consecutively included in the prospective Vienna Cirrhosis Study (VICIS) (NCT03267615). Referral to HVPG measurement was based on reasonable suspicion of ACLD as a result of radiological and laboratory results and/or previous clinical symptoms associated with PHT according to national and international consensus recommendations. 19 , 20
Patients with pre‐ or post‐hepatic causes of PHT, non‐cirrhotic PHT, hepatocellular carcinoma beyond Milan criteria, acute decompensation or non‐elective hospitalization, liver metastases or a history of transjugular intrahepatic portosystemic shunt (TIPS) implantation or liver transplantation were excluded (Figure S1). Patients receiving concomitant treatment with non‐selective beta‐blockers were not included in the analysis. More specifically, patients either had never received non‐selective beta‐blockers (NSBB) or paused NSBB intake at least 5 days before HVPG measurement. Relevant clinical information and laboratory parameters were collected from patients’ medical records. cACLD was defined as the absence of decompensating events prior to or at the time point of HVPG measurement, ie ascites, hepatic encephalopathy and variceal bleeding. 21 Furthermore, vibration‐controlled transient elastography (VCTE; Fibroscan®) was performed instantaneously prior to HVPG measurement and considered for analysis when meeting reliability criteria as previously published. 22
2.2. HVPG measurements
HVPG measurements were performed by trained physicians of the Vienna Hepatic Hemodynamic Lab following a defined standard operating procedure 23 in fasted condition. Briefly, the right internal jugular vein was punctured by ultrasound guidance under local anaesthesia. A catheter introducer set (8.5 F, Arrow International) was inserted using Seldinger technique. The liver vein was cannulated by an angled balloon occlusion catheter (Medical University of Vienna/Medizintechnik Pejcl, Austria). 24 Adequate placement and wedge position were verified by X‐ray after injection of contrast agent while the balloon was inflated. At least three measurements of free and wedged hepatic vein pressure were performed to assess HVPG. No adverse events requiring medical intervention were recorded for patients included in this study.
2.3. Analysis of ELF score components
The components of the ELF score (TIMP1, HA, PIIINP) were measured in serum—obtained from the catheter introducer sheath placed in the internal jugular vein during HVPG measurements—by in vitro diagnostic CE‐certified chemiluminescence immunoassays using the respective assay kits on an Advia Centaur CP analyser (Siemens Healthcare Diagnostics Inc) in an ISO 15189 accredited laboratory at the Department of Laboratory Medicine, Medical University of Vienna. The measurement ranges were for TIMP1 3.5‐1300 ng/mL, for HA 1.6‐1000 ng/mL, and for PIIINP 0.5‐150 ng/mL. The total coefficients of variation according to CLSI Guideline EP5‐A2 were ≤6.0, ≤7.7 and ≤6.5 for TIMP1, HA and PIIINP respectively.
The final score was calculated as previously reported 5 : ELF score = 2.494 + 0.846 ln(CHA) + 0.735 ln(CPIIINP) + 0.391 ln(CTIMP1). ELF component measurements were performed by technicians at the department of Laboratory Medicine at the Medical University of Vienna blinded to the clinical and hemodynamic data of the subjects.
2.4. Statistics
Statistical analyses were performed using ibm spss Statistics 26 (IBM) and GraphPad Prism 8 (GraphPad Software). Continuous variables are reported as mean ± standard error of the mean (SEM) or median and IQR, and categorical variables are presented as numbers (n) and proportions (%) of patients. Comparisons of continuous variables were performed using Student's t‐test or Mann‐Whitney U test, as applicable. Categorical variables were compared with chi‐squared or Fisher‘s exact test, as applicable. Receiver operating characteristic (ROC) curves were used to test the performance of ELF score to detect CSPH. Additionally, we assessed area under the receiver operating characteristics (AUROC), sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, as well as positive (PLR) and negative (NLR) likelihood ratios for the detection of CSPH and HRPH. The optimal cut‐off values were evaluated by Youden's index (sensitivity + specificity −1), whereas most inclusive cut‐offs achieving a sensitivity or specificity ≥90% were chosen for ruling‐out and ruling‐in CSPH and HRPH respectively. Furthermore, exploratory AUROC analyses were performed in subgroups of different disease aetiologies and patients with cACLD. A two‐sided P ≤ .05 was defined to denote statistical significance.
2.5. Ethics
This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments and approved by the local ethics committee of the Medical University of Vienna (EK1262/2017). All patients gave written informed consent to liver vein catheterizations and provided written consent to be enrolled in the VICIS study (NCT03267615). Study results are reported according to STARD guidelines for diagnostic studies (https://www.equator‐network.org/reporting‐guidelines/stard/).
3. RESULTS
3.1. Patient characteristics
The majority of patients in our study cohort were male (n = 135/201, 67%), and the prevalence of CSPH and HRPH was 88% (177/201) and 32% (65/201) respectively. ALD (n = 83, 41%) and viral hepatitis (n = 40, 20%) were the most common aetiologies of ACLD. The mean age was 56.8 ± 0.8 years (Table S1). Eighty‐five (42%) patients presented with cACLD (including n = 62, 73% with CSPH). Most patients (58%) had Child‐Turcotte‐Pugh (CTP) stage A, while 33% had CTP stage B and 9% had CTP stage C. Median model for end‐stage liver disease (MELD) score was 11 (9‐14).
Seventy‐five (37%) patients had no varices, 64 (32%) had small varices and 56 (28%) had large oesophageal varices, whereas variceal status was unknown in 6 (3%) patients. Seventeen (9%) patients had a history of variceal bleeding, 85 (42%) had a history of or current ascites and 38 (19%) had a history of or current signs of hepatic encephalopathy. Median HVPG was 17 (12‐21) mm Hg. Twenty‐four (12%) patients had subclinical PHT (PH, ie 6‐9 mm Hg), 112 (56%) showed an HVPG between 10 and 19 mm Hg, and 65 (32%) had HRPH.
3.2. Severity of PHT
Patient characteristics were compared by stratification according to severity of PHT, ie HVPG 6‐9 mm Hg, 10‐19 mm Hg and ≥ 20 mm Hg (Table 1). Aetiologies of liver disease differed significantly among HVPG groups with a continuous increase in ALD prevalence with rising severity of PHT (17% among patients with 6‐9 mm Hg, 38% with 10‐19 mm Hg and 54% with ≥20 mm Hg, respectively; P = .021).
Table 1.
