SUMMARY
Pregnancy necessitates physiological exposure, and often re-exposure, to foreign fetal alloantigens. The consequences after pregnancy are highly varied, with evidence of both alloimmunization and expanded tolerance phenotypes. We show that pregnancy primes the accumulation of fetal-specific maternal CD8+ T cells and their persistence as an activated memory pool after parturition. Cytolysis and the potential for robust secondary expansion occurs with antigen re-encounter in non-reproductive contexts. Comparatively, CDS+ T cell functional exhaustion associated with increased PD-1 and LAG-3 expression occurs with fetal antigen re-stimulation during subsequent pregnancy. PD-L1/LAG-3 neutralization unleashes the activation of fetal-specific CD8+ T cells, causing fetal wastage selectively during secondary but not primary pregnancy. Thus, CD8+ T cells with fetal alloantigen specificity persist in mothers after pregnancy, and protection against fetal wastage in subsequent pregnancies is maintained by their unique susceptibility to functional exhaustion. Together, distinct mechanisms whereby fetal tolerance is maintained during primary compared with subsequent pregnancies are demonstrated.
In Brief
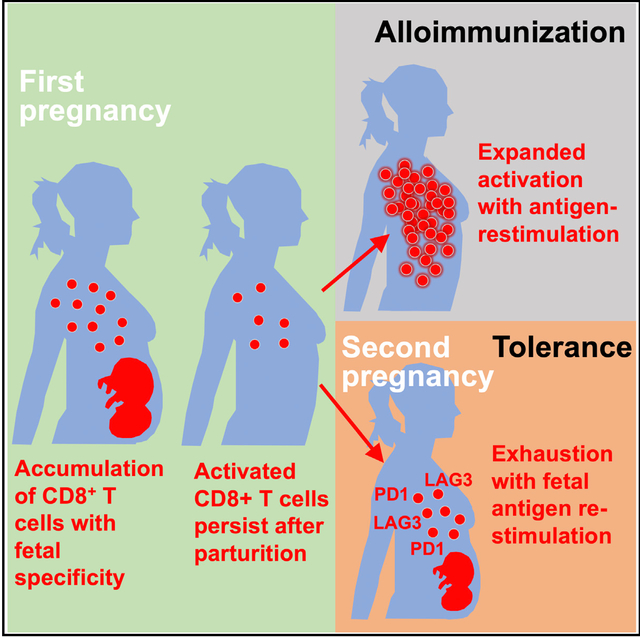
Expecting mothers are immunologically aware of, but do not reject, genetically foreign tissues of the developing fetus. Comparing tolerance occuring during first and subsequent pregnancies, Kinder et al. show that activated memory CD8+ T cells primed by prior pregnancy are uniquely prone to functional exhaustion with fetal antigen re-stimulation.
Graphical Abstract
INTRODUCTION
Expecting mothers are immunologically aware of genetically foreign paternal antigens expressed by the developing fetus (Ta furi et al., 1995; Van Rood et al., 1958). Sensitization or alloimmunization to fetal-expressed antigens persists in mothers after parturition, often with profound functional consequences (Deshmukh and Way, 2019; Porrett, 2018). Long-term survival of renal allografts in humans is reduced in cases of offspring-to-mother or husband-to-wife donor-recipient pairings (Ghafari, 2008). Increasing parity among female donors is also a consistent risk factor for graft-versus-host disease after stem cell transplantation (Flowers et al., 1990; Gale et al., 1987; Kollman et al., 2001). By contrast to these sensitization phenotypes, rates of preeclampsia and other idiopathic complications are reduced in secondary compared with primary pregnancies (Eskenazi et al., 1991; Hernández-Díaz et al., 2009). These protective benefits of prior pregnancy are partner specific since the incidence of preeclampsia rebounds to levels that are similar to that of the primary pregnancy in cases of subsequent pregnancy with a new partner (Feeney and Scott, 1980; Li and Wi, 2000; Robillard et al., 1993; Trupin et al., 1996; Tubbergen et al., 1999). Thus, pregnancy primes fetal-specific immunity capable of alloimmunization and tolerogenic phenotypes.
Sensitization to fetal antigens coincides with the systemic and decidual accumulation of maternal CD8+ T cells during pregnancy and the persistence of these cells after parturition (Deshmukh and Way, 2019; Linscheid and Petroff, 2013; Tilburgs and Strominger, 2013). In humans, the selective accumulation of activated CD8+ T cells with specificity to Y chromosome-encoded alloantigens is found in mothers who have given birth to male offspring (Lissauer et al., 2012; Piper et al., 2007; Powell et al., 2017; Verdijk et al., 2004). Donor monoclonal high-affinity CD8+ T cells with surrogate fetal-ovalbumin (OVA) specificity also expand and persist after pregnancy in mice, when transfer is initiated at late pregnancy time points (Barton et al., 2017). The activation of donor CD8+ T cells with fetal-OVA specificity before transfer drives fetal resorption in pregnancies sired by OVA-expressing male mice (Moldenhauer et al., 2017). However, the relevance of these findings is uncertain given the efficient clonal deletion of these same cells when adoptive transfer is initiated earlier during pregnancy (Erlebacher et al., 2007). In other words, few, if any, of these cells are expected to remain through late gestation and persist after parturition if efficient culling of fetal-specific CD8+ T cells occurs in early pregnancy. Considering the dominant effects of T cell receptor (TCR) affinity in peripheral CD8+ T cell tolerance or deletional anergy (Smith et al., 2014), tracking monoclonal donor T cells with fixed high affinity to cognate antigen may not accurately reflect the polyclonal response of endogenous CD8+ T cells. These limitations are highlighted by the lack of fetal-OVA-specific CD8+ T cell expansion in late pregnancy among an unmanipulated endogenous maternal repertoire using tetramer staining (Tay et al., 2013). However, these negative results, analyzing only a fraction of peripheral T cells, may also be biased by the low precursor frequency of endogenous T cells with specificity to any single defined antigen (Moon et al., 2007).
To more comprehensively investigate how mothers immunologically respond to fetal stimulation during pregnancy, major histocompatibility complex (MHC) class I tetramer staining was combined with the enrichment of tetramer-positive cells to identify even rare endogenous maternal CD8+ T cells with defined fetal specificity. This approach leverages the advantages of tracking endogenous maternal T cells, but simultaneously bypasses the limitations associated with sampling only a fraction of peripheral T cells. By refocusing these tools, a distinct pool of fetal-specific CD8+ T cells primed by pregnancy that persist after parturition is identified, and the molecular basis responsible for their context-specific alloimmunization versus tolerance phenotypes is demonstrated.
