Abstract
Purpose:
Scurvy, due to vitamin C deficiency, is commonly referenced as a “forgotten” or “historical” disease. A growing number of case reports challenge this notion. Bone health providers are often consulted early in the presentation of scurvy to evaluate musculoskeletal complaints resulting from impaired collagen production and disrupted endochondral bone formation. In this report, we describe two cases of childhood scurvy. Our objective is to summarize the key features of scurvy for bone health providers, with the goal of raising awareness and facilitating diagnosis in future cases.
Case Descriptions:
Case one occurred in a twelve-year-old non-verbal, non-ambulatory female on a ketogenic diet for refractory epilepsy. Clinical findings included hemarthrosis, transfusion dependent anemia, elevated inflammatory markers and epiphysiolysis. Magnetic resonance imaging (MRI) revealed multifocal bone marrow signal abnormalities and physeal irregularities. Case two occurred in a typically developing five-year old male presenting with limp and knee pain. Symptoms progressed despite casting and immobilization. Mild anemia, elevated inflammatory markers, and multi-focal marrow and physeal MRI abnormalities were identified. Subsequent dietary history revealed total absence of fruit or vegetable consumption. The diagnosis of scurvy was confirmed in both cases by undetectable plasma vitamin C concentrations. Treatment with vitamin C led to rapid clinical improvement.
Conclusion:
Scurvy can no longer be considered a historical diagnosis and should not be forgotten when evaluating children with musculoskeletal ailments. Early recognition of the signs, symptoms, and imaging findings of scurvy can reduce the clinical burden of this disease with the timely initiation of vitamin C therapy.
Keywords: Scurvy, vitamin C deficiency, osteopenia, magnetic resonance imaging, ketogenic diet, avoidant restrictive food intake disorder
Introduction
Endochondral bone formation is dependent upon vitamin C (ascorbic acid) through its actions in promoting collagen formation, osteoblast differentiation, and matrix deposition [1]. Humans lack the gulonolactone oxidase enzyme required to synthesize endogenous vitamin C and are therefore at risk of deficiency (scurvy) due to insufficient dietary intake or gastrointestinal malabsorption [2]. Musculoskeletal symptoms are a common presenting feature in children with scurvy. Yet in many cases, the diagnosis is not made in a timely manner and often not before expensive, invasive, and unnecessary medical testing has been performed. Such delays may be ascribed to the idea that scurvy is an uncommon cause of musculoskeletal symptoms in children, but could also be related to the erroneous notion that scurvy is a historical disease.
In this report, we describe two cases of scurvy in children presenting with musculoskeletal ailments. One child had severe developmental delay and required a medically prescribed ketogenic diet, the second patient was a typically developing child subsequently determined to have avoidant restrictive food intake disorder. Using the details of these cases, along with recent reports from the medical literature, we aim to provide guidance to clinicians involved in pediatric bone health care with the goal of raising awareness, expediting diagnosis, and reducing the burden of this disease. Permission for publication of clinical information was obtained from both families. IRB oversight was not required as this report did not meet the definition of human subjects research.
Case Descriptions
Case One:
A medically complex, non-verbal, non-ambulatory, ventilatory dependent 12-year-old female with history of cystic encephalomalacia, cerebral palsy and refractory epilepsy attributed to meningoencephalitis at eight months of age presented to orthopaedics with acute onset left knee swelling. Past history was notable for a medically prescribed ketogenic diet since the age of four years, secondary osteoporosis for which she received one course of pamidronate at eight years (discontinued because of acute phase reaction), and multiple instances of feeding intolerance and abdominal distension related to bacterial overgrowth and gut dysmotility. Initial radiographs demonstrated osteopenia, but no discernable fracture. Labs were notable for anemia and elevated inflammatory markers (Supplemental Table 1). Knee MRI was notable for signal abnormalities in the marrow (hypointensity on T1 and hyperintensity on T2 weighted images) and the distal femoral epiphysis, as well as a small joint effusion and soft tissue edema (Figure 1). These findings were initially interpreted as gelatinous conversion of bone marrow related to cachexia and chronic osteonecrosis.