Patient characteristics stratified by severity of portal hypertension
| N = 201 | HVPG 6‐9 mm Hg (n = 24) | HVPG 10‐19 mm Hg (n = 112) | HVPG ≥ 20 mm Hg (n = 65) | P‐value |
|---|---|---|---|---|
| Age (y) | 52.7 ± 2.3 | 56.8 ± 1.1 | 58.4 ± 1.5 | 0.129 |
| Sex (M, %) | 16 (67) | 74 (66) | 45 (69) | 0.910 |
| Etiology (n, %) | ||||
| ALD | 4 (17) | 43 (38) | 35 (54) | .021 |
| Viral | 8 (33) | 21 (19) | 11 (17) | |
| Other | 12 (50) | 48 (43) | 19 (29) | |
| Decompensation (n, %) | 1 (4) | 62 (55) | 53 (82) | <.001 |
| Varices (n, %) | ||||
| None | 18 (75) | 41 (37) | 11 (17) | <.001 |
| Small | 3 (13) | 25 (22) | 23 (35) | |
| Large | 1 (4) | 43 (38) | 30 (46) | |
| (Unknown) | 2 (8) | 3 (3) | 1 (1.5) | |
| Child score (points) | 5 (5‐5) | 6 (5‐7) | 7 (6‐9) | <.001 |
| MELD (points) | 8 (7‐12) | 10 (9‐14) | 12 (10‐15) | <.001 |
| Creatinine (mg/dL) | 0.73 (0.63‐0.87) | 0.77 (0.59‐1.02) | 0.71 (0.60‐0.97) | .847 |
| Sodium (mmol/L) | 140 (139‐142) | 139 (137‐141) | 137 (134‐140) | <.001 |
| Albumin (g/dL) | 40.8 (37.3‐42.4) | 37.2 (33.0‐40.7) | 36.0 (30.2‐39.0) | <.001 |
| INR | 1.2 (1.0‐1.3) | 1.3 (1.2‐1.5) | 1.4 (1.3‐1.6) | <.001 |
| Platelets (g/L) | 126 (90‐162) | 99 (70‐137) | 96 (57‐126) | .056 |
| TE (kPa) a | 16.3 (11.7‐20.4) | 27.7 (17.0‐43.0) | 62.2 (34.8‐75.0) | <.001 |
| ELF score (points) | 10.1 (9.6‐10.7) | 11.2 (10.4‐12.3) | 11.8 (11.1‐12.8) | <.001 |
| TIMP1 (ng/mL) | 251 (217‐301) | 297 (244‐421) | 409 (291‐495) | <.001 |
| PIIINP (ng/mL) | 11.5 (8.8‐16.2) | 17.9 (10.8‐28.3) | 20.6 (13.3‐33.1) | <.001 |
| HA (ng/mL) | 61.4 (43.4‐135.7) | 195.1 (106.0‐392.4) | 284.8 (174.9‐505.5) | <.001 |
Abbreviations: (ALD) Alcohol‐related liver disease; (ELF) enhanced liver fibrosis score; (HA) Hyaluronic acid(HVPG) hepatic venous pressure gradient; (INR) International normalized ratio; (M) male gender; (MELD) Model for end‐stage liver disease score; (PIIINP) Amino‐terminal propeptide of type III procollagen; (TIMP‐1) Tissue inhibitor matrix metalloproteinase‐1; (VCTE) vibration‐controlled transient elastography.
P‐values < .05 are indicated in bold.
Reliable TE results available in n = 21 (87.5%), n = 81 (72.3%) and n = 37 (56.9%) patients respectively.
Expectedly, the proportion of patients with decompensated cirrhosis (dACLD) increased with rising severity of PH (4%, 55% and 82%, respectively; P < .001). Consequently, median MELD and CTP score increased with HVPG (P < .001). ELF score increased significantly across HVPG strata, with a median ELF of 10.1 (9.6‐10.7) in patients with an HVPG of 6‐9mm Hg, 11.2 (10.4‐12.3) with 10‐19mm Hg and 11.8 (11.1‐12.8) with ≥20 mm Hg (P < .001).
3.3. Patient characteristics according to ELF score tertiles
Similarly, patient characteristics were compared after stratification by ELF score tertiles (T1‐T3): T1 was defined as ELF < 10.76, T2 as ELF between 10.76 and 11.96 and T3 as ELF > 11.96. CTP score and MELD increased in patients within higher ELF tertiles, as well as the proportion of patients with decompensation (all P ≤ .001; Table 2).
Table 2.
Patient characteristics stratified by enhanced liver fibrosis (ELF) scores
| N = 201 | Tertile 1 (n = 67) | Tertile 2 (n = 67) | Tertile 3 (n = 67) | P‐value |
|---|---|---|---|---|
| ELF < 10.76 | ELF 10.76‐11.96 | ELF > 11.96 | ||
| Age (y) | 54.7 ± 1.3 | 59.0 ± 1.6 | 56.8 ± 1.3 | .107 |
| Sex (M, %) | 49 (73) | 50 (75) | 36 (54) | .016 |
| Etiology (n, %) | ||||
| ALD | 12 (18) | 31 (46) | 39 (58) | <.001 |
| Viral | 23 (34) | 9 (13) | 8 (12) | |
| Other | 32 (48) | 27 (40) | 20 (30) | |
| Decompensation (n, %) | 22 (33) | 41 (61) | 53 (79) | <.001 |
| Varices (n, %) | ||||
| None | 31 (46) | 16 (24) | 23 (34) | .036 |
| Small | 11 (16) | 24 (36) | 16 (24) | |
| Large | 22 (33) | 26 (39) | 26 (39) | |
| (Unknown) | 3 (5) | 1 (1.5) | 2 (3) | |
| HVPG (mm Hg) | 12 (9‐18) | 18 (14‐21) | 19 (16‐21) | <.001 |
| Child score (points) | 5 (5‐5) | 6 (5‐7) | 8 (6‐10) | <.001 |
| MELD (points) | 9 (8‐12) | 10 (9‐13) | 14 (11‐17) | <.001 |
| TE (kPa) a | 18.8 (14.1‐28.1) | 30.1 (19.4‐46.5) | 48.0 (29.5‐70.0) | <.001 |
| Creatinine (mg/dL) | 0.77 (0.63‐0.96) | 0.74 (0.59‐1.06) | 0.67 (0.56‐0.90) | .222 |
| Sodium (mmol/L) | 140 (138‐142) | 138 (136‐140) | 138 (135‐140) | .001 |
| Albumin (g/dL) | 40.7 (38.7‐42.4) | 37.1 (33.3‐40.1) | 31.3 (28.1‐35.1) | <.001 |
| INR | 1.2 (1.1‐1.4) | 1.3 (1.2‐1.4) | 1.5 (1.4‐1.7) | <.001 |
| Platelets (g/L) | 98 (65‐133) | 105 (77‐141) | 101 (76‐132) | .258 |
Abbreviations: ALD, alcohol‐related liver disease; ELF, enhanced liver fibrosis score; HVPG, hepatic venous pressure gradient; INR, international normalized ratio; M, male gender; MELD, model for end‐stage liver disease score; VCTE, vibration‐controlled transient elastography.