RESULTS
Maternal CD8+ T Cells with Fetal Specificity Persist after Pregnancy
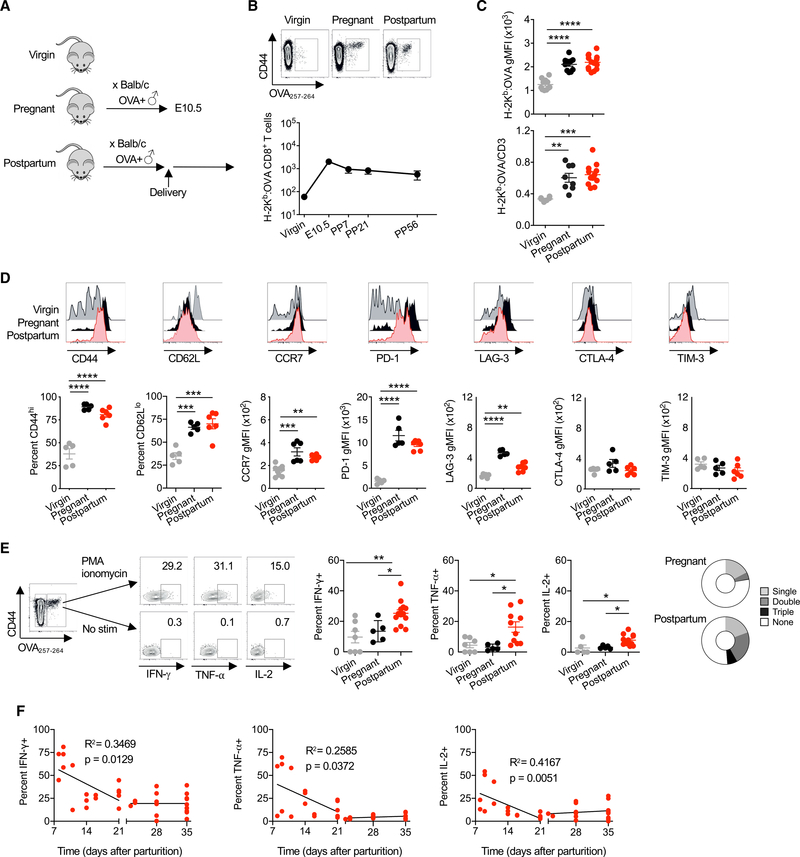
Given the limited availability of defined antigens among MHC haplotype alleles, BALB/c (H-2d) male mice with constitutive cell-surface OVA expression (Ehst et al., 2003; Moon et al., 2011) (Act-OVA mice) were used to sire allogeneic pregnancy in non-transgenic C57BL/6 (H-2b) females (Figure 1A). This mating scheme transforms OVA into a surrogate fetal antigen and allows the identification of endogenous, polyclonal fetal-specific (H-2Kb:OVA257–264) CD8+ T cells using MHC class I tetramer staining and enrichment (Erlebacher et al., 2007; Moldenhauer et al., 2009; Rowe et al., 2011). Using this approach, we show that pregnancy primes the ~50-fold expansion of maternal CD8+ T cells with fetal-OVA specificity by mid-gestation (embryonic day [E]10.5) in the spleen and peripheral lymph nodes (Figure 1B). The number of these cells contract following delivery and are maintained at >10-fold increased levels up to 8 weeks after parturition compared with cells of the same specificity in virgin control mice (Figure 1B).
Figure 1. Maternal CD8+ T Cells with Fetal Specificity Expand during Pregnancy and Persist after Parturition.
(A) Female mice on the C57BL/6 (H-2b) background were mated with Act-OVA male mice on the BALB/c (H-2d) background. For analysis, females were sacrificed either before mating (virgin), at embryonic day 10.5 (E10.5) (pregnant), or 21 days following delivery (postpartum).
(B) Number of H-2Kb:OVA257–264-specific CD8+ T cells in the spleen and pooled peripheral lymph nodes of female C57BL/6 virgin or mid-gestation (E10.5) mice compared with postpartum week 1 (PP7), week 3 (PP21), and week 8 (PP56) after allogeneic pregnancy sired by Act-OVA male on the BALB/c background.
(C) H-2Kb:OVA257–264 tetramer geometric mean fluorescence intensity (gMFI) and ratio of tetramer gMFI to CD3 gMFI for CD8+ T cells from female C57BL/6 virgin, mid-gestation (E10.5) pregnant, and PP21 after allogeneic pregnancy sired by Act-OVA male mice on the BALB/c background.
(D) Percentage of CD44hi, CD62Llo, CCR7, PD-1, LAG-3, CTLA-4, and TIM-3 expression levels by H-2Kb:OVA257–264 CD8+ T cells for each group of mice described in (C).
(E) Representative flow cytometry plots showing the identification of H-2Kb:OVA257–264 CD8+ T cells by tetramer staining, and their production of IFN-γ, TNF-α, or IL-2 following PMA-ionomycin stimulation compared with no stimulation controls for cells isolated from the spleen and pooled peripheral lymph nodes for each group of mice described in (C).
(F) Frequency of IFN-γ, TNF-α, or IL-2 producing H-2Kb:OVA257–264 CD8+ T cells from female C57BL/6 mice at the indicated time points following allogeneic pregnancies sired by Act-OVA male mice on the BALB/c background.
Data are from at least 3 independent experiments, each with similar results, with each point representing data from an individual mouse.
Bar, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001.
Phenotypic shifts were also observed for maternal CD8+ T cells primed by fetal antigen stimulation. Both during pregnancy and after parturition, maternal CD8+ T cells with fetal-OVA specificity showed increased affinity, based on H-2Kb:OVA tetramer staining intensity and avidity after normalization to TCR expression levels compared with cells of the same specificity in virgin control mice (Figure 1C). The majority of fetal OVA-specific CD8+ T cells during pregnancy and day 21 after parturition were CD44hi, indicating antigen experience (Figure 1D). However, the sustained downregulation of CD62L together with increased CCR7 expression blurred further classification as central or effector memory cells (Figure 1D) (Sallusto et al., 1999). Pregnancy-primed maternal CD8+ T cells also showed an increased expression of defined negative co-stimulatory molecules, including PD-1 and LAG-3, with expression remaining elevated after parturition, while others, including CTLA-4 and TIM-3, either did not change significantly during pregnancy or changes were not sustained after parturition (Figure 1D). Thus, primary pregnancy primes the expansion of fetal-specific CD8+ T cells that persist as a distinctive memory pool, with the enriched expression of molecules associated with activation and functional exhaustion.
Maternal Memory CD8+ T Cells Are Capable of Cytolysis and Effector Cytokine Production
To further investigate the functional properties of fetal-OVA-specific CD8+ T cells, their potential for cytokine production was evaluated. The production of canonical effector cytokines interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin-2 (IL-2) by H-2Kb:OVACD8+T cells following phorbol 12-myristate 13-acetate (PMA)-ionomycin stimulation was significantly increased by day 21 after parturition compared to cells of the same specificity in virgin or mid-gestation pregnant mice (Figure 1E). Maternal CD8+ T cells with fetal-OVA specificity also showed increased polyfunctionality in regard to the simultaneous production of multiple effector cytokines after parturition compared with cells of the same specificity during pregnancy (Figure 1E). Additional time course analysis showed cytokine production to be even higher early after delivery, stabilizing by day 21 after parturition (Figure 1F). Accordingly, subsequent experiments focus on day 21 postpartum, given the functionally stable pool of memory cells at this time point.
