Abstract
Diabetes is not only an endocrine but also a vascular disease. Cardiovascular complications are the leading cause of morbidity and mortality associated with diabetes. Diabetes affects both large and small vessels and hence diabetic complications are broadly classified as microvascular (retinopathy, nephropathy and neuropathy) and macrovascular (heart disease, stroke and peripheral arterial disease) complications. Endothelial dysfunction, defined as an imbalance of endothelium-derived vasoconstrictor and vasodilator substances, is a common denominator in the pathogenesis and progression of both macro and microvascular complications. While the pathophysiology of diabetic complications is complex, endothelin-1 (ET-1), a potent vasoconstrictor with proliferative, profibrotic, and proinflammatory properties, may contribute to many facets of diabetic vascular disease. This review will focus on the effects of ET-1 on function and structure of microvessels (retina, skin and mesenteric arteries) and macrovessels (coronary and cerebral arteries) and also discuss the relative role(s) of endothelin A (ETA) and ETB receptors in mediating ET-1 actions.
Keywords: cerebrovasculature, retina, diabetes, peripheral vasculature, endothelial function, endothelin, ETA receptor, ETB receptor, diabetic complications
1. Introduction
The incidence and prevalence of diabetes has risen steeply in the last decade [1, 2]. Not just the sheer number of patients but increased mortality and morbidity due to increased cardiovascular diseases associated with diabetes are of great concern [1]. The fact that there is an alarming increase in the number of younger patients diagnosed with type 2 diabetes intensifies this concern because development of these complications depend on the duration of the disease and the degree of glycemic control [2, 3]. While the ultimate goal is to prevent the development of diabetes and cure the disease, prevention and treatment of complications is equally important. Most if not all diabetic complications have a significant vascular component and hence traditionally classified as microvascular (nephropathy, retinopathy and neuropathy) and macrovascular (heart disease, stroke and peripheral arterial disease) [3, 4]. Accumulating evidence suggest that small vessel disease is also important for heart disease, stroke and neurodegenerative diseases such as dementia and Alzheimer’s disease in patients with diabetes [6–8]. Numerous studies have shown that glycemic control is an effective strategy in prevention and reduction of retinopathy and nephropathy, however, the impact of glycemic control on macrovascular complications and small vessel disease of the heart and brain is not known [3, 4]. There is a great need to develop new therapeutic approaches to reduce the burden of diabetic complications.
Endothelial dysfunction is a prominent feature of cardiovascular diseases and also plays an important role in both micro and macrovascular complications of diabetes [5, 6]. In early stages, the imbalance of increased vasoconstrictors like ET-1 and decreased bioavailability of vasodilator nitric oxide (NO) due to hyperglycemia-driven oxidative stress results in impaired vasorelaxation [7]. As the disease progresses, the prolonged loss of protective effects of NO and activation of the ET system leads to structural alterations, thrombosis and plaque development in the vessel wall [7, 8]. ET-1 is not only one of the most potent vasoconstrictors, but also stimulates proliferation of vascular smooth muscle cells (VSMCs), promotes fibrosis and inflammation [9]. These properties make ET-1 a likely candidate that play a major role in diabetic vascular complications. Given that the regulation of endothelial function may vary in different vascular beds, this review will briefly discuss the ET biology with relevance to diabetes and its complications and concentrate on our current understanding of the role of ET-1 on function and structure of multiple vascular beds including retinal, cerebral, coronary, mesenteric and peripheral circulation in diabetes.
2. The ET system
ET-1 is a potent vasoconstrictor and also stimulates cell proliferation [10]. ET-1 mediates its diverse effects via two distinct G protein-coupled receptor subtypes, ETA and ETB. As diagrammed schematically in Fig. 1, ETA receptors, localized mainly on VSMCs of blood vessels, is responsible for the contractile and proliferative response to ET-1 [11]. The role of ETB in vascular regulation is more complex. For instance, ETB receptors located on endothelial cells mediate vasodilatation via the release of relaxing factors such as NO and prostacyclin(PGI2). This receptor subtype can also lead to vasoconstriction when the receptors are located on VSMCs in certain vascular beds [11]. Thus, the net contractile effect of ET-1 depends mainly on overall ET receptor profile, defined as the relative density of ETA and ETB on smooth muscle cells to ETB receptors on endothelial cells. This duality of function of ET-1 underscores the importance of binding and function of both ET receptor subtypes.
Fig 1.
