Abstract
Objective
To analyze the time trends of nationwide diabetes incidence <15 years of age from 1989 until 2017 in Austria.
Methods
The Austrian Diabetes Incidence Study Group registers all newly diagnosed patients with diabetes mellitus <15 years of age in a prospective population‐based study. The diabetes type was classified on the basis of clinical and laboratory findings according to American Diabetes Association criteria. Time trends were estimated by Joinpoint analysis.
Results
1311 patients were diagnosed with type 1 diabetes (T1D) between 1989 and 1999 and 4624 patients with any type of diabetes (1999‐2017). T1D accounted for the majority of cases (94.2%), 1.8% were classified as type 2 (T2D) and 4.0% as other specific types of diabetes (1999‐2017). In the total cohort (age 0 to <15 years), a constant increase until 2012 (annual percent change [APC] 4.5, 95% confidence interval [CI]: 3.94, 5.06) was observed, followed by a leveling off with a corresponding drop (APC 0.28, 95%CI: −3.94, 4.69). This observation was mainly driven by the dynamic in the youngest age group (0‐4 years) with a steep increase until 2007 (APC 7.1, 95%CI: 5.05, 9.19) and a decrease from 2007 to 2017 (APC −0.86, 95%CI: 4.41, 2.82). No significant increase of T2D <15 years was detected. Over the observed time period (APC = 3.7, 95%CI: −0.30, 7.78).
Conclusions
The incidence of T1D is declining in young children aged 0 to 4 years, but is still rising in children 5 to 14 years in Austria. Incidence of T2D did not increase significantly and other specific types of diabetes occur twice as often compared to T2D.
Keywords: children, incidence rate, other specific types of diabetes, rotavirus vaccination, type 1 diabetes mellitus, type 2 diabetes mellitus
1. INTRODUCTION
According to the latest report from 2019, the International Diabetes Federation (IDF) estimates that around 98 200 children <15 years of age develop type 1 diabetes (T1D) each year worldwide. 1 The etiology of T1D is multifactorial; however, the specific roles for genetic susceptibility, environmental factors, the immune system, and β‐cells in the pathogenic processes underlying T1D remain unclear.
Most diabetes registries are focusing on T1D cases only and data for T2D or other specific types of diabetes are often lacking in this age group <15 years.1, 2
The variation of T1D incidence between different regions of the world 1 is also well known, with Finland and Sweden showing the highest incidence worldwide.3, 4 The latest publication from the EURODIAB‐group reported a 3.4% (95%CI: 2.8%, 3.9%) per annum increase in the T1D incidence rate across all 22 European countries for the time period 1989 to 2013. 2 There is no uniform global incidence trend in childhood T1D. Some regions for example Shanghai in China, a previously low incidence area reported a rapid increase of 14.2% (95%CI: 13.9, 14.6) annual incidence rate (1997‐2011) among children under 15 years of age. 5 Other regions described a non‐significant annual increase, like British Columbia, Canada 1.3% (95%CI: 0.0, 2.5) for the period of 2002 to 2013 and Australia 0.4% (95%CI: 0.1, 0.9) for the period 2000 to 2011.6, 7 In recent years, the Northern European countries (Norway, Sweden, and Finland) reported a leveling off of the previously observed steep increase of incidence for similar time periods.3, 4, 8, 9
Recently two scientific reports from Australia and the USA hypothesized the introduction of a rotavirus vaccination as possible explanation for the observed decrease in diabetes incidence rate.10, 11 In previous publications, enterovirus‐infections and rotavirus‐infections have been discussed as one potential cause for the ß‐cell‐destruction, 12 the reduction of rotavirus infections due to rotavirus vaccination may be an influencing factor, however, so far the literature remains controversial.13, 14, 15, 16
Incidence trends for diabetes mellitus type 2 (T2D) in children have documented a steep increase in some regions like Middle Eastern Arab Countries, Japan or the U.S.,17, 18, 19, 20 but this T2D‐epidemic has not been observed in Europe so far.19, 21, 22, 23 The range of T2D‐incidence in youth has been summarized in a review article from 2013, ranging from zero cases in the Netherlands to 5300 per 100 000 in Pima Indians in the USA. 19
The Austrian diabetes incidence study group has previously reported a significant annual increase of 5.7% per year for T1D childhood incidence for the time period 1999 to 2007, but for T2D cases no significant increase could be found. 22
This most recent dataset provides an update of the Austrian national prospective incidence trends for the years 1989 to 2017, providing a nation‐wide follow up information on T1D but also including data on T2D and other specific types of diabetes in youth <15 years. Additionally, we investigated the potential effect of the introduction of a rotavirus vaccination in our country, since rotavirus vaccination has been described to be associated with T1D incidence in recent publications.10, 11
2. METHODS
The Austrian Diabetes Incidence Study Group has been collecting data of all newly diagnosed diabetes onsets from 0 to <15 years of age since 1989, which are prospectively registered by a network of all pediatric hospitals, covering the whole country of Austria. Following data were collected: age, sex, date of diagnosis, clinical symptoms, height, weight, laboratory data at onset (glucose, pH, HCO3, ketones, HbA1c, and diabetes‐specific antibodies), and family history of diabetes. The Incidence Study Groups performs biannual for data collection.