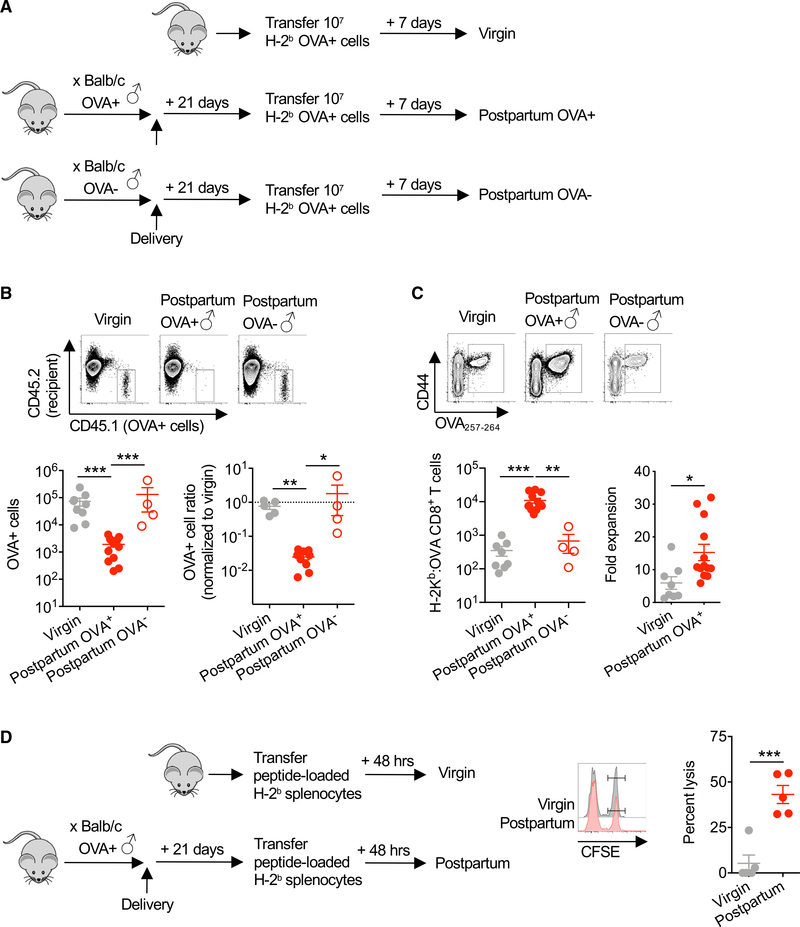
To more precisely address how mothers immunologically respond to intact fetal cells, the persistence and CD8+ T cell response to adoptively transferred splenocytes from Act-OVA transgenic mice were evaluated (Figure 2A). We found sharply more accelerated elimination of OVA+ cells in females with prior pregnancy sired by OVA-expressing males (Figure 2B). By day 7 after transfer, OVA+ (CD45.1+) cells were reduced by ~100-fold in mothers with prior pregnancy sired by OVA-expressing males as compared with virgin control mice (Figure 2B). The accelerated rejection of OVA+ cells paralleled the increased accumulation of fetal-OVA-specific CD8+ T cells in mice with prior pregnancy sired by OVA-expressing males (Figure 2C). These differences reflect accelerated expansion and are not explained by the elevated number of OVA-specific CD8+ T cells in postpartum mice given significantly increased fold expansion in postpartum compared with virgin mice before OVA+ cell transfer (Figure 2C). The accelerated elimination of adoptively transferred OVA+ cells and more robust secondary expansion of fetal-OVA-specific CD8+ T cells in postpartum mice also reflect an antigen-specific response to fetal-OVA stimulation since both were overturned in mice with prior pregnancy sired by OVA− non-transgenic males (Figures 2B and 2C). Antigen-specific CD8+ T cells mediate cytolysis and accelerated elimination of OVA+ cells since splenocytes loaded with the H-2Kb class I-restricted 0VA257–264 peptide were selectively eliminated after transfer into mice with prior pregnancy sired by OVA-expressing males (Figure 2D). Thus, despite the enriched expression of molecules associated with both activation and functional exhaustion, CD8+ T cells retained after parturition are functionally capable of alloimmunization with antigen re-stimulation.
Figure 2. Pregnancy-Primed CD8+ T Cells Are Cytolytic and Capable of Robust Secondary Re-expansion.
(A) Schematic showing transfer of splenocytes from Act-OVA mice on the C57BL/6 (H-2b) background into each group of mice, including virgin control and PP21 mice after allogeneic pregnancy sired by Act-OVA (postpartum OVA+) or non-transgenic male mice on the BALB/c background.
(B) Absolute number (left) or normalized ratio (right) of OVA+ splenocytes recovered from the spleen and pooled peripheral lymph nodes 7 days following transfer into each group of mice described in (A).
(C) Number of H-2Kb:OVA257–264 CD8+ T cells in the spleen and pooled peripheral lymph nodes and fold expansion from pre-transfer levels for mice described in (A).
(D) Schematic showing transfer peptide-loaded target cells, their relative persistence, and calculated percent lysis of target cells loaded with OVA257–264 (CFSEhi) compared with irrelevant control peptide (Zika virus NS5 227–234 peptide; CFSElo) 48 h following adoptive transfer into female virgin compared with PP21 mice after allogeneic pregnancy sired by Act-OVA males on the BALB/c background.
Data are from at least 3 independent experiments, each with similar results, with each point representing data from an individual mouse.
Bar, mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.005.
Maternal Memory CD8+ T Cell Hyporesponsiveness during Secondary Pregnancy
The risk of several idiopathic pregnancy complications, including preeclampsia, is reduced in second pregnancy (Deshmukh and Way, 2019; Eskenazi et al., 1991; Hernández-Díaz et al., 2009; Trupin et al., 1996). These protective benefits of prior pregnancy occur in a partner-specific manner, suggesting that while pregnancy primes alloimmunization to fetal antigens, tolerogenic responses are regained or even reinforced during subsequent pregnancy. One explanation for why sensitization predominates when fetal-alloantigen responses are evaluated in non-reproductive contexts is that physiological changes specific to pregnancy are required to stimulate hyporesponsive phenotypes for maternal immune components primed in prior pregnancy.
