Abstract
Objective
High magnetic resonance imaging (MRI)–detected inflammation is associated with greater progression and poorer outcomes in rheumatoid arthritis (RA). This analysis aimed to determine if baseline MRI inflammation was related to clinical response and remission in the Assessing Very Early Rheumatoid arthritis Treatment (AVERT) study.
Methods
AVERT was a phase IIIb, randomized, controlled trial with a 12‐month, double‐blind treatment period enrolling patients with early (≤2 years' duration), anti‐citrullinated peptide–positive methotrexate (MTX)‐naive RA. In this post hoc analysis, patients in the abatacept plus MTX (n = 114) and MTX (n = 111) arms with available MRI results were stratified into low and high baseline MRI inflammation groups based on previously developed cutoffs of synovitis and osteitis on unilateral hand–wrist contrast‐enhanced MRI. Simplified Disease Activity Index (SDAI) remission (≤3.3), Clinical Disease Activity Index (CDAI) remission (≤2.8), Boolean remission, and Disease Activity Score in 28 joints using the C‐reactive protein level (<2.6) were assessed.
Results
Overall, 100 of 225 patients (44.4%) had high baseline MRI inflammation. In patients with high baseline MRI inflammation, a significantly greater proportion achieved remission at 12 months with abatacept plus MTX versus MTX across SDAI (45.1% versus 16.3%; P = 0.0022), CDAI (47.1% versus 20.4%; P = 0.0065), and Boolean indices (39.2% versus 16.3%; P = 0.0156). In patients with low baseline MRI inflammation, remission rates were not significantly different with abatacept plus MTX versus MTX (SDAI: 39.7% versus 32.3%; P = 0.4961).
Conclusion
In seropositive, MTX‐naive patients with early RA and presence of objectively measured high inflammation by MRI, indicating poor prognosis, remission rates were higher with abatacept plus MTX treatment versus MTX.
Introduction
Rheumatoid arthritis (RA) is an immune‐mediated disease, characterized by systemic, chronic joint inflammation that leads to structural damage 1. While improving the signs and symptoms of RA is essential, and clinical remission is a key goal of treatment, in order to reduce long‐term disability, preventing the progression of structural joint damage is important 2, 3.
Significance & Innovations.
Among patients with active early rheumatoid arthritis (RA), high levels of objective magnetic resonance imaging (MRI)–detected inflammation at baseline were indicative of which patients were more likely to achieve clinical remission with treatment with abatacept plus methotrexate versus methotrexate.
Objective identification of inflammation using MRI in RA may be added to the several predictive biomarkers used to aid treatment decisions.
When starting biologic therapy, the ability to identify patients with a greater likelihood of structural progression is important to minimize disease impact through personalizing RA treatment approaches. Objective measures of inflammation, such as magnetic resonance imaging (MRI), may provide information on top of clinical assessments 4. MRI allows assessment of synovitis more accurately than clinical evaluation alone and can detect subclinical levels of inflammation 5, 6.
MRI has the potential to be a predictive imaging biomarker, providing objective information that could inform treatment decisions, such as when tapering of biologic therapy should be considered 7. In a 5‐year follow‐up study of patients with RA, MRI was shown to be able to identify bone erosions before these become evident on radiographs 8. Recent data from a secondary analysis of 2 randomized clinical trials suggested that it may be possible to stratify patients as progressors or nonprogressors based on their level of MRI inflammation after 24 weeks of treatment and that attainment of a low MRI inflammation score may predict a lack of structural disease progression independently of attaining clinical remission 9.
Abatacept is an immunomodulator that disrupts the continuous cycle of T cell activation that characterizes RA and inhibits B cell–derived autoantibody and proinflammatory cytokine production 10. In the Assessing Very Early Rheumatoid arthritis Treatment (AVERT) trial, a significantly greater proportion of patients receiving abatacept plus methotrexate (MTX) compared with MTX achieved Disease Activity Score in 28 joints using the C‐reactive protein level (DAS28‐CRP) remission (DAS28‐CRP score <2.6) at 12 months (P = 0.010) 1. Numerically greater reductions in synovitis, osteitis, and erosion scores from baseline were also seen with abatacept plus MTX compared with MTX 1.
Modern RA treatment recommendations highlight the need to aim for remission 11, 12. A means of predicting the achievement of clinical remission would be of great value to patients and their treating physicians in the clinical setting. As such, the aim of this post hoc analysis was to determine the proportion of patients in each treatment arm of the AVERT study achieving clinical remission over 12 months, stratified by baseline MRI‐detected inflammation status, as an objective measure of inflammation. We investigated whether a greater benefit of more intensive (combination) therapy would be observed among those with high MRI inflammation at baseline.
