Summary
Pediatric allogeneic hematopoietic cell transplantation (HCT) practices differ from those of adults, particularly the heterogeneity of transplantable nonmalignant diseases and the lower incidence of graft‐versus‐host disease (GVHD). Several guidelines regarding the management of acute (a) GVHD in adult HCT have been published. We aimed to capture the real‐life approaches for pediatric aGVHD prophylaxis/treatment, and data from 75/193 (response rate 39%) EBMT centers (26 countries) were included, representing half (48%) of the pediatric EBMT‐HCT activity. Results with ≥75% approval from respondents (74/75) for GVHD prophylaxis after myeloablative HCT for malignancies partially contradict published guidelines: Single‐agent cyclosporine A (CsA) was used for matched sibling donor HCT in 47%; blood CsA levels were reported lower; the relapse risk in malignant diseases influenced GVHD prophylaxis with early withdrawal of CsA; distinct longer duration of CsA was employed in nonmalignant diseases. Most centers used additional anti‐thymocyte globulin for matched unrelated and mismatched donor HCT, but not for matched siblings. Regarding prophylaxis in nonmyeloablative conditioning (mainly for nonmalignant diseases), responses showed broad heterogeneity. High conformity was found for first‐line treatment; however, results regarding steroid‐refractory aGVHD indicate an earlier diagnosis in children. Our findings highlight the need for standardized pediatric approaches toward aGVHD prophylaxis/treatment differentiated for malignant and nonmalignant underlying diseases.
Keywords: acute GVHD, hematopoietic cell transplantation, pediatrics, prophylaxis, treatment
Introduction
Pediatric allogeneic hematopoietic stem cell transplantation (HCT) is an established treatment for a range of malignant and nonmalignant diseases. Graft‐versus‐host disease (GVHD) remains one of the main barriers to the success of HCT. Acute GVHD (aGVHD) and subsequent chronic GVHD, including associated long‐term sequelae, may be more devastating in children who are still growing and developing [1, 2, 3, 4].
Both preventive and treatment strategies for pediatric aGVHD vary, and the optimal practice is not well defined. Protocols, which may superficially appear similar, often harbor significant dissimilarities, challenging the interpretation of published outcome data, particularly if combined adult and pediatric data sets with different age‐groups and underlying diagnoses are evaluated. GVHD prophylaxis varies according to drug, dosage, route of administration, and duration. Steroids are almost universally used as first‐line treatment for aGVHD, but the details and definitions of steroid refractoriness (SR) vary considerably. Since pediatric HCT is performed for a wide range of underlying diseases, and pediatric aGVHD differs from adult aGVHD in terms of incidence, severity, and response to treatment, the Pediatric Diseases Working Party (PDWP) of the European Society of Blood and Marrow Transplantation (EBMT) surveyed centers performing pediatric HCT and collated information on prophylactic and treatment strategies in aGVHD. The present study aimed to harmonize this data with a view to standardizing strategies and compare our results with the published mainly adult recommendations [5, 6, 7]. We wanted to investigate the variations in the definition of SR‐aGVHD in use in daily clinical practice, which is important to the GVHD community [8]. Furthermore, we aimed to identify areas of heterogeneity and disagreement to improve design and feasibility of future interventional pediatric studies.
Materials and methods
In November 2018, we updated a survey that was initially performed in 2014 in which 193 EBMT centers performing pediatric HCT were invited to participate. The first questionnaire included 80 questions relating to practices in aGVHD prophylaxis in myeloablative or reduced‐intensity conditionings for malignant and nonmalignant diseases, segregated regarding stem cell source, donor specifics, HLA‐matching, and graft manipulation; furthermore, respondents were asked for practice patterns regarding first, second and subsequent treatment line both for acute and chronic GVHD, including details about the use of monoclonal and polyclonal antibodies. Based on the answers of the first questionnaire, and after discussion within the board of the PDWP of the EBMT, we then designed a focused second questionnaire (25 questions), which was sent to the same centers that had completed the first questionnaire. This second survey focused on aGVHD prophylaxis regarding (i) myeloablative for mainly malignant underlying diseases or reduced‐intensity conditionings for mainly nonmalignant diseases, (ii) donor details [matched sibling donors (MSD), matched unrelated donors (MUD), and mismatched donors (MMD), including haplo‐identical donors], and (iii) stem cell source (peripheral blood stem cells, bone marrow; excluding cord blood) and aGVHD first‐ and second‐line treatment only, including definitions of steroid‐refractory aGVHD. Details of haplo‐identical HCT and post‐transplant cyclophosphamide were not included, as we aimed for the most common settings. The response rate for some individual questions varied and is indicated by the denominator in the text. The results of the second questionnaire were discussed in an expert workshop of the PDWP of the EBMT in November 2018 in Frankfurt.