P‐values < .05 are indicated in bold.
Reliable TE results available in n = 59 (87.5%), n = 49 (88.1%) and n = 53 (79.1%) patients, respectively.
Furthermore, HVPG increased significantly across tertiles: median HVPG 12 (9‐18) mm Hg in T1, 18 (14‐21) mm Hg in T2 and 19 (16‐21) mm Hg in T3 (P < .001). The presence of varices was significantly different between groups stratified by ELF tertiles in the overall cohort, however, a similar proportion of patients had large varices (33% vs 39% vs 39%, respectively; P = .036).
3.4. Correlation between ELF score and its single components with HVPG
ELF score and its single components correlated with HVPG in the overall cohort (Spearman's Rho 0.443, 0.32‐0.55; P < .001; Figure 1A‐D). Among the single ELF components, HA displayed the strongest correlation with HVPG (Rho 0.419, 0.29‐0.53; Table 3, Table S2).
Figure 1.
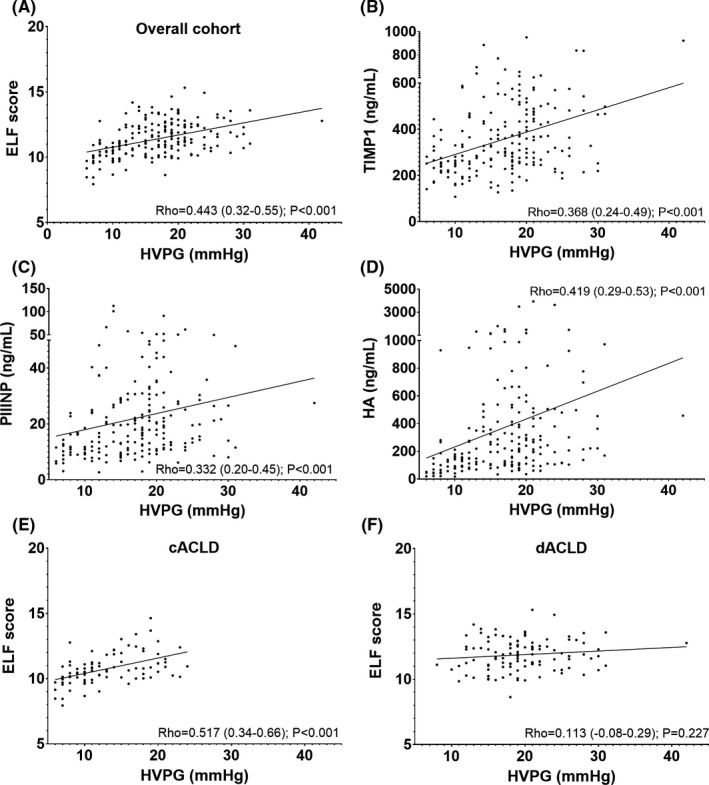
Correlation of enhanced liver fibrosis (ELF) score with hepatic venous pressure gradient (HVPG). Abbreviations: cACLD, compensated advanced chronic liver disease; dACLD, decompensated advanced chronic liver disease; ELF, enhanced liver fibrosis score; HA, hyaluronic acid; HVPG, hepatic venous pressure gradient; mm Hg, millimetres of mercury; PIIINP, amino‐terminal propeptide of type III procollagen; Rho, Spearman's Rho; TIMP‐1, tissue inhibitor matrix metalloproteinase‐1
Table 3.
Correlation of enhanced liver fibrosis (ELF) score and its single components with hepatic venous pressure gradient (HVPG)
| Selection | Parameter | Rho | 95%CI | P‐value |
|---|---|---|---|---|
| Overall (N = 201) | ELF | 0.443 | 0.321‐0.551 | <.001 |
| TIMP1 | 0.368 | 0.238‐0.485 | <.001 | |
| PIIINP | 0.332 | 0.199‐0.453 | <.001 | |
| HA | 0.419 | 0.294‐0.530 | <.001 | |
| ALD (N = 83) | ELF | 0.281 | 0.063‐0.473 | .010 |
| TIMP1 | 0.235 | 0.014‐0.434 | .033 | |
| PIIINP | 0.213 | −0.010 to 0.415 | .053 | |
| HA | 0.283 | 0.065‐0.475 | .010 | |
| VIRAL (N = 40) | ELF | 0.449 | 0.151‐0.673 | .004 |
| TIMP1 | 0.279 | −0.045 to 0.550 | .081 | |
| PIIINP | 0.328 | 0.009‐0.586 | .039 | |
| HA | 0.488 | 0.200‐0.699 | .001 | |
| OTHER (N = 78) | ELF | 0.391 | 0.178‐0.569 | <.001 |
| TIMP1 | 0.323 | 0.102‐0.514 | .004 | |
| PIIINP | 0.197 | −0.034 to 0.407 | .084 | |
| HA | 0.402 | 0.191‐0.578 | <.001 | |
| HVPG 6‐9 (N = 24) | ELF | 0.569 | 0.203‐0.796 | .004 |
| TIMP1 | −0.147 | −0.529 to 0.284 | .493 | |
| PIIINP | 0.559 | 0.189‐0.790 | .005 | |
| HA | 0.475 | 0.076‐0.743 | .019 | |
| HVPG 10‐19 (N = 112) | ELF | 0.304 | 0.120‐0.468 | .001 |
| TIMP1 | 0.247 | 0.058‐0.418 | .009 | |
| PIIINP | 0.273 | 0.087‐0.441 | .004 | |
| HA | 0.268 | 0.081‐0.437 | .004 | |
| HVPG ≥ 20 (N = 65) | ELF | −0.023 | −0.273‐0.229 | .853 |
| TIMP1 | 0.020 | −0.232 to 0.270 | .874 | |
| PIIINP | −0.105 | −0.347 to 0.149 | .404 | |
| HA | 0.021 | −0.231 to 0.271 | .867 | |
| Child‐A (N = 116) | ELF | 0.450 | 0.286‐0.588 | <.001 |
| TIMP1 | 0.261 | 0.077‐0.438 | .005 | |
| PIIINP | 0.237 | 0.052‐0.407 | .010 | |
| HA | 0.471 | 0.311‐0.605 | <.001 | |
| Child‐B/C (N = 85) | ELF | 0.014 | −0.206 to 0.233 | .896 |
| TIMP1 | 0.129 | −0.093 to 0.338 | .241 | |
| PIIINP | −0.003 | −0.222 to 0.216 | .979 | |
| HA | −0.009 | −0.227 to 0.211 | .938 | |
| cACLD (N = 85) | ELF | 0.517 | 0.335‐0.661 | <.001 |
| TIMP1 | 0.227 | 0.062‐0.468 | .010 | |
| PIIINP | 0.291 | 0.076‐0.479 | .007 | |
| HA | 0.535 | 0.358‐0.675 | <.001 | |
| dACLD (N = 116) | ELF | 0.113 | −0.076 to 0.294 | .227 |
| TIMP1 | 0.186 | −0.002 to 0.361 | .046 | |
| PIIINP | 0.045 | −0.144 to 0.231 | .633 | |
| HA | 0.127 | −0.062 to 0.307 | .174 |
Abbreviations: 95%CI, 95% confidence interval; ALD, Alcohol‐related liver disease; cACLD, compensated advanced chronic liver disease; dACLD, decompensated advanced chronic liver disease; ELF, enhanced liver fibrosis score; HA, hyaluronic acid; HVPG, hepatic venous pressure gradient; PIIINP, Amino‐terminal propeptide of type III procollagen; Rho, Spearman's Rho; TIMP‐1, Tissue inhibitor matrix metalloproteinase‐1.