Patients and Methods
AVERT study design and procedures
AVERT was a phase IIIb, randomized, active‐controlled trial of 24 months with a 12‐month, double‐blind treatment period (NCT01142726) and has been described previously 1. Briefly, eligible patients were 18 years or older with early, active RA (persistent symptoms for ≤2 years), DAS28‐CRP ≥3.2, active clinical synovitis of ≥2 joints for at least 8 weeks (regardless of any current treatment), and anti–citrullinated peptide‐2 antibody positivity. Patients were MTX naive or had received MTX (≤10 mg/week) for ≤4 weeks with no MTX for 1 month prior to enrollment 1.
During the 12‐month treatment period, patients were stratified based on corticosteroid use at baseline (yes/no) and randomized 1:1:1 to receive abatacept plus MTX, abatacept monotherapy, or MTX. Abatacept was administered subcutaneously at 125 mg/week without an intravenous loading dose. MTX was initiated at 7.5 mg/week and titrated to 15–20 mg/week within 6–8 weeks (≤10 mg/week permitted in patients with intolerance) 1. Patients with a worsening of disease requiring additional or amended therapy during the treatment period were discontinued from study participation. Only patients who were receiving abatacept plus MTX or MTX alone and who had available MRI data were included in the current post hoc analysis. The abatacept monotherapy arm was not powered for efficacy analyses in AVERT, and as such these patients were not included in the current analyses.
The AVERT study was conducted in accordance with good clinical practice and the Declaration of Helsinki. All patients provided written informed consent. The protocol and amendments were approved by the appropriate institutional review boards/independent ethics committees at each site. Bristol Myers Squibb (the sponsor) provided the study drug, designed the study, conducted the study in collaboration with the principal investigators, collected the data, monitored the conduct of the study, and performed statistical analyses, including the post hoc analyses reported here.
MRI assessments
Contrast‐enhanced 1.5T MRI of metacarpophalangeal joints 1–5 of the patient's most clinically active hand and wrist, based on clinical assessment of synovitis, was performed at baseline, 6 months, and 12 months 13. The same hand and wrist were imaged at each assessment. MRI inflammation was scored using the Outcome Measures in Rheumatology Rheumatoid Arthritis Magnetic Resonance Imaging Scoring (RAMRIS) method 14 by 2 independent central readers who were blinded to treatment arm and using a randomized order of time points. The scores of both assessors were averaged, and the top 5% of discrepancies for total change in score relative to baseline were adjudicated by consensus review. Only scans from baseline and 12 months were used in this analysis.
Post hoc analysis
Patients were stratified into “low” and “high” baseline MRI inflammation groups (Figure 1) based on previous analyses, which demonstrated that patients with high baseline MRI inflammation had an increased risk of structural damage progression compared with those with low baseline inflammation 9, 15. Inflammation was measured using RAMRIS scores. Low inflammation was defined as synovitis ≤3 or osteitis ≤3 and combined ≤9 (synovitis plus osteitis [double‐weighted]), and high inflammation was defined as synovitis >3 and osteitis >3 or combined >9 (synovitis plus osteitis [double‐weighted]). As in prior studies, osteitis was double‐weighted in quantification of inflammation due to a greater ability to predict structural damage progression and greater effect on erosion development versus synovitis 9, 15.
Figure 1.
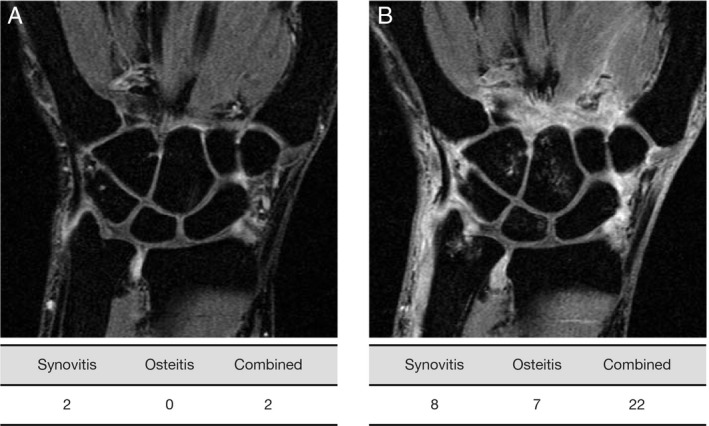
Contrast‐enhanced 1.5T magnetic resonance image (MRI) of wrist with low (A) and high (B) MRI‐detected inflammation. Low MRI inflammation: synovitis ≤3 or osteitis ≤3 and combined ≤9 (synovitis plus osteitis 2×). High MRI inflammation: synovitis >3 and osteitis >3 or combined >9 (synovitis plus 2× osteitis).