Data were analyzed at the EBMT PDWP Data Office in Paris, France. For statistical analyses IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, NY, USA) were used. Pearson coefficient correlation was utilized to determine correlations between at least two continuous variables. Data were analyzed using R version 2.13.0 and exported to Excel 2013 version 15.0 (Microsoft® Excel).
Results
A total of 75/193 (39%) centers participated, representing almost half (48%) of the allogeneic pediatric HCT activity within the EBMT. Ninety‐two percent (69/75) of the responding centers transplanted pediatric patients only and six centers transplanted both adults and children. The distribution of center size seemed quite balanced with 46% (34/75) of centers performing more than the mean number of pediatric HSCTs per year 2014 according to the EBMT registry. We were able to include data about practice patterns from 67% (26/39) of countries registered within the EBMT in 2014 for performing pediatric HCT. The numbers of responding centers from each participating country are shown in Fig. 1.
Figure 1.
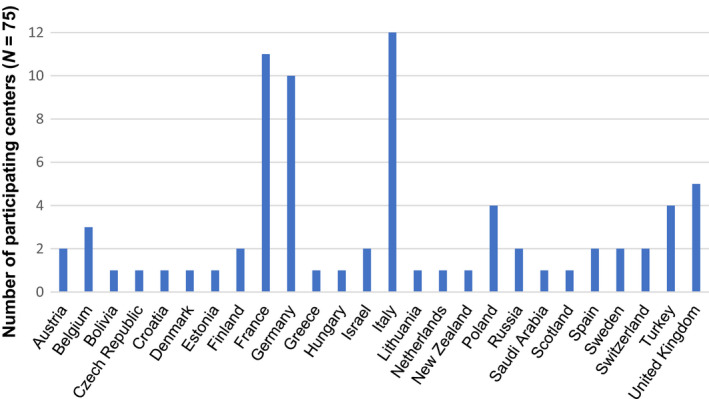
Participating countries (N = 26) of 39 (67%) countries registered within the European Society of Blood and Marrow Transplantation in 2014 for performing pediatric hematopoietic cell transplantation and number of participating centers. Responding centers (N = 75 of 75 participating centers).
GVHD prophylaxis in HCT with myeloablative conditioning for mainly malignant underlying diseases
MSD
Of 74 respondents to this question, cyclosporine A (CsA) alone was routinely used by 47% (35/74), and a combination of CsA and a short course of methotrexate (MTX) was used by 45% (33/74) of the responding centers when bone marrow (BM) was the stem cell source. Of note, the use of peripheral blood as the stem cell source (PBSC) did not change the choice of prophylaxis in 73% (54/74) of the centers, although details were not provided. Additional anti‐thymocyte globulin (ATG) was given in 21% (16/74) of the centers.
MUD
A combination of CsA and MTX was used by 95% (45/47) of the 47 responding centers when BM was the stem cell source. Again, in the majority of responding centers (85%, 40/47) the use of PBSC did not change GVHD prophylaxis. ATG was added to this regimen by 81% (38/47) of the centers.
MMD
A combination of CsA and MTX was used by 88% (65/74) of the 74 responding centers, and ATG was added in 96% (71/74). Ex vivo T‐cell depletion (TCD) was employed by 67% (50/74) of the centers. The use of PBSC in MMD did not influence the choice of prophylaxis in 93% (69/74) of the centers.
ATG
ATG for GVHD prophylaxis in myeloablative conditioning (MAC) HCT for malignancies was used in MUD and MMD HCT in 81% (60/74) and 96% (71/74) of the 74 responding centers, respectively (Table 1). ATG was used in MSD HCT in 21% (16/74) of the centers. More specifically, centers were asked to provide details of ATG administration. Of 71 respondents, 48% (34/71) used thymoglobulin (Genzyme, Cambridge, MA, USA), 27% (19/71) used grafalon (Neovii Biotech, Lexington, MA, USA), and 25% (18/71) used both agents. The median daily dose of thymoglobulin was 2.5 mg/kg, which was usually given on three consecutive days (starting day between day −7 and day −3). The median daily dose of grafalon was 10 mg/kg, which was also usually given on three consecutive days (starting day between day −6 to and day −3). Among the ATG schedules in MSD, MUD, or MMD HCT, no meaningful differences were reported.
Table 1.