P‐values < .05 are indicated in bold.
After stratifications according to ACLD aetiology, we specifically analysed correlations in patients with ALD and viral hepatitis. Median HVPG was higher in ALD than viral hepatitis patients (19 [16‐21] mm Hg vs 14 [10‐20] mm Hg, respectively; P = .001). The correlation coefficients were markedly lower in patients with ALD as compared to viral hepatitis or other aetiologies. PIIINP showed no significant association with HVPG in ALD patients, while TIMP1 did not correlate with HVPG in patients with viral hepatitis.
Similar correlation analyses of the HVPG and ELF were performed in subgroups according to severity of PHT: 6‐9 mm Hg, 10‐19 mm Hg and ≥20 mm Hg respectively. Interestingly, ELF score (Rho = 0.569, P = .004), PIIINP (Rho = 0.559; P = .005) and HA (Rho = 0.475, P = .019) showed the strongest correlation in patients with subclinical PHT. ELF score (Rho = 0.304, P = .001) and its single components significantly correlated with HVPG in stratum 10‐19 mm Hg, while no ELF parameter (eg ELF Rho = −0.023; P = .853) showed a meaningful correlation with HVPG in patients with HRPH.
Furthermore, patients were stratified by CTP stage and absence/presence of prior events of hepatic decompensation (Figure 1E,F). No correlation between ELF parameters and HVPG was found in patients with CTP stage B/C. Similarly, ELF showed no significant correlation in decompensated (dACLD) patients. Conversely, all parameters continuously increased with PHT severity in patients within CTP stage A and within cACLD patients. Again, in these patients with persevered liver function, ELF score (Rho = 0.450 for CTP‐A and Rho = 0.517 for cACLD; P < .001, respectively) and HA (Rho = 0.471 for CTP‐A and Rho = 0.535 for cACLD; P < .001, respectively) displayed the strongest association with HVPG.
3.5. Detection of clinically significant PHT by ELF
AUROC analysis was performed to assess whether ELF score and/or its single components accurately detect CSPH (HVPG ≥ 10 mm Hg). In the overall study population, ELF displayed an AUROC of 0.833 (0.75‐0.92; P < .001, Table 4). CSPH could be ruled‐out with an ELF cut‐off at <9.7 with 97% sensitivity and 38% specificity. Ruling‐in of CSPH with ELF score ≥11.1 achieved 61% sensitivity and 92% specificity, with a PPV of 98%. The optimal single ELF cut‐off (by Youden's index) was 10.5, yielding 81% sensitivity and 75% specificity and PPV of 96% and NPV of 35% respectively. However, these ELF cut‐offs yielded relevant proportions of false‐positive and false‐negative rates (Table S2).
Table 4.