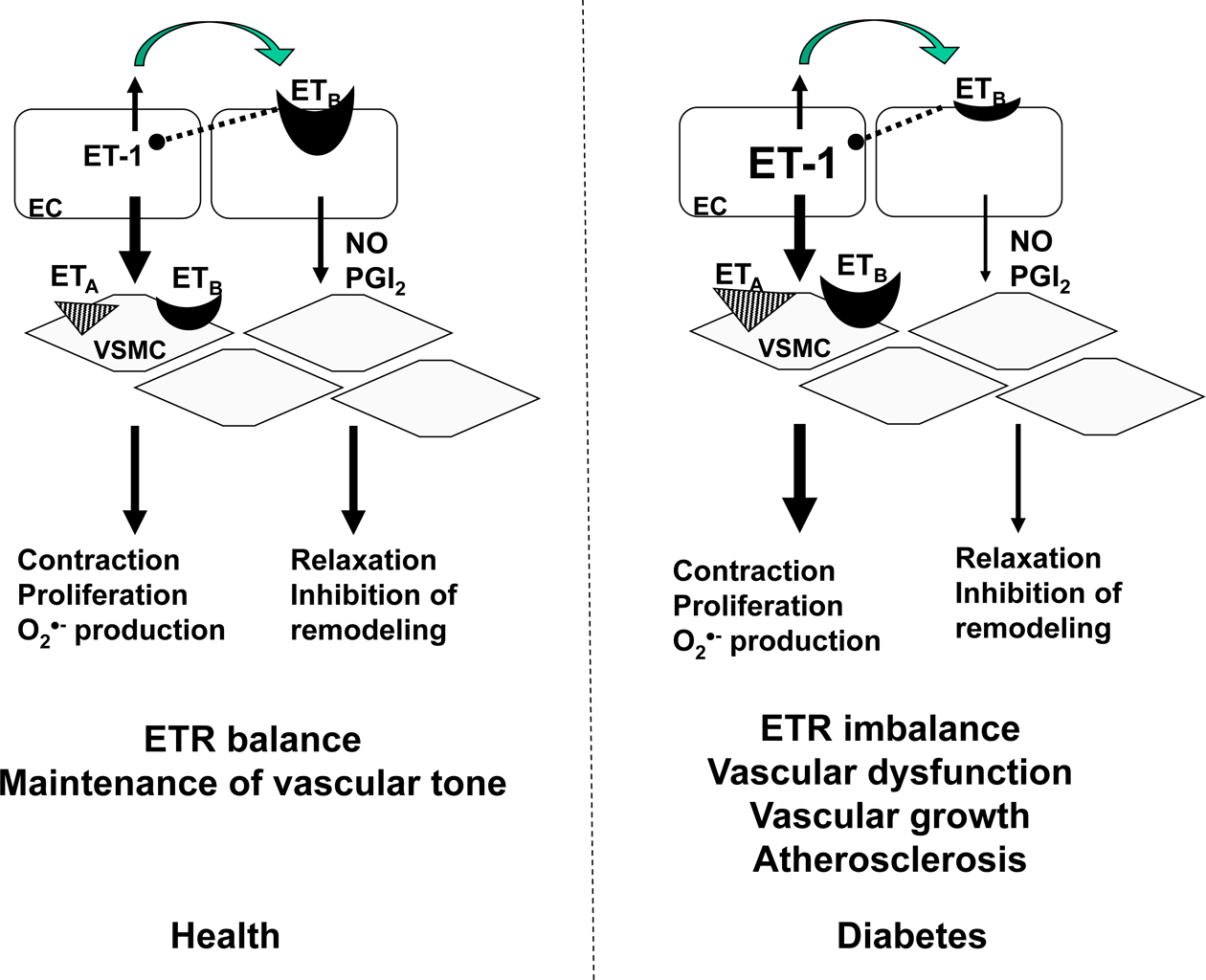
The vascular ET system. Under physiological conditions, majority (80%) of ET-1 produced by endothelial cells is secreted towards the underlying VSMC. The balance of EC ETB and VSMC ETA and ETB receptors in healthy blood vessels is critical for regulation of vascular tone. In diabetes, ET-1 production as well as VSMC ETA and ETB receptors are increased favoring a more contractile and proliferative phenotype leading to complications of diabetes. EC, endothelial cells; NO, nitric oxide, O2●-, superoxide; PGI2, prostacyclin; VSMC, vascular smooth muscle.
Several studies have suggested that endothelial ETB receptors may be vasculoprotective [12–14]. First, selective blockade of ETB receptors exacerbates ischemic brain damage [13]. Second, ETB receptor deficiency augments neuronal damage upon exposure to hypoxia-ischemia in vivo [15]. Third, inhibition of the ETB receptor system in a knock-out mouse model or pharmacological blockade by an ETB antagonist leads to enhanced intimal hyperplasia observed in carotid arteries after injury induced by ligation [12]. Moreover, ETB is involved in the clearance of ET peptides, since blockade of this receptor subtype leads to increased plasma levels of ET-1 in animals and humans [10]. Activation of this receptor subtype on endothelial cells also exerts a negative feedback on ET-1 synthesis by endothelial cells [16]. Thus, any decrease in endothelial ETB receptors would result in increased ET-1 biosynthesis, decreased clearance, and diminished production of NO and PGI2, all of which result in unopposed ETA activation. These studies clearly demonstrate favorable effects of ETB receptor activation under normal conditions. However, the expression profile of ETB receptors and ET receptor ratios may change and dictate detrimental effects of ET-1 in diabetes.
A potential role of ET-1 in various cardiovascular diseases such as hypertension and heart failure fueled the development of ET receptor antagonists as new line of therapeutics. Within five years of discovery of ET-1, an orally active ET receptor antagonist was reported and shortly after, bosentan, the dual ETA and ETB receptor antagonist, was introduced for the treatment of pulmonary hypertension. This was followed by a number of receptor subtype selective and nonselective (dual) antagonists [17]. Despite this rapid pace and accumulating evidence which show that ET-1 contributes to the development of various cardiovascular disorders and related complications, clinical trials with ET receptor antagonists in cardiovascular diseases have been rather disappointing [17]. Part of these negative results may stem from the complexity of ET receptor expression and interaction in various tissues under physiological and pathological conditions as briefly discussed above.
3. Diabetes and ET-1
Several lines of evidence suggest that the ET-1 system may contribute to diabetic vascular disease. Plasma ET-1 levels are elevated in patients with type 1 or type 2 diabetes [18–20]. A significant correlation has been observed between plasma ET-1 levels and diabetic complications. ET-1 levels are higher in patients with microalbuminuria, elevated glycosylated hemoglobin (HbA1c) concentrations retinopathy [18, 19].