Figure 1: Imaging findings in two children with scurvy.
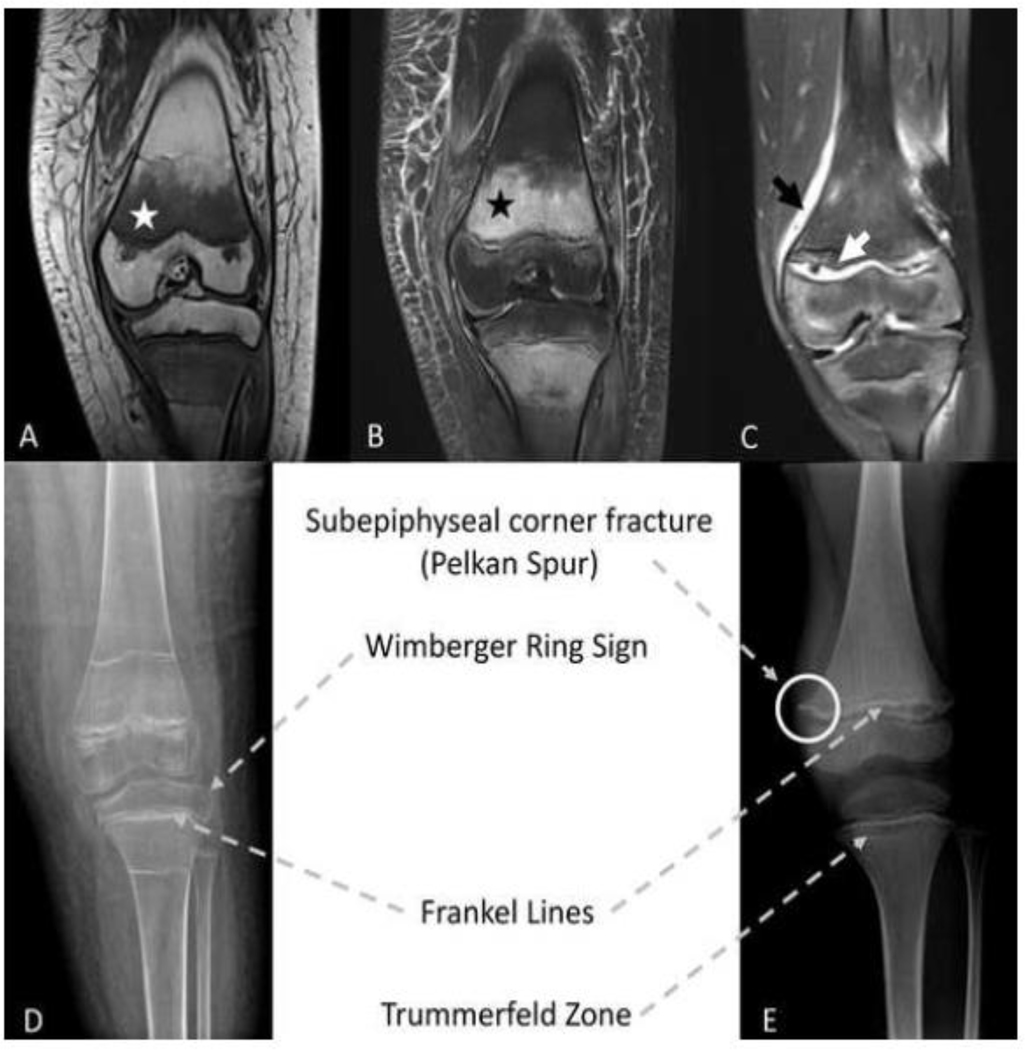
T1 (A) and T2 (B) weighted magnetic resonance imaging (MRI) images in 12-year old female. White and black stars indicate the classic findings of scurvy including hypointense marrow signal on T1 and hyperintense marrow signal on T2. (C) T2 weighted MRI image in 5-year old male. Black arrows indicate periosteal enhancement, white arrows indicate widening and irregularity at the growth plate. Classic radiographic findings of scurvy in a 12-year old female (D) and 5-year old male (E). Note - the dense metaphyseal bands visible adjacent to the distal femoral and proximal tibial physes in (D) likely represent past bisphosphonate treatment.