Disease activity at 12 months was compared between the abatacept plus MTX and MTX treatment groups within the high and low baseline MRI inflammation subgroups. Patients were assessed using the following measures: Simplified Disease Activity Index (SDAI) remission (≤3.3), Clinical Disease Activity Index (CDAI) remission (≤2.8), Boolean remission (tender joint count ≤1, swollen joint count ≤1, CRP level ≤1 mg/dl, and patient's global assessment of disease activity ≤1 [visual analog scale, range 0–10 cm]), and DAS28‐CRP <2.6.
Statistical analyses
Post hoc analyses were carried out using the intent‐to‐treat population, which included all patients who received at least 1 dose of the study drug. Only patients receiving abatacept plus MTX or MTX alone with available MRI results were included in the current post hoc analysis. Outcomes at 12 months were compared between treatment arms using statistical tests: chi‐square tests for categorical variables (or exact method if there were <5 patients) and Student's t‐tests for numerical variables.
Results
Patients
Of the 351 patients enrolled and randomized to treatment in the AVERT study, 119 were randomized to receive abatacept plus MTX and 116 to receive MTX. Of these 235 patients, 225 (95.7%) had baseline MRI data available: 114 of 119 patients (95.8%) in the abatacept plus MTX arm and 111 of 116 (95.7%) in the MTX arm. Baseline MRI scans were not available for 10 of 235 patients (4.3%).
Baseline characteristics of the AVERT study have been reported previously 1. Briefly, patients had a mean RA symptom duration of 0.58 years in the abatacept plus MTX group and 0.50 years in the MTX group. The patient population had highly inflammatory disease: mean tender joint count 14.0 and 12.8, swollen joint count 11.2 and 10.7, CRP level 18.1 mg/liter and 17.3 mg/liter, and mean DAS28‐CRP 5.5 and 5.3 in the abatacept plus MTX and MTX groups, respectively.
At baseline, 125 of 225 patients (55.6%) were classified as having low MRI inflammation, and 100 of 225 (44.4%) as having high MRI inflammation (Table 1). A total of 51 of 114 patients (44.7%) in the abatacept plus MTX arm and 49 of 111 patients (44.1%) in the MTX arm were classified as having high MRI inflammation at baseline.
Table 1.
Baseline magnetic resonance imaging (MRI) inflammation and disease activity measures by baseline MRI inflammation status and by treatment groupa
| Low MRI inflammation | High MRI inflammation | |||
|---|---|---|---|---|
| Abatacept plus MTX (n = 63) | MTX (n = 62) | Abatacept plus MTX (n = 51) | MTX (n = 49) | |
| MRI‐detected inflammation measures | ||||
| Synovitis | 2.6 ± 2.0 | 3.1 ± 2.4 | 9.2 ± 3.6 | 9.3 ± 3.6 |
| Osteitis | 0.3 ± 0.6 | 0.2 ± 0.5 | 10.0 ± 9.7 | 10.7 ± 9.8 |
| Combinedb | 3.1 ± 2.4 | 3.5 ± 2.7 | 29.3 ± 21.3 | 30.7 ± 21.3 |
| Disease activity measures | ||||
| SDAI | 39.9 ± 17.4 | 43.5 ± 24.6 | 76.9 ± 44.9 | 61.1 ± 35.2 |
| CDAI | 34.0 ± 14.7 | 32.1 ± 15.0 | 43.4 ± 16.2 | 37.7 ± 18.1 |
| DAS28‐CRP | 5.1 ± 1.1 | 5.0 ± 1.3 | 6.2 ± 1.2 | 5.6 ± 1.4 |
Values are the mean ± SD. MTX = methotrexate; SDAI = Simplified Disease Activity Index; CDAI = Clinical Disease Activity Index; DAS28‐CRP = Disease Activity Score in 28 joints using the C‐reactive protein level.
Osteitis double weighted.