In vitro studies also support possible upregulation of ET-1 by hyperglycemia and insulin [21, 22]. We have shown increased plasma and tissue ET-1 in type 2 diabetic Goto-Kakizaki (GK) rats [23]. In streptozotocin (STZ)-induced type 1 diabetes, mesenteric ET-1 levels are greater than those measured control animals [24]. Furthermore, elements that are required for ET-1 synthesis are upregulated in diabetes [25, 26, 27]. These studies suggest that the upregulation of ET-1 and its receptors may be involved in vascular complications of diabetes. Numerous studies have tested this hypothesis further using pharmacological interventions to block the activation of ET receptors as discussed below. Since the role of ET-1 in diabetic nephropathy has been the subject of several review articles [28–31], this review will focus on retinal, cerebral, coronary and peripheral vascular function and structure in diabetes.
3.1. ET-1 and the retina
Endothelial cells as well as nonvascular cells in the retina produce ET-1 and also express ET receptors [8]. Studies have suggested that ET-1 impairs autoregulation of retinal blood flow which can cause hyperperfusion promoting formation of retinal microaneurysms and edema [32, 33]. On the other hand, it is also possible that ET-1-mediated vasoconstriction can trigger a hypoxic state which later leads to pathological angiogenesis as seen in diabetic retinopathy. Deng et al had reported that there is an upregulation of ET-1 and ETA receptors in the retina of type 1 diabetic rats and this was associated with increased resistivity index, an indicator of vasoconstriction. Treatment with the dual receptor antagonist bosentan prevented these changes [34]. The ET system was found to be upregulated in type 2 diabetes as well [35]. The same group recently reported that glucose-induced ET-1 expression is regulated by extracellular signal related kinase 5 (ERK5) in the endothelial cells and retina of diabetic rats [36]. An interesting recent study reported that ETA receptor antagonism by atrasentan partially prevents diabetes-induced decrease in retinal flow rate and wall shear rate indicating that ET-1 mediates early decreases in blood flow [37]. It has to be recognized that all these intervention studies were performed in experimental models. In diabetic patients with retinopathy plasma ET-1 levels are increased [38], however, Ogata et al. reported lower vitreous ET-1 levels in patients with proliferative retinopathy [39]. Future studies are needed to prove causality and develop ET receptor antagonists as a therapeutic modality in diabetic retinopathy.
3.2. ET-1 and the cerebral circulation
Maintenance of cerebral blood flow across a wide pressure range is critical for brain perfusion and this is achieved by the regulation of vascular tone by myogenic, neuronal, and ligand-dependent mechanisms. However, alterations to this system may be detrimental and could contribute to cerebrovascular disease. While an acute interruption of cerebral blood flow may cause stroke, chronic impairment of perfusion is associated with neurodegenerative diseases like dementia and Alzheimer’s disease, all of which are more common in patients with diabetes. Studies have demonstrated increased myogenic tone in experimental diabetes [40–42]. In addition to increased basal tone, cerebral arteries from diabetic animals exhibit diminished endothelium derived relaxation [40, 43, 44]. Increasing evidence suggests that ET-1 is involved in the pathology of cerebrovascular disease [45]. Contractile responses to ET-1 is augmented in the rat and rabbit basilar arteries of type 1 diabetic rats, respectively [46, 47], and augmented myogenic tone is decreased after ET receptor antagonism [40] in type 1 diabetes. We have shown that basilar arteries from GK rats, a model of type 2 diabetes, exhibit increased sensitivity to ET-1 [48]. Endothelium-dependent relaxation was also impaired in this model. ETA receptor blockade restored relaxation to control values in the GK animals and selective ETB blockade caused paradoxical constriction in diabetes. This study suggested that there may be an upregulation of VSMC ETB receptors and that both endothelial and VSMC ETB receptors are involved in the regulation of vascular function in diabetes. This study also prompted the question: “Is this paradoxical constriction mediated by activation of unoccupied ETA receptors in the presence of an ETB antagonist or is it due to the loss of vasculoprotective effects of ETB receptors?” In a recent study, we used dual antagonist bosentan to address this issue and hypothesized that dual blockade is not as effective as selective ETA antagonism since bosentan negates the beneficial effects of ETB receptor activation [49]. In contrast to our hypothesis, we found dual antagonism to be as effective as selective ETA blockade and concluded that when blocked simultaneously with the ETA receptor, the ETB receptor antagonism is protective by improving cerebrovascular dysfunction in diabetes.
ET-1 is not only a potent vasoconstrictor but also involved in vascular remodeling [12, 50]. We have reported that diabetes is associated with remodeling of middle cerebral arteries and this response is partially blocked by ETA receptor antagonism suggesting that this may be a preventive therapeutic approach [23]. Given that genetic or pharmacological inhibition of ETB receptors worsen vascular remodeling in a wire injury model, we hypothesized that diabetes decreases protective endothelial ETB receptors contributing to vascular remodeling and antagonism of this receptor exacerbates changes in the vascular structure. To address this question, control and diabetic rats were treated with selective ETB antagonist A192621 and dual antagonist bosentan for 4 weeks starting at the onset of diabetes. Endothelial and VSMC ET receptors were also quantified. VSMC ETA and ETB receptors were increased in diabetes and this was prevented by chronic bosentan treatment. In contrast to our hypothesis, diabetes did not influence endothelial ETB receptors. Middle cerebral artery (MCA) wall thickness was also increased in diabetes. Selective ETB receptor antagonism with A192621 blunted and combined ETA and ETB receptor blockade with bosentan completely attenuated this response (manuscript under review). On the other hand, A192621 treatment augmented remodeling in control animals indicating a physiological protective role for this receptor subtype. The finding that bosentan treatment prevents changes in ET receptor profile suggests that ET-1 has a positive feedback on the expression of its receptors in the cerebrovasculature underscoring the fact that the ET receptor antagonism may yield different results in healthy and diseased states.