The completeness of case ascertainment was >93%, with a uniform completeness of ascertainment over time (1999‐2007: >93%, 2008‐2013:97%).2, 22 We use the capture‐recapture method recommended by the WHO DiaMond Project, 24 as described earlier25, 26, and is also used in the EURODIAB‐study. 2
Diabetes was classified by physicians on the basis of clinical and laboratory findings according to the American Diabetes Association (ADA) criteria relevant for the time periods.27, 28 From 1989 to 1998 only cases with T1D were registered, from 1999 onward information on T2D and other specific types of diabetes were collected in the same network using the ADA diagnostic criteria: a marked elevation of the blood glucose level confirms the diagnosis of diabetes, including a random plasma glucose concentration ≥ 200 mg/dL (11.1 mmol/L) or fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) in the presence of classical symptoms or an HbA1c of ≥6.5 rel% (47.5 mmol/mol). T1D was classified in cases with an additional presence of autoimmunity and/or ketones or diabetic ketoacidosis.
The classification of T2D was based on clinical and laboratory findings defined by the following inclusion criteria: Overweight at diagnosis (defined as body mass index >90th percentile); plus any two of the following risk factors: (a) Family history of type 2 diabetes in first‐ or second‐degree relative. (b) race/ethnicity. (c) Signs of insulin resistance or conditions associated with insulin resistance (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome, or small for‐gestational‐age birth weight). and (d) Maternal history of diabetes or gestational diabetes mellitus during the child's gestation. 28
All cases, initially classified by the reporting clinician as T2D, unclear (unclassifiable) type or other specific types of diabetes (eg, MODY [Maturity Onset Diabetes of the Young], CFRD [Cystic fibrosis related Diabetes]) were re‐evaluated. Physicians were asked for re‐evaluation according to ADA classification criteria, clinical follow‐up and long term course, treatment modalities, additional results (eg, genetics), and ethnical background, as it has been done in the previous Austrian Diabetes Incidence Study Group publications.21, 22, 26
The Austrian incidence data for study periods 1979 to 2005 and 1999 to 2007 have been published before.22, 26 This current report provides a follow‐up of the prospective population‐based Austrian Diabetes incidence data <15 years from the study period 2008 to 2017 and these data are presented in comparison to the previously published incidence data from 1989 to 2007. 22
We retrieved data on number of rotavirus vaccinations on a national level from the Federal Ministry of Labour, Social Affairs, Health, and Consumer Protection in order to investigate a possible association of T1D incidence with rotavirus vaccination introduced in our country in 2007. Since July 2007, rotavirus vaccinations have been subsidized in Austria for all children aged 7 weeks up to 6 months of life. Data of vaccination coverage on an individual level are not available in Austria. Vaccination coverage percentage was assessed with the rotavirus sales figures from the 2007 to 2017 assuming a complete course of vaccination per child (two doses for Rotarix and three doses for RotaTeq) and the numbers of children eligible for vaccination according to their age. 29
2.1. Statistical analysis
Time trends of age‐standardized rates as well as annual percent change and P values were estimated by Joinpoint analysis. 30 Maximum number of joinpoints was set to 2 Denominator values for calculating rates (ie, number of boys and girls ages 0 to <15 years) were obtained from the National Population Registry (Statistics Austria). 29 Slopes of trends modeled by Joinpoint analysis are described by annual percent change (APC) and 95% confidence intervals. Subgroup analyses was performed according to sex and age group (0‐4, 5‐9, and 10‐14 years truncated) in patients with T1D as well as for yearly age‐groups between 1 and 5 years of age. 95% confidence intervals were calculated for proportions as well. Figures and all other calculations were done in SAS version 9.4. 31 Rates are given per 100 000 person years (PY).