Over the next few weeks, the knee swelling progressed, inflammatory markers increased, and anemia worsened. Suprapatellar joint aspiration was performed and revealed bloody fluid with <200 white blood cells/μL that was negative for gram stain and cultures. Nevertheless, she was treated with a three-week course of cefazolin and cephalexin for culture-negative arthritis. Her health continued to deteriorate, resulting in multiple hospital admissions for management of the hemarthrosis, transfusion dependent anemia (normocytic with reticulocytosis, hemoglobin nadir of 4.8 g/dL), and bleeding from gums and tracheostomy. Additional testing including a technetium-99 bone scan (notable for increased uptake in the left knee), repeat arthrocentesis, lyme serology, and anti-nuclear antibody did not reveal a diagnosis. Follow up imaging showed persistence of MRI abnormalities and progression of epimetaphyseal abnormalities on radiograph as described in Table 1 and shown in Figure 1.
Table 1:
Clinical features of scurvy
| Patient Risk Factors | Laboratory Findings |
| • Avoidant restrictive food intake disorders | • Microcytic anemia (| reticulocyte count) |
| - Autism, developmental delay | • ↑ inflammatory markers (ESR, CRP) |
| - Typically developing | • ↓ vitamin C (plasma/serum < 10 μmol/L) |
| • Gastrointestinal malabsorption | Radiographic findings |
| • Medically restricted diets | • Osteopenia |
| - Ketogenic diet | • Periosteal reaction |
| - Food allergies | • Metaphyseal spurs (“Pelken spurs”) |
| • Food scarcity | • Dense metaphyseal line (“Frankel’s line”) |
| Presenting signs/symptoms | • Lucent metaphyseal band (“Trümmerfeld zone”) |
| • Painful limp | |
| • Joint swelling (knees/ankles) | • Calcified epiphyseal ring (“Wimberger’s Ring”) |
| - Hemarthrosis | |
| • Gingivitis (bleeding gums) | • Epiphysiolysis |
| • Perifollicular petechiae / bruising | MRI findings |
| • Fatigue / malaise | • Low T1 and high T2 signal in marrow |
| • Alopecia | • Periosteal enhancement (hemorrhage) |
| • Corkscrew hairs | • Adjacent soft tissue enhancement (edema) |
| Consider Scurvy in Children with Musculoskeletal Ailments in the Following Scenarios: | |
| • Unexplained bleeding/bruising/hemarthrosis | • Metaphyseal irregularities in absence of abnormal bone mineral metabolism markers |
| • Inflammatory marker elevation out of proportion to injury | • Osteomyelitis not responsive to antibiotics / chronic recurrent multifocal osteomyelitis |
| • Unexplained limp, bone pain | |
CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging
Scurvy was eventually considered after multi-disciplinary review of imaging studies, three months following initial presentation, and confirmed by an undetectable plasma vitamin C concentration. Due to past intolerance of a commercially available ketogenic formula, the patient’s sole source of nutrition was an invariant blenderized 2:1 ketogenic diet consisting of chicken breast, avocado, heavy cream, and coconut oil supplemented with iodized salt, multi-vitamin/mineral supplement (Phlexy-vits, Nutricia), baking soda, fish oil and calcium carbonate. This diet was subsequently determined to have been providing her with 60–70% for the recommended dietary allowance (RDA) for vitamin C (45 mg/day) over the past four years [3]. Furthermore, vitamins were being mixed into formula with the sodium bicarbonate, which may have reduced absorption. Vitamin C treatment at 500 mg/day for one month, followed by 250 mg/day thereafter, resulted in rapid improvement in symptoms. Notably, no further red blood cell transfusions were required. Follow up radiographs four weeks later showed mild epiphysiolysis, which healed with conservative therapy.