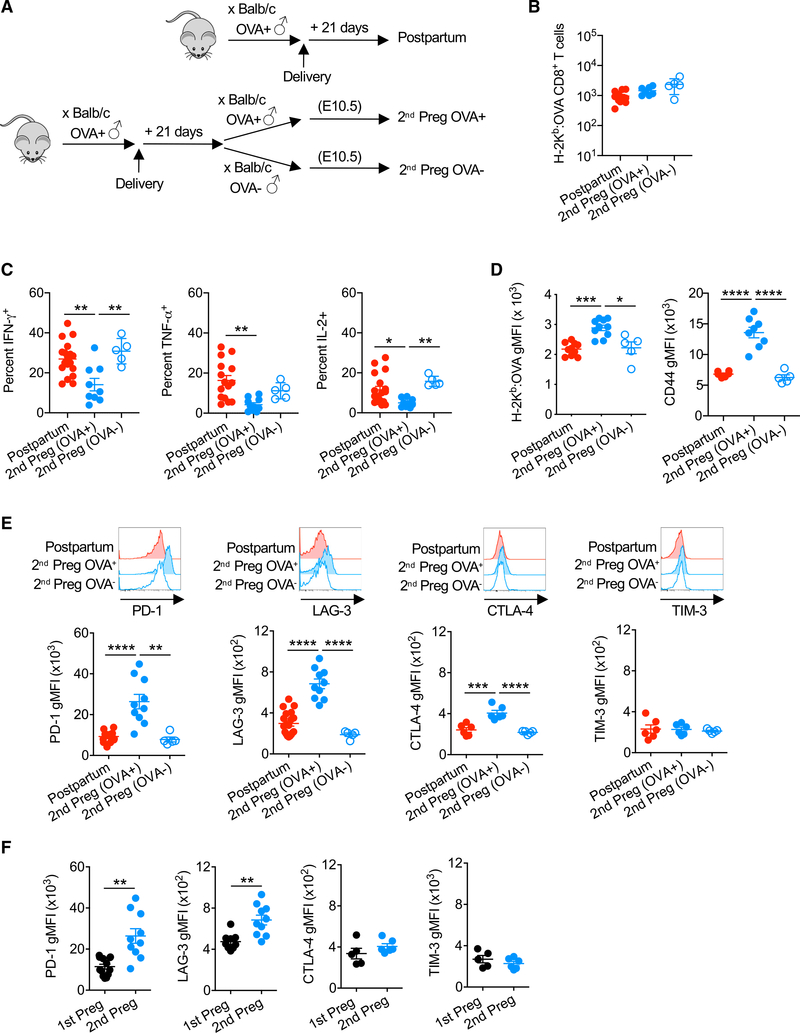
To investigate whether such functionally distinct recall responses occur in response to fetal antigen re-exposure during pregnancy, fetal-OVA-specific CD8+ T cells primed by primary OVA+ pregnancies were tracked during secondary allogeneic pregnancy sired by OVA+ compared with OVA− male mice (Figure 3A). In striking contrast to the ~50-fold expansion of fetal-specific CD8+ T cells during primary pregnancy (Figure 1B), fetal-OVA-specific CD8+T cells did not significantly accumulate further with fetal-OVA stimulation during secondary pregnancy (Figure 3B). The production of effector cytokines after PMA-ionomycin stimulation was also significantly reduced among maternal CD8+ T cells with fetal-OVA specificity during secondary pregnancy compared with postpartum mice (Figure 3C). Blunted effector cytokine production by OVA-specific CD8+ T cells required fetal-OVA re-stimulation during secondary pregnancy since the production of IFN-γ, TNF-α, and IL-2 each remained at levels comparable to postpartum mice during secondary pregnancy sired by non-transgenic, OVA− male mice (Figure 3C). These hyporesponsiveness phenotypes are also not explained by ignorance of fetal antigen re-stimulation since both tetramer binding avidity and CD44 expression levels were further increased among memory CD44hi, antigen experience fetal-OVA-specific CD8+ T cells selectively during secondary pregnancy sired by OVA+ mice (Figure 3D).
Figure 3. Maternal CD8+ T Cell-Blunted Responsiveness to Fetal Antigen Re-stimulation during Secondary Pregnancy.
(A) C57BL/6 (H-2b) females 21 days following allogeneic mating with Act-OVA male mice on the BALB/c background were compared to pregnant mice mid-gestation during secondary pregnancy sired by Act-OVA or non-transgenic male mice on the BALB/c background.
(B) Number of H-2Kb:OVA257–264 CD8+ T cells in the spleen and pooled peripheral lymph nodes of mice described in (A).
(C) Percentage of IFN-γ-, TNF-α-, or IL-2-producing H-2Kb:OVA257–264 CD8+ T cells following PMA-ionomycin stimulation for each group of mice described in (A).
(D) H-2Kb:OVA257_264 tetramer staining intensity and CD44 expression levels by H-2Kb:OVA257_264 CD8+ T cells for each group of mice described in (A).
(E) PD-1, LAG-3, CTLA-4, and TIM-3 expression levels by H-2Kb:OVA257_264 CD8+ T cells for each group of mice described in (A).
(F) Relative expression of PD-1, LAG-3, CTLA-4, and TIM-3 among H-2Kb:OVA257–264 CD8+ T cells mid-gestation during primary or secondary pregnancy, each sired by Act-OVA male mice on the BALB/c background.
Data are from at least 3 independent experiments, each with similar results, with each point representing data from an individual mouse.
Bar, mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001.
The molecular basis for this functional shift among memory CD8+ T cells was evaluated by comparing their expression of cell-intrinsic exhaustion molecules with fetal antigen re-stimulation during secondary pregnancy. Consistent with the blunted expansion of CD8+ T cells with fetal-OVA re-stimulation during secondary pregnancy, these cells showed a sharply increased expression of PD-1, LAG-3, and CTLA-4 co-inhibitory molecules, whereas the expression of TIM-3 remained unchanged (Figure 3E). These phenotypic shifts in OVA-specific CD8+ T cells require fetal antigen re-stimulation during pregnancy, since the expression of each remained comparable to cells in postpartum mice during secondary pregnancy sired by non-OVA-expressing male mice (Figure 3E). The expression of PD-1 and LAG-3 was also significantly increased by maternal CD8+ T cells during secondary compared with primary pregnancy, whereas the expression levels of other markers (CTLA-4, TIM-3) remained similar (Figure 3F). Thus, the hyporesponsiveness of maternal memory CD8+ T cells to fetal antigen re-stimulation is associated with selectively increased levels of PD-1 and LAG-3 expression during secondary compared with primary pregnancy.
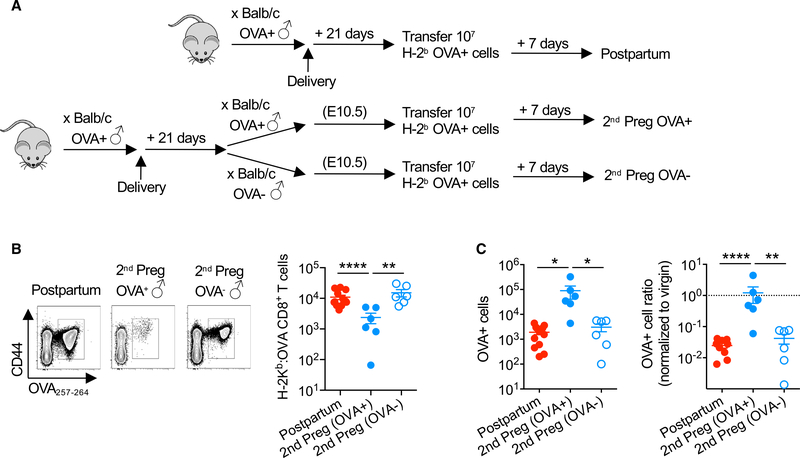
To further investigate this discordant response of CD8+ T cells in postpartum mice to OVA antigen re-stimulation in the context of secondary pregnancy (Figure 3B) compared with adoptively transferred OVA+ cells (Figure 2C), the response when these stimulation parameters are combined was evaluated (Figure 4A). We found that the accumulation of fetal-OVA-specific CD8+ T cells in response to stimulation by intravenously transferred OVA+ cells is sharply reduced during secondary pregnancies sired by OVA-expressing males compared with postpartum control mice (Figure 4B). In turn, the accelerated elimination of OVA+ cells in postpartum recipients is also overturned when transfer is initiated during secondary allogeneic pregnancy sired by OVA-expressing male mice (Figure 4C). These hyporesponsiveness phenotypes among CD8+ T cells during secondary pregnancy require fetal-OVA stimulation since the blunted re-expansion of OVA-specific CD8+ T cells and the dampened elimination of OVA+ cells each rebound to levels that are comparable to those of postpartum mice during secondary pregnancy sired by nonOVA-expressing male mice (Figures 4B and 4C). Thus, active sensing of fetal-expressed antigens during secondary pregnancy blunts the expansion and cytolytic function of maternal CD8+ T cells primed by prior pregnancy.
Figure 4. Fetal Antigen Stimulation during Secondary Pregnancy Overrides Cytolysis and Re-expansion of Maternal Memory CD8+ T Cells.