Vaccination coverage percentage was calculated by the sum of the number of vaccinations divided by two or three depending on the type of vaccination and then by the number of newborns in the corresponding year. The significance level was set to 5%.
3. RESULTS
3.1. Basic characteristics
During the study period, 1989 to 2017 a total of 5935 new cases with diabetes mellitus <15 years of age were diagnosed. Basic characteristics of 4624 cases collected for the period 1999 to 2017 (all types of diabetes) are provided in Table 1. The majority of 4356 (94.2%) patients were classified as T1D, 83 patients (1.8%) as T2D, and 185 patients (4.0%) as other specific types of diabetes (MODY, CFRD, and other rare forms). T2D cases were significantly older and predominantly female compared to T1D cases and other specific types of diabetes. The background population of children <15 years of age in Austria was 1.338.323 in 1989 and 1.263.740 in 2017.
TABLE 1.
Basic characteristics of the study population classified as type 1 (T1D), type 2 (T2D), and other specific types of diabetes (*P < .05 comparing T1D and T2D)
| 1999‐2017 | T1D | T2D | Other specific types of diabetes |
|---|---|---|---|
| n | 4356 | 83 | 185 |
| % | 94.2 | 1.8 | 4.0 |
| % Females (95%CI) | 46.1 (44.7, 47.3) | 60.3 (49.5, 71.2)* | 50.3 (42.8, 57.7) |
| Age at diagnosis [y, mean (95%CI]) | 8.5 (8.3, 8.6) | 12.7 (12.3, 13.1)* | 9.5 (8.9, 10.1) |
3.2. T1D incidence trends
For the total cohort (age 0 to <15 years), a significant constant increase until the year 2012 (APC 4.5, 95%CI: 3.9, 5.1) was observed, followed by a leveling off with a corresponding drop of APC (APC 0.3, 95%CI: 3.9, 4.7). This observation was mainly driven by the dynamic of the youngest age group (0‐4 years) with a steep increase until 2007 (APC 7.1, 95%CI: 5.1, 9.2) and a decreased rate from 2007 to 2017 (APC −0.9, 95%CI: −4.4, 2.8). Observed age adjusted rates are shown in Supplementary Table S1, annual percentage changes (APC's) in Table 2 and the incidence trends of T1D from 1989 to 2017 in different age groups are presented in Figure 1. Since July 2007, rotavirus vaccination has been subsidized in Austria for all children from week 7 up to 6 months of life (<7 months). Over the time the vaccination coverage percentage showed a steep increase, peaking in 2010, but remained >75% afterwards (Figure 2). Joinpoint analysis for yearly subgroups of age shows that the younger the children the earlier the stagnation and drop of time trend occurs. Confidence intervals for time point of joinpoints are wide because of small sample size within 1‐year age groups so that time point of joinpoint is estimated with little precision. We omitted the age group below 1 year, because the numbers are very small.
TABLE 2.
Annual percentage changes (APC's), corresponding 95%CI and P value by age groups, sex, and type of diabetes (*P < .05)
| T1D age (years) | Lower endpoint | Upper endpoint | APC | Lower CI | Upper CI | P value |
|---|---|---|---|---|---|---|
| 0‐4 | 1989 | 2007 | 7.1* | 5.1 | 9.2 | .001 |
| 2007 | 2017 | −0.9 | −4.4 | 2.8 | .629 | |
| 5‐9 | 1989 | 2017 | 4.1* | 3.5 | 4.7 | .001 |
| 10‐14 | 1989 | 2017 | 3.8* | 3.3 | 4.2 | .001 |
| 0‐14 | 1989 | 2012 | 4.5* | 3.9 | 5.1 | .001 |
| 2012 | 2017 | 0.3 | −3.9 | 4.7 | .895 | |
| T2D 0‐14 | 1999 | 2017 | 3.7 | −0.30 | 7.8 | .068 |
Note: Number and time of joinpoints in time trends were selected by Joinpoint regression modeling.