Post hoc analyses
Baseline
Among patients with high MRI inflammation at baseline, the mean ± SD combined inflammation measure was 29.3 ± 21.3 in the abatacept plus MTX group and 30.7 ± 21.3 in the MTX group. Among patients with low MRI inflammation at baseline, the mean ± SD combined inflammation measure was 3.1 ± 2.4 for patients in the abatacept plus MTX group and 3.5 ± 2.7 for patients in the MTX group. All disease activity scores (SDAI, CDAI, and DAS28‐CRP) were numerically higher in patients with high MRI inflammation at baseline versus those with low inflammation (Table 1).
12 months
Among patients with high MRI inflammation at baseline, the proportion of patients that achieved remission was significantly greater in the abatacept plus MTX group than in the MTX group for SDAI (45.1% versus 16.3%; P = 0.0022), CDAI (47.1% versus 20.4%; P = 0.0065), and Boolean (39.2% versus 16.3%; P = 0.0156) indices. Numerical differences in the proportions of patients achieving a DAS28‐CRP score of <2.6 were seen (60.8% versus 40.8%; P = 0.0667) (Figure 2). In those with low MRI inflammation at baseline, the proportion who achieved remission at month 12 based on any of the clinical remission criteria (SDAI, CDAI, Boolean, or DAS28‐CRP) was numerically, but not significantly, higher in the abatacept plus MTX group than in the MTX group (Figure 2).
Figure 2.
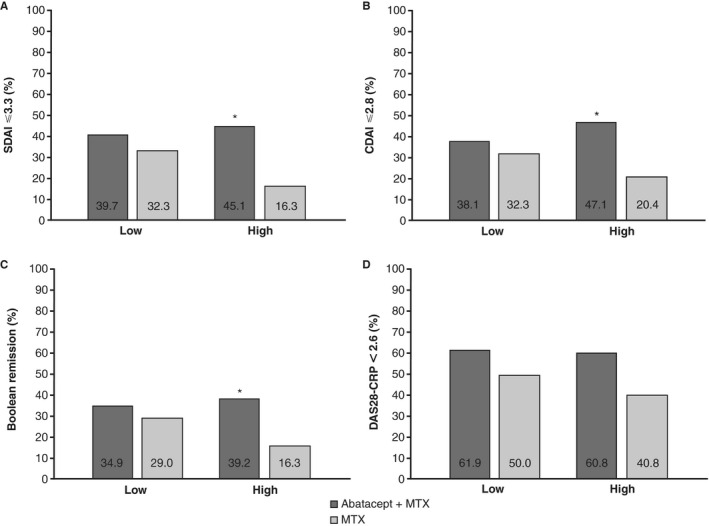
Efficacy outcomes at 12 months by baseline magnetic resonance imaging (MRI) inflammation status and by treatment group (post hoc analyses). Proportion of patients with Simplified Disease Activity Index (SDAI) score ≤3.3 (A), Clinical Disease Activity Index (CDAI) score ≤2.8 (B), Boolean remission (tender joint count ≤1, swollen joint count ≤1, C‐reactive protein [CRP] level ≤1 mg/dl, and patient global assessment of disease activity ≤1) (C), and Disease Activity Score in 28 joints using the CRP level (DAS28‐CRP) <2.6 (D). For low MRI inflammation at baseline, n = 63 for abatacept plus methotrexate (MTX), and n = 62 for MTX. For high MRI inflammation at baseline, n = 51 for abatacept plus MTX, and n = 49 for MTX. Normal approximations were used if the number of patients with SDAI score ≤3.3, CDAI score ≤2.8, Boolean remission, or DAS28‐CRP score <2.6 was ≥5, otherwise an exact method was used. * = P < 0.05 for abatacept plus MTX versus MTX; SDAI P = 0.0022, CDAI P = 0.0065, Boolean remission P = 0.0156.
Discussion
In this post hoc analysis of the AVERT study, patients with early RA and a high MRI inflammation status at baseline who were treated with abatacept plus MTX were statistically more likely to achieve clinical remission than those treated with MTX. In contrast, among those with low MRI inflammation at baseline, remission rates were numerically, but not significantly, higher in the abatacept plus MTX and MTX groups, and no statistically significant differences were observed for any outcome measure.
