Summary
Background
Previous studies have identified an inverse association between melanoma and smoking; however, data from population‐based studies are scarce.
Objectives
To determine the association between smoking and socioeconomic (SES) on the risk of development of melanoma. Furthermore, we sought to determine the implications of smoking and SES on survival.
Methods
We conducted a population‐based case–control study. Cases were identified from the Welsh Cancer Intelligence and Surveillance Unit (WCISU) during 2000–2015 and controls from the general population. Smoking and SES were obtained from data linkage with other national databases. The association of smoking status and SES on the incidence of melanoma were assessed using binary logistic regression. Multivariate survival analysis was performed on a melanoma cohort using a Cox proportional hazard model using survival as the outcome.
Results
During 2000–2015, 9636 patients developed melanoma. Smoking data were obtained for 7124 (73·9%) of these patients. There were 26 408 controls identified from the general population. Smoking was inversely associated with melanoma incidence [odds ratio (OR) 0·70, 95% confidence interval (CI) 0·65–0·76]. Smoking was associated with an increased overall mortality [hazard ratio (HR) 1·30, 95% CI 1·09–1·55], but not associated with melanoma‐specific mortality. Patients with higher SES had an increased association with melanoma incidence (OR 1·58, 95% CI 1·44–1·73). Higher SES was associated with an increased chance of both overall (HR 0·67, 95% CI 0·56–0·81) and disease‐specific survival (HR 0·69, 95% CI 0·53–0·90).
Conclusions
Our study has demonstrated that smoking appeared to be associated with reduced incidence of melanoma. Although smoking increases overall mortality, no association was observed with melanoma‐specific mortality. Further work is required to determine if there is a biological mechanism underlying this relationship or an alternative explanation, such as survival bias.
What's already known about this topic?
Previous studies have been contradictory with both negative and positive associations between smoking and the incidence of melanoma reported.
Previous studies have either been limited by publication bias because of selective reporting or underpowered.
What does this study add?
Our large study identified an inverse association between smoking status and melanoma incidence.
Although smoking status was negatively associated with overall disease survival, no significant association was noted in melanoma‐specific survival.
Socioeconomic status remains closely associated with melanoma. Although higher socioeconomic populations are more likely to develop the disease, patients with lower socioeconomic status continue to have a worse prognosis.
Short abstract
Linked Comment: Thompson and Friedman. Br J Dermatol 2020; 182:1080.
Plain language summary available online
Although there is a wealth of knowledge on the association of melanoma with risk factors such as ultraviolet light exposure, skin type and genetics,1 the relationship between tobacco smoke and melanoma is less clear. Tobacco smoke is a type 1 carcinogen, associated with 18 types of cancer.2 Song et al.3 reported a moderate inverse association between melanoma and smoking in a meta‐analysis of two cohort studies. This association was observed in both ex‐smokers and current smokers in men, but not women. A larger meta‐analysis, including 23 studies, reported a similar inverse association.4 Both papers reported significant limitations, notably publication bias because of selective reporting in the published studies. Furthermore, confounding variables were not included in the analysis.
A recent, prospective cohort study has further explored the association. After adjusting for potential confounding factors, no association was observed between current smoking and melanoma [odds ratio (OR) 1·01, 95% confidence interval (CI) 0·64–1·61].5 Although the study addressed the aforementioned limitations by adjusting for confounding factors, the study was significantly underpowered; only a small proportion of the cohort developed melanoma and the average follow‐up duration was short (3·5 years).
The relationship between socioeconomic status and melanoma, on the other hand, is well established in the literature, with research dating back to the 1980s.6, 7 Those in higher income or higher educational groups are at an increased risk of developing melanoma, attributed to greater exposure to lifestyle factors, such as sun holidays and tanning bed use.8 However, once diagnosed, those with a lower socioeconomic status have a worse prognosis, a finding seen across multiple jurisdictions with different healthcare systems.8 Understanding and addressing this worsened prognosis is therefore a clear public health priority.9, 10, 11
In this paper we describe the largest study investigating the association of smoking and melanoma published to date. We have used the power of routinely collected data to overcome limitations of previous studies and investigate the prognostic implications of smoking in this patient cohort. Furthermore, we sought to investigate the association of socioeconomic status on the incidence and survival of melanoma.
Patients and methods
The described study has been reported in accordance with the Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement (see Table S1 in the Supporting Information).12 The study was conducted in two stages. In stage one, a case–control study was performed to assess the relationship between smoking and the development of melanoma. In stage two, a cohort study was conducted to determine the association between smoking and survival within the melanoma cohort (Fig. 1).
Figure 1.
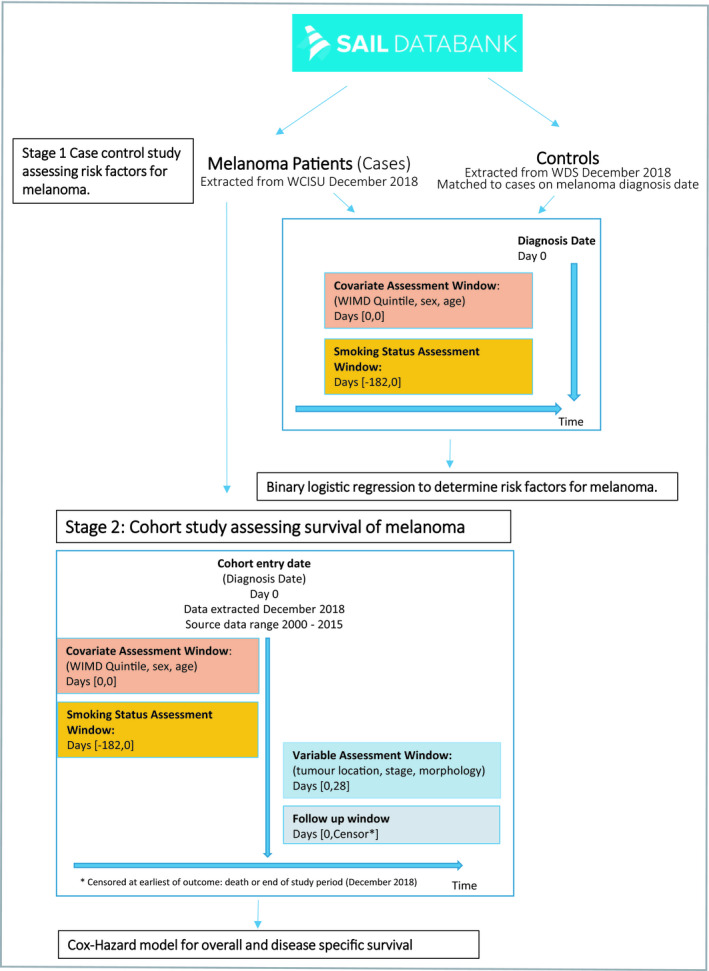
Study design. SAIL, Secure Anonymised Information Linkage; WCISU, Welsh Cancer Intelligence and Surveillance Unit; WDS, Welsh Demographic Service; WIMD, Welsh Index of Multiple Deprivation.