FIGURE 1.
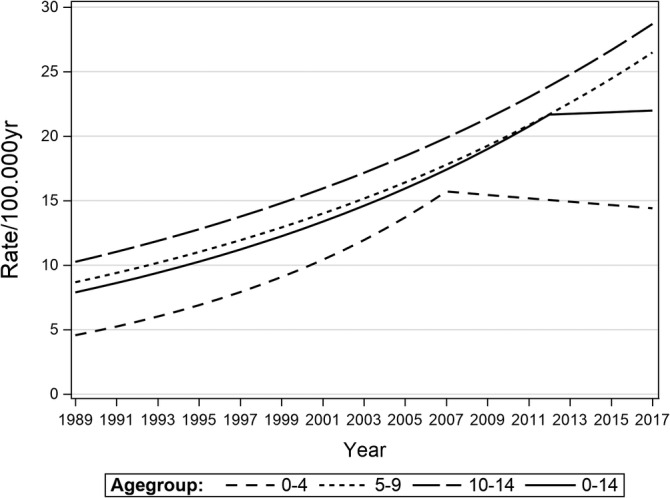
T1D incidence trends in different age groups (0‐4, 5‐9,10‐14 years, total cohort) for the time period from 1989 to2017
FIGURE 2.
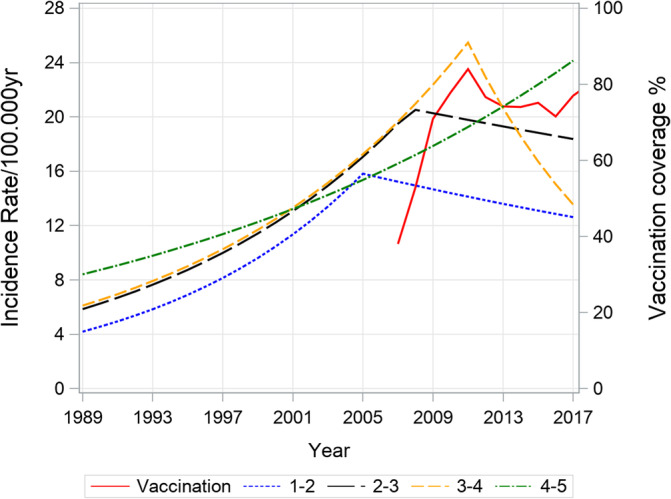
Age standardized incidence rate of T1D (age groups:1‐<2,2‐<3,3‐<4,4‐<5 years) and rotavirus vaccinationcoverage percentage
3.3. Sex‐specific T1D incidence trends
The sex‐specific T1D incidence trends increased monotonically over the observed period with a male predominance (Supplementary Table S1 and Table 2), P = .001 for both sexes.
3.4. T2D Incidence trends
Since 1999 the Austrian Diabetes Incidence Study Group additionally collects the non‐T1D‐cases for the same age group 0 to <15 years. The incidence rate of T2D remained very low (range 0.07‐0.74/100.000 PY; Supplementary Table S1). A slight but not significant increase (APC = 3.7, 95% CI: −0.3, 7.8) was observed over a period of 19 years (Figure 3 and Table 2).
FIGURE 3.
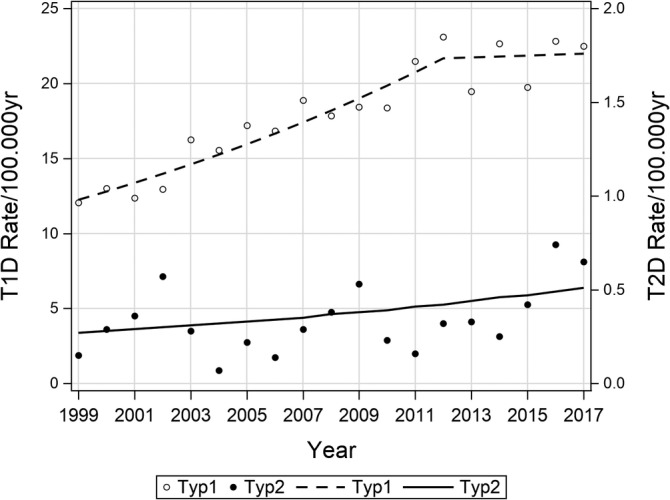
Age‐standardized incidence rate of T1D and T2D from 1999 to 2017
4. DISCUSSION
The results of this nation‐wide population‐based report on diabetes incidence trends for the time period 1989 to 2017 have to be taken into context with other recent reports about childhood diabetes incidence trends.
