Abstract
The proper formation of the stratum corneum, involves a highly choreographed sequence of events in which the granular cell becomes a desiccated corneocyte. In this issue, Akinduro et al (2016) detail the molecular machinery underlying the removal of the nucleus (nucleophagy), during the end-stages of keratinization. They provide evidence that nucleophagy is induced when keratinocytes differentiate and that a failure in the initiation of nucleophagy is associated with parakeratosis.
Epidermis functions as a dynamic barrier protecting the organism from a variety of external insults as well as excessive fluid loss. In order to fulfill its protective function, keratinocytes undergo a specific process of differentiation (keratinization) culminating in the formation of the stratum corneum. Keratinization initiates when the relatively undifferentiated, keratin filament-filled, proliferating basal cells receive cues to differentiate and migrate. Asymmetric division of the proliferating basal cells plays a key role in activating the basal to suprabasal fate switch (Fuchs, 2008; Lopez-Pajares et al., 2013). Furthermore, a coordinated activating and silencing of various genes occurs during both the early and late stages of keratinization(Gdula et al., 2013) and references therein). The appearance of small, membrane-bound granules (lamellar granules) at the apical surface of the lowermost suprabasal (spinous) cells is one of the first morphological indicators of differentiation (Lavker, 1976) and references therein). The next major differentiation product is filaggrin, which is first observed in the granular cells (Steinert et al., 1981). The elaboration of the modified cornfield envelope represents the last of the differentiation products(Candi et al., 2005; Steinert and Marekov, 1995). Keratin filaments, lamellar granules, filaggrin and the components necessary for the modified corneocyte envelope accumulate in the granular cells and are subsequently either discharged (lamellar granules) or modified to form the filament/matrix complex that comprises the bulk of the flattened corneocyte. Another important event is the remodeling and eventual dissolution of the nucleus that takes place during the keratinization process(Gdula et al., 2013). In the paper by Akinduro et al. (2016), we now begin to understand the molecular machinery that underlies the end-stage events in the life of the keratinocyte nucleus (nucleophagy).
Autophagy
The first clue that lytic processes (autophagy) might be involved in the morphological transformation of granular cells into corneocytes comes from a series of ultrastructural studies in the early 1970’s (Lavker, 1974; Lavker and Matoltsy, 1970). Using the ruminal epithelium (a keratinizing epithelium) as a model, the events occurring in the intermediate stages in transition from granular to horny cells were described. The initial event was an increase in autolysosomes in the granular cells. That lytic events triggered the initial stages leading to cornification (Lavker and Matoltsy, 1970), was the observation that mitochondrial remnants within the lysosome/autolysosomes (possibly echoing a process of what is now termed mitophagy) resided in the granular cells (Lavker, 1974; Lavker and Matoltsy, 1970; Morioka et al., 1999).This preceded the disappearance of the metabolic organelles (mitochondria, Golgi, endoplasmic reticulum) and the destruction of nuclei (Fig. 1) (Lavker, 1974; Lavker and Matoltsy, 1970). As cornification advanced, the nucleus was one of the last structures to undergo degradation possibly via nucleophagy, although that term was not used at the time (Lavker and Matoltsy, 1970). Using multicolor confocal microscopy, image analysis and mathematical modeling a clearer picture of nuclear changes during keratinization has evolved(Gdula et al., 2013). In this issue of the JID, Akinduro et al. (2016) utilized comprehensive genetic, molecular and cellular biological approaches and demonstrated that nucleophagy serves as a means of nuclear removal, critical for keratinocyte differentiation.
Figure 1.