(A) Schematic showing the transfer of splenocytes from Act-OVA mice on the C57BL/6 (H-2b) background into each group of mice, including PP21 mice after allogeneic pregnancy sired by Act-OVA male mice on the BALB/c background, and pregnant mice mid-gestation during secondary pregnancy sired by Act-OVA or non-transgenic male mice on the BALB/c background.
(B) Number of H-2Kb:OVA257–264 CD8+ T cells and 7 days following intravenous transfer of B6 (H-2b) Act-OVA (OVA+) splenocytes for each group of mice described in (A).
(C) Absolute number (left) or normalized ratio (right) of Act-OVA (OVA+) splenocytes recovered from the spleen and pooled peripheral lymph nodes 7 days following adoptive transfer for each group of mice described in (A).
Data are from at least 3 independent experiments, each with similar results, with each point representing data from an individual mouse.
Bar, mean ± SEM. *p < 0.05, **p < 0.01, and ****p < 0.001.
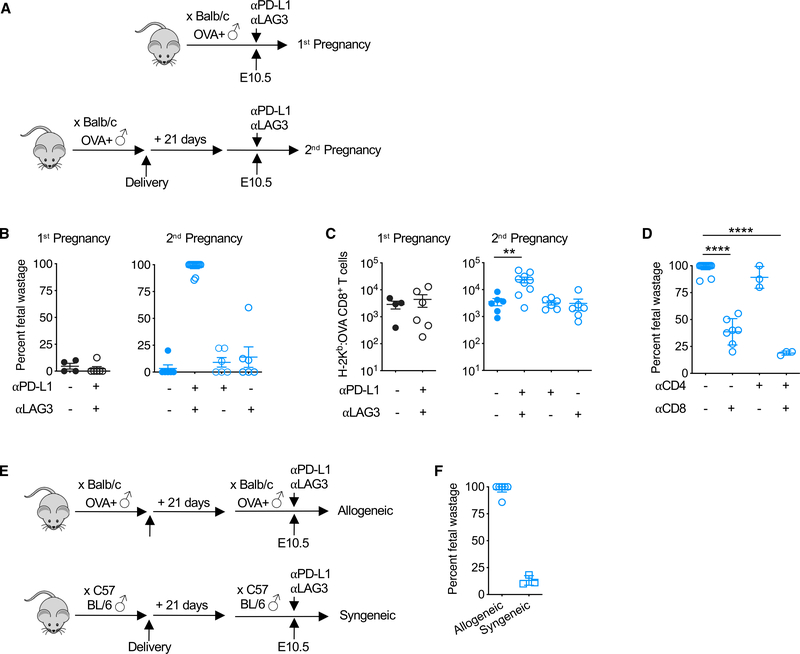
PD-1 and LAG-3 Impair CD8+ T Cell Activation Selectively during Secondary Pregnancy
To investigate the functional significance of selectively amplified PD-1 and LAG-3 expression by maternal T cells during secondary pregnancy, the effects of neutralizing these molecules on pregnancy outcomes and the accumulation of fetal-specific CD8+ T cells were evaluated (Figure 5A). The administration of PD-L1 and LAG-3 neutralizing antibodies at mid-gestation during secondary but not primary pregnancy triggered near-complete fetal wastage within 72 h (Figure 5B). Fetal wastage induced by PD-L1/LAG-3 functional blockade was associated with the unleashed re-expansion of fetal-OVA-specific CD8+ T cells during secondary pregnancy, but it had no significant impact on the accumulation of these cells during primary pregnancy (Figure 5C). Simultaneously neutralizing both PD-L1 and LAG-3 was required, since neither fetal wastage nor re-expansion of CD8+ T cells with fetal-OVA specificity occurred during secondary pregnancy, when mice were treated only with neutralizing antibodies against PD-L1 or LAG-3 (Figures 5B and 5C). Fetal wastage triggered by PD-L1/LAG-3 blockade during secondary pregnancy directly reflects the unleashed activation of fetal-specific CD8+ T cells, since fetal wastage was significantly reduced with the co-depletion of maternal CD8+ T cells with PDL1/LAG-3 functional blockade (Figure 5D). These pregnancy outcome phenotypes are also not explained by potential PD-L1/ PD-1 or LAG-3 direct stimulation of CD4+T-cells sincethe depletion of these cells neither overturned fetal wastage induced by PD-L1/ LAG-3 blockade in CD8+ T cell sufficient mice nor the protection against fetal wastage in CD8+ T cell depleted mice (Figure 5D). However, fetal wastage triggered by PD-L1 /LAG-3 blockade was overturned in secondary syngeneic pregnancies sired by MHCidentical male and female mice, suggesting that the maternal recognition of genetically foreign fetal alloantigens drives the necessity for CD8+ T cell functional exhaustion that protects against fetal wastage (Figures 5E and 5F). Thus, progressively increased levels of PD-1 and LAG-3 are expressed by maternal CD8+ T cells with fetal stimulation during secondary as compared with primary allogeneic pregnancy, and these co-inhibitory molecules protect against fetal wastage by suppressing the activation of memory maternal CD8+ T cells primed by prior pregnancy. These results highlight distinct strategies for how tolerance is achieved by naive and memory T cells in response to fetal antigen stimulation during first pregnancy compared with subsequent pregnancies.
Figure 5. PD-1 and LAG-3 Restrict Re-expansion of Fetal-Specific CD8+ T Cells Selectively during Secondary Pregnancy.
(A) Schematic showing the administration of αPD~L1 and/or αLAG-3 antibodies mid-gestation (E10.5) during primary or secondary pregnancies sired by Act-OVA male mice on the BALB/c background.
(B) Percentage of fetal wastage 3 days after the administration of αPD-L1 and/or αLAG-3 antibodies for mice described in (A).
(C) Number of H-2Kb:OVA257–264 CD8+ T cells in the spleen and pooled peripheral lymph nodes for each group of mice described in (A).
(D) Fetal wastage 3 days after the administration of αPD-L1/LAG-3 antibodies with or without co-administration of αCD8- and/or αCD4-depleting antibody during secondary pregnancy sired by Act-OVA male mice on the BALB/c background.
(E) Schematic showing the administration of αPD-L1 and/or LAG-3 antibodies mid-gestation (E10.5) during allogeneic compared with syngeneic secondary pregnancies.
(F) Percentage of fetal wastage 3 days following αPD-L1/LAG-3 antibody treatment mid-gestation for groups of mice described in (E).
Data are from at least 3 independent experiments, each with similar results, with each point representing data from an individual mouse.
Bar, mean ± SEM. **p < 0.01 and ****p < 0.0001.
DISCUSSION
Pregnancy remains an immunological marvel and paradox. Despite numerous mechanisms implicated in protecting allogeneic fetal tissues against premature rejection, our core knowledge of how pregnancy works immunologically remains rudimentary. In this regard, important clues can be uncovered by investigating parity, and the potential differences in how naive compared with activated immune cells respond to fetal antigen stimulation. In line with the accelerated activation and expansion of protective memory immune cells with specificity to genetically foreign microbial invaders (Blattman et al., 2000; Harty and Badovinac, 2008; Jameson and Masopust, 2009), placental mammals have likely also developed teleologically conserved pathways for remembering beneficial fetal alloantigens and enforcing fetal tolerance during subsequent exposures.