Case Two:
A previously healthy five-year-old male presented to orthopaedics for evaluation of right knee pain and limp. Parents initially attributed symptoms to a minor injury from jumping off a couch four weeks prior. No fracture was seen on radiographs. Initial labs were notable for mildly low hemoglobin and hematocrit and elevated erythrocyte sedimentation rate (Supplemental Table 1). MRI of the right knee was obtained and interpreted as consistent with an age-indeterminate physeal fracture based upon widening and irregularity of growth plate, increased signal at distal femoral metaphysis, and a small amount of adjacent soft tissue edema. He was placed in a cylinder cast and restricted from weight-bearing. Cholecalciferol 2000 international units daily was started for mild vitamin D deficiency.
Over the next several months, symptoms generalized to involve both lower extremities and progressed in severity such that he was unable to ambulate and relied on his upper extremities to rise from sitting (Gower’s sign). Evaluation by pediatric neurology was notable for findings of proximal muscle weakness, right quadriceps atrophy, and diminished patellar reflexes, yet creatinine kinase and nerve conduction studies were normal. Further consultations with rheumatology, bone health, and genetics were performed in consideration of possible diagnoses including chronic recurrent multi-focal osteomyelitis, juvenile idiopathic arthritis, amplified musculoskeletal pain syndrome, metabolic bone disease, heavy metal poisoning, and an inborn error of metabolism. Naproxen and levo-carnitine were started empirically.
In the setting of further clinical deterioration, the patient was admitted to the pediatric hospitalist service for coordination of care. Scurvy was ultimately suspected, six months from initial presentation, after team review of radiograph and whole body MRI images revealed systemic epimetaphyseal abnormalities characteristic of vitamin C deficiency including growth plate widening and irregularities and subepiphyseal corner fractures (radiographs) and multi-focal signal abnormalities and marrow enhancement with periosteal reaction and soft tissue edema (MRI), Figure 1. Further dietary history revealed near absence of fruit, vegetable, or juice consumption resulting in vitamin C intake below the RDA of 25 mg/day [3]. The diagnosis was confirmed by an undetectable vitamin C level in plasma. Vitamin C therapy was initiated at a dose of 200 mg daily for one week, followed by 100 mg daily thereafter. Clinical symptoms rapidly improved and patient began therapy with a food psychologist to address the underlying avoidant restrictive food intake disorder.
Discussion
Although commonly referred to as a “forgotten disease”, our review of the English language literature identified at least 50 published cases of scurvy in pediatric patients over the past two decades. Many of these reports, including our own, are notable for a significant delay in diagnosis despite the involvement of multi-disciplinary teams of pediatric providers and sub-specialists at academic medical centers. Patients were often subjected to costly and superfluous investigations (technetium bone scans, computed tomography, whole body MRIs, skin, bone, and marrow biopsies, nerve conduction studies) before a diagnosis was reached [4–10]. Importantly, because scurvy is readily reversible with vitamin C therapy, delays in diagnosis contributed to unnecessary pain, suffering, and clinical morbidity in affected patients.
The history of scurvy and its association with sailors, citrus fruits, and the early clinical trials of James Lind is well ensconced in medical lore [11]. This, plus the widespread availability of fresh fruits, vegetables and vitamin fortified foods in the developed world, may contribute to provider perception of scurvy as a historical disease. Furthermore, the signs, symptoms, and radiographic findings of scurvy, especially when viewed in isolation, can mimic more widely known conditions including growth plate injury, osteomyelitis, and neuromuscular, rheumatologic or hematologic disease. A summary of clinical features that should raise suspicion for scurvy is provided in Table 1. The suggested clinical approach for potential cases of scurvy is provided in Supplemental Figure 1.