In accordance with other registries2, 7, 18, 32 the Austrian T1D incidence has increased significantly over a long time period.22, 26 In this current report diabetes incidence rates showed a constant, significant annually increase of 4.5% in all age groups until 2007. From 2007 onwards for the first time a decrease could be observed driven in the youngest age group (0‐4 years). This dynamic consecutively influenced the incidence of the total cohort resulting in a plateau at high level.
Whereas the US,11, 18 Canada, 7 France 33 as well as other countries like Croatia, 34 Poland, 35 and Kuwait 36 reported an increase in incidence for similar recent time periods, a plateau, respectively, a leveling off has been seen in Ireland, 37 Finland, 3 and Sweden 4 as well as Japan. 20
In concordance to our observation a decrease in incidence in the youngest age group (0‐4 years) was seen in the US from 2002 to 2009 18 and in Croatia for girls only from 2004 to 2012. 34
A coherent explanation for the observed decrease is missing so far. The pathogenesis of T1D remains still unclear, although a role for rota‐ and enterovirus infections has been postulated.12, 38 Therefore, a vaccination against rotavirus might be of protective value. Two recent publications from Australia and the USA hypothesized that rotavirus vaccination could play a role in prevention of diabetes.10, 11 Both studies reported a decrease in diabetes incidence after the introduction of a rotavirus vaccination and drew the conclusion, that rotavirus vaccination might decrease diabetes incidence in childhood. Rogers et al. 11 were able to investigate their cohort on an individual level, while Perrett et al. 10 reported data on a aggregated national level.
In contrast, two recent Finnish studies as well as a nationwide, population‐based cohort study in Finnish children up to the age of 6 years did not find an effect of Rotavirus vaccination on Diabetes risk. 13
Furthermore, there was no difference in T1D incidence in Finish children between rotavirus vaccination or placebo group in the Rotavirus Efficacy and Safety Trial. 16 Also, criticism concerning the Australian data was raised, stating that ecologic fallacy cannot be excluded, statistical methods applied may not be appropriate 15 and that the abrupt, rather than gradual decrease in incidence at the time of rotavirus vaccine implementation suggests the presence of a time‐varying confounder. 14 The differences reported in the studies from the US, Australia, and Finland are questioning whether geographic variation of genetic background and environmental risk factors might modify the potentially positive effect of rotavirus vaccination on T1D incidence. 16
The reasons behind the leveling off in Austrian incidence trends remains unclear and cannot be answered without additional individual information on rotavirus vaccination status. However, the observed change in incidence trend shows a timely coincidence with the introduction of nation‐wide rotavirus vaccination and contributes to the ongoing controversial discussion about the causal effect of rotavirus vaccination.
Rapid growth and obesity are other early life factors being implicated in the possible etiology of T1D. The prevalence of overweight and obesity was 15%, respectively, 4% in Austrian children between 3 and 6 years in 2003. 39 More recent or longitudinal data on percentage of overweight and obesity in young Austrian children are lacking. However, data for Central European children 2 to 13 years (1999‐2016) provide evidence that the epidemic of overweight/obesity shows a plateau. 40
In the current study the incidence of T2D (range 0.07‐0.74/100000 PY) remained stable on a low level from 1999 to 2017. Similarly, other European countries have reported also low rates of T2D. Recent studies found prevalence rates of 0.6 in Danish children 41 and 1.2 in Irish children below 16 years of age 42 and 2.4 in South Germany. 43 In line with our study, prevalence rates remained unchanged in Denmark between 2002 and 2014 and South‐Germany between 2004/2005 and 2016.42, 43, 44 In the UK, the incidence was 0.72/100000 PY (2015‐2016) with a significant increase in the subgroup of girls and of children with South‐Asian ethnicity only. 45
In contrast to Europe, data from the US, 46 Canada, 47 and Japan 48 report higher incidence and prevalence rates of T2D with incidences between 5.2 and 12.5/100000 PY and an annual increase rate between 3.7% and7.2%. The incidence of T2D in Tokyo, Japan increased in the period between 1975 and 2015, interestingly a decrease could be observed in the most recent years. 48
The differences between the central European countries and the Northern American and Asian countries seem to be mainly explained by the lower percentage of people with high risk ethnic background in central European countries.
