Abstract
Cell morphology and tissue integrity are essential for embryogenesis. Caveolins are membrane proteins that induce the formation of surface pits called caveolae that serve as membrane reservoirs for cell and tissue protection during development. In vertebrates, caveolin 1 (Cav1) and caveolin 3 (Cav3) are required for caveola formation. However, the formation of caveola and the function of caveolins in invertebrates are largely unknown. In this study, three caveolins, Cav‐a, Cav‐b, and CavY, are identified in the genome of the invertebrate chordate Ciona spp. Based on phylogenetic analysis, Cav‐a is found to be closely related to the vertebrate Cav1 and Cav3. In situ hybridization shows that Cav‐a is expressed in Ciona embryonic notochord and muscle. Cell‐free experiments, model cell culture systems, and in vivo experiments demonstrate that Ciona Cav‐a has the ability to induce membrane curvature at the plasma membrane. Knockdown of Cav‐a in Ciona embryos causes loss of invaginations in the plasma membrane and results in the failure of notochord elongation and lumenogenesis. Expression of a dominant‐negative Cav‐a point mutation causes cells to change shape and become displaced from the muscle and notochord to disrupt tissue integrity. Furthermore, we demonstrate that Cav‐a vesicles show polarized trafficking and localize at the luminal membrane during notochord lumenogenesis. Taken together, these results show that the invertebrate chordate caveolin from Ciona plays crucial roles in tissue integrity and morphology by inducing membrane curvature and intracellular vesicle trafficking during embryogenesis.
Keywords: caveolin, Ciona spp., lumen formation, membrane curvature, morphogenesis
Abbreviations
- ANISEED
ascidian network for in situ expression and embryological data
- APEX
soybean ascorbate peroxidase
- BFA
brefeldin A
- BHK
baby hamster kidney
- Cav
caveolin
- ECM
extracellular matrix
- EF1α
elongation factor 1 alpha
- EGFP
enhanced green fluorescent protein
- FBS
fetal bovine serum
- FRAP
fluorescence recovery after photobleaching
- GBP
GFP binding peptides
- hpf
hours post fertilization
- KO
knockout
- L
lumen
- L
lysine
- LTE
Leishmania tarentolae extract
- MBP
maltose‐binding protein
- MEF
mouse embryonic fibroblast
- MO
morpholino
- MUSCLE
multiple sequence comparison by Log‐Expectation
- mu
muscle
- no
notochord
- P
proline
- RAxML
randomized axelerated maximum likelihood
- spp
species
- TEM
transmission electron microscopy
- WGA
wheat germ agglutination
1. INTRODUCTION
Membrane curvature is an important characteristic of the cell. Curved membrane domains are present at the plasma membrane as well as in internal organelles,1 playing essential roles in maintaining normal cell morphology.2 Caveolae represent one type of curved membrane domain at the plasma membrane.3 Caveolae are small invaginations of the plasma membrane that appear as bulb‐like structures by electron microscopy.4 The flattening of caveolae provides a reservoir of membrane to protect cells during mechanical stretching or hypo‐osmotic shock as cells change the shape.5 Thus, they act as buffers against force to protect cells from membrane damage and loss of cell‐cell contact.6 Endothelial, muscle, and fat cells have a high abundance of caveolae and these cells are subjected to comparatively high mechanical forces.7 Large numbers of caveolae are also found in the notochord of zebrafish embryos where they act as mechanoprotective agents during embryonic development.8, 9
At the plasma membrane, the distinctive bulb‐like structure of caveolae is generated by two layers of proteins: the inner one is made up of integral membrane proteins termed caveolins, forming a putative polygonal lattice, while the outer layer comprises a filamentous coat of cavins.10 Both caveolins and cavins are required for caveola formation through the induction of membrane curvature.11 It is worth noting that cavins are absent in the invertebrate genome.12 Three paralogues of caveolins, caveolin1 (Cav1), caveolin2 (Cav2), and caveolin3 (Cav3), are present in the mammalian genome, with distinct expression patterns in various tissue types.13 Cav1 and Cav2 are coexpressed in many nonmuscle cells whereas Cav3 is muscle‐specific and has a role in muscle development in vertebrates.14, 15 Only Cav1 and Cav3 can drive caveola formation,16, 17, 18 suggesting that Cav1 has a major function in nonmuscle cells. Moreover, the caveolins are involved in several other cellular processes such as membrane trafficking via endocytosis and transcytosis, cell migration, extracellular matrix remodeling, tissue regeneration, lipid metabolism, and homeostasis.19 Surprisingly, caveolin‐knockout mice are viable and fertile, showing that caveolins are not essential for survival.7 However, caveolin‐KO mice show several pathological symptoms.20, 21, 22, 23, 24 Mutations of caveolin genes have been linked to a number of diseases in humans.7
Evolutionary analysis of caveolins reveals that they are related to each other through common ancestry, indicating that they have conserved functions.16 The expression of vertebrate caveolins in bacteria has been shown to form caveolae‐like structures.17 Surprisingly, the expression of Caenorhabditis elegans caveolins could not induce the formation of caveola‐like structures in mammalian cells,16 in a model bacterial system,17 or in a cell‐free system.18 Until now, no visible caveolae structures have been observed in invertebrate tissues,12 with the exception of the description of a caveola‐like structure in C. elegans.25 In contrast, the expression of caveolins from Apis mellifera in Cav1‐knockout mammalian cells caused the formation of caveolae.16 The current data suggest that the functional differentiation of caveolins might exist between the vertebrates and invertebrate lineages.
Here, we showed that without cavin, ascidian caveolin induced membrane curvature to form plasma membrane invaginations, which were morphologically different from vertebrate caveolae. Knockdown of caveolin in Ciona embryos caused the loss of a distinct class of plasma membrane invaginations, identifying these structures as caveolin‐dependent invertebrate caveolae. Loss of caveolin caused defects in muscle and notochord elongation, and thus affected tail morphogenesis. Furthermore, we examined the effects of a Cav‐a dominant‐negative mutant, expressed under its own promoter in the muscle and notochord. Similarly, a short tail phenotype was produced as a consequence of compromised function of Cav‐a in the muscle and notochord. We also found that caveolin vesicles were involved in lumen opening in the process of notochord lumenogenesis. Thus, our results demonstrate that Cav‐a plays crucial roles in tail morphogenesis and notochord lumen formation in Ciona embryos.
2. MATERIALS AND METHODS
2.1. Experimental animals
We used Ciona spp. in our experiments. C. savignyi and C. intestinalis were collected from Qingdao and Rongcheng harbour bay (Shandong Province, China) and was maintained in a seawater tank with aeration and constant illumination in the laboratory. The dissection of adult animals for the extraction of gametes was carried out with the help of scissors, while the developmental stages were considered as Hotta's classification.26 After fertilization and dechorionation the embryos were cultured at 16°C.