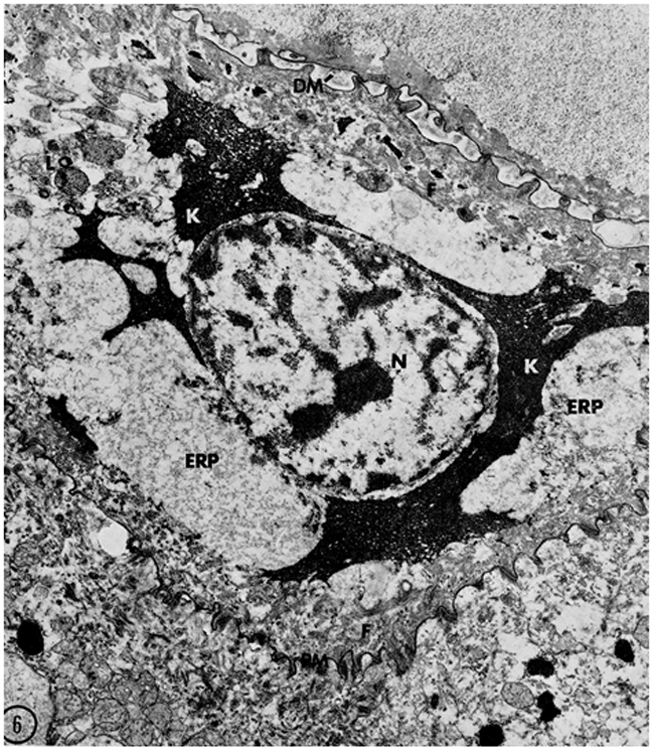
A keratinocyte in an advanced stage of transformation from a granular cell to a corneocyte. Lysosomes/autophagosomes (L) are present in the cytoplasm. Filaggrin (K) is spread throughout the cytoplasm intermingling with keratin filaments (F). The thickened, modified corneocyte envelope (PM) is tightly attached to the lower granular cell. The nucleus (N), last of the recognizable organelles, shows signs of degradation. Reprinted from ©Lavker and Matoltsy, 1970, J Cell Biology, 44:501–512
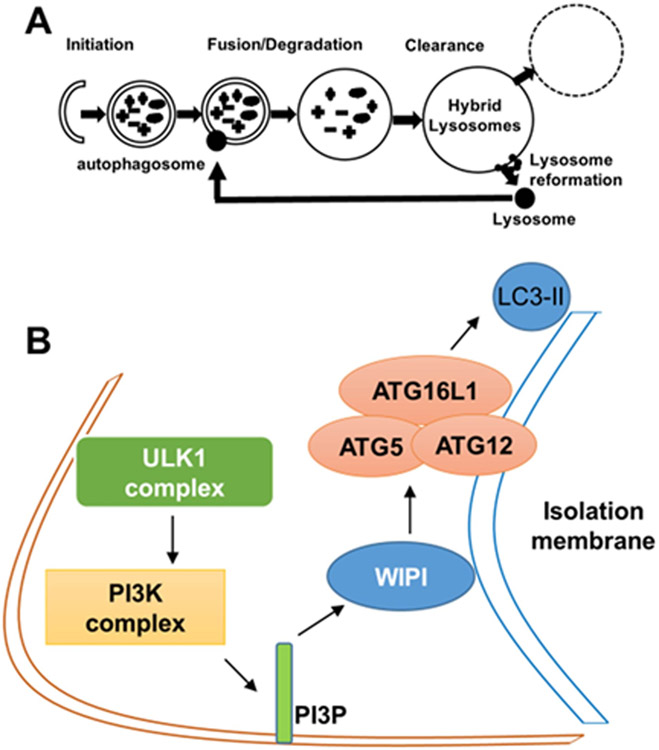
Autophagy is a process of self-cannibalism in which cellular material is engulfed within double-membraned autophagosomes for ultimate digestion (initiation; Fig. 2A). The initial steps in autophagy involve the formation of an isolation membrane (Fig. 2A). Genes associated with this event include: unc-51 like autophagy activating kinase 1 (ULK1) complex; phosphatidylinositol-3-kinase (PI3K) complex (VPS34/Beclin1/ATG14L); and WD repeat domain phosphoinositide interacting 1 (WIPI1). Activation of these complexes leads to the association of ATG16L1 with the ATG5-ATG12 conjugate. The ATG5-ATG12-ATG16L1 complex then adds phosphatidylethanolamine (PE) to the C-terminus of the microtubule associated protein 1 light chain 3 (LC3) protein promoting the elongation of the isolation membrane and subsequent autophagosome formation (Fig. 2B). Thus, Beclin1 and the ATG genes are related to the initial formation of the autophagosomes and LC3 is a well-accepted autophagosome marker. At the late stage of autophagy, autophagosomes fuse with lysosomes resulting in autolysosomes, which function in degradation (Fig. 2A). Recently, studies are starting to detail the steps involved in the end stage of autophagy, in which the autolysosomes are recycled to yield lysosomes via autophagic lysosome reformation (ALR; Fig. 2A) (Chen and Yu, 2013).
Figure 2.