To investigate fetal tolerance in this dynamic physiological context, an instructive preclinical model of allogeneic mating was developed, allowing longitudinal tracking of fetal-specific CD8+ T cells primed by primary pregnancy after parturition and during secondary pregnancy. We show that primary fetal alloantigen stimulation during pregnancy primes the expansion and retention of maternal CD8+ T cells with fetal specificity, and the capacity for cytolysis and accelerated secondary expansion after antigen re-exposure outside of pregnancy. These findings are consistent with prior studies in mice showing more rapid rejection of skin allografts after pregnancy sired by males expressing matched alloantigens (Barton et al., 2017), and in humans, the persistence of cytolytic CD8+ T cells with specificity to Y chromosome-encoded alloantigens in the peripheral blood of mothers who have previously given birth to male offspring (Lissa uer et al., 2012; Piper etal., 2007; Verdijketal., 2004). Our results expand upon these observations by showing that these maternal memory CD8+ T cells are also poised to adopt a functionally exhausted phenotype in response to fetal antigen re-stimulation in the physiological context of secondary pregnancy. The hyporesponsivness of these memory maternal CD8+ T cells is mediated by cell-intrinsic PD-1 and LAG-3 expression, since functionally neutralizing these molecules reinvigorates the activation of fetal-specific CD8+T cells, causing fetal wastage selectively during secondary but not primary pregnancy.
Since hemizygous OVA-expressing males were used to sire allogeneic pregnancies, near-complete fetal wastage after PDL1/LAG-3 neutralization highlights the importance of maternalfetal alloantigen mismatch that occurs independently of OVA expression by offspring. Further enforcing this point is sharply reduced fetal wastage during secondary syngeneic compared with secondary allogeneic pregnancy despite PD-L1/LAG-3 functional blockade. We have also previously shown that fetal alloreactive FOXP3+ regulatory CD4+ T cells persist in mice after primary pregnancy, and have shown the association between their accelerated re-expansion with fetal antigen re-stimulation and protection against fetal wastage in secondary pregnancy (Rowe et al., 2012). However, the susceptibility of maternal CD8+ T cells to functional re-invigoration during secondary pregnancy and the persistence of these phenotypes despite co-depletion of CD4+ T cells highlight CD8+ T cell-intrinsic programming also promotes partner-specific resiliency against complications during secondary compared with primary pregnancy.
These findings provide a potential unifying explanation for the current discrepancies in importance for PD-1/PD-L1 in prior studies under seemingly indistinguishable experimental conditions. For example, PD-1/PD-L1 -mediated maternal T cell quiescence has been described to be essential for averting fetal wastage (Guleria et al., 2005) and yet completely dispensable for normal pregnancy outcomes in other studies (Taglauer et al., 2009). Our findings that prior pregnancy and maternal exposure to fetal alloantigens dictate the importance of co-inhibitory molecules such as PD-1/PD-L1 in fetal tolerance provides a conceptual framework for interpreting these discordant results by considering the often-overlooked parameter of parity. In turn, the need for simultaneously blocking PD-1 and LAG-3 to unleash the activation of sensitized maternal CD8+ T cells is consistent with the hierarchical model of CD8+ T cell exhaustion mediated by multiple co-inhibitory molecules previously demonstrated during persistent infection and cancer (Blackburn et al., 2009; McLane et al., 2019).
The present knowledge of how CD8+ T cells respond to foreign antigens is largely based on characterizing their expansion and function after infection or stimulation under other pro-inflammatory conditions, followed by antigen re-exposure (Blattman et al., 2000; Harty and Badovinac, 2008; Jameson and Masopust, 2009). However, pregnancy is arguably an equal, if not more universal, alloimmunization event as compared with microbial infection. Accordingly, investigating the maternal response to fetal alloantigen during first and subsequent pregnancies in comparison to classical models of CD8+ T cell priming after acute infection reveals some similarities along with interesting differences in how these cells respond to repeated foreign antigen stimulation. For example, the expansion of antigen-specific CD8+ T cells occurs with a comparable tempo after fetal stimulation during primary pregnancy compared with acute infection conditions, both reaching peak levels within the first 7–10 days. Antigen-specific CD8+ T cells are similarly retained as an activated memory pool capable of cytolysis and effector cytokine production after antigen elimination with parturition or pathogen clearance. Outside the context of pregnancy, mothers are also capable of the rapid clearance of fetal cells or tissues upon antigen re-stimulation, with more accelerated CD8+T cell re-expansion that parallels the response during secondary pathogen challenge.
A distinguishing feature of maternal CD8 T cells primed by pregnancy is their blunted responsiveness to fetal re-stimulation during subsequent pregnancy. Memory CD8+ T cells primed by prior pregnancy fail to re-expand appreciably with fetal antigen re-stimulation during secondary pregnancy; instead, they respond by upregulating cell-intrinsic PD-1 and LAG-3 expression. PD-L1/LAG-3 functional neutralization unleashes their activated expansion, causing near-complete fetal wastage. Thus, while pregnancy-induced memory CD8+ T cells are capable of more robust activation-expansion compared with naive cells, they are also poised for hyporesponsiveness in what appears to represent a context-dependent “exhaustion prone” phenotype. Based on these results highlighting specialized mechanisms whereby fetal tolerance is maintained during secondary compared with primary pregnancy, establishing the gene expression and epigenetic marks that control this sharp detour from alloimmunization to exhaustive memory, and the parameters during secondary pregnancy that activate these functional changes in CD8+ T cell phenotype, represent important next steps for future study. These findings will be critical for understanding the molecular signals that may promote enhanced partner-specific protection from complications such as preeclampsia during secondary pregnancy and provide an immunological framework for addressing other reproductive complications such as infertility, including secondary infertility after a first successful pregnancy (Brazdova et al., 2016; Deshmukh and Way, 2019; Robertson and Sharkey, 2016). Considering the importance of reproductive fitness in trait selection, it is perhaps not surprising that investigating immune cells during pregnancy reveals additional facets for how they naturally work. By extension, understanding how reproductive tolerance is achieved can open up innovative strategies for improving pregnancy outcomes, with likely application to other translational contexts such as transplantation and autoimmunity, in which long-lasting antigen-specific tolerance is desired.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Sing Sing Way (singsing.way@cchmc.org).
Materials Availability
All unique reagents generated in this study are available from the Lead Contact.
Data and Code Availability
This study did not result in any datasets or custom code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
C57BL76 (H-2b; CD45.2), BALB/c (H-2d), and B6-Ly5.1 (H-2b; CD45.1) mice (6–8 weeks old) were purchased from NCI colony at Charles River Laboratories. Act-OVA transgenic mice that constitutively express recombinant OVA protein behind the β-actin promoter have been described (Ehst et al., 2003). All mice were housed under specific pathogen-free conditions with a 12 hour on/ off light cycle at Cincinnati Children’s Hospital. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of Cincinnati Children’s Hospital (protocol no. 2018–0022).
METHOD DETAILS
Experimental Matings
For primary pregnancies, hemizygous Act-OVA male mice backcrossed on the BALB/c or non-transgenic BALB/c males (6–8 weeks) were introduced to C57BL/6 or B6-Ly5.1 virgin (6–8 weeks) mice for 24 hours and visualization of the copulation plug was determined as embryonic day (E) 0.5. For secondary pregnancies, postpartum females (> 14 days) were randomly selected to mate with BALB/c Act-OVA or non-trangenic male mice (6–8 weeks). Fetal wastage was assessed as in utero resorption or fetal death together with placental friability.