Diagnostic accuracy of enhanced liver fibrosis (ELF) score and vibration‐controlled transient elastography (VCTE) for clinically significant portal hypertension (CSPH) and high‐risk portal hypertension (HRPH)
| AUROC | 95%CI | P‐value | Cut‐off | SENS | SPEC | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|---|---|
| CSPH—Overall cohort | ||||||||||
| ELF | 0.833 | 0.75‐0.92 | <.001 | Youden: 10.5 | 0.81 | 0.75 | 0.96 | 0.35 | 3.24 | 0.25 |
| In: 11.1 | 0.61 | 0.92 | 0.98 | 0.24 | 7.63 | 0.42 | ||||
| Out: 9.7 | 0.97 | 0.38 | 0.92 | 0.6 | 1.56 | 0.08 | ||||
| TE a | 0.834 | 0.76‐0.91 | <.001 | Youden: 23.2 | 0.70 | 0.95 | 0.99 | 0.36 | 14.8 | 0.31 |
| In: 23.2 | 0.70 | 0.95 | 0.99 | 0.36 | 14.8 | 0.31 | ||||
| Out: 11.8 | 0.95 | 0.29 | 0.88 | 0.5 | 1.33 | 0.18 | ||||
| CSPH—ALD | ||||||||||
| ELF | 0.978 | 0.94‐1.00 | .001 | Youden: 10.3 | 0.95 | 1 | 1 | 0.5 | — | 0.05 |
| In: 10.3 | 0.95 | 1 | 1 | 0.5 | — | 0.05 | ||||
| Out: 9.9 | 0.99 | 0.5 | 0.98 | 0.67 | 1.98 | 0.02 | ||||
| CSPH—VIRAL | ||||||||||
| ELF | 0.629 | 0.42‐0.84 | 0.265 | — | — | — | — | — | — | — |
| CSPH—cACLD | ||||||||||
| ELF | 0.759 | 0.64‐0.87 | <.001 | Youden: 10.5 | 0.69 | 0.78 | 0.90 | 0.49 | 3.14 | 0.4 |
| In: 11.1 | 0.42 | 0.91 | 0.93 | 0.37 | 4.67 | 0.64 | ||||
| Out: 9.6 | 0.97 | 0.26 | 0.78 | 0.75 | 1.31 | 0.12 | ||||
| TE a | 0.743 | 0.62‐0.86 | .002 | Youden: 23.2 | 0.59 | 0.95 | 0.95 | 0.5 | 11.7 | 0.43 |
| In: 23.2 | 0.59 | 0.95 | 0.95 | 0.5 | 11.7 | 0.43 | ||||
| Out: 11.8 | 0.89 | 0.30 | 0.75 | 0.55 | 1.27 | 0.36 | ||||
| HRPH—Overall cohort | ||||||||||
| ELF | 0.677 | 0.60‐0.75 | <.001 | Youden: 11.1 | 0.75 | 0.56 | 0.45 | 0.83 | 1.70 | 0.45 |
| In: 13.0 | 0.18 | 0.9 | 0.48 | 0.7 | 1.80 | 0.91 | ||||
| Out: 10.1 | 0.97 | 0.26 | 0.39 | 0.95 | 1.31 | 0.12 | ||||
| TE a | 0.813 | 0.74‐0.89 | <.001 | Youden: 27.4 | 0.92 | 0.59 | 0.45 | 0.95 | 2.23 | 0.14 |
| In: 67.1 | 0.43 | 0.91 | 0.64 | 0.82 | 4.9 | 0.62 | ||||
| Out: 27.4 | 0.92 | 0.59 | 0.45 | 0.95 | 2.23 | 0.14 | ||||
Abbreviations: 95%CI, 95% confidence interval; ALD, alcohol‐related liver disease; AUROC, area under the receiver operating characteristics; cACLD, compensated advanced chronic liver disease; CSPH, Clinically significant portal hypertension; ELF, enhanced liver fibrosis score; HRPH, high‐risk portal hypertension; NLR, negative likelihood ratio; PLR, positive likelihood ratio; VCTE, vibration‐controlled transient elastography.
P‐values < .05, PPV and NPV ≥ 0.90, PLR > 7 and NRL < 0.1 are indicated in bold.
Reliable VCTE results available in n = 139 (69.2%) patients in the overall cohort, and n = 66 (77.6%) patients with cACLD.
Among ELF single components, HA achieved the best performance with an AUROC of 0.828 (0.73‐0.92; P < .001). In contrast, TIMP1 and PIIINP showed less diagnostic value to detect CSPH: AUROC 0.722 (0.63‐0.81; P < .001) and 0.748 (0.66‐0.83; P < .001) respectively (Table S3).
Furthermore, AUROC was analysed by forming subgroups according to ACLD aetiology. In patients with ALD, ELF performed excellent regarding the detection of CSPH with an AUROC 0.978 (0.94‐1.00; P = .001, Table 4). Ruling‐out of CSPH with an ELF score < 9.9 achieved 99% sensitivity, 50% sensitivity, 98% PPV and 67% NPV. ELF score ≥ 10.3 was the optimal cut‐off for ruling‐in CSPH (95% sensitivity, 100% specificity, 100% PPV and 50% NPV) in ALD patients. In contrast, ELF was not able to discriminate between subclinical PHT and CSPH in patients with viral hepatitis (AUROC 0.629; P = .265).
Finally, we assessed the predictive value for non‐invasive CSPH diagnosis in patients with cACLD (Table 2, Figure 2A). In this cohort, ELF AUROC was 0.759 (0.64‐0.87; P < .001), with an optimal cut‐off at 10.5 that detected CSPH with 69% sensitivity, 78% specificity, 90% PPV and 49% NPV. Ruling‐in of CSPH in cACLD at an ELF score ≥11.1 had 93% PPV with 42% sensitivity and 91% specificity. Moreover, CSPH could be ruled‐out in cACLD patients by an ELF cut‐off at <9.6 with 97% sensitivity, 26% specificity, 78% PPV and 75% NPV.
Figure 2.
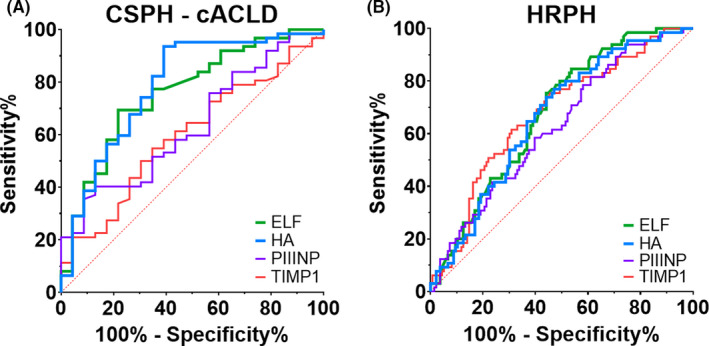
Area under the receiver operating characteristics (AUROC) for detection of clinically significant portal hypertension (CSPH) and high‐risk portal hypertension (HRPH). (A) CSPH in patients with compensated liver disease (cACLD) and (B) HRPH in the overall cohort. Abbreviations: cACLD, compensated advanced chronic liver disease; CSPH, Clinically significant portal hypertension; ELF, Enhanced liver fibrosis score; HA, Hyaluronic acid; HRPH, High‐risk portal hypertension; PIIINP, Amino‐terminal propeptide of type III procollagen; TIMP‐1, Tissue inhibitor matrix metalloproteinase‐1
In patients with cACLD, HA was the only single parameter suited to detect CSPH, with a slightly but non‐significantly better performance than ELF score: AUROC 0.787 (0.67‐0.90; P < .001). Of note, among the single ELF components HA performed reasonable for CSPH prediction in cACLD, however, HA still had limited predictive value for ruling‐in and ruling‐out CSPH (Table S3 and Paragraph S1).
3.6. Detection of high‐risk PHT by ELF
Similar to AUROC analysis for detection of CSPH, we tested whether ELF and its components accurately detected HRPH (HVPG ≥ 20 mm Hg; Table 4). In the overall cohort, all parameters displayed statistically significant, however, weak potential for the non‐invasive diagnosis of HRPH: AUROC 0.677 (0.60‐0.75; P < .001) for ELF, 0.673 (0.59‐0.75; P < .001) for TIMP1, 0.625 (0.55‐0.71; P = .004) for PIIINP, and 0.663 (0.59‐0.74; P < .001) for HA (Figure 2B). Non‐invasive ruling‐in of HRPH by ELF and its single components was suboptimal, however, ruling‐out of HRPH by an ELF score < 10.1 yielded a NPV of 95% (39% PPV, 97% sensitivity and 26% specificity).