Overview of methods
Analyses of primary and secondary care National Health Service data and national administrative data for 2000–2015 in Wales, U.K. (population 3·1 million) were performed. In instances where relevant data were unavailable from a single source, multiple datasets were linked. Data were retrieved from six national databases (Table 1). In Wales, population‐level de‐identified person‐based health and socioeconomic administrative datasets are collated and linked within the Secure Anonymised Information Linkage (SAIL) databank.13, 14, 15 Robust policies, structures and controls are in place to protect privacy through a reliable matching and anonymization process, achieved in conjunction with the NHS Wales Informatics Service using a split‐file multiple‐encryption approach described in detail in previous published work.14
Table 1.
List of databases used and their description
| Database | Description |
|---|---|
| Annual District Death Extract | Collected from the Office for National Statistics, containing death registration information, relating to Welsh residents including those who died outside of Wales. |
| Outpatient Dataset for Wales | Administrative and clinical data obtained from outpatient appointments in Wales. |
| Patient Episode Database for Wales | Administrative and clinical data for all hospital admissions, including diagnosis and operations performed. |
| Welsh Cancer Intelligence and Surveillance Unit | The national cancer registry for Wales. Captures all Welsh patients with melanoma from a number of sources: multidisciplinary team data, pathology data, other routine data sources in Wales and the English cancer registry. |
| Welsh Longitudinal General Practice | Administrative and clinical data from all patient visits to a general practitioner. |
| Welsh Demographic Service Dataset | Administrative data about individuals resident or registered in Wales that have used National Health Service services. |
Cases
In Wales, all patients with a diagnosis of melanoma are recorded in the Welsh Cancer Intelligence and Surveillance Unit (WCISU) register. Cases were identified from WCISU using International Classification of Disease 10 (ICD‐10) codes C43·0‐C43·9 and morphology codes according to the International Classification of Diseases for Oncology (ICDO‐3) 8720‐8790.16 Patients with melanoma in situ were not included in the study as either cases or controls. Demographic information was assessed at the date of diagnosis. Melanoma‐specific variables (tumour location, stage and morphology) were assessed at the date of diagnosis.
Controls
Four sets of general population controls were randomly selected from the Welsh Demographic Service Dataset. Controls were not matched to cases. Both cases and controls needed to be alive and resident in Wales on the date of melanoma diagnosis. To increase the power of the study we aimed to have four controls for every case.17
Smoking status
Self‐reported smoking status, for cases and controls were obtained from the Welsh Longitudinal General Practice (WLGP) data, as recorded during patients’ consultations with their general practitioner in primary care, using Read codes that have been previously validated18 (Table S2; see Supporting Information). Patients were defined as either a nonsmoker (for lifelong nonsmokers), ex‐smoker (for those that had previously smoked) or current smokers. The smoking assessment window extended from the melanoma diagnosis date to 6 months prior. Where serial assessments were available, the smoking record most recent to the diagnosis was selected. Where ‘nonsmoker’ was recorded, the WLGP dataset was explored to establish whether the individual had previously been classified as a smoker. In such circumstances, the individual was classed as an ex‐smoker.
Socioeconomic status
Socioeconomic status was measured using the Welsh Index of Multiple Deprivation (WIMD) version 2001, a measure based on the Index of Multiple Deprivation and used as the official measure of socioeconomic status for the Welsh Government.19 Individual scores are based upon a person's postal address. Wales is divided into 1896 lower‐layer super‐output areas (LSOAs) following the 2001 Census, each consisting of approximately 1600 people. The WIMD scores for each LSOA are calculated from weighted scores from eight domains of socioeconomic status (income, employment, health, education, access to services, community safety, physical environment and housing socioeconomic status). Each LSOA in Wales has been ranked according to its WIMD score and grouped into quintiles, with quintile five being the highest socioeconomic status and one being the lowest.
Mortality data
Data relating to mortality, including cause of death for the melanoma cohort were obtained from the Annual District Death Extract dataset, which contains the diagnostic codes listed on patient's death certificates, held within the SAIL Databank.
Charlson Comorbidity Index
The Charlson Comorbidity Index is a widely used measure of comorbidity. An overall score is calculated from a list of conditions, each of which has been allocated a weight of between one and six based upon its adjusted relative risk of 1‐year mortality.20
Ethical approval
Study approval was granted by the SAIL Databank independent Information Governance Review Panel (project 0593). Data held within the SAIL Databank are made available to researchers in an anonymized format and are therefore not subject to data protection legislation. SAIL follows all relevant legislative and regulatory frameworks in using population data for research.
Statistical analysis
Case–control (stage one)
Descriptive statistics were used to characterize the melanoma cases and controls by smoking status and stage at diagnosis (cases only). An unconditional binary logistic regression model was used to calculate ORs with 95% CIs for the association with melanoma. Sex, socioeconomic status and age at the time of diagnosis (as a continuous variable) were incorporated into the statistical model as confounders.
Cohort study (patients with melanoma only) (stage two)
In this stage of the study only those with a diagnosis of melanoma were included (Fig. 1). Overall survival was calculated as the time from melanoma diagnosis to the time of death (outcome) or the end of the study (December 2018). Melanoma‐specific survival was calculated as the time from melanoma diagnosis to the date of death from melanoma, or the end of the study for patients still alive (December 2018). Cases with missing variables were excluded from this aspect of the study.
Kaplan–Meier curves were generated for smoking status and socioeconomic status, with curves compared using the log‐rank test. A Cox hazard proportional regression model was used to determine the association between smoking and mortality in the melanoma cohort. Sex, socioeconomic status, melanoma stage at diagnosis and age at diagnosis as a continuous variable were incorporated into the model as confounders. Both overall survival (deaths from any cause) and melanoma‐specific survival (defined on their death registration held within the Annual District Death Extract) were analysed in the melanoma cohort. All data were analysed using IBM SPSS Statistics for Windows (Released 2017, version 25·0, IBM Corp, Armonk, NY, U.S.A.). Statistical significance was assumed with a P < 0·05.