The re-emergence of scurvy in the pediatric population has been described in children with autism and developmental delay where selective eating due to sensory characteristics is common [6, 7, 9, 12–14]. Cases of scurvy in typically developing children, as in our second case, are unusual but not unprecedented [8, 15]. Bone health providers are well-versed in querying calcium and vitamin D intake, but may not have the resources to conduct the detailed dietary history needed to uncover other nutrient deficiencies. Likewise, parental reports of selective eating are commonplace in pediatric practice and may be overlooked. When selective eating is accompanied by poor growth, weight loss, or clinically significant nutrient deficiencies, involvement of a pediatric registered dietitian is essential and referral to a behavioral health provider for consideration of avoidant restrictive food intake disorder is required [16]. A finding of vitamin C (or any micronutrient) deficiency should prompt further evaluation for the coexistence of other macro- and micronutrient deficiencies (i.e. protein, calcium, vitamin D, zinc, vitamin K, thiamine) that could adversely affect bone health. Additionally, it should be noted that scurvy is not exclusively a pediatric disorder, as evidenced by recent reports in adults with malabsorption [17], disordered eating [18] and social isolation [19].
Ketogenic diets, characterized by marked restriction in carbohydrates (including vitamin C containing fruits), are increasingly prescribed by neurologists to manage refractory epilepsy in children and are gaining favor among patients (and their parents) afflicted with other conditions including diabetes [20, 21]. We are aware of two previously reported cases of scurvy in children treated with a ketogenic diet [22, 23]. An unusual feature of our index case was the finding of an undetectable plasma vitamin C concentration despite daily consumption of a vitamin C containing supplement. We speculate that co-administration of sodium bicarbonate [24] and the history of bacterial overgrowth led to malabsorption of vitamin C resulting in severe deficiency. Further study is needed to determine if there are clinical situations (such as the ketogenic diet) where vitamin C requirements may be greater than what is needed for the general population. In the meantime, patients and providers should be aware of the risk of micronutrient deficiency with the ketogenic diet and seek the consultation of a dietician.
Conclusion
As evidenced by our cases and in recent publications, it is no longer appropriate to view scurvy as a historical disease. Scurvy should be considered when musculoskeletal ailments follow an atypical course or when accompanied by unexplained bleeding, anemia, or elevated inflammatory markers. A high degree of suspicion, coupled with early involvement of a dietitian and careful review of radiograph and MRI images with an experienced radiologist will expedite diagnosis, reduce unnecessary medical testing, and limit the clinical suffering often seen with this disease.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health: K23DK114477 (DRW)
Footnotes
Disclosures: Edward D. Alten, Apeksha Chaturvedi, Melissa Cullimore, Anne A. Fallon, Leeann Habben, Inna Hughes, Natasha T. O’Malley, Homaira Rahimi, Danielle Renodin-Mead, Brianne L. Schmidt, Geoffrey A. Weinberg, and David R. Weber all declare that they have no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Aghajanian P, Hall S, Wongworawat MD, Mohan S (2015) The roles and mechanisms of actions of vitamin c in bone: New developments. J Bone Miner Res 30 (11):1945–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikimi M, Yagi K (1991) Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr 54 (6 Suppl):1203s–1208s [DOI] [PubMed] [Google Scholar]
- 3.National institutes of health, office of dietary supplements. Vitamin c: Fact sheet for professionals. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ Accessed 9/20/2019