3.7. Detection of varices by ELF
Similarly, we performed an exploratory analysis whether ELF was able to detect the presence of any varices or varices needing treatment (VNT). Median time span between gastroscopy and hepatic vein catheterization was 0.9 (IQR 0.0‐2.6) months, and 55 (27%) patients had gastroscopy on the same day as HVPG/ELF measurement. However, ELF had no value for the detection of any varices or VNT as the AUROCs were only 0.580 (P = .065) and 0.552 (P = .228) respectively (Figure S2).
3.8. Diagnostic value of vibration‐controlled transient elastography
Reliable VCTE results were obtained in 139 (69%) patients, with a median stiffness of 28.1 (17.6‐53.2) kPa. Stratified by PHT severity, median stiffness was 16.3 (11.7‐20.4) kPa in patients with HVPG 6‐9 mm Hg (n = 21, 88%), 27.7 (17.0‐43.0) kPa in patients with HVPG 10‐19 mm Hg (n = 81, 72%) and 62.2 (34.8‐75.0) kPa in patients with HVPG ≥ 20 mm Hg (n = 37, 57%; P < .001 for stiffness comparison between HVPG strata), respectively (Table 1). Correlation between VCTE and ELF was Rho = 0.591 (95%CI 0.47‐0.69, P < .001) and Rho = 0.668 (95%CI 0.56‐0.75, P < .001) between VCTE and HVPG in the overall cohort (Figure S3).
In the overall study cohort, VCTE AUROC was 0.834 (95%CI 0.76‐0.91, P < .001; Figure S3) for prediction of CSPH, which was nearly identical to ELF (AUROC 0.833). In 66 cACLD patients, VCTE had an AUROC of 0.743 (95% CI 0.62‐0.86, P = .002), also being comparable to ELF (AUROC 0.759) for diagnosis of CSPH. Ruling‐out CSPH by VCTE at <11.8 kPa achieved 94% sensitivity (29% specificity, PPV 88%, NPV 50%) in the overall cohort, and 89% sensitivity but only 55% NPV (30% specificity, 75% PPV) in cACLD patients. CSPH could be ruled‐in at >23.2 kPa with 95% specificity (59% sensitivity, 99% PPV, 36% NPV) in the overall cohort, and 95% specificity and 95% PPV (70% sensitivity, 50% NPV) in cACLD.
4. DISCUSSION
This prospective study of 201 patients undergoing HVPG and simultaneous ELF score measurement is by now the largest analysis investigating whether the ELF score can adequately predict CSPH or HRPH in patients with ACLD, while also specifically investigating the diagnostic accuracy of ELF within compensated cirrhosis (cACLD).
ELF score comprises three serum parameters—TIMP1, PIIINP and HA—that reflect fibrosis and matrix remodelling. 5 While the presence of advanced liver fibrosis is closely linked to the risk of PHT, additional dynamic components determine the severity of PHT and, thus, measurement of HVPG is the current diagnostic gold‐standard to detect CSPH.
The identification of non‐invasive predictors of CSPH represents an unmet clinical need for patients with cACLD 18 , 20 in order to facilitate therapeutic management. However, in patients with cACLD, several serum biomarkers that relate to liver fibrosis and PHT have not been evaluated in the setting of strictly cACLD or have only shown weak‐to‐moderate associations with HVPG, which limits their diagnostic value. 25 , 26
Since studies have confirmed that ELF score accurately predicts liver fibrosis, ELF depicts an alternative to elastography‐based assessment of liver fibrosis, as recently demonstrated for NASH. 8 However, along with restricted availability in non‐tertiary centres, elastography is sometimes limited by obesity and presence of ascites. Not surprisingly, it was also found that ELF single components relate to PHT. 27 , 28 , 29 Interestingly, Thabut et al demonstrated that correlation of Fibrotest (which comprises five biomarkers related to fibrosis) with HVPG was markedly better (Pearson's r = .58) when including patients without PHT, while correlation was weak in patients with HVPG ≥ 6 mm Hg (Pearson's r = .23). 30 However, the clinical value of this correlation of Fibrotest with HVPG in a population without PHT is limited. In contrast, our study assessed ELF only in patients with an HVPG ≥ 6mm Hg and in this setting, the ELF score showed a considerably stronger correlation with HVPG, as previously observed for Fibrotest. Still, we also observed a decrease in the correlation strength between ELF and its single components with increasing HVPG strata. Vizzutti et al reported similar findings for transient elastography, ie a weaker correlation between liver stiffness and HVPG in more pronounced PHT. 31 Another study that primarily investigated whether quantitative magnetic resonance imaging could predict CSPH that also included patients without PHT, indicated an accurate correlation between ELF and HVPG (Pearson's r = .758), but no significant correlation was found in patients with CSPH. 32
Importantly, ELF, Fibrotest and elastography primarily reflect parameters related to hepatic fibrosis, while the development of hyperdynamic circulation that further aggravates PHT in patients with ACLD 33 —and especially in decompensated cirrhosis (dACLD)—where the severity of PHT is not sufficiently captured by using fibrosis markers. This pathophysiological explanation was underlined by the lack of correlation between HVPG and ELF in our patients with HRPH.
Considering the diagnostic performance for CSPH screening of other non‐invasive fibrosis scores, the Fibrotest had an AUROC of 0.79 for detecting severe PHT (HVPG ≥12 mm Hg) in patients with cirrhosis, 30 however, again as many as 14% of the included patients did not have PHT. Accordingly, mean/median HVPG was considerably lower 30 than in our study.