Results
Between 2000 and 2015, 9636 patients were diagnosed with melanoma in Wales.
Stage one: case–control study
Patient demographics and clinical characteristics of the cases and controls are outlined in Table 2. Data relating to smoking status were available for 7124 (73·9%) of the melanoma cohort; 1460 current smokers (20·5%), 3065 (43·0%) ex‐smokers and 2599 (36·5%) nonsmokers.
Table 2.
Demographic and clinical characteristics of the case and control participants
| Parameter | Cases (n = 7124) | Controls (n = 26 408) | P‐value |
|---|---|---|---|
| Age, median (IQR) | 63·0 (50·0–74·0) | 43·0 (26·0–60·0) | |
| Age group | |||
| < 20 | 46 (0·7) | 3980 (16·2) | ≤ 0·001 |
| 20–29 | 262 (3·7) | 3866 (15·7) | |
| 30–39 | 488 (6·9) | 3898 (15·8) | |
| 40–49 | 833 (11·7) | 4230 (17·2) | |
| 50–59 | 1312 (18·4) | 3801 (15·5) | |
| 60–69 | 1582 (22·2) | 3180 (12·9) | |
| 70–79 | 1654 (23·2) | 2230 (9·1) | |
| 80–89 | 974 (13·7) | 1030 (4·2) | |
| > 90 | 144 (2·0) | 193 (0·8) | |
| Sex | |||
| Male | 3489 (49·0) | 12 735 (51·8) | 0·26 |
| Female | 3635 (51·0) | 13 673 (55·6) | |
| WIMD quintile | |||
| 1 | 1010 (14·18) | 5502 (22·4) | ≤ 0·001 |
| 2 | 1202 (16·87) | 5329 (21·7) | |
| 3 | 1464 (20·6) | 5333 (21·7) | |
| 4 | 1446 (20·3) | 4797 (19·5) | |
| 5 | 1996 (28·0) | 5447 (22·1) | |
| Unspecified | 6 (0·1) | 0 (0·0) | |
| Smoking status | |||
| Nonsmoker | 2599 (36·5) | 10 128 (41·2) | ≤ 0·001 |
| Ex‐smoker | 3065 (43·0) | 7326 (29·8) | |
| Current smoker | 1460 (20·5) | 8954 (36·4) | |
Data are n (%) unless otherwise indicated. IQR, interquartile range; WIMD, Welsh Index of Multiple Deprivation.
Smoking
After adjusting for sex, age and socioeconomic status, current smokers had 30% reduced odds for developing melanoma compared with nonsmokers, (OR 0·70, 95% CI 0·65–0·76) (Table 3). There was no association between being an ex‐smoker or nonsmokers and melanoma (OR 1·05, 95% CI 0·98–1·12).
Table 3.
Univariable logistic regression assessing risk factors for melanoma
| Variable | P‐value | Odds ratio (95% CI) | |
|---|---|---|---|
| Age | ≤ 0·001 | 1·04 | (1·04–1·05) |
| Smoking status | |||
| Nonsmokers | Reference | ||
| Ex‐smokers | 0·17 | 1·05 | (0·98–1·12) |
| Smokers | ≤ 0·001 | 0·70 | (0·65–0·76) |
| Men | 0·26 | 0·97 | (0·92–1·02) |
| WIMD | |||
| Q1 (lowest socioeconomic status) | Reference | ||
| Q2 | 0·09 | 1·09 | (0·97–1·20) |
| Q3 | ≤ 0·001 | 1·20 | (1·09–1·32) |
| Q4 | ≤ 0·001 | 1·30 | (1·18–1·43) |
| Q5 (highest socioeconomic status) | ≤ 0·001 | 1·58 | (1·44–1·73) |
CI, confidence interval; WIMD, Welsh Index of Multiple Deprivation; Q, quintile.
Socioeconomic status
We observed an inverse relationship between socioeconomic status and melanoma, whereby patients from higher socioeconomic WIMD quintiles were more likely to develop melanoma. Those in the highest socioeconomic quintile (WIMD 5) were 1·58 times more likely to develop melanoma as opposed to the lowest (HR 1·58, 95% CI 1·44–1·73) (Table 3).
Stage two: survival analysis of the melanoma cohort
Demographic data
Table 4 displays the demographics of the melanoma cohort. The median age at diagnosis was higher in nonsmokers (66·7 years) and ex‐smokers (64·5 years) than in current smokers (62·4 years). Socioeconomic status had significant variation among groups, with the current and ex‐smokers being more likely to have lower socioeconomic status WIMD quintiles. Stage at diagnosis was not significantly different between smoking groups or socioeconomic status. No differences between the mean Charlson Comorbidity Index scores were noted between the smoking groups or between WIMD quintiles (Table 4).
Table 4.