2.2. Molecular cloning and qRT‐PCR
The caveolin sequences were searched in the Ghost Database for C. savignyi. The putative ORF of caveolin‐a (Cav‐a), caveolin‐b (Cav‐b), and caveolin Y (CavY) were identified and amplified by PCR using cDNA from the larval stage for Cav‐a and Cav‐b, and cDNA from the adult stage for CavY (primers in Table S1). Then, they were subcloned and sequenced to confirm the identity. The total RNA was extracted from the larvae and adults using the RNAiso plus reagent according to the manufacturer's instructions (Takara Bio Inc). The integrity and quality of total RNA was determined by agarose gel electrophoresis and Nanodrop spectrophotometry (Thermo Fisher). First‐strand cDNA was synthesized using 0.5 µg total RNA by reverse transcriptase (Takara Bio Inc). At least one primer for Cav‐a, Cav‐b, and CavY (Table S1) was placed between exons to avoid genomic DNA amplification. The qRT‐PCR was performed using the SYBER Green PCR Master Mix (Roche) on Light Cycler 480 (Roche). The expression level of Cav‐a, Cav‐b, and CavY was normalized using EF1α as a reference.
2.3. Plasmid constructions
The 2, 1.5, and 1 kb DNA sequence upstream of the Cav‐a gene from C. savignyi was amplified by PCR and subcloned into Kpn1 and BamH1 restriction sites of the EGFP1 vector (Clontech) for promoter analysis. Cav‐a was amplified by PCR and subcloned into the BamH1 site of the Cav‐a2kb>EGFP plasmid. Inverse PCR was performed to remove five base pairs and ligated by infusion reaction to create a Cav‐a>Cav‐a::EGFP expression plasmid. To create notochord specific expression vector, 2 kb promoter from the upstream region of the brachyury gene was amplified by the PCR method, and then subcloned into Kpn1 and BamH1 sites to construct an expression vector Bra>mCherry and Bra>EGFP. The full length of Cav‐a was amplified by the PCR method. Cav‐a was subcloned into BamH1 site of Bra>mCherry plasmid. Inverse PCR was performed to remove five base pairs and ligated by infusion reaction to create the Bra>Cav‐a::mCherry. The full length of Cav‐a was subcloned into the pEGFP‐1 vector to generate the cmv>Cav‐a::EGFP expression plasmid.
To generate caveolin (Homo sapiens or C. intestinalis) expression vectors yielding N‐terminal fusion of GFP, the coding sequences (Cav1 H. sapiens, Q03135; Cav‐a C. intestinalis; ENSCINT00000002782) were synthesized from Integrated DNA Technologies (IA, USA) with flanking recombination sequences (att L1 and att L2), and cloned into the pDONOR221 vector by BP recombination (Invitrogen, Australia), followed by LR recombination (Invitrogen, Australia) to clone the encoding ORF into the destination vector: pCellFree‐N‐terminal‐GFP. All PCR primers are listed in Table S1.
2.4. Mutagenesis
The N‐terminal and C‐terminal deletion mutants of Cav‐a from C. savignyi were PCR amplified and subcloned into the BamH1 site of the Cav‐a‐2kb>EGFP plasmid. Five base pairs were removed by inverse PCR and then ligated by infusion reaction to create the Cav‐a>Cav‐aΔ1‐117::EGFP and Cav‐a>Cav‐aΔ160‐236::EGFP expression plasmids. For point mutant plasmids, the proline at position 189 of Cav‐a was changed to leucine by site‐directed mutagenesis. Two expression plasmids, Cav‐a>Cav‐a::EGFP and Bra>Cav‐a::mCherry were subjected to site‐directed mutagenesis using standard protocol to create Cav‐a>Cav‐aP189L::EGFP and Bra>Cav‐aP189L::mCherry expression plasmids. The PCR primers are listed in Table S1.
2.5. In situ hybridization and immunohistochemistry
Whole‐mount in situ hybridization was performed as described previously27 using a Roche kit. Embryos were collected at the desired stage and fixed in 4% paraformaldehyde in seawater for 2 hours at room temperature. They were then dehydrated in ethanol series and stored in methanol at −20°C until use. After in situ hybridization, the embryos were mounted in 50% glycerol and observed under a light microscopy. Full length Cav‐a from C. savignyi was used to produce antibody. Embryos were fixed by 4% paraformaldehyde in seawater for 2 hours at room temperature. After fixation and blocking, 1/1000 dilution of Cav‐a antibody was used as a primary antibody overnight at room temperature. Similarly, 1/1000 dilution of Alexa 566‐conjugated anti‐mouse IgG goat antibody was used as the secondary antibody. Then, the embryos were counterstained with wheat germ agglutinin (WGA, Molecular Probe) in 1/500 dilution. After washing, the embryos were mounted in VECTASHIELD with DAPI (Vector Laboratories) before microscopic observation.
2.6. Phylogenetic analysis
Similar caveolin sequences from different animal taxa were searched using BLAST28 in the NCBI, ENSEMBL, and ANISEED databases (Table S2). After collecting the full‐length caveolin amino acid sequences, we generated multiple sequence alignment with Clustal Omega. For phylogenetic analysis, these sequences were first subjected to multiple sequence alignment using MUSCLE29 and then curated by G block to remove any gaps in the alignments.30 The G block‐curated sequences were then used to build a phylogenetic tree using the maximum likelihood method by RAxML.31 In addition, the LG substitution model was opted as a model predicted by ProtTest332 for the sequences in the phylogenetic analysis with 100 bootstraps estimation.
2.7. Electroporation
Electroporation was followed according to the method described previously33 with some modifications. After fertilization, the dechorionated eggs (200 µL) were mixed with 60‐80 µg of plasmids in 100 µL final volume adjusted with MilliQ water and was electroporated with 400 µL of 0.96 M d‐Mannitol using a Gene Pulser Xcell System (BIO‐RAD) in 0.4 cm cuvettes. The exponential protocol was used with 50 V and 1000 µF as a parameter. Once electroporated, the fertilized eggs were washed, and cultured at 16°C.
2.8. Morpholino microinjection
The Cav‐a morpholino (Cav‐a‐MO–5′TTCTGTTCGTTGTGTTCACTCATTG‐3′) and control morpholino (Control MO–5′‐TTGTCTTCCTTGTCTTGACTCATTG‐3′) were purchased from Gene Tools. The stock solution of 1 µM was prepared by dissolving morpholino in sterile water. The dechorionated eggs were microinjected at 0.5 µM morpholino concentration as described previously.34 The dechorionated eggs were fertilized after microinjection and cultured at 16°C. The efficiency of knockdown was verified by Cav‐a antibody staining.
2.9. Rescue experiments
Dechorionated Ciona eggs were injected with Cav‐a morpholino and codon‐modified Cav‐a mRNA. Codon‐modified Cav‐a mRNA was synthesized using the primers listed in Table S1. Final concentrations of 0.5 µM Cav‐a MO and 135 ng/µL of codon‐modified Cav‐a mRNA were used. Dechorionated eggs were fertilized after microinjection and cultured at 16°C.
2.10. Transmission electron microscopy (TEM)
Ciona embryos were collected and fixed in 2.5% glutaraldehyde in PBS and 0.05% ruthenium red (Sigma) for 90 minutes on ice. The embryos were then washed with PBS three times and postfixed in 1.5% OsO4 in PBS for 150 minutes on ice. Then, the embryos were washed with PBS further three times. Dehydration of the embryos was carried out in an ethanol series, before embedding in Epon. Ultrathin sections were viewed without further staining or were stained with uranyl acetate and lead citrate. Sections were viewed in a JEOL JEM‐1200 or JEOL 1011 transmission electron microscope.