Autophagy and its initiation. (A) A schematic representation of the stages of autophagy. (B) A schematic representation of the initial steps in autophagy involving the formation of an isolation membrane. In this process, unc-51 like autophagy activating kinase 1 (ULK1) complex activates the phosphatidylinositol-3-kinase (PI3K) complex (VPS34/Beclin1/ATG14L). VPS34-derived PI3P recruits double FYVE-containing protein 1 (DFCP1/ZFYVE1) and WD repeat domain phosphoinositide interacting 1 (WIPI1) to the outer membrane of autophagosomes, which causes the association of the ATG5/ATG12 conjugate with ATG16L1. The ATG5/ATG12/ATG16L1 complex then adds phosphatidylethanolamine group (PE) to the C-terminus of the microtubule associated protein 1 light chain 3 (LC3) protein promoting the elongation of the isolation membrane and autophagosome formation.
Autophagy related genes in epidermis
To establish the molecular basis by which autophagy functions in epidermis, Akinduro et al. (2016) immunohistologically examined detailed expression patterns of several critical genes involved in the initiation of autophagy in epidermis. Among them, LC3, the well-accepted autophagosome marker, was shown to be robustly expressed in the granular layers. Such strong expression was persistent in neonatal and adult mice. Akinduro et al. (2016) also examined LC3 expression in human epidermis and observed a similar granular layer distribution. These findings indicate a high degree of autophagy in granular layers, which is consistent with the reports of excessive numbers of LC3 puncta in the upper layers of GFP-LC3 transgenic mouse epidermis as well as in the upper layers of EGFP-LC3-transduced 3D organotypic raft cultures of normal human epidermal keratinocytes (Moriyama et al., 2014; Rossiter et al., 2013). Additionally, Akinduro et al. (2016) found that autophagy related genes, including LC3, ATG5-ATG12, WIPI1, and ULK1, were upregulated at E16.5 when the granular layer is first observed. These findings suggest that autophagic processes play a role in the terminal phases of keratinization and for the first time indicate a major role for nucleophagy in keratinization.
Nucleophagy in keratinocyte differentiation
While the morphological evidence for nucleophagy in keratinocyte terminal differentiation has been known for over 45 years (Lavker, 1974; Lavker and Matoltsy, 1970), our understanding of the molecular components underlying these observations was scant. Akinduro et al. (2016) observed a dramatic increase in misshaped nuclei within differentiated keratinocytes. At morphologically irregular regions of such misshaped nuclei, they detected a colocalization of autophagic vesicle markers and nuclear materials. Furthermore, knockdown of autophagy-essential genes significantly reduced the cells with nucleophagic vesicles in differentiated keratinocytes; with negligible effect on proliferating keratinocytes. Finally, to validate the above observations, Akinduro et al. (2016) found that some of the LC3 puncta in the granular layers were coincident with areas of misshaped nuclei. Collectively, this is strong evidence that nucleophagy plays a key role in nuclear removal during keratinocyte terminal differentiation in vitro and in vivo.
Autophagy and Psoriasis
The links between nucleophagy and human disease are only just emerging. Parakeratosis, retention of nuclei in the stratum corneum, is one of characteristics of psoriasis. Akinduro et al. (2016) showed that LC3, WIPI1 and ULK1 were decreased in the parakeratotic region of epidermis from patients with psoriasis, and suggested that a failure in nucleophagy may contribute to the pathogenesis of this disease. Several studies have suggested that autophagy is impaired in psoriasis. For example, several single nucleotide polymorphisms (SNPs) in ATG16L1 gene, which interacts with ATG5/ATG12 to mediate the conjugation of PE to LC3, were associated with a susceptibility to psoriasis (Douroudis et al., 2012). Furthermore, p62, which was degraded by autophagy and is well-accepted as a negative marker for autophagy flux, was dramatically upregulated in the epidermis of psoriatic skin (Lee et al., 2011). Such p62 accumulation indicates an inhibition of autophagy flux in psoriatic epidermis. Taken together, Akinduro et al. (2016) and others (Douroudis et al., 2012; Lee et al., 2011) have provided evidence that an impairment in nucleophagy may contribute to the etiology of psoriasis. In support of this idea, UVB therapy (Qiang et al., 2013), retinoids (Rajawat et al., 2010), and vitamin D analogs (Hoyer-Hansen et al., 2005), which are used for the treatment of psoriasis, can induce autophagy.