Tetramer Staining and Enrichment
For tracking CD8+ T cells with H-2Kb:0VA257–264 specificity, single cell suspensions were prepared from the spleen and peripheral (axillary, brachial, cervical, inguinal, mesenteric, pancreatic, para-aortic/uterine) lymph nodes. Cells were stained with PE-conjugated H-2Kb:0VA257–264 MHC class I tetramer and enriched with anti-PE microbeads (Miltenyi Biotec). Bound fractions were eluted and stained with fluorochrome-labeled cell surface antibodies outlined in the Key Resources Table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE/Cy5 anti-mouse/human CD11b Antibody, Clone M1/70 | BioLegend | Cat# 101210; RRID: AB_312793 |
| PE/Cy5 anti-mouse/human CD45R/B220 Antibody, Clone RA3-B62 | BioLegend | Cat# 103210; RRID: AB_312995 |
| PE/Cy7 anti-mouse CD62L Antibody, Clone MEL-14 | BioLegend | Cat# 104418; RRID: AB_313103 |
| Brilliant Violet 605 anti-mouse Antibody, Clone 29F.1A12 | BioLegend | Cat# 135220; RRID: AB_2562616 |
| APC anti-mouse CD152 (CTLA-4) Antibody, Clone UC10–4B9 | BioLegend | Cat# 106310; RRID: AB_2087653 |
| Alexa Fluor 700 anti-mouse/human CD44 Antibody, Clone IM7 | BioLegend | Cat# 103026; RRID: AB_493713 |
| Brilliant Violet 650 anti-mouse IFN-gamma Antibody, Clone XMG1.2 | BioLegend | Cat# 505832; RRID: AB_2734492 |
| APC anti-mouse TNF-alpha Antibody, Clone MP6-XT22 | BioLegend | Cat# 506308; RRID: AB_315429 |
| Brilliant Violet 421 anti-mouse IL-2 Antibody, Clone JES6–5H4 | Biolegend | Cat# 503826; RRID: AB_2650897 |
| PE/Cy7 anti-mouse CD45.1 Antibody, Clone A20 | BioLegend | Cat# 110730; RRID: AB_1134168 |
| Brilliant Violet 421 anti-mouse CD45.2 Antibody, Clone 104 | BioLegend | Cat# 109832; RRID: AB_2565511 |
| eFluor 450 anti-mouse CD4 Antibody, Clone GK1.5 | Thermo Fisher Scientific | Cat# 48–0041-82; RRID: AB_10718983 |
| APC-eFluor 780 anti-mouse CD8alpha Antibody, Clone 53–6.7 | Thermo Fisher Scientific | Cat# 7–0081-82; RRID: AB_1272185 |
| PE/Cy5 anti-mouse CD11c Antibody, Clone N418 | Thermo Fisher Scientific | Cat# 15–0114-82; RRID: AB_468717 |
| PE/Cy5 anti-mouse F4/80 Antibody, Clone BM8 | Thermo Fisher Scientific | Cat# 15–4801-82; RRID: AB_468798 |
| PE/Cy7 anti-mouse CD197 (CCR7) Antibody, Clone 4B12 | Thermo Fisher Scientific | Cat# 25–1971-82; RRID: AB_469652 |
| FITC anti-mouse CD223 (LAG-3) Antibody, Clone C9B7W | Thermo Fisher Scientific | Cat# 11–2231-82; RRID: AB_2572484 |
| eFluor 450 anti-mouse CD366 (TIM3) Antibody, Clone 8B.2C12 | Thermo Fisher Scientific | Cat# 48–5871-82; RRID: AB_2574081 |
| FITC anti-mouse CD4 Antibody, Clone GK1.5 | Thermo Fisher Scientific | Cat# 11–0041-85; RRID: AB_464893 |
| PE/Cy7 anti-mouse CD8alpha Antibody, Clone 53–6.7 | Thermo Fisher Scientific | Cat# 25–0081-82; RRID: AB_469584 |
| PE/Cy7 anti-mouse CD3epsilon Antibody, Clone 145–2C11 | Thermo Fisher Scientific | Cat# 25–0031-82; RRID: AB_469572 |
| InVivoPlus anti-mouse CD4 Antibody Clone GK1.5 | BioXCell | Cat# BP0003–1; RRID: AB_1107636 |
| InVivoPlus anti-mouse CD8alpha Antibody, Clone 2.43 | BioXCell | Cat# BP0061; RRID: AB_1125541 |
| InVivoPlus anti-mouse PD-L1 Antibody, Clone 10F.9G2 | BioXCell | Cat# BP0101; RRID: AB_10949073 |
| InVivoPlus anti-mouse LAG-3 Antibody, Clone C9B7W | BioXCell | Cat# BP0174; RRID: AB_10949602 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| CFSE | Thermo Fisher Scientific | 65–0850-84 |
| Cell Stimulation Cocktail (500X) | Thermo Fisher Scientific | 00–4975-03 |
| H-2Kb:OVA257–64 MHC Class I Monomer | NIH Tetramer Core | N/A |
| Streptavidin, R-Phycoerythrin Conjugate (SAPE) | Thermo Fisher Scientific | S866 |
| Critical Commercial Assays | ||
| Fixation/Permeabilization Solution Kit | BD Biosciences | 554714 |
| Anti-PE Microbeads | Miltenyi | Cat# 130–048-801; RRID: AB_244373 |
| Experimental Models: Organisms/Strains | ||
| BALB/c-Act-2W1S/OVA | Laboratory of Marc Jenkins | Published in Moon et al., 2011; Backcrossed 10 generations to BALB/c background |
| C57BL/6NCr | Charles River Laboratories (NCI) | #556 |
| Balb/cAnNCr | Charles River Laboratories (NCI) | #555 |
| Software and Algorithms | ||
| FlowJo | BD Biosciences | https://www.flowjo.com |
| GraphPad Prism | GraphPad Software | https://www.graphpad.com |
Intracellular Cytokine Staining
For cytokine staining, cells were incubated with PMA/ionomycin (eBioscience) and Brefeldin A (GolgiPlug, BD Biosciences) in complete DMEM for 5 hours. Negative controls for this assay were incubated with Brefeldin A + DMEM alone. Cells were then washed and incubated with H-2Kb:OVA257–264 for MHC Class I tetramer staining as outlined above. Bound and unbound cell fractions were then fixed and permeabilized (BD Cytofix/Cytoperm) and stained with anti-cytokine antibodies described in the key resource table. Cells were analyzed on a FACSCanto cytometer (BD Biosciences) and analyzed using FlowJo (TreeStar) software.
Cell Transfer, Neutralization and Depletion
To measure cytotoxicity, splenocytes from congenic (CD45.2+) mice were labeled with either high (1 μM) or low (50 nM) CFSE, pulsed with OVA257–264 peptide (50nM CFSE) or irrelevant Zika virus, NS5227–234 peptide (1 μM CFSE), intravenously transferred at a 1:1 ratio into mice on the CD45.1 background, and harvested 48 hours thereafter. To investigate the response to cells expressing intact antigen, splenocytes from Act-OVA mice on the CD45.1 congenic background were intravenously transferred into CD45.2 recipients, and the persistence of adoptively-transferred CD45.1 + cells enumerated 7 days thereafter. For PD-1/LAG-3 blockade, purified anti-mouse PD-L1 (10F.9G2) antibody (BioXcell) and anti-mouse LAG-3 (C9B7W) antibodies (BioXcell) were administered intraperitoneally (500 μg each antibody per mouse) at mid-gestation during allogeneic pregnancy. For T cell depletion, purified anti-mouse CD8 (2.43) and/or anti-mouse CD4 (GK1.5) antibody (BioXcell) were administered intraperitoneally (500 μg per mouse) one day prior to administration of anti-PD-L1/LAG-3 antibodies.