Abatacept in combination with MTX has been shown to be significantly more effective in the treatment of RA than MTX, with a good safety and tolerability profile 1. To our knowledge, this is the first analysis to examine the effects of abatacept treatment in combination with MTX in a patient cohort stratified by a poor prognostic factor based on objective baseline MRI inflammation status. Our findings demonstrated that abatacept plus MTX was more effective than MTX at inducing remission in patients with RA irrespective of baseline levels of MRI inflammation, but that the benefit seen with combination therapy was greater in patients with higher baseline inflammation than in those with lower baseline inflammation. Notably, greater differences in the proportions of patients achieving remission with abatacept plus MTX versus MTX were seen in the group with high MRI inflammation at baseline, a feature indicative of poor prognosis. Thus, we speculate that presence of high MRI inflammation and potentially other poor prognostic factors could be used to risk‐stratify patients and determine who might derive the greatest benefit from initiation of abatacept therapy. However, caution must be exercised during this stratification, and MRI inflammation must be considered in the context of other biomarkers to prevent suboptimal treatment of patients considered to have a better prognosis (patients with low MRI inflammation). Although seropositive patients with high objective disease activity at baseline are at a greater risk for poor outcomes than those without these factors, patients without such factors still endure a prognosis that is life altering. Thus, patients considered to have a better prognosis should still be treated according to the current treatment guidelines, and objective assessment of inflammation in these seronegative patients may enable further patient stratification and the selection of appropriate patients for therapy escalation.
The current findings are important because biomarkers, such as MRI, that may help to determine which individuals are most likely to benefit from a particular therapy are of great interest. However, the high cost of MRI and its accessibility in daily clinical care are currently limiting factors in its adoption for use in routine clinical practice. The cost of MRI should be considered in the context of the costs avoided by preventing prescription of a specific biologic therapy to those patients who would be less likely to benefit from them, and furthermore by potentially preventing the consequences of inadequate disease control. The current study used a clinical trial cohort and MRI to demonstrate the potential utility of objectively measuring inflammation to identify patients who are likely to derive benefit from a particular therapy. Translation into clinical practice will depend on a variety of factors: the cost of MRI in the context of the costs that would be avoided by preventing prescription of a specific biologic therapy to patients less likely to benefit from it (including the management of adverse events); assessment of the risks and longer‐term benefits associated with treating patients with high inflammation with early intensive therapy; advances in technologies allowing for better routine quantification of inflammation; and the increasing use of modern imaging technologies more suited to routine use than MRI. The current study is a first step in understanding how MRI might have value in helping to identify these patients. More studies are still required in other populations with RA and varying clinical disease activity.
The prognostic and predictive value of MRI has been demonstrated in other contexts. For example, in a study of 143 patients with RA in clinical remission while receiving biologic disease‐modifying antirheumatic drugs (bDMARDs), MRI was an independent predictor of successful tapering. The study demonstrated that the use of >1 previous bDMARD, male sex, and low baseline MRI combined inflammation or combined damage score were all independent predictors of successful tapering 7.
The limitations of the current study should be considered. These were post hoc analyses, and the AVERT study was not designed specifically to answer the questions posed, nor was it powered to investigate these questions. In particular, the abatacept monotherapy arm was not powered for efficacy analyses in AVERT, and as such these patients were not included in the current analyses. Although this study provides a proof of concept, based on the use of MTX and a biologic agent, an approach employing the treat‐to‐target strategy with concomitant temporary glucocorticoids would also provide valuable insights into appropriate treatment regimens. While the inclusion of patients with only a narrow range of disease activity scores (DAS28‐CRP ≥3.2) allowed MRI inflammation to be assessed independently of disease activity, future studies with broader clinical disease activity inclusion criteria would be of value. In addition, clinical remission was only assessed at a single time point. It is important to consider that only the hand and wrist were imaged for each patient rather than multiple joints, and misclassification of MRI activity may occur to some degree in individuals with oligoarticular and asymmetrical disease; however, all MRI predictive validity work has been conducted using the single‐hand scanning as performed in this study. Further validation of the threshold for high versus low MRI inflammation is required; in particular, analyses in a long‐term cohort are needed to determine the best weight of osteitis versus synovitis to gauge severity of joint inflammation. Finally, tenosynovitis was not assessed in this study and may add to the imaging evaluation.
In conclusion, these post hoc analyses of the AVERT study showed that patients with early RA and a high level of MRI inflammation at baseline were more likely to achieve clinical remission with abatacept plus MTX compared with MTX. MRI as a measure of inflammation can provide added value as an objective assessment of disease to influence clinical decision making and guide the more precise use of therapies to treat RA.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Conaghan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Ahmad, Østergaard, Emery, Conaghan.
Acquisition of data
Emery, Durez.
Analysis and interpretation of data
Ahmad, Baker, Østergaard, Emery, Durez, Ye, Banerjee, Conaghan.