The incidence of other specific types of diabetes is twice as high as T2D in Austria (4% vs 1,8%). In recent years, the knowledge and assessment of genetic tests for other specific types of diabetes have been improved, for example, the genetic screening for MODY or neonatal diabetes has been implemented. Therefor distinguishing between different types of diabetes became easier.
Our prevalence data for T2D and MODY are comparable with data sets in neighboring regions like South‐Germany. 44 Since life expectancy in patients with cystic fibrosis has improved dramatically with newer treatment methods. More patients have been diagnosed with either CFRD or post‐transplant diabetes as they get older. This leads to an increase of patients classified as other specific types of diabetes.
5. CONCLUSION
This Austrian population‐based study shows a strong rise in the diabetes incidence trends observed over nearly 30 years from 1989 to 2017. The previously reported constant increase for T1D has clearly leveled off due to a constant incidence rate in the youngest age group <5 years since 2007. The reasons behind this leveling off remain unclear. However, the introduction of a nation‐wide rotavirus vaccination shows a timely coincidence suggesting the vaccination might play a role in the observed decrease of T1D incidence. The T2D incidence remains low whereas other specific types of diabetes were diagnosed twice as often, compared to T2D.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
B.R.M. is the coordinator of the Austrian Diabetes incidence group, managed the data, wrote the manuscript. M.F., E.F.R., and S.E.H. were involved in data recruitment, read and edited the manuscript. T.W. performed the statistical analyses, generated the graphs, read, and edited the manuscript.
Supporting information
TABLE S1: Observed age‐adjusted rate per 100 000 PY over an observation period of 29 years (1989‐2017) for T1D and 19 years (1999‐2017) for T2D in children and adolescents younger than 15 years of age.
ACKNOWLEDGEMENTS
We acknowledge the input of all members of the Austrian Diabetes Incidence Study group. A list of all current members is attached in the Appendix. The study was in part supported by Novo Nordisk Austria and Sanofi‐Aventis Austria. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
APPENDIX 1.
Additional Austrian Diabetes Incidence Study Group Members
Bauer M., Beran E., Bognar M., Bonfig W., Brugger M., Farid G., Glas K., Hassan J., Jäger A., Judmaier S., Kaderschabek N., Kitzler P., Klingbacher S., Kovacs U., Lindauer S., Lück U., Mayrhofer R., Neuhauser M., Niederseer R., Piringer G., Plank R., Polland V., Prchla C., Reichle D., Rojacher T., Schermann P., Schober J., Seick‐Barbarini D., Seiwald M., Sickl E., Steigleder‐Schweiger C., Wakolbinger G., Wutzl H., Zanier U., and Zimmerer F.
Rami‐Merhar B, Hofer SE, Fröhlich‐Reiterer E, Waldhoer T, Fritsch M, for the Austrian Diabetes Incidence Study Group. Time trends in incidence of diabetes mellitus in Austrian children and adolescents <15 years (1989‐2017). Pediatr Diabetes. 2020;21:720–726. 10.1111/pedi.13038
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13038.