A previous study (84% CSPH; 58% cACLD) showed that ELF score is able to predict CSPH, however, yielded a low AUROC of 0.68. 15 Conversely, Sandahl et al showed that combining ELF with soluble CD163 achieved an AUROC of 0.91 to predict CSPH in a training cohort and an AUROC of 0.90 in a validation cohort. 16 ELF alone (AUROC 0.88; 90% CSPH) performed slightly better in the study of Sandahl et al as compared to our study (ELF AUROC 0.833; 88% CSPH). Of note, we excluded patients who received concomitant treatment with NSBB at the time of HVPG measurement, while concomitant NSBB therapy was not an exclusion criterium in the study by Sandahl et al This selection criterium might be relevant since we previously found that liver stiffness (also a marker of “static fibrosis”) and HVPG showed better correlation under NSBB therapy—which is explained by their inhibitory effect on the hyperdynamic circulation as the “dynamic” component or PHT. 34
Importantly, ELF was able to rule‐in CSPH at a cut‐off >11.1 with a PPV of 98%. However, this ELF CSPH cut‐off requires validation in independent cohorts, since the related diagnostic indices may have been affected by the high prevalence of CSPH in our study population. Unfortunately, ELF cut‐offs for ruling‐out CSPH failed to provide clinically meaningful diagnostic value.
Of note, ELF showed an even better diagnostic accuracy for diagnosing CSPH in patients with ALD (AUROC 0.978), as compared to patients with viral hepatitis (AUROC 0.629), while the correlation between ELF and HVPG was considerably weaker in patients with ALD. This counterintuitive observation is explained by more pronounced severity of PHT in ALD patients, who simply had a very high pretest probability of CSPH as compared to viral liver disease. The high AUROC among ALD patients may, thus, be a consequence of the disproportionally high proportion of easy‐to‐classify patients with advanced disease in this subgroup. At the same time, there is a “loss‐of‐correlation” between ELF and HVPG in patients with CSPH (ie the higher HVPG strata), which explains the weaker correlation between ELF and HVPG in ALD patients, in whom CSPH was highly common. Accordingly, the observed differences in AUROC values are primarily a consequence of patient characteristics and should not be interpreted as evidence for aetiology‐dependent differences in the diagnostic performance of the test. Thus, differences in discriminative ability of ELF to detect CSPH in patients with ALD or viral liver disease cannot be fully answered by this study, mostly because of the limited number of ALD patients with only subclinical PHT.
While the ELF was of limited accuracy for ruling‐in HRPH, an ELF cut‐off < 10.1 could, however, rule‐out HRPH with a high NPV of 95%. This finding may be useful for clinical decision‐making regarding TIPS implantation. For example, early or pre‐emptive TIPS implantation has shown favourable impact on patients with acute variceal bleeding (AVB), 35 and is currently recommended in patients with AVB if the HVPG is ≥20 mm Hg. 19 There also might be clinical applicability of ELF in patients with ascites evaluated for TIPS in order to assess if the refractoriness of ascites is mostly caused by HRPH. However, acute clinical events such as AVB or infections may impact on ELF score via dysregulation of ELF parameters. In our study, patients with acute decompensation and non‐elective admission at the time of HVPG measurement were excluded. Therefore, we think that ELF score yields best results for the diagnosis of HRPH (and also for CSPH) if the test is performed under stable conditions without acute decompensation or acute‐on‐chronic liver failure (ACLF).
Furthermore, liver stiffness measurement by VCTE had similar diagnostic value for prediction of CSPH as compared to ELF both in the overall cohort (AUROC 0.834 and 0.833 respectively) and patients with cACLD (AUROC 0.743 and 0.759 respectively). Previous elastography studies commonly reported better performance of VCTE for cACLD, however, this may be attributed to lower prevalence of CSPH. 36 , 37 , 38
Importantly, HA performed best in most analyses among all single ELF components, especially in patients with cACLD, which might be explained by its physical‐mechanical molecular properties. HA—as an essential component of liver ECM—might correlate more directly with the mechanical component of increased intrahepatic resistance than PIIINP and TIMP1. In contrast, PIIINP in serum was recently discussed to either reflect synthesis or degradation of type III collagen, 39 while TIMP1 poses as a regulatory protein for proteinases that are involved in degradation of extracellular matrix. 27
Shortcomings of this study include the overrepresentation of patients with CSPH which may have impacted the cut‐off for differentiating between subclinical PHT and CSPH. Furthermore, there was a considerable rate of patients with prior decompensation in our study population, in whom the presence of CSPH is highly probable and the clinical relevance of non‐invasive tests for CSPH is less meaningful. However, the latter limitation was addressed by an additional analysis in the specific cohort of cACLD patients providing information regarding the diagnostic value of ELF for CSPH in the setting of compensated disease.
In summary, ELF significantly correlates with HVPG and may be used to rule‐in CSPH (cut‐off > 11.1) and rule‐out HRPH (cut‐off < 10.1). However, there remains a considerable diagnostic “grey area” where ELF cannot provide a clinically meaningful categorization of PHT severity and HVPG measurements are still necessary to diagnose CSPH.
ETHICS APPROVAL
This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments and approved by the local ethics committee of the Medical University of Vienna (EK1262/2017). All patients gave written informed consent to liver vein catheterizations and provided written consent to be enrolled in the VICIS study (NCT03267615).
CONFLICT OF INTEREST
BeSi received travel support from AbbVie and Gilead. RM received speaker honoraria from Abbott, DiaSorin and Siemens. BeSc received travel support from Abbvie and Gilead. PS received speaking honoraria from Bristol‐Myers Squibb and Boehringer‐Ingelheim, consulting fees from PharmaIN, and travel support from Falk and Phenex Pharmaceuticals. TB received travel support from AbbVie, Bristol‐Myers Squibb, and Medis, as well as speaker fees from Bristol‐Myers Squibb. DB received travel support from Abbvie and Gilead. MP is an investigator for Bayer, BMS, Lilly and Roche; he received speaker honoraria from Bayer, BMS, Eisai and MSD; he is a consultant for Bayer, BMS, Ipsen, Eisai, Lilly, MSD and Roche; he received travel support from Bayer and BMS. MT received speaker fees from BMS, Falk Foundation, Gilead, Intercept and MSD; advisory board fees from Albireo, BiomX, Boehringer Ingelheim, Falk Pharma GmbH, Genfit, Gilead, Intercept, MSD, Novartis, Phenex and Regulus. He further received travel grants from Abbvie, Falk and Gilead and Intercept and unrestricted research grants from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda. MM has served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, WL Gore & Associates and Janssen. TR received grant support from Abbvie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare and Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept and Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim, Gilead, MSD and Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche. AS, EE, RP and AFS declare no conflict of interest.