- 4.Bingham AC, Kimura Y, Imundo L (2003) A 16-year-old boy with purpura and leg pain. J Pediatr 142 (5):560–563 [DOI] [PubMed] [Google Scholar]
- 5.Brennan CM, Atkins KA, Druzgal CH, Gaskin CM (2012) Magnetic resonance imaging appearance of scurvy with gelatinous bone marrow transformation. Skeletal Radiol 41 (3):357–360 [DOI] [PubMed] [Google Scholar]
- 6.Duggan CP, Westra SJ, Rosenberg AE (2007) Case records of the massachusetts general hospital. Case 23–2007. A 9-year-old boy with bone pain, rash, and gingival hypertrophy. N Engl J Med 357 (4):392–400 [DOI] [PubMed] [Google Scholar]
- 7.Ma NS, Thompson C, Weston S (2016) Brief report: Scurvy as a manifestation of food selectivity in children with autism. J Autism Dev Disord 46 (4):1464–1470 [DOI] [PubMed] [Google Scholar]
- 8.Nastro A, Rosenwasser N, Daniels SP, Magnani J, Endo Y, Hampton E, Pan N, Kovanlikaya A (2019) Scurvy due to selective diet in a seemingly healthy 4-year-old boy. Pediatrics 144 (3):e20182824 [DOI] [PubMed] [Google Scholar]
- 9.Swed-Tobia R, Haj A, Militianu D, Eshach O, Ravid S, Weiss R, Aviel YB (2019) Highly selective eating in autism spectrum disorder leading to scurvy: A series of three patients. Pediatr Neurol 94:61–63 [DOI] [PubMed] [Google Scholar]
- 10.Vaezipour N, Leibundgut K (2018) Nonalimental scurvy with relapse symptoms after stopping oral vitamin c supplementation. Pediatrics 142 (2):e20172139 [DOI] [PubMed] [Google Scholar]
- 11.Baron JH (2009) Sailors’ scurvy before and after james lind--a reassessment. Nutr Rev 67 (6):315–332 [DOI] [PubMed] [Google Scholar]
- 12.Kinlin LM, Blanchard AC, Silver S, Morris SK (2018) Scurvy as a mimicker of osteomyelitis in a child with autism spectrum disorder. Int J Infect Dis 69:99–102 [DOI] [PubMed] [Google Scholar]
- 13.Gulko E, Collins LK, Murphy RC, Thornhill BA, Taragin BH (2015) Mri findings in pediatric patients with scurvy. Skeletal Radiol 44 (2):291–297 [DOI] [PubMed] [Google Scholar]
- 14.Weinstein M, Babyn P, Zlotkin S (2001) An orange a day keeps the doctor away: Scurvy in the year 2000. Pediatrics 108 (3):E55 [DOI] [PubMed] [Google Scholar]
- 15.Frank BS, Runciman M, Manning WA, Ivy DD, Abman SH, Howley L (2019) Pulmonary hypertension secondary to scurvy in a developmentally typical child. J Pediatr 208: 291–291.e292 [DOI] [PubMed] [Google Scholar]
- 16.Bryant-Waugh R (2019) Avoidant/restrictive food intake disorder. Child Adolesc Psychiatr Clin N Am 28 (4):557–565 [DOI] [PubMed] [Google Scholar]
- 17.Lipner S (2018) A classic case of scurvy. Lancet (London, England) 392 (10145):431–431 [DOI] [PubMed] [Google Scholar]
- 18.Urueña-Palacio S, Ferreyro BL, Fernández-Otero LG, Calo PD (2018) Adult scurvy associated with psychiatric disorders and breast feeding. BMJ case reports 2018 bcr2017223686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levavasseur M, Becquart C, Pape E, Pigeyre M, Rousseaux J, Staumont-Sallé D, Delaporte E (2015) Severe scurvy: An underestimated disease. European journal of clinical nutrition 69 (9):1076–1077 [DOI] [PubMed] [Google Scholar]
- 20.Joshi SM, Singh RK, Shellhaas RA (2013) Advanced treatments for childhood epilepsy: Beyond antiseizure medications. JAMA Pediatr 167 (1):76–83 [DOI] [PubMed] [Google Scholar]
- 21.Lennerz BS, Barton A, Bernstein RK, et al. (2018) Management of type 1 diabetes with a very low-carbohydrate diet. Pediatrics 141 (6):e20173349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willmott NS, Bryan RA (2008) Case report: Scurvy in an epileptic child on a ketogenic diet with oral complications. Eur Arch Paediatr Dent 9 (3):148–152 [DOI] [PubMed] [Google Scholar]
- 23.Ahmad SA, Al Thobiti TA, El Toum M, Al Harbi F (2018) Florid scurvy in an autistic child on a ketogenic diet. Pediatr Emerg Care [published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Hawthorne BE, Storvick CA (1948) Effect of sodium bicarbonate and ammonium chloride on ascorbic acid metabolism of adults. Proc Soc Exp Biol Med 67 (4):447–449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