REFERENCES
- 1.IDF DIABETES ATLAS Ninth edition 2019 [Internet]. https://diabetesatlas.org/en/resources/
- 2. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989‐2013: a multicentre prospective registration study. Diabetologia. 2019;62:408‐417. [DOI] [PubMed] [Google Scholar]
- 3. Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:428‐429. [DOI] [PubMed] [Google Scholar]
- 4. Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60:577‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Z, Sun C, Wang C, et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997‐2011. Acta Diabetol. 2014;51:947‐953. [DOI] [PubMed] [Google Scholar]
- 6. Haynes A, Bulsara MK, Bower C, Jones TW, Davis EA. Regular peaks and troughs in the Australian incidence of childhood type 1 diabetes mellitus (2000‐2011). Diabetologia. 2015;58:2513‐2516. [DOI] [PubMed] [Google Scholar]
- 7. Fox DA, Islam N, Sutherland J, Reimer K, Amed S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19:501‐505. [DOI] [PubMed] [Google Scholar]
- 8. Ludvigsson J. Increasing incidence but decreasing awareness of type 1 diabetes in Sweden. Diabetes Care. 2017;40(10):e143‐e144. [DOI] [PubMed] [Google Scholar]
- 9. Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G. Incidence of type 1 diabetes in Norway among children aged 0‐14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian childhood diabetes registry. Diabetologia. 2014;57:57‐62. [DOI] [PubMed] [Google Scholar]
- 10. Perrett KP, Jachno K, Nolan TM, Harrison LC. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatr. 2019;173:280‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogers MAM, Basu T, Kim C. Lower incidence rate of type 1 diabetes after receipt of the rotavirus vaccine in the United States, 2001‐2017. Sci Rep. 2019;9:7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honeyman MC, Coulson BS, Stone NL, et al. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319‐1324. [DOI] [PubMed] [Google Scholar]
- 13. Vaarala O, Jokinen J, Lahdenkari M, Leino T. Rotavirus vaccination and the risk of celiac disease or type 1 diabetes in Finnish children at early life. Pediatr Infect Dis J. 2017;36:674‐675. [DOI] [PubMed] [Google Scholar]
- 14. Vajravelu ME, Tamaroff J, Shults J. Role of rotavirus vaccination in decline in incidence of type 1 diabetes. JAMA Pediatr. 2019;173:893. [DOI] [PubMed] [Google Scholar]
- 15. Rosenbauer J, Castillo K, Bächle C. Role of rotavirus vaccination in decline in incidence of type 1 diabetes. JAMA Pediatr. 2019;173(9):893–894. 10.1001/jamapediatrics.2019.2466. [DOI] [PubMed] [Google Scholar]
- 16. Hemming‐Harlo M, Lähdeaho ML, Mäki M, Vesikari T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J. 2019;38:539‐541. [DOI] [PubMed] [Google Scholar]
- 17. Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. 2016;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazeli Farsani S, van der Aa MP, van der Vorst MMJ, Knibbe CAJ, de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471‐1488. [DOI] [PubMed] [Google Scholar]
- 20. Tajima N, Morimoto A. Epidemiology of childhood diabetes mellitus in Japan. Pediatr Endocrinol Rev. 2012;10:44‐50. [PubMed] [Google Scholar]
- 21. Rami B, Schober E, Nachbauer E, Waldhör T. Type 2 diabetes mellitus is rare but not absent in children under 15 years of age in Austria. Eur J Pediatr. 2003;162:850‐852. [DOI] [PubMed] [Google Scholar]
- 22. Schober E, Waldhoer T, Rami B, Hofer S. Incidence and time trend of type 1 and type 2 diabetes in Austrian children 1999‐2007. J Pediatr. 2009;155:190‐193.e1. [DOI] [PubMed] [Google Scholar]
- 23. Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82:247‐255. [DOI] [PubMed] [Google Scholar]
- 24. Karvonen M Viik‐Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes. Diabetes Care 2000;23:1516–1526. [DOI] [PubMed] [Google Scholar]
- 25. Schober E, Schneider U, Waldhör T, Tuomilehto J. Increasing incidence of IDDM in Austrian children: a nationwide study 1979‐1993. Diabetes Care. 1995;18:1280‐1283. [DOI] [PubMed] [Google Scholar]
- 26. Schober E, Rami B, Waldhoer T. Steep increase of incidence of childhood diabetes since 1999 in Austria. Time trend analysis 1979‐2005. A nationwide study. Eur J Pediatr. 2008;167:293‐297. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Type 2 diabetes in children and adolescents. Pediatrics. 2000;105:671‐680. [DOI] [PubMed] [Google Scholar]
- 28. American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8‐S16. [DOI] [PubMed] [Google Scholar]
- 29.Statistik Austria [Internet]. www.statistik.at
- 30.Joinpoint Regression Program, Version 4.7.0.0.; Statistical Research and Applications Branch, National Cancer Institute.
- 31.SAS version 9.4. SAS Institute Inc., Cary, NC.