Supporting information
Simbrunner B, Marculescu R, Scheiner B, et al. Non‐invasive detection of portal hypertension by enhanced liver fibrosis score in patients with different aetiologies of advanced chronic liver disease. Liver Int. 2020;40:1713–1724. 10.1111/liv.14498
REFERENCES
- 1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749‐1761. [DOI] [PubMed] [Google Scholar]
- 2. Ripoll C, Groszmann R, Garcia–Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481‐488. [DOI] [PubMed] [Google Scholar]
- 3. Ripoll C, Groszmann RJ, Garcia‐Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50(5):923‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47(2):455‐460. [DOI] [PubMed] [Google Scholar]
- 5. Parkes J, Guha IN, Roderick P, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2011;18(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg WMC, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127(6):1704‐1713. [DOI] [PubMed] [Google Scholar]
- 7. Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut‐off values. J Hepatol. 2013;59(2):236‐242. [DOI] [PubMed] [Google Scholar]
- 8. Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: Baseline data from the STELLAR trials. Hepatology. 2019;70(5):1521‐1530. [DOI] [PubMed] [Google Scholar]
- 9. Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the enhanced liver fibrosis test vs fibrotest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018;154(5):1369‐1379. [DOI] [PubMed] [Google Scholar]
- 10. Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non‐alcoholic fatty liver disease. Gastroenterology. 2009;136(1):160‐167. [DOI] [PubMed] [Google Scholar]
- 11. Mayo MJ, Parkes J, Adams‐Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48(5):1549‐1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vesterhus M, Hov JR, Holm A, et al. Enhanced liver fibrosis score predicts transplant‐free survival in primary sclerosing cholangitis. Hepatology. 2015;62(1):188‐197. [DOI] [PubMed] [Google Scholar]
- 13. Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59(9):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 14. Irvine KM, Wockner LF, Shanker M, et al. The enhanced liver fibrosis score is associated with clinical outcomes and disease progression in patients with chronic liver disease. Liver Int. 2016;36(3):370‐377. [DOI] [PubMed] [Google Scholar]
- 15. Hametner S, Ferlitsch A, Ferlitsch M, et al. The VITRO Score (Von Willebrand Factor Antigen/Thrombocyte Ratio) as a new marker for clinically significant portal hypertension in comparison to other non‐invasive parameters of fibrosis including ELF test. PLoS ONE. 2016;11(2):e0149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandahl TD, McGrail R, Møller HJ, et al. The macrophage activation marker sCD163 combined with markers of the Enhanced Liver Fibrosis (ELF) score predicts clinically significant portal hypertension in patients with cirrhosis. Aliment Pharmacol Ther. 2016;43(11):1222‐1231. [DOI] [PubMed] [Google Scholar]
- 17. Mauro E, Crespo G, Montironi C, et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67(5):1683‐1694. [DOI] [PubMed] [Google Scholar]
- 18. Mandorfer M, Montagnani M, Lisotti A, et al. Non‐invasive diagnostics for portal hypertension: a comprehensive review. Semin Liver Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 19. Reiberger T, Püspök A, Schoder M, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129(Suppl 3):135‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743‐752. [DOI] [PubMed] [Google Scholar]
- 21. Angeli P, Bernardi M, Villanueva C, et al. Practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406‐460. [DOI] [PubMed] [Google Scholar]
- 22. Schwabl P, Bota S, Salzl P, et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35(2):381‐390. [DOI] [PubMed] [Google Scholar]
- 23. Reiberger T, Schwabl P, Trauner M, Peck‐Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and transjugular liver biopsy. J Visualized Exp. 2020: e58819. In‐press. [DOI] [PubMed] [Google Scholar]
- 24. Ferlitsch A, Bota S, Paternostro R, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int. 2015;35(9):2115‐2120. [DOI] [PubMed] [Google Scholar]
- 25. Martinez SM, Fernández‐Varo G, González P, et al. Assessment of liver fibrosis before and after antiviral therapy by different serum marker panels in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2011;33(1):138‐148. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen‐khac E, Chatelain D, Tramier B, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non‐invasive laboratory tests. Aliment Pharmacol Ther. 2008;28(10):1188‐1198. [DOI] [PubMed] [Google Scholar]
- 27. Busk TM, Bendtsen F, Nielsen HJ, Jensen V, Brünner N, Møller S. TIMP‐1 in patients with cirrhosis: relation to liver dysfunction, portal hypertension, and hemodynamic changes. Scand J Gastroenterol. 2014;49(9):1103‐1110. [DOI] [PubMed] [Google Scholar]
- 28. Kropf J, Gressner AM, Tittor W. Logistic‐regression model for assessing portal hypertension by measuring hyaluronic acid (hyaluronan) and laminin in serum. Clin Chem. 1991;37(1):30‐35. [PubMed] [Google Scholar]
- 29. Gressner AM, Tittor W, Negwer A, Pick‐Kober KH. Serum concentrations of laminin and aminoterminal propeptide of type III procollagen in relation to the portal venous pressure of fibrotic liver diseases. Clin Chim Acta. 1986;161(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 30. Thabut D, Imbert‐bismut F, Cazals‐hatem D, et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26(3):359‐368. [DOI] [PubMed] [Google Scholar]
- 31. Vizzutti F, Arena U, Romanelli RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV‐related cirrhosis. Hepatology. 2007;45(5):1290‐1297. [DOI] [PubMed] [Google Scholar]
- 32. Palaniyappan N, Cox E, Bradley C, et al. Non‐invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol. 2016;65(6):1131‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villanueva C, Albillos A, Genescà J, et al. Development of hyperdynamic circulation and response to beta‐blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63(1):197‐206. [DOI] [PubMed] [Google Scholar]
- 34. Reiberger T, Ferlitsch A, Payer BA, et al. Non‐selective beta‐blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47(5):561‐568. [DOI] [PubMed] [Google Scholar]
- 35. Bucsics T, Schoder M, Goeschl N, et al. Re‐bleeding rates and survival after early transjugular intrahepatic portosystemic shunt (TIPS) in clinical practice. Dig Liver Dis. 2017;49(12):1360‐1367. [DOI] [PubMed] [Google Scholar]
- 36. Abraldes JG, Bureau C, Stefanescu H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study. Hepatology. 2016;64(6):2173‐2184. [DOI] [PubMed] [Google Scholar]
- 37. Bureau C, Metivier S, Peron JM, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27(12):1261‐1268. [DOI] [PubMed] [Google Scholar]
- 38. Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102‐111.e1. [DOI] [PubMed] [Google Scholar]
- 39. Nielsen MJ, Nedergaard AF, Sun S, et al. The neo‐epitope specific PRO‐C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5(3):303‐315. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