For Apex experiments in cultured mammalian cells, BHK cells and caveolin‐1 knockout mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% FBS, 1% Pen/Strep at 37°C, and 5% CO2. Cells were seeded in 6‐well plates and transient transfections were carried out with Lipofectamine 3000 (Invitrogen). The constructs cmv>Cav‐a::EGFP and GFP‐nanobody‐APEX2 were cotransfected in BHK cells and CAV1−/− MEFs, and then cultured for 24 hours. The BHK cells and MEFs were collected and then processed for EM following established protocols.35 Sections were then viewed as described above.
2.11. Cell‐free expression experiments
GFP‐tagged synthetic caveolins from C. intestinalis were translated in a cell‐free system that utilizes a Leishmania tarentolae extract (LTE) and then purified by gradient floatation fraction assay as previously described in detail.18 For Immuno‐gold labeling, the Glow‐discharged carbon coated grid was placed on a sample droplet for 20 minutes, followed by blocking with 1% casein in PBS 3 times for 5 minutes. After that, the grid was incubated with sequential antibody droplets as follows, (a) GFP nanobody‐MBP (60 μg/mL) for 30 minutes, (b) anti‐MBP antibody rabbit IgG (at 1:100 dilution) (New England Biolabs, UK) for 30 minutes, and (c) 10 nm colloidal gold‐conjugated protein A (at 1:50 dilution) (Cell Microscopy Center, Department of Cell Biology University Medical Center Utrecht The Netherlands). Each time, excess antibodies were washed‐off through blocking buffer incubation (3 times for 5 minutes). For negative staining, the grid was washed with PBS (5 times for 5 minutes) and fixed with 4% PFA in PBS for 10 minutes, followed by water wash (5 times for 5 minutes) and staining with methylcellulose and uranyl acetate (9:1) for 10 minutes. For electron tomography, Dual‐axis tilt electron tomography was performed in a TECNAI 12 (Philips) microscopy at an acceleration voltage of 120 kV (−60° to +60° at 2° incremental sampling). This instrument is equipped with a 4 × 4 k LC‐1100 Direct Electron under the control of Serial EM (Boulder, USA) at 2‐fold binning. The densities of membranes were reconstructed from tilt series using weighted back‐projection in IMOD (Boulder, USA).
2.12. Light microscopy, time‐lapse movies, and FRAP assays
Imaging for the bright‐field, differential interference contrast, and fluorescence was carried out using a Nikon ECLIPSE Ni. Confocal images were taken by Nikon A1 confocal microscope with identical settings and the same exposure time using either 20x or 40x objectives (1.2 NA). The FRAP experiments were carried out on the apical membrane of notochord cells in stage 23 embryos. The Cav‐a‐tGFP signal was photobleached by illumination with a 405‐nm diode laser (50 mW) and the images were captured by continuous recording. All images were handled with Adobe Photoshop, Adobe Illustrator, and Image J.
2.13. Quantification and statistical analysis
All measurements in the image data were carried out by NIS elements version 4.50 and ImageJ software. The graphs were generated in either Microsoft Excel 14.0.4 or GraphPad Prism7. For statistical analysis, we used two‐tailed unpaired t tests in Microsoft Excel 14.0.4. The statistically significant graphs are presented as mean ± standard deviation with p values indicated by asterisks (*P < .05, **P < .01, ***P < .001, and ****P < .0001). All figures were prepared using Adobe Photoshop and Adobe illustrator, and all movies were handled with Image J.
3. RESULTS
3.1. Evolutionarily conserved caveolins in ascidian C. savignyi
We used vertebrate caveolin and cavin sequences to blast against the Ciona genome. No significant hit results for cavins were obtained in the default settings. In contrast, caveolins are readily detected using the same search conditions. Consistent with a previous result,12 we conclude that Ciona, like other invertebrates, lacks cavins. The three identified Ciona caveolins paralogues are named as Cav‐a, Cav‐b, and CavY. The Cav‐a and Cav‐b genes are located on the same chromosome, while the CavY gene is located on a different chromosome, as revealed by mapping of Cav‐a, Cav‐b, and CavY sequences to the genome. Examination of the gene structure showed that Cav‐a has two introns and three exons, but Cav‐b has only one intron and two exons. Similarly, CavY has two introns and three exons (Figure S1A). We also performed multiple sequence alignments of the caveolin proteins and found that all Ciona caveolins have three characteristic domains; oligomerization, scaffolding, and intramembrane domain. The mammalian “caveolin signature motif,” FEDVIAEP,36 was also conserved in Ciona caveolins (asterisks in Figure S1B). The full open reading frames of three caveolins after amplification showed that the Cav‐a sequence was longer than that of Cav‐b and CavY (Figure S1C).
To understand the relationship and origin of ascidian caveolins, evolutionary analysis was performed by building a phylogenetic tree across metazoans (Figure 1A). Lower vertebrates have four caveolins, Cav1, Cav2, Cav3, and CavY, while in mammals only Cav1, Cav2, and Cav3 are present in their genomes.16 Our phylogenetic analysis illustrated that the vertebrate Cav1, Cav2, and Cav3 clustered together, which confirmed that they were closely related and these sequences probably duplicated in the vertebrate genomes after separation from the tunicates. Our results also showed that Ciona Cav‐a (green arrowheads) from the tunicate group (box in Figure 1A) clustered with the vertebrate Cav1 (red arrow) and Cav3 groups, suggesting that Cav‐a from the tunicates might be functionally conserved with the vertebrates’ Cav1. The origin of Cav‐b and CavY is less clear. They seemed to have originated early, during the evolution of the caveolin gene as indicated by their long branches in the phylogenetic tree (Figure 1A). We have compared the sequence of Cav‐a from Ciona with vertebrate Cav1. The identity and similarity values were 46% and 72%, respectively (Figure S2). In addition, the mRNA expression levels of Cav‐a and Cav‐b were comparatively high during the embryonic stage, but not in the case of CavY, which showed high expression levels in the adult stage only (Figure 1B). In situ hybridization revealed that Cav‐a was initially expressed only in the muscle, but later in both muscle and notochord (Figure 1C). No positive signal was detected by the Cav‐a sense probe (Figure S3).
Figure 1.

Evolutionarily analysis and mRNA expression of caveolin genes. A, Phylogenetic analysis of caveolins. The maximum likelihood analysis for the phylogenetic tree was carried out using the LG model for amino acid substitution. The gaps and missing data were eliminated during tree constructions. The box area denotes the cluster of Cav‐a, Cav‐b, and CavY. The green arrowheads indicated Ciona Cav‐a, while the red arrow indicated human Cav1. The scale bar represents the substitution rate. B, Expression of caveolin mRNA in C. savignyi. The Cav‐a and Cav‐b are highly expressed during the embryonic stage. The expression level of CavY is low at the embryonic stage, but becomes high in adults. C, The Cav‐a mRNA signal is detected only in the muscle (red arrowhead) at the early tailbud stage, but later it is detected both in the muscle (red arrowhead) and notochord (green arrows) at mid‐tailbud stage and larval stage. Scale bar represents 100 µm
3.2. Cav‐a is localized in muscle and notochord in ascidian embryos and larvae
To explore the in vivo function of Ciona Cav‐a, we first raised a monoclonal antibody and performed immunohistochemistry to detect the localization of endogenous Cav‐a during embryogenesis. Initially, at 11 hours post‐fertilization (hpf), Cav‐a was localized only in the muscle (yellow arrowhead), but was not detected in the notochord (Figure 2A). However, at 14 hpf, when the embryo developed into an arch shape due to bending of the tail region, the Cav‐a showed strong signals both in the muscle (yellow arrowhead) and notochord (white arrow). Just before lumen formation at 17 hpf, Cav‐a was localized at the center of the lateral domain (white arrow in Figure 2A) presumably forming the apical domain in the notochord cell. Later at 22 hpf, when the lumen was fully expanded in the Ciona notochord, Cav‐a was found to be enriched at the luminal membrane of the notochord (white arrow in Figure 2A) and labeled punctate structures on the membrane of the muscle cell (yellow arrowhead in Figure 2A). The embryos were also counterstained with wheat germ agglutination (WGA) conjugated with fluorescent dye to show the epithelia and notochord. Cav‐a promoter analysis gave similar results (Figure S4) to immunohistochemistry and in situ hybridization experiments. The dual expression patterns of Cav‐a in the muscles and notochord of Ciona embryos imply that its function might be related to the morphogenesis of these tissues.