Demographic characteristics of the melanoma cohort
| Characteristic | Total (n = 9636) | Unknown (n = 2512) | Nonsmoker (n = 2599) | Ex‐smoker (n = 3065) | Current smoker (n = 1460) | χ2 P‐value |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 64·3 (50·5–75·5) | 62·6 (48·9–75·0) | 66·7 (51·9–77·0) | 64·5 (51·4–75·4) | 62·4 (48·5–73·37) | |
| Age group | ||||||
| 0–9 | < 5 (0·1)a | < 5 (0·2)a | 0 (0) | 0 (0) | 0 (0) | |
| 10–19 | 46 (0·5) | 13 (0·5) | 20 (0·8) | < 5 (0·2)a | 9 (0·6) | |
| 20–29 | 327 (3·4) | 82 (3·3) | 97 (3·7) | 57 (1·9) | 91 (6·2) | |
| 30–39 | 726 (7·5) | 180 (7·2) | 221 (8·5) | 154 (5·0) | 171 (11·7) | |
| 40–49 | 1242 (12·9) | 291 (11·6) | 406 (15·6) | 266 (8·7) | 279 (19·1) | |
| 50–59 | 1615 (16·8) | 385 (15·3) | 480 (18·5) | 417 (13·6) | 333 (22·8) | |
| 60–69 | 2103 (21·8) | 536 (21·3) | 552 (21·2) | 716 (23·4) | 299 (20·5) | |
| 70–79 | 2085 (21·6) | 571 (22·7) | 471 (18·1) | 850 (27·7) | 193 (13·2) | |
| 80–89 | 1257 (13·0) | 368 (14·6) | 294 (11·3) | 515 (16·8) | 80 (5·5) | |
| 90–99 | 230 (2·4) | 82 (3·3) | 57 (2·2) | 86 (2·8) | 5 (0·3) | |
| > 100 | < 5 (0·1)a | 0 (0) | < 5 (0·2)a | 0 (0) | 0 (0) | |
| Sex | ||||||
| Men | 4750 (49·3) | 1261 (50·2) | 1161 (44·7) | 1661 (54·2) | 667 (45·7) | ≤ 0·001 |
| Women | 4886 (50·7) | 1251 (49·8) | 1438 (55·3) | 1404 (45·8) | 793 (54·3) | |
| WIMD quintile | ||||||
| 1 (lowest socioeconomic status) | 1300 (13·5) | 290 (11·5) | 269 (10·4) | 450 (14·7) | 291 (19·9) | ≤ 0·001 |
| 2 | 1662 (17·2) | 460 (18·3) | 382 (14·7) | 508 (16·6) | 312 (21·4) | |
| 3 | 1951 (20·2) | 487 (19·3) | 507 (19·5) | 669 (21·8) | 288 (19·7) | |
| 4 | 2169 (22·5) | 723 (28·8) | 558 (21·5) | 606 (19·8) | 282 (19·3) | |
| 5 (highest socioeconomic status) | 2547 (26·4) | 551 (21·9) | 881 (33·9) | 828 (27·0) | 287 (19·7) | |
| Unspecified | 7 (0·1) | 0 (0) | < 5 (0·1)a | < 5 (0·2)a | < 5a | |
| Charlson Comorbidity score, mean | 4·27 | 4·62 | 4·06 | 4·18 | 4·21 | 0·69 |
| Location | ||||||
| Head and neck | 1836 (19·1) | 521 (20·7) | 451 (17·4) | 649 (21·2) | 216 (14·8) | ≤ 0·001 |
| Upper limb | 2071 (21·5) | 497 (19·8) | 758 (29·2) | 662 (21·6) | 466 (31·9) | |
| Lower limb | 2370 (24·6) | 593 (23·6) | 593 (22·8) | 685 (22·3) | 319 (21·8) | |
| Trunk | 2884 (29·9) | 706 (28·1) | 698 (26·9) | 956 (31·2) | 395 (27·1) | |
| Unspecified | 476 (4·9) | 195 (7·8) | 99 (3·8) | 113 (3·7) | 64 (4·4) | |
| Stage | ||||||
| 1 | 4216 (43·8) | 900 (35·8) | 1220 (46·9) | 1484 (48·4) | 612 (41·9) | 0·06 |
| 2 | 1837 (19·1) | 488 (19·4) | 473 (18·2) | 676 (22·1) | 200 (13·7) | |
| 3 | 319 (3·3) | 100 (4·0) | 82 (3·2) | 95 (3·1) | 42 (2·9) | |
| 4 | 125 (1·3) | 30 (1·2) | 39 (1·5) | 35 (1·1) | 21 (1·4) | |
| Unspecified | 3139 (32·6) | 994 (39·6) | 785 (30·2) | 775 (25·3) | 585 (40·1) | |
| Morphology | ||||||
| Melanoma – not otherwise specified | 3122 (32·4) | 954 (38·0) | 798 (30·7) | 844 (27·5) | 526 (36·0) | ≤ 0·001 |
| Superficial spreading melanoma | 4129 (42·8) | 887 (35·3) | 1221 (47·0) | 1367 (44·6) | 654 (44·8) | |
| Nodular melanoma | 1578 (16·4) | 436 (17·4) | 387 (14·9) | 561 (18·3) | 194 (13·3) | |
| Melanoma in lentigo maligna | 466 (4·8) | 124 (4·9) | 109 (4·2) | 187 (6·1) | 46 (3·2) | |
| Otherb | 347 (3·6) | 111 (4·4) | 84 (3·2) | 106 (3·5) | 40 (2·7) | |
Data are n (%) unless otherwise indicated. IQR, interquartile range; WIMD, Welsh Index of Multiple Deprivation. aResults under five are not released from Secure Anonymised Information Linkage via disclosure control policies, to ensure privacy protection adherence. bBalloon cell melanoma, regressing melanoma, amelanotic melanoma, melanoma in junctional naevus, acral lentiginous melanoma, desmoplastic melanoma, melanoma in giant pigment naevus, mixed epithelial and spindle cell, epithelioid cell, spindle cell – not otherwise specified, spindle cell type A.
Mortality
A total of 3103 (32·2%) patients with melanoma died during the study period. Of these, 1688 (54·4%) died from melanoma (melanoma listed as the primary cause of death on their death certificate) and 1415 (45·6%) deaths were unrelated to melanoma. For patients who died from any cause, median time to death was 2·36 years. For patients who died of melanoma, median time to death was 1·73 years.
Univariate survival analysis
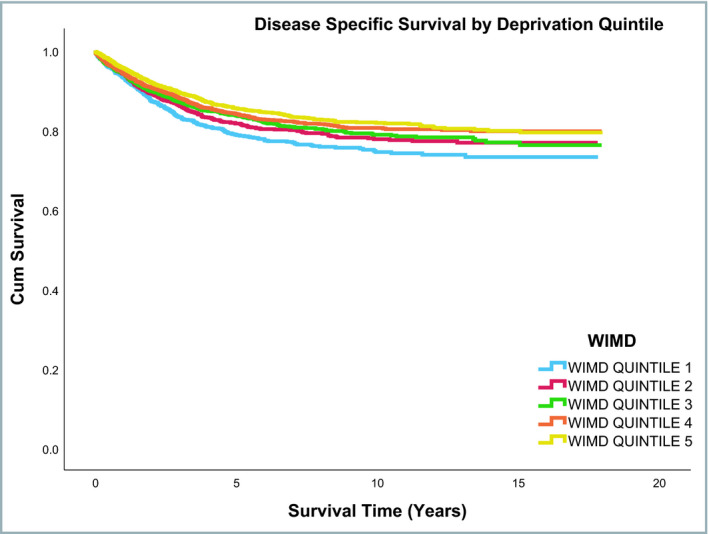
Median follow‐up duration of the entire cohort was 5·22 years (range: 0–18 years). Overall survival rates were different across the three smoking status groups, with ex‐smokers having lower survival than current or nonsmokers (P ≤ 0·001). In contrast, no difference was observed across the three smoking status groups for disease‐specific mortality (P = 0·88). Overall and melanoma‐specific survival rates by smoking status are shown in Table S3 (see Supporting Information). Figures 2 and 3 shows the overall and disease‐specific survival curves, respectively, by smoking status. Overall and disease‐specific survival rates differed significantly across the WIMD quintiles (Table S4; see Supporting Information). Figures 4 and 5 show the overall and disease‐specific survival curves, respectively, by socioeconomic status.