3.3. ET-1 and mesenteric circulation
Since mesenteric arteries are considered to be resistance vessels contributing to the regulation of blood pressure, function and structure of mesenteric arteries in diabetes has been the subject of many studies. In an early study Makino et al. reported that in type 1 diabetes, plasma and tissue ET-1 levels are increased, and ET-1-induced contraction is reduced due to desensitization of the ETA receptors [51, 52]. Other studies including our own have demonstrated increased sensitivity and reactivity to ET-1 in type 1 [53] and type 2 diabetes [54–59]. We have established that the mesenteric circulation in type 2 diabetic GK rats is hyperreactive to the potent vasoconstrictor ET-1 and shows impaired relaxation to ACh in an NO-dependent manner [58]. In a follow-up study, we tested the hypothesis that ETA antagonism would improve vascular function by attenuating constrictor responses to ET-1 and improving relaxation governed by NO, whereas ETB blockade would further exacerbate ET-1 vasoconstriction and decrease relaxation in a dose-dependent manner. As predicted, ETA antagonism attenuated hyperreactivity in diabetes but results with ETB receptor antagonism were not as clear. Selective blockade with a lower dose of A192621 augmented vasoconstriction in controls while it had no further effect on ET-1 hyperreactivity in diabetes suggesting that in control animals blockade of ETB receptor worsens reactivity. The higher dose of A192621 showed an ETA-like effect and decreased vasoconstriction in diabetes. These studies suggest an interaction between receptor subtypes and warrants further investigation.
Several studies suggest that ET-1 interacts with other contractile pathways to stimulate a hyperactive state in diabetes. In type 1 diabetes, ET-1 has been suggested to interact with thromboxane A2 to mediate vasoconstriction in a vascular bed-specific manner [53]. Matsumoto et al. recently reported that short term angiotensin II receptor type1 receptor blockade normalizes the ET-1-mediated contractility in type 2 diabetes [60]. Same group also reported that MEK/ERK pathway mediated enhanced contraction to ET-1 in diabetes [54]. From a therapeutic stand point, it was suggested that peroxisome proliferator-activated receptor (PPAR)γ agonists (thiazolidinediones) that are heavily used to treat diabetes may improve vascular dysfunction by regulating ET-1 expression [55]. Interestingly, all the aforementioned studies so far have been conducted in male animals. Only one study investigated the vascular function in female diabetic mice and reported that ET-1-induced contractions are increased in female type 1 diabetic mice [61]. Given the differences in cardiovascular disease manifestation in males and females, it is of great interest to investigate gender differences in vascular dysfunction in diabetes in future studies.
Not only function but the structure of mesenteric arteries is dysregulated in diabetes [62]. Gilbert et al. reported that there is mast cell infiltration, vascular hypertrophy and increased growth factor expression in type 1 diabetes. They also showed that dual ET antagonism with bosentan significantly reduced vascular remodeling, matrix deposition and epidermal growth factor but not transforming growth factor expression [63]. Our results in type 2 diabetes demonstrated increased vascular remodeling indicated by greater media:lumen associated with increased collagen deposition [14]. ETA receptor blockade prevented this increase whereas ETB receptor antagonism caused further thickening of the medial layer. Accordingly, collagen deposition was reversed by ETA receptor blockade but exacerbated with ETB receptor antagonism. All together, these results suggest that ET-1 contributes to the remodeling of mesenteric resistance arteries in diabetes via activation of ETA receptors and that ETB receptors provide vasculoprotective effects. These findings also emphasize the differences in the ET system in different vascular beds. The same treatment with the ETB receptor antagonist, as discussed above, did not worsen but blunted vascular remodeling in the cerebral circulation (manuscript under review). This may be due to the differences in ET receptor subtype distribution and highlights the complexities of using ET receptor antagonists in clinical studies.
3.4. ET-1 and coronary circulation
The role of ET-1 on coronary circulation and on heart function in diabetes also deserves mention. Kamata el al. investigated the responses to several vasoconstrictors in a type 1 model of diabetes using isolated perfused hearts and reported that coronary artery constriction to low concentrations of ET-1 is exaggerated in diabetes potentially due to the alterations in the voltage-gated calcium channels [64]. Another study reported increased sensitivity to ET-1 as well as a rapid vasoconstriction to big ET-1 in diabetic hearts [65]. Verma and colleagues studied vascular responses to ET-1 and provided evidence that coronary hyperreactivity to ET-1 is normalized in bosentan treated type 1 diabetic animals [66]. Another group also reported increased contractility to ET-1 that is associated with augmented protein kinase C(PKC) activation in again type 1 diabetes [67]. Katakam et al. extended these studies to an insulin resistance model. ET-1-induced vasoconstriction is reduced in obese Zucker rats and this stems from increased ETB-mediated nitric oxide generation and uncoupling of calcium signaling [68].
As in other vascular beds, ET-1 signaling can contribute to structural changes in the coronary vascular bed. ET-1 is associated with the development of atherosclerosis via stimulation of VSMC growth, migration, matrix remodeling and growth factor expression [69–72]. Furthermore, the ET system is upregulated in atherosclerotic lesions in humans as well as in experimental models [73–76]. ET receptor antagonism markedly reduces atherosclerotic lesions in low density lipoprotein (LDL) receptor and Apo-E knock-out mice [77, 78]. Several studies investigated the distribution of ET receptors in the coronary circulation. Human right coronary artery has predominantly ETA receptors [79–81]. On the other hand, left coronary artery has relatively greater number of ETB receptors [82]. Interestingly, ETB receptors are increased on atherosclerotic left anterior descending coronary artery [83]. Lee and colleagues reported that ET-1-mediated calcium signaling and tyrosine phoshorylation are increased in diabetic and dyslipidemic pigs with coronary artery disease. The same study also showed that statin treatment can inhibit ET upregulation and signaling [84]. Given that atherosclerotic coronary artery disease is increased up to 6-fold in patients with diabetes, ET-1 may be involved in the development of advanced atherosclerosis in diabetes.