Figure 2.

Localization of Cav‐a in Ciona embryos. A, Localization of Cav‐a at different developmental stages of Ciona embryos by immunohistochemistry. At 11 hours postfertilization (hpf), Cav‐a is localized in the muscle only (white arrowhead). At 14 hpf, Cav‐a is localized at both the muscle (white arrowhead) and notochord cells (white arrow). At the beginning of lumen formation (17 hpf), Cav‐a is localized at the center of the lateral domain (white arrow) of the notochord cell and the membrane of the muscle cell (white arrowhead). At 22 hpf, Cav‐a is localized at the luminal membrane (arrow) and at the membrane of the muscle cells (arrowhead). The green images show counterstaining with wheat germ agglutination (WGA) at different stages of Ciona embryos. DAPI is shown in the merged image. B, Schematic diagrams of the domain organization of the Cav‐a, C‐terminal, and N‐terminal deletions of Cav‐a mutant. Blue, yellow, and red rectangles indicate oligomerization, scaffolding, and intramembrane domains, respectively. C, Wild‐type, C‐terminal, and N‐terminal deletions of Cav‐a GFP fusion constructs are expressed in the living embryos. White arrows Cav‐a is localized at the luminal membrane domain of the notochord cell (white arrows) and caveolar vesicles are visible in the muscle cell (yellow arrows). The C‐terminal deletion of Cav‐a (Cav‐a∆160‐236‐EGFP) is mislocalized in the nuclei of notochord cells and the muscle cells (blue arrows) at 20 hpf. The N‐terminal deletion of Cav‐a (Cav‐a∆1‐117‐EGFP) shows normal membrane localization in the notochord and muscle cells at 20 hpf. Large vacuoles are observed at the muscle cells (red arrow). Scale bar represents 10 µm. D, Caveolae‐like structures are present in the Ciona embryonic muscle and notochord. Caveolae‐like “U” shape structure (red arrows in two top panels) and long tubular membrane invagination (blue arrows) are present at the apical luminal membrane of the notochord. Caveolae‐like structure is also observed in the muscle (red arrows in bottom panels). Scale bar is 0.2 µm. no = notochord, L = lumen, and mu = muscle. E, Quantification of caveolae (n = 10) shape in Ciona. Bulb diameter is indicated by the red arrow, neck diameter by the black arrow, and depth of caveolae by the blue arrow
Comparison of the Ciona caveolins with the vertebrate caveolins suggests the presence of three distinct caveolin domains: oligomerization, scaffolding, and intramembrane. We generated wild‐type, C‐terminal, and N‐terminal deletion mutants of Cav‐a GFP fusion proteins (Figure 2B) to examine which domain is required for its subcellular localization in living embryos. The results showed that the C‐terminal deleted mutant, Cav‐a∆160‐236‐EGFP, was localized in the nucleus of both the notochord (blue arrow) and muscle cell (blue arrow) at 20 hpf (Figure 2C). In contrast, the N‐terminal deleted mutant, Cav‐a∆1‐117‐EGFP fusion protein localized normally in both the notochord (white arrow) and muscle cell at 20 hpf (Figure 2C). Strikingly, the N‐terminal deleted mutant of Cav‐a caused the formation of a large number of giant vesicles in the muscles (red arrow, Figure 2C). These observations suggest that the C‐terminal region of Cav‐a is essential for its plasma membrane localization, while N‐terminal is required for its cytoplasmic trafficking.
Plasma membrane localization of Ciona caveolin is consistent with the localization of vertebrate caveolin that generates the caveolae structure in the plasma membranes. To visualize the structure in the plasma membrane of Ciona embryonic notochord and muscle tissues, we performed TEM of the wild‐type Ciona embryos. The results revealed invaginated uncoated structures at the apical luminal membrane in notochord cell (red arrows in Figure 2D) and muscle cells (red arrows in Figure 2D). Those structures resembled vertebrate caveolae, but without a distinct “neck” shape (Figure 2D). We quantified and compared the caveolae shape in Ciona (Figure 2E) and zebrafish (Figure S5A,B), and found that the “neck” of Ciona caveolae is wider than that in zebrafish. No caveolae rosette‐like structures were observed in our electron microscopy data. This observation indicates that vertebrate caveolae‐like structures are present in the Cav‐a expressed tissues in Ciona embryos but these structures have distinct morphology. Interestingly, this morphology is virtually identical to the few remaining caveolar structures observed in the zebrafish notochord upon knockout of the notochord Cavin1 isoform, Cavin1b.9
3.3. Cav‐a forms caveola‐like structures by inducing membrane curvature
In order to determine whether Ciona Cav‐a generates those caveolae‐like structures in the plasma membrane, we first expressed Cav‐a::GFP and Cav‐b::GFP in a LTE cell‐free system and examined the effect of the Cav‐a protein on endogenous membranes within the lysate.18 The results showed that Ciona Cav‐a but not Cav‐b induced membrane curvature similar to that induced by human Cav1 (red circle in Figure 3A). We then expressed Ciona caveolin fusion proteins in baby hamster kidney (BHK) cells and found that the expressed protein did not colocalize with endogenous cavin1 (Figure 3B). Cav‐b::EGFP also showed no colocalization with cavin1 (Figure 3C). These results suggest that unlike mammalian cavins and one tested invertebrate caveolin from the honey bee,11 Ciona Cav‐a could not recruit the endogenous cavin1 protein.
Figure 3.

Ciona Cav‐a does not recruit vertebrate cavin‐1, but induces membrane curvature in a Leishmania tarentolae extract (LTE) cell‐free system. A, In vitro curvature generation by Ciona Cav‐a in LTE. Successive virtual cross‐section of reconstructed tomograms highlights Ciona Cav‐a‐induced membrane curvatures, which resemble mammalian CAV1‐induced membrane curvatures. Red circles show CAV1‐induced curvature. Yellow circles show the 10 nm gold labeling. Scale bars in A, 20 nm. B‐C, Expression of Cav‐a::EGFP (B) and Cav‐b::EGFP (C) in baby hamster kidney (BHK) cells. White arrows show the punctate distribution in the BHK cells. White arrows show the punctate structure of cavin1 at the plasma membrane in the Cav‐a‐expressed cells. The Cav‐a::EGFP and cavin1 do not colocalize (white arrows in B). White arrows show the ring structure of Cav‐b::EGFP in the cytoplasm, which does not colocalize with cavin1 (white arrows in C)
We further examined the trafficking of Ciona caveolins by EM in two mammalian cell culture model systems. Cav‐a GFP was detected by APEX staining. In BHK cells, the peroxidase reaction product was found associated with cytoplasmic vesicles (red arrowheads in Figure 4A) and the invaginations at the surface plasma membrane (blue arrows in Figure 4B) that were far larger than caveolae (150 nm vs approximately 65 nm for caveolae). This was confirmed by caveolin knockout (Cav1−/−) MEFs; similar curved structures were detected at the plasma membrane (blue arrowhead in Figure 4E), suggesting that Ciona Cav‐a can form large curved domains at the plasma membrane with distinct morphology similar to the caveolae. In contrast, the Cav‐b APEX reaction product was found within the intraluminal vesicles of multivesicular endosomes and diffusely localized at the plasma membrane in BHK cells (red arrowhead in Figure 4C) and KO MEFs (red arrowhead in Figure 4F,G) with no association with regions of high plasma membrane curvature. Expression of vertebrate Cav1 GFP and APEX2‐nanotrap in mammalian cells was used as a control (Figure S6).