Figure 2.
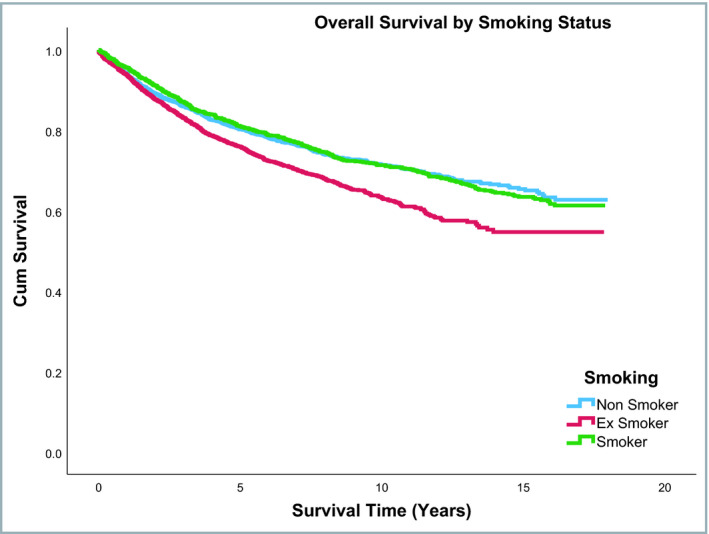
Overall survival by smoking status.
Figure 3.
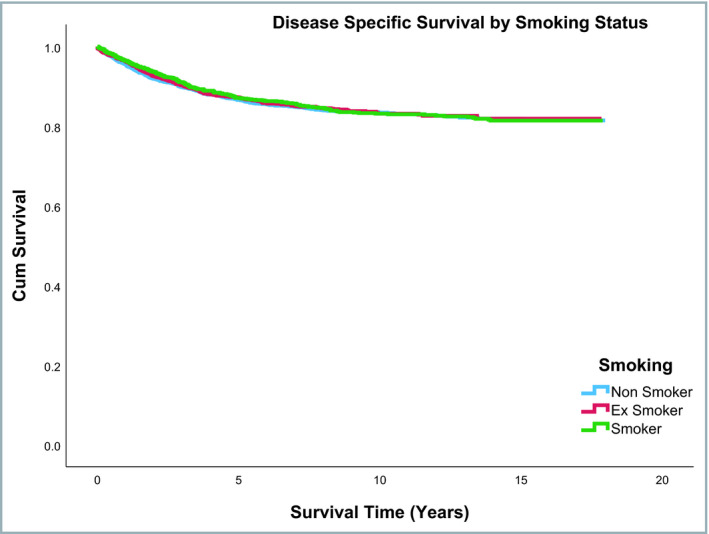
Disease‐specific survival rates by smoking status.
Figure 4.
Overall survival by socioeconomic status. WIMD, Welsh Index of Multiple Deprivation.
Figure 5.
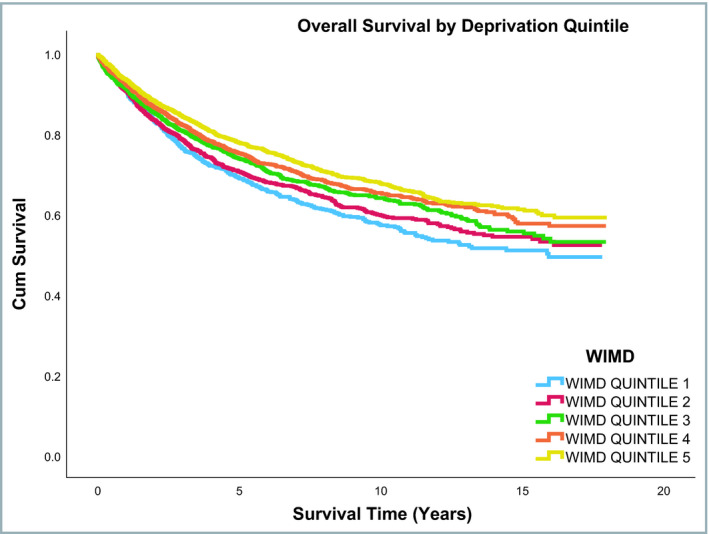
Disease‐specific survival by socioeconomic status. WIMD, Welsh Index of Multiple Deprivation.
Multivariable survival analysis
After adjusting for the aforementioned factors, current smokers had an increased overall risk of death as compared with nonsmokers (HR 1·30, 95% CI 1·09–1·55) (Table 5). There was no association between current smoking and melanoma‐specific mortality. Increased odds of survival was noted in the highest socioeconomic WIMD quintile (quintile 5), compared with the lowest (quintile 1) (HR 0·67, 95% CI 0·56–0·81). A similar trend was observed with disease‐specific mortality (HR 0·69, 95% CI 0·53–0·90).
Table 5.