Since this review focuses on the vasculature, ET-1 effects on cardiac myocytes and matrix are not discussed but it needs to be stated that ET-1 can also be involved in the development and progression of diabetic cardiomyopathy [45, 85–88]. A recent very interesting study reported that endothelial-cell derived ET-1 causes cardiac fibrosis via stimulation of the transition of endothelial cells to mesenchymal origin fibroblasts [89].
ET-1 and peripheral circulation
Skin ulcerations and impaired wound healing are important clinical problems in diabetes. Microvascular disease is an important component of these issues. As recently reviewed by Kalani, the pathogenesis of diabetic skin microagiopathy is complex [7]. Reduced microvascular reactivity and increased blood flow through arteriovenous shunts impair nutritive capillary circulation. An intriguing study showed that ETA receptor antagonism can increase nutritive skin microcirculation profoundly in patients with type 2 diabetes [90]. Augmented ET-1 vasoconstriction of precapillary resistance vessels has been suggested to increase capillary pressure leading to arteriovenous shunting. Therefore, a reduction in capillary pressure may improve this situation and increase nutritive capillary blood flow [91]. A pilot study investigated the potential use of ET receptor antagonism in patient with critical limb ischemia and showed that local infusion of ETA receptor antagonist increased oxygen tension in the foot and toe circulation indicative of improved nutritive capillary blood flow [92]. A recent study reported decreased ET-1-mediated constriction in microvessels isolated from the chest wall skeletal muscle of diabetic patients undergoing coronary bypass surgery. Authors speculated this response may contribute to vasomotor dysfunction and subsequent tissue edema in patients with diabetes [93].
4. Conclusions
ET-1 is an important vasoactive factor with pleitropic actions. This brief review summarizes our current understanding of ET-1 actions on function and structure of multiple vascular beds that are relevant to diabetic complications. While these mostly experimental studies strongly suggest a role for ET-1 in the pathogenesis and progression of diabetic vascular disease and ET receptor antagonists may have important therapeutic applications, direct evidence would come from human studies with these antagonists. However, failed trials with ET receptor antagonists due to increased side effects prevent us from moving forward [94]. It is clear that ET receptor biology is quite complex and may differ in health and disease states. A better understanding of the ET receptors and interaction with other therapeutic targets for vascular disease such as statins, thiazolidones and angiotensin II receptor blockers may offer an alternative strategy.
Acknowledgements
Adviye Ergul is the recipient of American Heart Association Established Investigator Award (0740002N) and VA Merit Award.
References
- 1.American Diabetes Association. National diabetes fact sheet. http://www.diabetes.org/diabetes-statistics.jsp
- 2.Calcutt NA, Cooper ME, Kern TS, and Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akalin S, Berntorp K, Ceriello A, Das AK, Kilpatrick ES, Koblik T, Munichoodappa CS, Pan CY, Rosenthall W, Shestakova M, Wolnik B, Woo V, Yang WY, and Yilmaz MT. Intensive glucose therapy and clinical implications of recent data: a consensus statement from the Global Task Force on Glycaemic Control. Int J Clin Pract. 2009;63:1421–1425 [DOI] [PubMed] [Google Scholar]
- 4.Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, Krahenbuhl S, and Diem P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006;152:27–38 [DOI] [PubMed] [Google Scholar]
- 5.Versari D, Daghini E, Virdis A, Ghiadoni L, and Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32 Suppl 2:S314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262:173–183 [DOI] [PubMed] [Google Scholar]
- 7.Kalani M The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc Health Risk Manag. 2008;4:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam HC. Role of endothelin in diabetic vascular complications. Endocrine. 2001;14:277–284 [DOI] [PubMed] [Google Scholar]
- 9.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29 [DOI] [PubMed] [Google Scholar]
- 10.Webb ML and Meek TD. Inhibitors of endothelin. Med Res Rev. 1997;17:17–67 [DOI] [PubMed] [Google Scholar]
- 11.Ergul A Development of endothelin receptor antagonists as potential therapeutic agents. Exp Opin Ther Patents. 2003;13:33–44 [Google Scholar]
- 12.Murakoshi N, Miyauchi T, Kakinuma Y, Ohuchi T, Goto K, Yanagisawa M, and Yamaguchi I. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106:1991–1998 [DOI] [PubMed] [Google Scholar]
- 13.Chuquet J, Benchenane K, Toutain J, MacKenzie ET, Roussel S, and Touzani O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke. 2002;33:3019–3025 [DOI] [PubMed] [Google Scholar]
- 14.Sachidanandam K, Portik-Dobos V, Harris AK, Hutchinson JR, Muller E, Johnson MH, and Ergul A. Evidence for vasculoprotective effects of ETB receptors in resistance artery remodeling in diabetes. Diabetes. 2007;56:2753–2758 [DOI] [PubMed] [Google Scholar]
- 15.Siren A-L, Lewczuk P, Hasselblatt M, Dembowski C, Schilling L, and Ehrenreich H. Endothelin B receptor deficiency augments neuronal damage upon exposure to hypoxia-ischemia in vivo. Brain Research. 2002;945:144–149 [DOI] [PubMed] [Google Scholar]