Figure 4.

Ultrastructural characterization of Ciona Cav‐a expressed in mammalian cells. GFP‐tagged Ciona Cav‐a and Cav‐b were coexpressed with GFP‐nanobody‐APEX2, respectively, in BHK cells (A‐C) or in CAV1−/− MEFs (D‐G), and then processed for electron microscopy. The DAB reaction product is associated with intracellular compartments including endosomes (green arrows in C, End) and numerous intracellular vesicles (red arrowheads in A, B, and E). At the plasma membrane (PM), the DAB reaction product is associated with uncoated vesicles/pits of approximately 150 nm diameter in both Cav‐a expressed BHK cells (blue arrows in panel B shows the surface connection of caveolae and the dashed line indicates PM) and CAV1 KO MEFs (blue arrows in E). In Cav‐b expressed BHK cells and CAV1 KO MEFs, peroxidase reaction products were diffused at the plasma membrane and were not observed to be associated with membrane curvature (red arrowheads in C and F‐G). N, nucleus. Bars 1 µm
All these results strongly suggest that Ciona Cav‐a has the ability to form caveola‐like structures by inducing the curvature of the plasma membrane.
3.4. Cav‐a protects the morphology of the muscle and notochord cells and affects tail morphogenesis
We used microinjection of Cav‐a morpholinos to knock down its expression in whole muscle and notochord tissues. The Cav‐a antibody staining confirmed the efficacy of our morpholino probe (Figure S7A,B′). Inhibition of Cav‐a expression by morpholino injection caused the failure of notochord lumen formation (Figure 5A; compare to control embryos with fully expanded lumens), disappearance of caveolae‐like structures in muscle and notochord (Figure 5B), and the tail length was significantly reduced in the Cav‐a knockdown embryos (Figure 5C). The phenotypes were rescued in Cav‐a MO rescue experiments (Figure 5A,C). These observations indicate that caveolae‐like structures in Ciona embryos are Cav‐a dependent and it plays crucial roles in Ciona tail morphogenesis. We thus further analyzed the locomotive behaviors of Ciona embryos and observed that the Cav‐a MO larvae lost the ability to swim. Their tails could not swing freely to drive forward movement. Cav‐a MO larvae just vibrated subtly and remained where they were (Supporting Information Movies S1 and S2).
Figure 5.

Cav‐a is required for tail elongation and notochord lumen formation. A, Phenotypes of Cav‐a knockdown by morpholino microinjection and rescued embryo. The upper panel is Control Cav‐a morpholino, Cav‐a morpholino‐injected embryos, and phenotype rescued embryos. Enlarged image in lower panel shows visible lumen (L) in the Control Cav‐a morpholino injected and rescued embryos. The abnormal muscle (red arrowhead) and notochord (red arrow) cells with no lumen are observed in Cav‐a morpholino injected embryo. Scale bars are 100 and 10 µm, respectively. no = notochord, and mu = muscle. B, TEM pictures of Ciona embryos. Invaginated caveolae‐like membrane structures in muscle and notochord cells (red arrow) in control morpholino‐injected embryos. In Cav‐a morpholino‐injected embryos, no caveolae‐like structures are detected in muscle and notochord cells. Scale bar 500 nm. C, Quantitative data on tail length of nontreated, control, and Cav‐a morpholino injected and rescued embryos. Asterisk (****) indicates the significant difference (P < .0001) and ns indicates the nonsignificant difference. D, Expression of Cav‐a‐P189L construct in Ciona living embryos. Aggresome‐like structures appear near the nucleus at 15 and 20 hpf in notochord and muscle cells (white arrows). The morphology of the Cav‐a‐P189L‐expressed notochord cell is abnormal (yellow arrows). Scale bar is 10 µm. E, The phenotypes of nontreated, Cav‐a::EGFP‐ and Cav‐a‐‐P189L‐expressed Ciona embryos at 20 hpf. Scale bar is 100 µm. F, Quantitative data on tail length of nontreated, Cav‐a::EGFP‐ and Cav‐a‐P189L‐expressed embryos. Asterisk (****) indicates the significant difference (P < .0001) and ns indicates the nonsignificant difference
To confirm the phenotype seen in Cav‐a morpholino knockdown embryos, we made a point mutation (Cav‐a‐P189L) by replacing proline at position 189 with lysine (equivalent to mammalian CAV1P132L or CAV3P104L). This site has been demonstrated to play a crucial role in the structural stability of the caveolin proteins37 with mutations in this region causing the proteins to act as dominant‐negative mutants.38 Expression of the Cav‐a‐P189L::EGFP mutant under Cav‐a own promoter, produced noticeable phenotypes, consistent with a dominant negative effect in Ciona embryos (Figure 5D). The Cav‐a‐P189L::EGFP fusion protein formed aggresome‐like structures near the nucleus in both muscle and notochord cells at 15 and 20 hpf (white arrows in Figure 5D). Importantly, notochord cells expressing the mutant protein did not align in the same row with other wild‐type cells and showed abnormal round morphology and no lumen (yellow arrows in Figure 5D). This observation prompted us to utilize the brachyury promoter to drive the expression of the Cav‐a‐P189L point mutant, specifically in the notochord, and to make a time‐lapse movie to examine what happens to those mutant cells in the notochord during morphogenesis. Intriguingly, we found that the mutant notochord cell became round and was squeezed out from the notochord line by the neighboring wild‐type cells (Figure S8). This phenomenon was never observed in wild‐type Cav‐a::EGFP expressing cells.
Muscle cells expressing Cav‐a‐P189L::EGFP also displayed abnormal morphology (white arrows in Figure 5D). We observed that the tail lengths of mutant embryos were shorter than that in control embryos (Figure 5E; quantitated in Figure 5F). Analysis of swimming behavior demonstrated that mutant larvae displayed limited movements, similar to the Cav‐a MO ones (Supporting Information Movie S3 and S4). These observations demonstrated that Cav‐a is required for locomotive ability in Ciona larvae, most probably by maintaining tissue mechanics.
Taken together, these results indicate that Ciona Cav‐a is required for the maintenance of cell shape, tissue integrity, and, ultimately, for tail morphogenesis.