Cox model for overall and disease‐specific survival
| Variable | Overall mortality | Disease‐specific mortality | ||||
|---|---|---|---|---|---|---|
| P‐value | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | |||
| Sex | ||||||
| Men | Reference | Reference | ||||
| Women | ≤ 0·001 | 1·28 | (1·13–1·46) | 0·01 | 1·35 | (1·12–1·62) |
| Smoking status | ||||||
| Nonsmoker | Reference | Reference | ||||
| Ex‐Smoker | 0·93 | 1·00 | (0·87–1·14) | 0·20 | 0·88 | (0·73–1·07) |
| Smoker | 0·03 | 1·30 | (1·09–1·55) | 0·25 | 1·15 | (0·91–1·45) |
| WIMD quintile | ||||||
| 1 (Lowest socioeconomic status) | Reference | Reference | ||||
| 2 | 0·75 | 0·97 | (0·80–1·18) | 0·93 | 0·99 | (0·75–1·30) |
| 3 | 0·01 | 0·78 | (0·65–0·95) | 0·09 | 0·79 | (0·60–1·04) |
| 4 | 0·04 | 0·75 | (0·62–0·91) | 0·08 | 0·78 | (0·59–1·03) |
| 5 (highest socioeconomic status) | ≤ 0·001 | 0·67 | (0·56–0·81) | 0·01 | 0·69 | (0·53–0·90) |
| Charlson Comorbidity Index | 0·08 | 1·01 | (1·00–1·02) | 0·517 | 1·00 | (0·99–1·02) |
| Location | ||||||
| Trunk | Reference | Reference | ||||
| Lower limb | 0·10 | 0·86 | (0·72–1·02) | ≤ 0·001 | 0·79 | (0·63–1·01) |
| Upper limb | 0·01 | 0·73 | (0·61–0·88) | ≤ 0·001 | 0·62 | (0·48–0·79) |
| Head and neck | 0·48 | 0·94 | (0·80–1·11) | 0·06 | 0·80 | (0·63–1·01) |
| Unspecified | 0·28 | 1·21 | (0·86–1·70) | 0·83 | 1·05 | (0·67–1·64) |
| Stage | ||||||
| 1 | Reference | Reference | ||||
| 2 | ≤ 0·001 | 2·48 | (2·15–2·86) | ≤ 0·001 | 6·24 | (4·95–7·88) |
| 3 | ≤ 0·001 | 3·65 | (2·96–4·59) | ≤ 0·001 | 11·48 | (8·52–15·48) |
| 4 | ≤ 0·001 | 11·78 | (8·76–15·53) | ≤ 0·001 | 32·55 | (22·73–46·61) |
| Agea | ≤ 0·001 | 1·06 | (1·05–1·06) | ≤ 0·001 | 1·02 | (1·02–1·03) |
| Morphology | ||||||
| Superficial spreading melanoma | Reference | Reference | ||||
| Nodular melanoma | 0·96 | 1·15 | (0·98–1·35) | 0·08 | 1·23 | (0·98–1·54) |
| Melanoma in lentigo maligna | 0·70 | 1·05 | (0·81–1·37) | 0·02 | 0·43 | (0·21–0·89) |
| Otherb | 0·12 | 1·25 | (0·95–1·67) | 0·50 | 1·16 | (0·76–1·74) |
| Unspecified | 0·01 | 1·24 | (1·05–1·47) | 0·04 | 1·28 | (1·01–1·62) |
CI, confidence interval; WIMD, Welsh Index of Multiple Deprivation. aAge was included as a continuous variable in the model; bBalloon cell melanoma, regressing melanoma, amelanotic melanoma, melanoma in junctional naevus, acral lentiginous melanoma, desmoplastic melanoma, melanoma in giant pigment naevus, mixed epithelial and spindle cell, epithelioid cell, spindle cell – not otherwise specified, spindle cell type A.
Men had an increased risk of overall and melanoma‐specific death compared with women (overall HR 1·28, 95% CI 1·13–1·46; disease‐specific HR 1·35, 95% CI 1·12–1·62). Tumour location was an important predictor of survival. For overall survival, tumours located on the upper limb were associated with increased survival compared with those on the trunk (HR 0·73, 95% CI 0·61–0·88), with no association between tumours on the head and neck, and lower limbs. With regards to melanoma‐specific mortality, tumours located on the trunk were associated with an increased risk of mortality when compared with those in other locations.
Age was associated with a small increased risk of overall and melanoma‐specific mortality (overall HR 1·06, 95% CI 1·05–1·06, P ≤ 0·001; disease‐specific HR 1·02, 95% CI 1·01–1·03, P ≤ 0·001). Melanoma morphology was not associated with overall survival, however, melanoma‐specific mortality was increased in those with nodular melanoma (HR 1·23, 95% CI 0·98–1·54) whereas those with lentigo maligna melanoma had improved survival (HR 0·43, 95% CI 0·21–0·89). The Charlson Comorbidity Index was not association with overall (HR 1·01, 95% CI 1·00–1·02) or melanoma‐specific survival (HR 1·00, 95% CI 0·99–1·02).
Discussion
We found that smokers were less likely to develop melanoma in this population‐based, case–control study, but that their overall survival was reduced. After controlling for age, sex, socioeconomic status, tumour location, morphology and stage, the smoking group had an increased risk of death from all causes as compared with the nonsmoking group. However, when investigating melanoma‐specific mortality, no association was observed.
The mechanism responsible for the observed protective association of smoking on the risk of developing melanoma is not yet known, but several plausible hypotheses exist. Some authors hypothesize that the accumulation of nicotine in cells containing melanin suppresses the inflammatory response to ultraviolet B.21, 22, 23 Additionally, as smoking increases elastosis, it has been hypothesized that elastosis formation is protective of melanoma.24 Alternative explanations include earlier deaths in current and ex‐smokers leading to survival bias, whereby those exposed to smoking die before being at risk of developing melanoma.
Melanoma is not the only condition where smoking has been shown to have a favourable association, others include Parkinson disease and ulcerative colitis.25, 26 The protective association in Parkinson disease has been attributed to nicotine's ability to prevent brain damage and dopamine depletion. The depletion of dopamine occurs in the substantia nigra, an area of the brain populated by melanocytes. It is therefore plausible that Parkinson disease and melanoma share similar pathogenesis.27 Numerous studies have demonstrated an increased risk of melanoma in patients with Parkinson disease and vice versa.28 The inverse association of smoking and the risk of developing ulcerative colitis is well reported in the literature; however, the pathogenesis is less well understood.29
The relationship with smoking status has been investigated for nonmelanoma skin cancers. In a prospective cohort study of over one million participants, current smokers were found to have a reduced risk of developing basal cell carcinoma (BCC). Similar to our study, this ‘protective’ association was not observed in ex‐smokers. Squamous cell carcinoma (SCC) is conversely more common in smokers.30 The Notch pathway, which functions broadly in specifying cell fates during embryogenesis and adult life, has a key role in linking the control of epidermal differentiation and proliferation.31 Aberrant Notch signalling leads to skin cancer, although with different associations with different skin cancer types.31 For melanoma, nodular and superficial BCC, Merkel carcinoma and SCC in sun‐protected sites increased Notched signalling has an oncogenic effect. However, for basosquamous BCC and SCC on sun‐exposed sites increased signalling has an oncosuppressive effect. The Notch pathway has been found to be downregulated in smokers, which could provide a further explanation on the protective association of smoking on melanoma and nodular BCC and the higher risk of SCC on sun‐exposed sites.31, 32, 33, 34
Although we observed that smokers appeared to be at reduced risk of melanoma, their overall survival was reduced. This finding is not surprising given the strong relationship between smoking and other life‐limiting conditions, such as the majority of cancers and cardiorespiratory disease. However, consistent with the potential protective influence of smoking on melanoma development, the risk of death from melanoma was not different between the smokers and nonsmokers after adjusting for age, sex, stage of disease, morphology, socioeconomic status and tumour location. This might imply that smoking does not affect the disease progression of melanoma. This is however, not consistent with the work of Jones et al., who identified that at presentation, smokers had an increased risk of lymph node metastasis.35 The discrepancy may be explained by the fact that their study did not control for socioeconomic status. In addition, Jones et al. reported an association between smoking status and Breslow thickness at presentation. In our study we did not have data on Breslow thickness; however, smoking status was not associated with stage at presentation.