- 16.Sanchez R, MacKenzie A, Farhat N, Nguyen TD, Stewart DJ, Mercier I, Calderone A, and Thorin E. Endothelin B receptor-mediated regulation of endothelin-1 content and release in cultured porcine aorta endothelial cell. J Cardiovasc Pharmacol. 2002;39:652–659 [DOI] [PubMed] [Google Scholar]
- 17.Battistini B, Berthiaume N, Kelland NF, Webb DJ, and Kohan DE. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drug. Exp Biol Med (Maywood). 2006;231:653–695 [PubMed] [Google Scholar]
- 18.Collier A, Leach JP, McLellan A, Jardine A, Morton JJ, and Small M. Plasma endothelin-like immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care. 1992;15:1038–1040 [DOI] [PubMed] [Google Scholar]
- 19.Haak T, Jungmann E, Felber A, Hillmann U, and Usadel KH. Increased plasma levels of endothelin in diabetic patients with hypertension. Am J Hypertens. 1992;5:161–166 [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Ghatei MA, Lam HC, O’Halloran DJ, and Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310 [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Ohnaka K, Takayanagi R, Umeda F, and Nawata H. Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990;267:16–18 [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y, Kasai K, Nakamura T, Emodo T, and Shimoda S-I. Effects of glucose and insulin on immunoreactive endothelin-1 release from cultured bovine endothelial cells. Metabolism. 1991;40:165–169 [DOI] [PubMed] [Google Scholar]
- 23.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, and Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644 [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, Miyamori I, Yoneda T, and Takeda R. Production of endothelin-1 from the mesenteric arteries of streptozotocin induced diabetic rats. Life Sci. 1991;48:2553–2556 [DOI] [PubMed] [Google Scholar]
- 25.Khamaisi M, Dahan R, Hamed S, Abassi Z, Heyman SN, and Raz I. Role of protein kinase C in the expression of endothelin converting enzyme-1. Endocrinology. 2009;150:1440–1449 [DOI] [PubMed] [Google Scholar]
- 26.Khamaisi M, Raz I, Shilo V, Shina A, Rosenberger C, Dahan R, Abassi Z, Meidan R, Lecht S, and Heyman SN. Diabetes and radiocontrast media increase endothelin converting enzyme-1 in the kidney. Kidney Int. 2008;74:91–100 [DOI] [PubMed] [Google Scholar]
- 27.Su W, Dai DZ, Liu HR, Na T, and Dai Y. Upregulated endothelin system in diabetic vascular dysfunction and early retinopathy is reversed by CPU0213 and total triterpene acids from Fructus Corni. Clin Exp Pharmacol Physiol. 2007;34:1228–1233 [DOI] [PubMed] [Google Scholar]
- 28.Turgut F and Bolton WK. Potential new therapeutic agents for diabetic kidney disease. Am J Kidney Dis. 2010;55:928–940 [DOI] [PubMed] [Google Scholar]
- 29.Neuhofer W and Pittrow D. Endothelin receptor selectivity in chronic kidney disease: rationale and review of recent evidence. Eur J Clin Invest. 2009;39 Suppl 2:50–67 [DOI] [PubMed] [Google Scholar]
- 30.Pittrow D and Kirch W. Update on endothelin-related diseases. Eur J Clin Invest. 2009;39 Suppl 2:1–2 [DOI] [PubMed] [Google Scholar]
- 31.Sorokin A and Kohan DE. Physiology and pathology of endothelin-1 in renal mesangium. Am J Physiol Renal Physiol. 2003;285:F579–589 [DOI] [PubMed] [Google Scholar]
- 32.Kohner EM, Patel V, and Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–607 [DOI] [PubMed] [Google Scholar]
- 33.Pang IH and Yorio T. Ocular actions of endothelins. Proc Soc Exp Biol Med. 1997;215:21–34 [DOI] [PubMed] [Google Scholar]
- 34.Deng DX, Evans T, Mukherjee K, Downey D, and Chakrabarti S. Diabetes-induced vascular dysfunction in the retina: role of endothelins. Diabetologia. 1999;42:1228–1234 [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarti S, Gan X, Merry A, Karamzyn M, and Sima A. Augmented rentinal endothelin-1, endothelin-3, endothelinA and endothelinB gene expression in chronic diabetes. Curr Eye Res. 1998;17:301–307 [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Feng B, Chen S, Zuo Y, and Chakrabarti S. Glucose-induced endothelin-1 expression is regulated by ERK5 in the endothelial cells and retina of diabetic rats. Can J Physiol Pharmacol. 2010;88:607–615 [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Yadav AS, Leskova W, and Harris NR. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp Eye Res;91:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamura M, Ohgawara H, Naruse M, Suzudi N, Iwasaki N, Naruse K, Hori S, Demura H, and Omori Y. Increased plasma endothelin in NIDDM patients with retinopathy. Diabetes Care. 1992;15:1396–1397 [DOI] [PubMed] [Google Scholar]
- 39.Ogata M, Naruse M, Iwasaki N, Katoh S, Ohta Y, Hori S, Demura H, and Iwamoto Y. Immunoreactive endothelin levels in the vitreous fluid are decreased in diabetic patients with proliferative retinopathy. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S378–379 [DOI] [PubMed] [Google Scholar]
- 40.Dumont AS, Dumont RJ, McNeill JH, Kassell NF, Sutherland GR, and Verma S. Chronic endothelin antagonism restores cerebrovascular function in diabetes. Neurosurgery. 2003;52:653–660; discussion 659–660 [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann PA, Knot HJ, Stevenson AS, and Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004 [DOI] [PubMed] [Google Scholar]
- 42.Didion SP, Lynch CM, Baumbach GL, and Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347 [DOI] [PubMed] [Google Scholar]
- 43.Mayhan W Impairment of endothelium-dependent dilation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989;256:H621–H625 [DOI] [PubMed] [Google Scholar]
- 44.Arrick DM, Sharpe GM, Sun H, and Mayhan WG. Diabetes-induced cerebrovascular dysfunction: role of poly(ADP-ribose) polymerase. Microvasc Res. 2007;73:1–6 [DOI] [PubMed] [Google Scholar]