3.5. Cav‐a vesicles transport to apical membrane required for notochord lumen opening and expansion
Antibody immunofluorescence staining showed that Cav‐a is localized at the lateral domain at 17 hpf and transported to the apical domain during notochord lumen expansion (22 hpf) (Figure 2A). To analyze the dynamics of Cav‐a localization, we expressed Cav‐a‐GFP in the notochord. Cav‐a‐GFP localized in the perinuclear region at 14 hpf (Figure 6A). At 19 hpf, when the lumen started to open and grow, Cav‐a‐GFP formed vesicles (white arrowheads) and accumulated at the apical membrane domains (Figure 6B). At 21 hpf, when the notochord lumen became fully expanded, it was specifically enriched at the apical domains (Figure 6C). To observe the dynamics of Cav‐a‐GFP in the membrane, we performed fluorescence recovery after photobleaching (FRAP) experiments (Figure 6D). Fluorescence in the bleached region (red rectangle in Figure 6D) was recovered approximately 80% within 5 minutes after bleaching, while its neighboring region (dark yellow rectangle in Figure 6D) did not show a significant change (Figure 6E). This demonstrated that luminal membrane‐localized caveolin was recruited from the cytoplasm (white arrowheads in Figure 6D, Supporting Information Movie S5), indicating that Cav‐a vesicles transport to the apical membrane during notochord lumenogenesis.
Figure 6.

Cav‐a vesicle transport to the luminal membrane and is essential for notochord lumen formation. A, Cav‐a‐tGFP localizes at the peri‐nucleus in Ciona embryos at 14 hpf. B, At 19 hpf, Cav‐a‐tGFP presents as vesicles in the cytoplasm (white arrowheads) and accumulates at the apical membrane domains. C, At 21 hpf, Cav‐a‐tGFP specially enriches at the apical domains. D, FRAP experiment in the notochord cell's apical membrane. Red and dark yellow rectangles show the bleached and its neighbor regions of Cav‐a‐tGFP at the luminal membrane, respectively. The white line demarcates between two adjacent notochord cells (no1 and no2). E, The recovery of fluorescence after bleaching of Cav‐a‐tGFP (red line) and the change of fluorescence intensity in its neighbor region (dark yellow line). F, Phenotypes of Ciona notochord tubulogenesis after BFA treatment. At 21, 23, and 26 hpf, there is no lumen present in BFA‐treated groups, while lumen (L) is present in the controls and the tubular structure (T) is formed at 26 hpf in the control group. Scale bars are 10 µm
Next, we examined whether vesicle trafficking mechanisms contribute to lumen formation in the Ciona notochord. Therefore, we utilized Brefeldin A (BFA), a chemical inhibitor that acts on the endoplasmic reticulum and the Golgi complex, to block vesicle trafficking.39 Ciona embryos treated with BFA at 16 hpf (before lumen formation) showed no visible lumen, as compared to the controls (Figure 6F), suggesting that the vesicle trafficking is indeed required for notochord lumen formation and contributes to embryonic tail morphogenesis.
4. DISCUSSION
Caveolins are evolutionarily conserved proteins, which have been demonstrated to play crucial roles in vertebrate development and cell physiology. However, their functions have not yet been fully investigated in invertebrates. Here, we have revealed that Ciona Cav‐a, in the absence of cavin proteins, has the ability to induce membrane curvature. We show that Ciona Cav‐a plays essential roles in protecting muscle and maintaining notochord cell integrity and is required for tail morphogenesis in urochordate embryogenesis. Knockdown of Cav‐a or expression of a dominant‐negative mutant form of Cav‐a causes a short tail and results in a loss of the lumen in the notochord cells of Ciona embryos (Figure 7).
Figure 7.
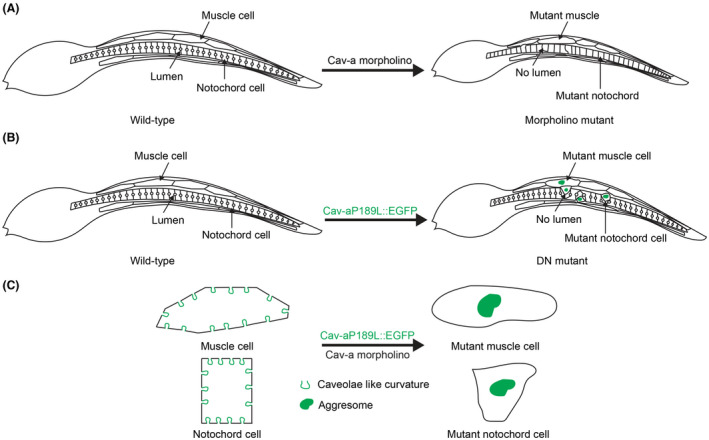
Working model of Cav‐a in ascidian cell and tissue morphology. A, The schematic diagram depicting the phenotypes of Cav‐a morpholino‐injected embryos. The MO embryo displays abnormal muscle and notochord cell morphology with no notochord lumen and short tail length. B, The schematic diagram of the phenotypes of Cav‐a dominant‐negative mutant expressed Ciona embryos. The aggresomes are presented (Green) in muscle and notochord cells in Cav‐aP189L::EGFP expressing embryos. The mutant notochord cell is squeezed out by neighboring wild‐type cells with no lumen formation. C, At the cellular level, the mutant cell phenotype is displayed with loss of caveolae‐like structure by forming aggresome (Green), and loss of normal cellular architecture in both muscle and notochord cells
4.1. Ascidian Cav‐a forms membrane curvature in both in vitro and in vivo experiments
It is known that the vertebrate caveolins can generate caveola structures40 but the existence of caveolae in invertebrates is less clear. Some invertebrate caveolins, such as those from C. elegans, cannot generate caveolae‐like structures when expressed in mammalian cells,16 but this differs from caveolin from the honey bee, A. mellifera, which was shown to generate caveolae when expressed in the same system and could recruit the endogenous cavin‐1 protein, despite no genes encoding cavin proteins being evident in the bee or other invertebrate genomes.16 The cavin proteins are known to be critical for the generation of caveolae in vertebrates41 but it is also apparent that in model systems mammalian caveolin alone can generate membrane curvature.17, 18 In the urochordate animal Ciona, our results using a cell‐free system, as well as in vivo experimental results, revealed that Cav‐a was recruited to the plasma membrane, and generated membrane invaginations that were morphologically similar to vertebrate caveolae, but with a wider “neck”. The loss of these structures in Cav‐a knockdown Ciona embryos provides strong evidence that these wide‐necked “caveolae” are the invertebrate counterpart of mammalian caveolae. Interestingly, this morphology is almost identical to caveolae of the zebrafish notochord in embryos lacking the notochord cavin1 isoform9; the number of caveolae is greatly decreased upon the loss of notochord Cavin1b but the remaining caveolae have similar morphology to those in the Ciona embryos. We, therefore, speculate that this morphology might be due to the lack of cavins in Ciona, as in other invertebrates.