Consistent with the published literature we found that the risk of developing melanoma was positively associated with socioeconomic status in our study.1 The underlying explanation is poorly understood and likely to be complex and multifactorial. Socioeconomic status is closely linked with lifestyle factors such as travel, sunbed use and hobbies that are also associated with sunlight exposure, with the literature supporting the notion that those that are more affluent have greater exposure to lifestyle factors that increase melanoma incidence.1, 8 Our study also demonstrated that those in the highest socioeconomic status group were less likely to smoke.
Despite higher socioeconomic status being associated with an increased risk of melanoma development, lower socioeconomic status is associated with poorer survival once diagnosed. This relationship was observed in both overall and disease‐specific survival rates. This is consistent with the broader health literature where it has been shown that lower socioeconomic status is associated with premature mortality from a number of conditions such as cardiovascular disease, respiratory disease and some malignancies.36 In previous studies, low socioeconomic status has been associated with later stage of melanoma diagnosis; however, this was not observed in this study. Our results may be explained by the measure used to classify socioeconomic status, the WIMD score. One of the seven domains used to determine the WIMD quintile is health, which is determined by the number of limiting long‐term illnesses, the all‐cause death rate, cancer incidence and birthweight. Patients within the low socioeconomic status group may therefore have other attributable factors influencing survival.
Limitations of this study included missing data, the lack of information available on ethnicity and ultraviolet light exposure. As with any population‐based study, missing data prevented analysis on the total cohort. Data were missing for some of the cohort on smoking status and stage of disease. Smoking status was obtained from the WLGP, as recorded during patient's consultations with their general practitioner. To date, the WLGP covers 80% of general practices across Wales. Of the 2512 patients for whom smoking data were absent, 2431 (96·8%) belonged to general practices not contributing data to the SAIL Databank. It is therefore assumed that data for this variable were missing at random and would not bias the results.
Additionally, information was not available on the quantity of tobacco smoked by participants. The Read codes listed in the Table S2 (see Supporting Information) do capture some information on the amount of smoking. In practice, these codes were rarely utilized by general practitioners, with the majority simply recording 137R (current smoker) and therefore we were unable to provide meaningful results. This is a substantial limitation as the cumulative exposure to tobacco was not assessed, thus it was not possible to calculate a dose–response relationship.
When stage of melanoma was not recorded in the WCISU data, and could not be obtained from other linked data, these data were missing. To assess the effect of this missingness, a sensitivity analysis was performed. Missing data were incorporated into the regression model as a separate category for stage. This was found not to affect the statistical significances outlined in the results section.
A further limitation of population‐based studies using routinely collected data is incomplete control of confounding, that of data that are not specified, incompletely captured or misclassified, namely tumour location (relating to ICD‐10 Code C43·9 melanoma unspecified) and tumour morphology [M7203 – MM NOS (melanoma – not otherwise specified)]. The classification codes used to extract smoking status from general practitioner data have shown to classify 8·6% ex‐smokers as never smokers. Any misclassification would not significantly bias the results.
Ethnicity is only available on special request within the SAIL Databank and was therefore not incorporated into the statistical model. In Wales, population statistics reveal that 95% of the population are white and therefore the significance of ethnicity on the results would be minimal.37
In conclusion, this is the largest study to date indicating that smoking has an inverse relationship on the risk of developing melanoma. While the detrimental repercussions of smoking are well documented, further work is required to uncover the mechanism underlying this relationship, including further assessment about survival bias. If a biological association seems likely, this could lead to the development of novel prevention and treatment options, opening up a new wave of medical therapy for melanoma. Furthermore, this work reinforces the ongoing association between melanoma and socioeconomic status. Despite numerous public health strategies, higher socioeconomic groups continue to have a higher incidence of melanoma, however, lower socioeconomic status is related to poor survival once melanoma is diagnosed. The implications of these results, in a country such as the U.K. where health care is free to all, are significant. Further work is required to investigate the barriers to care that may exist for the lowest socioeconomic status group so that policies can be implemented to prevent healthcare inequality and improve melanoma outcomes for all.
Supporting information
Table S1 The Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement.
Table S2 List of Read codes used for smoking status in this study.
Table S3 Overall and disease‐specific survival rates by smoking status.
Table S4 Overall and disease‐specific survival rates by socioeconomic status.
Acknowledgments
This study makes use of the Secure Anonymised Information Linkage (SAIL) Databank. We would like to acknowledge all the data providers who make anonymized data available for research.
Funding sources This work was supported by an iGrant from Tenovus Cancer Care. The ReconRegen research group is supported by the Royal College of Surgeons of England, the British Association of Plastic, Reconstructive and Aesthetic Surgeons and the Swansea Bay University Health Board. Health Data Research UK, which receives its funding from HDR UK Ltd (grant ref: NIWA1) funded by the U.K. Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and the Wellcome Trust. This work was also supported by an ESRC award establishing the Administrative Data Research Centre Wales (ES/L007444/1). S.M.L. reports grants from Wellcome Senior Clinical Fellowship in Science (205039/Z/16/Z) during the conduct of the study. Funders played no role in the study design, methods, data collection, data analysis, manuscript preparation or publication.
Conflicts of interest None to declare.