- 45.Khan ZA and Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003;81:622–634 [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto T, Yoshiyama S, Kobayashi T, and Kamata K. Mechanisms underlying enhanced contractile response to endothelin-1 in diabetic rat basilar artery. Peptides. 2004;25:1985–1994 [DOI] [PubMed] [Google Scholar]
- 47.Alabadi JA, Miranda FJ, Llorens S, Centeno JM, Marrachelli VG, and Alborch E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur J Pharmacol. 2004;486:289–296 [DOI] [PubMed] [Google Scholar]
- 48.Harris AK, Elgebaly MM, Li W, Sachidanandam K, and Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Sachidanandam K, and Ergul A. Comparison of selective versus dual endothelin receptor antagonism on cerebrovascular dysfunction in diabetes. Neurol Res. 2010;33:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah N, Touyz R, Reudelhuber T, and Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240 [DOI] [PubMed] [Google Scholar]
- 51.Makino A and Kamata K. Time-course changes in plasma endothelin-1 and its effects on the mesenteric arterial bed in streptozotocin-induced diabetic rats. Diabetes Obes Metab. 2000;2:47–55 [DOI] [PubMed] [Google Scholar]
- 52.Makino A and Kamata K. Elevated plasma endothelin-1 level in streptozotocin-induced diabetic rats and responsiveness of the mesenteric arterial bed to endothelin-1. Br J Pharmacol. 1998;123:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arikawa E, Cheung C, Sekirov I, Battell ML, Yuen VG, and McNeill JH. Effects of endothelin receptor blockade on hypervasoreactivity in streptozotocin-diabetic rats: vessel-specific involvement of thromboxane A2. Can J Physiol Pharmacol. 2006;84:823–833 [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto T, Ishida K, Nakayama N, Kobayashi T, and Kamata K. Involvement of NO and MEK/ERK pathway in enhancement of endothelin-1-induced mesenteric artery contraction in later-stage type 2 diabetic Goto-Kakizaki rat. Am J Physiol Heart Circ Physiol. 2009;296:H1388–1397 [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto T, Kobayashi T, and Kamata K. Relationships among ET-1, PPARgamma, oxidative stress and endothelial dysfunction in diabetic animals. J Smooth Muscle Res. 2008;44:41–55 [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto T, Kakami M, Noguchi E, Kobayashi T, and Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H1480–1490 [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto T, Noguchi E, Kobayashi T, and Kamata K. Mechanisms underlying the chronic pioglitazone treatment-induced improvement in the impaired endothelium-dependent relaxation seen in aortas from diabetic rats. Free Radic Biol Med. 2007;42:993–1007 [DOI] [PubMed] [Google Scholar]
- 58.Sachidanandam K, Harris A, Hutchinson J, and Ergul A. Microvascular versus macrovascular dysfunction in type 2 diabetes: differences in contractile responses to endothelin-1. Exp Biol Med (Maywood). 2006;231:1016–1021 [PubMed] [Google Scholar]
- 59.Sachidanandam K, Elgebaly MM, Harris AK, Hutchinson JR, Mezzetti EM, Portik-Dobos V, and Ergul A. Effect of chronic and selective endothelin receptor antagonism on microvascular function in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;294:H2743–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto T, Ishida K, Taguchi K, Kobayashi T, and Kamata K. Short-term angiotensin-1 receptor antagonism in type 2 diabetic Goto-Kakizaki rats normalizes endothelin-1-induced mesenteric artery contraction. Peptides. 2010;31:609–617 [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto T, Kakami M, Kobayashi T, and Kamata K. Gender differences in vascular reactivity to endothelin-1 (1–31) in mesenteric arteries from diabetic mice. Peptides. 2008;29:1338–1346 [DOI] [PubMed] [Google Scholar]
- 62.Rumble JR, Cooper ME, Cox AJ, Soulis T, Wu L, Youssef S, Jasik M, Jerums G, and Gilbert RE. Vascular hypertrophy in experimental diabetes: Role of advanced glycation end products. J Clin Invest. 1997;99:1016–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert RE, Rumble JR, Cao Z, Cox AJ, van Eeden P, Allen TJ, Kelly DJ, and Cooper ME. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ Res. 2000;86:158–165 [DOI] [PubMed] [Google Scholar]
- 64.Kamata K, Ozawa Y, Kobayashi T, and Matsumoto T. Effect of long-term streptozotocin-induced diabetes on coronary vasoconstriction in isolated perfused rat heart. J Smooth Muscle Res. 2008;44:177–188 [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto T, Ozawa Y, Taguchi K, Kobayashi T, and Kamata K. Diabetes-associated changes and role of N epsilon-(carboxymethyl)lysine in big ET-1-induced coronary vasoconstriction. Peptides. 2010;31:346–353 [DOI] [PubMed] [Google Scholar]
- 66.Verma S, Arikawa E, Lee S, Dumont AS, Yao L, and McNeill JH. Exaggerated coronary reactivity to endothelin-1 in diabetes: reversal with bosentan. Can J Physiol Pharmacol. 2002;80:980–986 [DOI] [PubMed] [Google Scholar]
- 67.Tickerhoof MM, Farrell PA, and Korzick DH. Alterations in rat coronary vasoreactivity and vascular protein kinase C isoforms in Type 1 diabetes. Am J Physiol Heart Circ Physiol. 2003;285:H2694–2703 [DOI] [PubMed] [Google Scholar]
- 68.Katakam PV, Snipes JA, Tulbert CD, Mayanagi K, Miller AW, and Busija DW. Impaired endothelin-induced vasoconstriction in coronary arteries of Zucker obese rats is associated with uncoupling of [Ca2+]i signaling. Am J Physiol Regul Integr Comp Physiol. 2006;290:R145–153 [DOI] [PubMed] [Google Scholar]