Unanswered nucleophagy questions
Most of our knowledge about nucleophagy comes from studies on lower eukaryotes such as yeast. Detailed mechanisms of nucleophagy in mammals remain unclear. Akinduro et al. (2016) showed that WIPI1 and ULK1 were required for nucleophagy during keratinocyte terminal differentiation in vitro. However, to better understand how nucleophagy is initiated and regulated during terminal differentiation of keratinocytes, further investigations on the molecular mechanisms of nucleophagy are necessary. For example, Akinduro et al. (2016) showed that Beclin1, a key component of the PI3K complex, which mediates nucleation at early stage of autophagy, was increased at E16.5. However, this protein was not localized within the epidermal granular layers. This raises the question of whether Beclin1 is essential for autophagy/nucleophagy in the epidermal granular layers. While nuclear changes and nucleophagy have thus far been primarily keratinocyte-centric, this raises the question about other cell types such as fibroblasts? A form of nucleophagy has been reported in mouse embryonic fibroblasts carrying lamin mutations as well as in wild-type cells (Park et al., 2009). We anticipate that the present paper by Akinduro et al (2016) will stimulate investigations of nucleophagy in other skin cells. Furthermore, studies on autophagy in general have been heavily focused on the initiation stage; whereas, end-stage events are just starting to be recognized. Akinduro et al (2016) nicely detailed the early events in nucleophagy; however, the events taking place during the end stages of nucleophagy are unclear. Nonetheless, the present study by Akinduro et al (2016) should stimulate further investigations on the roles of autophagy in keratinocyte biology as well as diseases of abnormal keratinization.
Clinical.
Inhibition of nucleophagy when keratinocytes differentiate is associated with parakeratosis.
The therapies that can induce nucleophagy may be considered to treat skin diseases with parakeratosis, such as psoriasis.
Studies on the molecular basis of nucleophagy regulation in epidermal differentiation are needed to identify the small molecules that promote keratinization.
References
- Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6:328–40. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu L (2013) Autophagic lysosome reformation. Exp Cell Res 319:142–6. [DOI] [PubMed] [Google Scholar]
- Douroudis K, Kingo K, Traks T, Reimann E, Raud K, Ratsep R, et al. (2012) Polymorphisms in the ATG16L1 gene are associated with psoriasis vulgaris. Acta Derm Venereol 92:85–7. [DOI] [PubMed] [Google Scholar]
- Fuchs E (2008) Skin stem cells: rising to the surface. J Cell Biol 180:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdula MR, Poterlowicz K, Mardaryev AN, Sharov AA, Peng Y, Fessing MY, et al. (2013) Remodeling of three-dimensional organization of the nucleus during terminal keratinocyte differentiation in the epidermis. J Invest Dermatol 133:2191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M (2005) Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ 12:1297–309. [DOI] [PubMed] [Google Scholar]
- Lavker RM (1974) Horny cell formation in the epidermis of Rana pipiens. J Morphol 142:365–77. [DOI] [PubMed] [Google Scholar]
- Lavker RM (1976) Membrane coating granules: the fate of the discharged lamellae. J Ultrastruct Res 55:79–86. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Matoltsy AG (1970) Formation of horny cells: the fate of cell organelles and differentiation products in ruminal epithelium. J Cell Biol 44:501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Shin DM, Yuk JM, Shi G, Choi DK, Lee SH, et al. (2011) Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol 186:1248–58. [DOI] [PubMed] [Google Scholar]
- Lopez-Pajares V, Yan K, Zarnegar BJ, Jameson KL, Khavari PA (2013) Genetic pathways in disorders of epidermal differentiation. Trends Genet 29:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka K, Takano-Ohmuro H, Sameshima M, Ueno T, Kominami E, Sakuraba H, et al. (1999) Extinction of organelles in differentiating epidermis. Acta Histochemica Et Cytochemica 32:465–76. [Google Scholar]
- Moriyama M, Moriyama H, Uda J, Matsuyama A, Osawa M, Hayakawa T (2014) BNIP3 plays crucial roles in the differentiation and maintenance of epidermal keratinocytes. J Invest Dermatol 134:1627–35. [DOI] [PubMed] [Google Scholar]
- Park YE, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, et al. (2009) Autophagic degradation of nuclear components in mammalian cells. Autophagy 5:795–804. [DOI] [PubMed] [Google Scholar]
- Qiang L, Wu C, Ming M, Viollet B, He YY (2013) Autophagy controls p38 activation to promote cell survival under genotoxic stress. J Biol Chem 288:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajawat Y, Hilioti Z, Bossis I (2010) Autophagy: a target for retinoic acids. Autophagy 6:1224–6. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Konig U, Barresi C, Buchberger M, Ghannadan M, Zhang CF, et al. (2013) Epidermal keratinocytes form a functional skin barrier in the absence of Atg7 dependent autophagy. J Dermatol Sci 71:67–75. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Cantieri JS, Teller DC, Lonsdale-Eccles JD, Dale BA (1981) Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A 78:4097–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN (1995) The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem 270:17702–11. [DOI] [PubMed] [Google Scholar]