Role of the Study Sponsor
Bristol Myers Squibb facilitated the study design and reviewed and approved the manuscript prior to submission. The authors had full access to the study data, contributed to the interpretation of the results, and had ultimate control over the decision to publish and the final version of the manuscript submitted for publication. Professional medical writing and editorial assistance was provided by Lola Parfitt, MRes, at Caudex and was funded by Bristol Myers Squibb. Publication of this article was not contingent upon approval by Bristol Myers Squibb.
ClinicalTrials.gov identifier: NCT01142726.
The views expressed herein are those of the authors and not necessarily those of the NHS, the NIHR, the UK Department of Health, or the US government and Department of Veterans Affairs.
Supported by Bristol Myers Squibb. Dr. Baker's work was supported by the US Department of Veterans Affairs (Clinical Science Research & Development Career Development Award IK2 CX000955). Drs. Emery and Conaghan's work was supported in part by the NIHR Leeds Biomedical Research Centre.
Drs. Ahmad, Ye, and Banerjee own stock or stock options in Bristol Myers Squibb. Dr. Baker has received consulting fees from Bristol Myers Squibb (less than $10,000). Dr. Østergaard has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Hospira, Janssen, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche, and UCB (less than $10,000 each). Dr. Emery has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly and Company, Gilead, MSD, and Novartis (less than $10,000 each). Dr. Durez has received consulting fees, speaking fees, and/or honoraria from Bristol Myers Squibb, Eli Lilly and Company, Merck, and Sanofi (less than $10,000 each). Dr. Conaghan has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Roche (less than $10,000 each). No other disclosures relevant to this article were reported.
References
- 1. Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barre E, et al. Evaluating drug‐free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active‐controlled AVERT study of 24 months, with a 12‐month, double‐blind treatment period. Ann Rheum Dis 2015;74:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Favalli EG, Becciolini A, Biggioggero M. Structural integrity versus radiographic progression in rheumatoid arthritis. RMD Open 2015;1:e000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lillegraven S, Prince FH, Shadick NA, Bykerk VP, Lu B, Frits ML, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 2012;71:681–6. [DOI] [PubMed] [Google Scholar]
- 4. Baker JF, Conaghan PG, Emery P, Baker DG, Ostergaard M. Relationship of patient‐reported outcomes with MRI measures in rheumatoid arthritis. Ann Rheum Dis 2017;76:486–90. [DOI] [PubMed] [Google Scholar]
- 5. Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease‐modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54:3761–73. [DOI] [PubMed] [Google Scholar]
- 6. Gandjbakhch F, Conaghan PG, Ejbjerg B, Haavardsholm EA, Foltz V, Brown AK, et al. Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol 2011;38:2039–44. [DOI] [PubMed] [Google Scholar]
- 7. Brahe CH, Krabbe S, Ostergaard M, Ornbjerg L, Glinatsi D, Rogind H, et al. Dose tapering and discontinuation of biological therapy in rheumatoid arthritis patients in routine care: 2‐year outcomes and predictors. Rheumatology (Oxford) 2019;58:110–9. [DOI] [PubMed] [Google Scholar]
- 8. Østergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, Pedersen‐Zbinden B, et al. New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum 2003;48:2128–31. [DOI] [PubMed] [Google Scholar]
- 9. Baker JF, Østergaard M, Emery P, Baker DG, Conaghan PG. Development and validation of rheumatoid arthritis magnetic resonance imaging inflammation thresholds associated with lack of damage progression. Clin Exp Rheumatol 2017;35:607–13. [PubMed] [Google Scholar]
- 10. Herrero‐Beaumont G, Martinez Calatrava MJ, Castaneda S. Abatacept mechanism of action: concordance with its clinical profile. Reumatol Clin 2012;8:78–83. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 12. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 13. Peterfy C, Burmester GR, Bykerk VP, Combe BG, DiCarlo JC, Furst DE, et al. Sustained improvements in MRI outcomes with abatacept following the withdrawal of all treatments in patients with early, progressive rheumatoid arthritis. Ann Rheum Dis 2016;75:1501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Østergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, et al. An introduction to the EULAR‐OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 2005;64 Suppl 1:i3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad HA, Baker JF, Østergaard M, Ye J, Emery P, Conaghan PG. Validating MRI‐detected inflammation thresholds predictive of structural damage progression in patients with rheumatoid arthritis in a randomized placebo‐controlled trial [abstract]. Ann Rheum Dis 2016;75:624. [Google Scholar]