- 32. Tran F, Stone M, Huang CY, et al. Population‐based incidence of diabetes in Australian youth aged 10‐18yr: increase in type 1 diabetes but not type 2 diabetes. Pediatr Diabetes. 2014;15:585‐590. [DOI] [PubMed] [Google Scholar]
- 33. Piffaretti C, Mandereau‐Bruno L, Guilmin‐Crepon S, Choleau C, Coutant R, Fosse‐Edorth S. Trends in childhood type 1 diabetes incidence in France, 2010‐2015. Diabetes Res Clin Pract. 2019;149:200‐207. [DOI] [PubMed] [Google Scholar]
- 34. Rojnic Putarek N, Ille J, Spehar Uroic A, et al. Incidence of type 1 diabetes mellitus in 0 to 14‐yr‐old children in Croatia: 2004 to 2012 study. Pediatr Diabetes. 2015;16:448‐453. [DOI] [PubMed] [Google Scholar]
- 35. Chobot A, Polanska J, Deja G, Jarosz‐Chobot P. Incidence of type 1 diabetes among Polish children ages 0‐14 years from 1989‐2012. Acta Diabetol. 2015;52:483‐488. [DOI] [PubMed] [Google Scholar]
- 36. Shaltout AA, Wake D, Thanaraj TA, et al. Incidence of type 1 diabetes has doubled in Kuwaiti children 0‐14 years over the last 20 years. Pediatr Diabetes. 2017;18:761‐766. [DOI] [PubMed] [Google Scholar]
- 37. Roche EF, McKenna AM, Ryder KJ, Brennan AA, O'Regan M, Hoey HM. Is the incidence of type 1 diabetes in children and adolescents stabilising? The first 6 years of a National Register. Eur J Pediatr. 2016;175:1913‐1919. 10.1007/s00431-016-2787-6. [DOI] [PubMed] [Google Scholar]
- 38. Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331‐2339. [DOI] [PubMed] [Google Scholar]
- 39. Elmafada I, Freisling H, Koenig J et al. Österreichischer Ernährungsbericht 2003. Austria: Institut für Ernährungswissenschaften, Universität Wien; 2003. [Google Scholar]
- 40. Garrido‐Miguel M, Cavero‐Redondo I, Álvarez‐Bueno C, et al. Prevalence and trends of overweight and obesity in European children from 1999 to 2016: a systematic review and meta‐analysis. JAMA Pediatr. 2019;173(10):e192430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oester IMB, Kloppenborg JT, Olsen BS, Johannesen J. Type 2 diabetes mellitus in Danish children and adolescents in 2014. Pediatr Diabetes. 2016;17:368‐373. [DOI] [PubMed] [Google Scholar]
- 42. O'Dea MI, O'Connell SM, O'Grady MJ. Prevalence and characteristics of paediatric type 2 diabetes in the Republic of Ireland. Diabet Med. 2017;34:1603‐1607. [DOI] [PubMed] [Google Scholar]
- 43. Neu A, Feldhahn L, Ehehalt S, et al. No change in type 2 diabetes prevalence in children and adolescents over 10 years: update of a population‐based survey in South Germany. Pediatr Diabetes. 2018;19:637‐639. [DOI] [PubMed] [Google Scholar]
- 44. Neu A, Feldhahn L, Ehehalt S, Hub R, Ranke MB. Type 2 diabetes mellitus in children and adolescents is still a rare disease in Germany: a population‐based assessment of the prevalence of type 2 diabetes and MODY in patients aged 20 years. Pediatr Diabetes. 2009;10:468‐473. [DOI] [PubMed] [Google Scholar]
- 45. Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35:737‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002‐2012. N Engl J Med. 2017;376:1419‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amed S, Islam N, Sutherland J, Reimer K. Incidence and prevalence trends of youth‐onset type 2 diabetes in a cohort of Canadian youth: 2002‐2013. Pediatr Diabetes. 2018;19:630‐636. [DOI] [PubMed] [Google Scholar]
- 48. Urakami T, Miyata M, Yoshida K, et al. Changes in annual incidence of school children with type 2 diabetes in the Tokyo metropolitan area during 1975‐2015. Pediatr Diabetes. 2018;19:1385‐1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1: Observed age‐adjusted rate per 100 000 PY over an observation period of 29 years (1989‐2017) for T1D and 19 years (1999‐2017) for T2D in children and adolescents younger than 15 years of age.