4.2. Cav‐a is required for the normal morphology of muscle and notochord during Ciona tail morphogenesis
Despite extensive work regarding caveolar biology in different organisms and cellular systems, the exact functions of caveolins remain elusive.7 There is also limited information on the function of invertebrate caveolins. We attempted to understand the function of Cav‐a from Ciona using morpholino and dominant negative approaches. The perturbation of Cav‐a function by morpholino injection led to severe disruption of tail morphogenesis of Ciona embryos. We observed that the embryo tail length was significantly shortened compared to the control and no lumen was formed in the notochord. In the vertebrate embryos, caveolin knockdown also caused a short tail phenotype.15, 42 There is accumulating evidence that caveolae flatten during mechanical stress. Perturbation of the function of caveolin leads to a decrease in the number of caveolae and, hence, the ability to flatten in response to membrane force, thus causing membrane damage and loss of cell and tissue integrity in vertebrates.8, 9, 43
We also expressed a point mutant of Cav‐a in a mosaic fashion in the notochord and muscle by electroporation and were able to identify a distinct phenotype, presumably as a consequence of the dominant negative effect of the mutant. Initially, the caveolin point mutant PL was linked to breast cancer in humans.44 Later studies showed that the caveolin PL mutant was able to inhibit the transport of wild‐type caveolins toward the plasma membrane by forming an aggresome structure near the nucleus, and thus acted in a dominant negative fashion.38, 45 In our studies, the Cav‐aP189L mutant formed an aggresome‐like structure near the nuclei of the muscle and notochord cells, presumably blocking Cav‐a from reaching the plasma membrane. As a result, the Cav‐a could not create membrane curvature to protect the cell during embryo morphogenesis. Strikingly, the mutant notochord cell was displaced by the neighboring wild‐type cells. The tail length was also decreased, similar to observations in Cav‐a knockdown embryos. Furthermore, the swimming ability was severely compromised in the mutant embryos.
Ciona Cav‐a is localized at the luminal membrane of the notochord cell and in vesicle‐like structures in the muscle and notochord cells. This localization presumably reflects its importance during the morphogenesis of Ciona embryos, since during this time the tail bends and the notochord cells show active movements causing the muscle and notochord to experience mechanical stress. Mechanoprotection can occur by flattening of membrane invaginations to allow the cell to change shape8, 9 or by fusion of vesicles into the membrane to increase the surface area.5 As the cooperation between the muscle and notochord is required to complete tail morphogenesis in Ciona,46 we suggest that Cav‐a has a role in tail morphogenesis by modulating either membrane curvature or vesicle trafficking to counter the stress experienced by the muscle and notochord cells.
4.3. Cav‐a vesicle trafficking plays roles in notochord lumen opening and formation
After intercalation, the notochord cell elongates and then separated extracellular lumen formation begins. Ultimately, the lumens grow in size and connect each other to form a single lumen tubular structure.47 However, little is known about how the extracellular lumen formation initiates. One possibility may be that initially, during lumen formation, membrane trafficking takes place which provides the necessary surface area for lumen formation and later the expansion of the lumen is driven by membrane transporters localized at the apical domain.48 During lumen expansion, the junctional actomyosin network regulates the shape of the lumen by exerting contractile force,49 and, as a consequence, the luminal membrane experiences a constant force, but how lumen morphology is maintained intact is not known. Vesicle trafficking is the major factor contributing the membrane to the apical domain for lumen formation during tubulogenesis.50 Recently, it was revealed that vesicles were trafficked from the basal membrane toward the apical membrane during lumen formation in Ciona notochord.51 FRAP has been used to determine the dynamics of the membrane proteins in diverse model organisms.52, 53, 54 The small region of plasma membrane was laser bleached and the fluorescence intensity of the bleached and its neighboring regions was recorded, respectively, to determine the sources of the recovered membrane proteins. In our experiment, the fluorescent intensity of Cav‐a::GFP at the bleached neighboring region did not decrease much, suggesting that the membrane‐localized Cav‐a::GFP is not supplied by the diffusion from the existent membrane‐localized population, but mainly through the vesicle trafficking from the cytoplasm. Thus, we demonstrated the fact that the Cav‐a vesicles are trafficked toward the apical luminal membrane. Abolishment of caveolin function or blocking of vesicle trafficking inhibits lumen formation and expansion, suggesting that Cav‐a at the luminal membrane serves to increase the surface area during lumen expansion to keep the lumen intact. Another possible scenario is that the Cav‐a act as a mechanosensor in the highly tensed apical membrane of the notochord. It might respond and regulate the membrane tension via flattening during apical membrane growth and lumen expansion. And the loss of this regulatory mechanism may have caused the failure of the lumen opening and growth.
5. CONCLUSIONS
In this study, we employed both in vitro and in vivo approaches to understand the function of caveolin from the invertebrate chordate Ciona during embryonic tail morphogenesis. Our findings revealed several interesting functions of invertebrate chordate caveolins. First, the invertebrate chordate caveolin, which is expressed without cavin proteins, has the ability to induce membrane curvature and form vertebrate caveolae‐like structures, but with a distinct morphology, at the plasma membrane. Second, we show that the invertebrate chordate caveolin maintains the morphology and integrity of muscle and notochord, and is required for tissue morphogenesis. Third, the invertebrate chordate caveolin is involved in notochord lumen formation through polarized vesicle trafficking. These findings provide new insights into the mechanisms involved in the protection and maintenance of tissue integrity during embryo morphogenesis and the evolutionary conservation of caveolin function.
6. COMPETING INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
B. Dong, R.G. Parton, and P. Bhattachan conceived and designed the research; P. Bhattachan, J. Rae, H. Yu, W. Jung, J. Wei, R.G. Parton, and B. Dong performed the research; B. Dong, R.G. Parton, and P. Bhattachan analyzed the data; B. Dong, P. Bhattachan, and R.G. Parton wrote the manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 31572352, 31771649), the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0302‐1), the Fundamental Research Funds for the Central Universities (201822016, 201762003), and the Taishan Scholar Program of Shandong Province, China (201502035). RGP is supported by Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowships (APP569452 and APP1150083) and NHMRC Program Grant (to RGP APP1037320) and by the Australian Research Council (ARC) Centre of Excellence in Convergent Bio‐Nano Science and Technology (Project Number CE140100036). The authors thank Kirill Alexandrov for advice regarding cell‐free expression experiments. We are also grateful to Dr Nick Ariotti for assistance with electron microscopy.
Bhattachan P, Rae J, Yu H, et al. Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. The FASEB Journal. 2020;34:1345–1361. 10.1096/fj.201901281R
Contributor Information
Robert G. Parton, Email: r.parton@imb.uq.edu.au.
Bo Dong, Email: bodong@ouc.edu.cn.