Plain language summary available online
References
- 1. Jiang AJ, Rambhatla PV, Eide MJ. Socioeconomic and lifestyle factors and melanoma: a systematic review. Br J Dermatol 2015; 172:885–915. [DOI] [PubMed] [Google Scholar]
- 2. Secretan B, Straif K, Baan R et al A review of human carcinogens; Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009; 10:1033–4. [DOI] [PubMed] [Google Scholar]
- 3. Song F, Qureshi AA, Gao X et al Smoking and risk of skin cancer: a prospective analysis and a meta‐analysis. Int J Epidemiol 2012; 41:1694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Z, Wang Z, Yu Y et al Smoking is inversely related to cutaneous malignant melanoma: results of a meta‐analysis. Br J Dermatol 2015; 173:1540–3. [DOI] [PubMed] [Google Scholar]
- 5. Dusingize JC, Olsen CM, Pandeya N et al Smoking and cutaneous melanoma: findings from the QSkin Sun and Health Cohort Study. Cancer Epidemiol Biomarkers Prev 2018; 27:874–81. [DOI] [PubMed] [Google Scholar]
- 6. Graham S, Marshall J, Haughey B et al An inquiry into the epidemiology of melanoma. Am J Epidemiol 1985; 122:606–19. [DOI] [PubMed] [Google Scholar]
- 7. Vagero D, Persson G. Risks, survival and trends of malignant melanoma among white and blue collar workers in Sweden. Soc Sci Med 1984; 19:475–8. [DOI] [PubMed] [Google Scholar]
- 8. Idorn LW, Wulf HC. Socioeconomic status and cutaneous malignant melanoma in Northern Europe. Br J Dermatol 2013; 170:787–93. [DOI] [PubMed] [Google Scholar]
- 9. Buchanan L. Slip, slop, slap, seek, slide – is the message really getting across? Dermatol Online J 2013; 19:19258. [PubMed] [Google Scholar]
- 10. Makin JK, Warne CD, Dobbinson SJ et al Population and age‐group trends in weekend sun protection and sunburn over two decades of the SunSmart programme in Melbourne, Australia. Br J Dermatol 2013; 168:154–61. [DOI] [PubMed] [Google Scholar]
- 11. Cancer Research UK . Be Clear on Cancer: Skin Cancer Campaign. London: Cancer Research UK, 2014. [Google Scholar]
- 12. Benchimol EI, Smeeth L, Guttmann A et al The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) Statement. PLOS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford DV, Jones KH, Verplancke JP et al The SAIL Databank: building a national architecture for e‐health research and evaluation. BMC Health Serv Res 2009; 9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones KH, Ford DV, Jones C et al A case study of the Secure Anonymous Information Linkage (SAIL) Gateway: a privacy‐protecting remote access system for health‐related research and evaluation. J Biomed Inform 2014; 50:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyons RA, Jones KH, John G et al The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak 2009; 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fritz A, Percy C, Jack A et al International Classification of Diseases for Oncology, 3rd edn Geneva: World Health Organization, 2003. [Google Scholar]
- 17. Grimes DA, Schulz KF. Compared to what? Finding controls for case‐control studies. Lancet 2005; 365:1429–33. [DOI] [PubMed] [Google Scholar]
- 18. Atkinson MD, Kennedy JI, John A et al Development of an algorithm for determining smoking status and behaviour over the life course from UK electronic primary care records. BMC Med Inform Decis Mak 2017; 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welsh Government . What is the Welsh Index of Multiple Deprivation and How Should it be Used? Cardiff: Welsh Government, 2014. [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL et al A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 21. Yerger VB, Malone RE. Melanin and nicotine: a review of the literature. Nicotine Tob Res 2006; 8:487–98. [DOI] [PubMed] [Google Scholar]
- 22. Mills CM, Hill SA, Marks R. Transdermal nicotine suppresses cutaneous inflammation. Arch Dermatol 1997; 133:823–5. [PubMed] [Google Scholar]
- 23. Mills CM, Hill SA, Marks R. Altered inflammatory responses in smokers. BMJ 1993; 307:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grant WB. Skin aging from ultraviolet irradiance and smoking reduces risk of melanoma: epidemiological evidence. Anticancer Res 2008; 28:4003–8. [PubMed] [Google Scholar]
- 25. Hernan MA, Takkouche B, Caamano‐Isorna F et al A meta‐analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002; 52:276–84. [DOI] [PubMed] [Google Scholar]
- 26. Thomas GAO, Rhodes J, Green JT et al Role of smoking in inflammatory bowel disease: implications for therapy. Postgrad Med J 2000; 76:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan T, Li X, Jankovic J. The association between Parkinson's disease and melanoma. Int J Cancer 2011; 128:2251–6. [DOI] [PubMed] [Google Scholar]
- 28. Huang P, Yang X‐D, Chen S‐D et al The association between Parkinson's disease and melanoma: a systematic review and meta‐analysis. Transl Neurodegener 2015; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohn Colitis 2014; 8:717–25. [DOI] [PubMed] [Google Scholar]
- 30. Pirie K, Beral V, Heath AK et al Heterogeneous relationships of squamous and basal cell carcinomas of the skin with smoking: the UK Million Women Study and meta‐analysis of prospective studies. Br J Cancer 2018; 119:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panelos J, Massi D. Emerging role of Notch signaling in epidermal differentiation and skin cancer. Cancer Biol Ther 2009; 8:1986–93. [DOI] [PubMed] [Google Scholar]
- 32. Balint K, Xiao M, Pinnix CC et al Activation of Notch1 signaling is required for β‐catenin–mediated human primary melanoma progression. J Clin Invest 2005; 115:3166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakayama K. Growth and progression of melanoma and non‐melanoma skin cancers regulated by ubiquitination. Pigment Cell Melanoma Res 2010; 23:338–51. [DOI] [PubMed] [Google Scholar]
- 34. Zhang M, Biswas S, Qin X et al Does Notch play a tumor suppressor role across diverse squamous cell carcinomas? Cancer Med 2016; 5:2048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones MS, Jones PC, Stern SL et al The impact of smoking on sentinel node metastasis of primary cutaneous melanoma. Ann Surg Oncol 2017; 24:2089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romeri E, Baker A, Griffiths C. Mortality by deprivation and cause of death in England and Wales, 1999‐2003. Health Stat Q 2006; 32:19–34. [PubMed] [Google Scholar]
- 37. Stats Wales . Ethnicity by Year And Ethnic Group. Cardiff: Welsh Government, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The Reporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement.
Table S2 List of Read codes used for smoking status in this study.
Table S3 Overall and disease‐specific survival rates by smoking status.
Table S4 Overall and disease‐specific survival rates by socioeconomic status.