QUANTIFICATION AND STATISTICAL ANALYSIS
For all experiments, female mice were randomly assigned to experimental groups. Differences between two groups were analyzed using the Student’s t test (normal distribution of data) or Mann Whitney test (data not normally distrusted), and for three of more groups the one-way ANOVA test (normal distribution of data) or the Kruskal-Wallis test (data not normally distrusted) (Prism, GraphPad). Temporal trends in cytokine production after delivery was analyzed using linear regression to determine presence of non-zero slope. For each analysis, a p value of ≤ 0.05 was taken as statistical significance.
Highlights.
Pregnancy primes CD8+ T cells with fetal specificity that persists after pregnancy
Memory CD8+ T cells are cytolytic and primed for effector cytokine production
Fetal-specific CD8+ T cells are functionally exhausted during secondary pregnancy
PD-1 and LAG-3 suppress CD8+ T cells selectively with fetal antigen re-stimulation
ACKNOWLEDGMENTS
We are indebted to Drs. Theresa Alenghat, Hitesh Deshmukh, and Chandra-shekhar Pasare, and Joseph Qualls for helpful suggestions. This work was supported by NIH through grants R01AI120202, R01AI124657, and DP1AI131080. S.S.W. is supported by the HHMI Faculty Scholars program, the March of Dimes Ohio Collaborative for Prematurity Research, and the Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Award.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Barton BM, Xu R, Wherry EJ, and Porrett PM (2017). Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J. Leukoc. Biol. 101, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, and Wherry EJ (2009). Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Sourdive DJ, Murali-Krishna K, Ahmed R, and Altman JD (2000). Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J. Immunol. 165, 6081–6090. [DOI] [PubMed] [Google Scholar]
- Brazdova A, Senechal H, Peltre G, and Poncet P (2016). Immune Aspects of Female Infertility. Int. J. Fertil. Steril. 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh H, and Way SS (2019). Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu. Rev. Pathol. 14, 185–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehst BD, Ingulli E, and Jenkins MK (2003). Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transplant. 3, 1355–1362. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, and Glimcher LH (2007). Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Fenster L, and Sidney S (1991). A multivariate analysis of risk factors for preeclampsia. JAMA 266, 237–241. [PubMed] [Google Scholar]
- Feeney JG, and Scott JS (1980). Pre-eclampsia and changed paternity. Eur. J. Obstet. Gynecol. Reprod. Biol. 11, 35–38. [DOI] [PubMed] [Google Scholar]
- Flowers ME, Pepe MS, Longton G, Doney KC, Monroe D, Witherspoon RP, Sullivan KM, and Storb R (1990). Previous donor pregnancy as a risk factor for acute graft-versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br. J. Haematol. 74, 492–496. [DOI] [PubMed] [Google Scholar]
- Gale RP, Bortin MM, van Bekkum DW, Biggs JC, Dicke KA, Gluckman E, Good RA, Hoffmann RG, Kay HE, Kersey JH, et al. (1987). Risk factors for acute graft-versus-host disease. Br. J. Haematol. 67,397–406. [DOI] [PubMed] [Google Scholar]
- Ghafari A (2008). Offspring-to-mother and husband-to-wife renal transplantation: a single-center experience. Transplant. Proc. 40, 140–142. [DOI] [PubMed] [Google Scholar]
- Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, and Sayegh MH (2005). A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, and Badovinac VP (2008). Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8, 107–119. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Toh S, and Cnattingius S (2009). Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 338, b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, and Masopust D (2009). Diversity in T cell memory: an embarrassment of riches. Immunity 37, 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, et al. (2001). Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood 98, 2043–2051. [DOI] [PubMed] [Google Scholar]
- Li DK, and Wi S (2000). Changing paternity and the risk of preeclampsia/ eclampsia in the subsequent pregnancy. Am. J. Epidemiol. 151, 57–62. [DOI] [PubMed] [Google Scholar]
- Linscheid C, and Petroff MG (2013). Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am. J. Reprod. Immunol. 69, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer D, Piper K, Goodyear O, Kilby MD, and Moss PA (2012). Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 189, 1072–1080. [DOI] [PubMed] [Google Scholar]
- McLane LM, Abdel-Hakeem MS, and Wherry EJ (2019). CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 37, 457–495. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, and Robertson SA (2009). Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 182, 8080–8093. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Diener KR, Hayball JD, and Robertson SA (2017). An immunogenic phenotype in paternal antigen-specific CD8+ T cells at embryo implantation elicits later fetal loss in mice. Immunol. Cell Biol. 95, 705–715. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, and Jenkins MK (2007). Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH 3rd, McClaren JL, Chu HH, Thomas PG, and Jenkins MK (2011). Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class ll-specific CD4+ T cells. Proc. Natl. Acad. Sci. USA 108, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, Martin WL, and Moss PA (2007). Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol. Reprod. 76, 96–101. [DOI] [PubMed] [Google Scholar]
- Porrett PM (2018). Biologic mechanisms and clinical consequences of pregnancy alloimmunization. Am. J. Transplant. 18, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, and Kilby MD (2017). Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J. Immunol. 199, 3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, and Sharkey DJ (2016). Seminal fluid and fertility in women. Fertil. Steril. 106, 511–519. [DOI] [PubMed] [Google Scholar]
- Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, and Papiernik E (1993). Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J. Reprod. Immunol. 24, 1–12. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, and Way SS (2011). Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, and Way SS (2012). Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, and Lanzavecchia A (1999). Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712. [DOI] [PubMed] [Google Scholar]
- Smith TR, Verdeii G, Marquardt K, and Sherman LA (2014). Contribution of TCR signaling strength to CD8+ T cell peripheral tolerance mechanisms. J. Immunol. 193, 3409–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafuri A, Alferink J, Möller P, Hämmerling GJ, and Arnold B (1995). Tcell awareness of paternal alloantigens during pregnancy. Science 270, 630–633. [DOI] [PubMed] [Google Scholar]
- Taglauer ES, Yankee TM, and Petroff MG (2009). Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 80, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay CS, Tagliani E, Collins MK, and Erlebacher A (2013). Cis-acting pathways selectively enforce the non-im mu nogen icity of shed placental antigen for maternal CD8 T cells. PLoS One 8, e84064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T, and Strominger JL (2013). CD8+ effector T cells at the fetalmaternal interface, balancing fetal tolerance and antiviral immunity. Am. J. Reprod. Immunol. 69, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupin LS, Simon LP, and Eskenazi B (1996). Change in paternity: a risk factor for preeclampsia in multi paras. Epidemiology 7, 240–244. [DOI] [PubMed] [Google Scholar]
- Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, and Dekker GA (1999). Change in paternity: a risk factor for preeclampsia in multiparous women? J. Reprod. Immunol. 45, 81–88. [DOI] [PubMed] [Google Scholar]
- Van Rood JJ, Eernisse JG, and Van Leeuwen A (1958). Leucocyte antibodies in sera from pregnant women. Nature 181, 1735–1736. [DOI] [PubMed] [Google Scholar]
- Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, Brand A, Mutis T, and Goulmy E (2004). Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood 103, 1961–1964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not result in any datasets or custom code.