REFERENCES
- 1. Jarsch IK, Daste F, Gallop JL. Membrane curvature in cell biology: an integration of molecular mechanisms. J Cell Biol. 2016;214(4):375‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dulhunty AF, Franzini‐Armstrong C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1975;250(3):513‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoeber M, Schellenberger P, Siebert CA, Leyrat C, Helenius A, Grunewald K. Model for the architecture of caveolae based on a flexible, net‐like assembly of Cavin1 and Caveolin discs. Proc Natl Acad Sci USA. 2016;113(50):E8069‐E8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1(5):445‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sinha B, Köster D, Ruez R, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144(3):402‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98‐112. [DOI] [PubMed] [Google Scholar]
- 7. Cheng JPX, Nichols BJ. Caveolae: one function or many? Trends Cell Biol. 2016;26(3):177‐189. [DOI] [PubMed] [Google Scholar]
- 8. Garcia J, Bagwell J, Njaine B, et al. Sheath cell invasion and trans‐differentiation repair mechanical damage caused by loss of caveolae in the zebrafish notochord. Curr Biol. 2017;27(13):1982‐1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim Y‐W, Lo HP, Ferguson C, et al. Caveolae protect notochord cells against catastrophic mechanical failure during development. Curr Biol. 2017;27(13):1968‐1981. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig A, Nichols BJ, Sandin S. Architecture of the caveolar coat complex. J Cell Sci. 2016;129(16):3077‐3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill MM, Bastiani M, Luetterforst R, et al. PTRF‐Cavin, a conserved cytoplasmic protein required for Caveola formation and function. Cell. 2008;132(1):113‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20(4):177‐186. [DOI] [PubMed] [Google Scholar]
- 13. Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minetti C, Bado M, Broda P, et al. Impairment of caveolae formation and T‐system disorganization in human muscular dystrophy with caveolin‐3 deficiency. Am J Pathol. 2002;160(1):265‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nixon SJ, Wegner J, Ferguson C, et al. Zebrafish as a model for caveolin‐ muscle disease; caveolin‐3 is required for myofibril organization and muscle cell patterning. Hum Mol Genet. 2005;14(13):1727‐1743. [DOI] [PubMed] [Google Scholar]
- 16. Kirkham M, Nixon SJ, Howes MT, et al. Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci. 2008;121(12):2075‐2086. [DOI] [PubMed] [Google Scholar]
- 17. Walser P, Ariotti N, Howes M, et al. Constitutive formation of caveolae in a bacterium. Cell. 2012;150(4):752‐763. [DOI] [PubMed] [Google Scholar]
- 18. Jung W, Sierecki E, Bastiani M, et al. Cell‐free formation and interactome analysis of caveolae. J Cell Biol. 2018;217(6):2141‐2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sohn J, Brick RM, Tuan RS. From embryonic development to human diseases: the functional role of caveolae/caveolin. Birth Defects Res Part C‐Embryo Today‐Rev. 2016;108(1):45‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park DS, Woodman SE, Schubert W, et al. Caveolin‐1/3 double‐knockout mice are viable, but lack both muscle and non‐muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160(6):2207‐2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Razani B, Engelman JA, Wang XB, et al. Caveolin‐1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121‐38138. [DOI] [PubMed] [Google Scholar]
- 22. Razani B, Wang XB, Engelman JA, et al. Caveolin‐2‐deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22(7):2329‐2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galbiati F, Engelman JA, Volonte D, et al. Caveolin‐3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin‐glycoprotein complex, and T‐tubule abnormalities. J Biol Chem. 2001;276(24):21425‐21433. [DOI] [PubMed] [Google Scholar]
- 24. Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin‐1 gene‐disrupted mice. Science. 2001;293(5539):2449‐2452. [DOI] [PubMed] [Google Scholar]
- 25. Roitenberg N, Bejerano‐Sagie M, Boocholez H, et al. Modulation of caveolae by insulin/IGF‐1 signaling regulates aging of Caenorhabditis elegans . EMBO Rep. 2018;19(8):e45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hotta K, Mitsuhara K, Takahashi H, et al. A web‐based interactive developmental table for the ascidian Ciona intestinalis, including 3D real‐image embryo reconstructions: I. From fertilized egg to hatching larva. Dev Dyn. 2007;236(7):1790‐1805. [DOI] [PubMed] [Google Scholar]
- 27. Wada S, Katsuyama Y, Yasugi S, Saiga H. Spatially and temporally regulated expression of the LIM class homeobox gene HRLIM suggests multiple distinct functions in development of the ascidian, Halocynthia‐roretzi . Mech Dev. 1995;51(1):115‐126. [DOI] [PubMed] [Google Scholar]
- 28. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403‐410. [DOI] [PubMed] [Google Scholar]
- 29. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564‐577. [DOI] [PubMed] [Google Scholar]
- 31. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics. 2014;30(9):1312‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best‐fit models of protein evolution. Bioinformatics. 2011;27(8):1164‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corbo JC, Levine M, Zeller RW. Characterization of a notochord‐specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis . Development. 1997;124(3):589‐602. [DOI] [PubMed] [Google Scholar]
- 34. Christiaen L, Wagner E, Shi W, Levine M. Microinjection of morpholino oligos and RNAs in sea squirt (Ciona) embryos. Cold Spring Harb Protoc. 2009;2009(12):pdb.prot5347. [DOI] [PubMed] [Google Scholar]
- 35. Ariotti N, Hall TE, Rae J, et al. Modular detection of GFP‐labeled proteins for rapid screening by electron microscopy in cells and organisms. Dev Cell. 2015;35(4):513‐525. [DOI] [PubMed] [Google Scholar]
- 36. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36(8):584‐595. [DOI] [PubMed] [Google Scholar]
- 37. Rieth MD, Lee J, Glover KJ. Probing the caveolin‐1 P132L mutant: critical insights into its oligomeric behavior and structure. Biochemistry. 2012;51(18):3911‐3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shatz M, Lustig G, Reich R, Liscovitch M. Caveolin‐1 mutants P132L and Y14F are dominant negative regulators of invasion, migration and aggregation in H1299 lung cancer cells. Exp Cell Res. 2010;316(10):1748‐1762. [DOI] [PubMed] [Google Scholar]
- 39. Mackenzie JM, Jones MK, Westaway EG. Markers for trans‐Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus‐infected cells. J Virol. 1999;73(11):9555‐9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 41. Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci. 2015;128(7):1269‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nixon SJ, Carter A, Wegner J, et al. Caveolin‐1 is required for lateral line neuromast and notochord development. J Cell Sci. 2007;120(13):2151‐2161. [DOI] [PubMed] [Google Scholar]
- 43. Cheng JPX, Mendoza‐Topaz C, Howard G, et al. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J Cell Biol. 2015;211(1):53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayashi K, Matsuda S, Machida K, et al. Invasion activating caveolin‐1 mutation in human scirrhous breast cancers. Can Res. 2001;61(6):2361‐2364. [PubMed] [Google Scholar]
- 45. Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152(5):1057‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Gregorio A, Harland RM, Levine M, Casey ES. Tail morphogenesis in the ascidian, Ciona intestinalis, requires cooperation between notochord and muscle. Dev Biol. 2002;244(2):385‐395. [DOI] [PubMed] [Google Scholar]
- 47. Dong B, Horie T, Denker E, et al. Tube formation by complex cellular processes in Ciona intestinalis notochord. Dev Biol. 2009;330(2):237‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng W, Nies F, Feuer A, Bocina I, Oliver D, Jiang D. Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis. Proc Natl Acad Sci USA. 2013;110(37):14972‐14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Denker E, Sehring IM, Dong B, Audisso J, Mathiesen B, Jiang D. Regulation by a TGF beta‐ROCK‐actomyosin axis secures a non‐linear lumen expansion that is essential for tubulogenesis. Development. 2015;142(9):1639‐1650. [DOI] [PubMed] [Google Scholar]
- 50. Bryant DM, Datta A, Rodriguez‐Fraticelli AE, Peranen J, Martin‐Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12(11):1035‐U1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mizotani Y, Suzuki M, Hotta K, et al. 14‐3‐3epsilona directs the pulsatile transport of basal factors toward the apical domain for lumen growth in tubulogenesis. Proc Natl Acad Sci USA. 2018;115(38):E8873‐E8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mvrakis M, Rikhy R, Lippincott‐Schwartz J. Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Dev Cell. 2008;16:93‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goehring N, Hoege C, Grill S, Hyman A. PAR proteins diffuse freely across the anterior–posterior boundary in polarized C. elegans embryos. J Cell Biol. 2011;193(3):583‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shi D, Usami F, Komatsu K, et al. Dynamics of planar cell polarity protein Vangl2 in the mouse oviduct epithelium. Mech Dev. 2016;141:78‐89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