- 69.Glassberg MK, Ergul A, Wanner A, and Puett D. Endothelin-1 promotes mitogenesis in airway smooth muscle cells. Am J Resp Cell Mol Biol. 1994;10:316–321 [DOI] [PubMed] [Google Scholar]
- 70.Komura I, Kurihara H, Sujiyama F, Takaku F, and Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Letters. 1988;238:249–252 [DOI] [PubMed] [Google Scholar]
- 71.Kohno M, Yokokawa K, Yasunari K, Kano H, Minami M, and Yoshikawa J. Effect of the endothelin family of peptides on human coronary artery smooth-muscle cell migration. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S84–89 [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, and Egido J. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97:125–134 [DOI] [PubMed] [Google Scholar]
- 73.Lerman A, Edwards BS, Hallet JW, Heublein DM, Sandberg SM, and Burnett SMJ. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001 [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi T, Miyauchi T, Iwasa S, Sakai S, Fan J, Nagata M, Goto K, and Watanabe T. Corresponding distributions of increased endothelin-B receptor expression and increased endothelin-1 expression in the aorta of apolipoprotein E-deficient mice with advanced atherosclerosis. Pathol Int. 2000;50:929–936 [DOI] [PubMed] [Google Scholar]
- 75.Lerman A, Webster MW, Chesebro JH, Edwards WD, Wei CM, Fuster V, and Burnett JC, Jr. Circulating and tissue endothelin immunoreactivity in hypercholesterolemic pigs. Circulation. 1993;88:2923–2928 [DOI] [PubMed] [Google Scholar]
- 76.Iwasa S, Fan J, Shimokama T, Nagata M, and Watanabe T. Increased immunoreactivity of endothelin-1 and endothelin B receptor in human atherosclerotic lesions. A possible role in atherogenesis. Atherosclerosis. 1999;146:93–100 [DOI] [PubMed] [Google Scholar]
- 77.Barton M, Haudenschild CC, d’Uscio LV, Shaw S, Munter K, and Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1998;95:14367–14372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Babaei S, Picard P, Ravandi A, Monge JC, Lee TC, Cernacek P, and Stewart DJ. Blockade of endothelin receptors markedly reduces atherosclerosis in LDL receptor deficient mice: role of endothelin in macrophage foam cell formation. Cardiovasc Res. 2000;48:158–167 [DOI] [PubMed] [Google Scholar]
- 79.Bacon CR, Cary NR, and Davenport AP. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ Res. 1996;79:794–801 [DOI] [PubMed] [Google Scholar]
- 80.Bacon CR and Davenport AP. Endothelin receptors in human coronary artery and aorta. Br J Pharmacol. 1996;117:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davenport AP, O’Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, Bacon CR, and Ferro A. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol. 1993;22 Suppl 8:S22–25 [DOI] [PubMed] [Google Scholar]
- 82.Elmoselhi AB and Grover AK. Endothelin contraction in pig coronary artery: receptor types and Ca(2+)-mobilization. Mol Cell Biochem. 1997;176:29–33 [PubMed] [Google Scholar]
- 83.Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M, Kiowski W, and Clozel J. Up-regulation of endothelin-b receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol. 1996;27:147–153 [DOI] [PubMed] [Google Scholar]
- 84.Lee DL, Wamhoff BR, Katwa LC, Reddy HK, Voelker DJ, Dixon JL, and Sturek M. Increased endothelin-induced Ca2+ signaling, tyrosine phosphorylation, and coronary artery disease in diabetic dyslipidemic Swine are prevented by atorvastatin. J Pharmacol Exp Ther. 2003;306:132–140 [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Evans T, Mukherjee K, Karmazyn M, and Chakrabarti S. Diabetes-induced myocardial structural changes: role of endothelin-1 and its receptors. J Mol Cell Cardiol. 2000;32:1621–1629 [DOI] [PubMed] [Google Scholar]
- 86.Chakrabarti S, Cukiernik M, Mukherjee S, and Chen S. Therapeutic potential of endothelin receptor antagonists in diabetes. Expert Opin Invest Drugs. 2000;9:2873–2888 [DOI] [PubMed] [Google Scholar]
- 87.Majumdar P, Chen S, George B, Sen S, Karmazyn M, and Chakrabarti S. Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab Res Rev. 2009;25:452–463 [DOI] [PubMed] [Google Scholar]
- 88.Chiu J, Xu BY, Chen S, Feng B, and Chakrabarti S. Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can J Physiol Pharmacol. 2008;86:365–372 [DOI] [PubMed] [Google Scholar]
- 89.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, and Hirata K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418 [DOI] [PubMed] [Google Scholar]
- 90.Settergren M, Pernow J, Brismar K, Jorneskog G, and Kalani M. Endothelin-A receptor blockade increases nutritive skin capillary circulation in patients with type 2 diabetes and microangiopathy. J Vasc Res. 2008;45:295–302 [DOI] [PubMed] [Google Scholar]
- 91.Fegan PG, Tooke JE, Gooding KM, Tullett JM, MacLeod KM, and Shore AC. Capillary pressure in subjects with type 2 diabetes and hypertension and the effect of antihypertensive therapy. Hypertension. 2003;41:1111–1117 [DOI] [PubMed] [Google Scholar]
- 92.Kalani M, Pernow J, Bragd J, and Jorneskog G. Improved peripheral perfusion during endothelin--a receptor blockade in patients with type 2 diabetes and critical limb ischemia. Diabetes Care. 2008;31:e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng J, Liu Y, Khabbaz KR, Hagberg R, Robich MP, Clements RT, Bianchi C, and Sellke FW. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2010;in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, and Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–459 [DOI] [PubMed] [Google Scholar]