Acute graft‐versus‐host disease prophylaxis in hematopoietic cell transplantation with myeloablative conditioning for mainly malignant underlying diseases. (A) Cyclosporine A (CsA); details with agreement in more than two‐thirds of the 74 responding centers of 75 participating centers are summarized. (B) Other agents than CsA.
| A. | MSD | MUD | MMD |
|---|---|---|---|
| aGVHD prophylaxis with CsA (N = 74) | |||
| CsA alone | 35/74 (47%) | ||
| CsA + MTX | 33/74 (45%) | 70/74 (95%) | 65/74 (88%) |
| Other | 6/74 (8%) | 4/74 (5%) | 9/74 (12%) |
| Additional ATG | 16/74 (21%) | 60/74 (81%) | 71/74 (96%) |
| TCD | 50/74 (67%) | ||
| No influence of SC‐source (BM/PBSC) | 54/74 (73%) | 63/74 (85%) | 69/74 (93%) |
| Regime of CsA administration (N = 75) | |||
| Start day −1 | 66/75 (90%) | ||
| i.v. | 64/75 (85%) | ||
| 2 doses | 63/75 (84%) | ||
| 3 mg/kg | 56/75 (75%) | ||
| CsA WB level | 71/75 (95%) | ||
| CsA WB level at C‐0 | 73/75 (97%) | ||
| Duration | Median 110 days (IQR 90) | ||
| WB target level below 200 ng/ml (N = 69) | 59/69 (85%) | ||
| WB target level <100 ng/ml | 9/59 (15%) | ||
| WB target level 100–150 ng/ml | 22/59 (37%) | ||
| WB target level 160–200 ng/ml | 20/59 (34%) | ||
| WB target level >200 ng/ml | 8/59 (14%) | ||
| Influence of relapse risk of underlying disease (N = 74) | 57/74 (77%) | ||
| Taper before discontinuation (N = 74) | 72/74 (97%) | ||
| Leucovorin rescue (N = 72) | 63/72 (87%) | ||
| i.v. | 61/63 (97%) | ||
| 24 h post‐MTX | 52/63 (82%) | ||
| B. aGVHD prophylaxis with other agents (N = 74) | N (%) of centers | Details | N (%) | Comments |
|---|---|---|---|---|
| Tacrolimus | 14/74 (19%) | i.v. | 13/14 (90%) | |
| WB level <10 ng/ml | 8/14 (60%) | Influence of relapse risk: 11/14 (80%) | ||
| WB level >10 ng/ml | 6/14 (40%) | |||
| MMF | 32/74 (43%) | Duration: median 45 days | Usually in combination with CNI: 28/32 (89%) | |
| Alemtuzumab | 16/71 (23%) | in MUD |
ATG, anti‐thymocyte globulin; BM, bone marrow; C‐0, lowest blood concentration reached before the next dose is administered; CNI, calcineurin inhibitor; CsA, cyclosporine A; i.v., intravenous; IQR, interquartile range; MAC, myeloablative conditioning; MMD, mismatched donor; MMF, mycophenolate mofetil; MSD, matched sibling donor; MTX, methotrexate; MUD, matched unrelated donor; PBSC, peripheral blood stem cell; TCD, T‐cell depletion; WB, whole blood.
Ex vivo TCD
T‐cell depletion was used by 67% (50/74) of the 74 responding centers, usually for MMD HCT. Within these centers, positive selection with anti‐CD34 antibodies was used in 78% (39/50) and negative selection in 44% (22/50). CD3/19 depletion was predominantly used (93%, 20/22), and only 7% (2/22) used “Alemtuzumab in the bag” (i.e., adding Alemtuzumab to the stem cell infusate [29]). Pharmacologic immunosuppression in addition to TCD was administered in 47% (35/74), mostly with CsA (86%, 30/35).
CsA administration
The details of CsA administration revealed a homogenous practice, and those with agreement in more than two‐thirds of the 75 responding centers are summarized (Table 1).
The particularities of CsA prophylaxis with lower concordance included initial doses of 2 mg/kg/day (11%, 8/75), 1 mg/kg/day (10%, 7/75), and 4–6 mg/kg/day (15%, 11/75). The dose and scheduling of short‐course MTX administration and leucovorin rescue varied as follows: 10 mg/m2 MTX on days +1, +3, and +6 in 37% (28/75); 15 mg/m2 MTX on day +1, and 10 mg/m2 MTX on days +3, +6, +11 in 27% (20/75). Furthermore, 25% (19/75) of the centers used the latter schedule with omitting MTX on day +11.
Targeted CsA blood concentrations at weeks 1, 2–4, and 8 were requested and 69 of 75 centers responded. We found that 88% (61/69) of the responding centers reported no difference in the target level within the first 8 weeks. Importantly, the majority of the centers (85%, 59/69) aimed for a post‐transplant target level <200 ng/ml with an equal distribution between 100–150 and 160–200 ng/ml. A trend toward a lower target concentration in MSD recipients could be surveyed (data not shown).
Of note, 77% (60/75) of the centers reported that the estimated relapse risk of the underlying malignant disease influenced CsA prophylaxis. This observation was supported by a clear difference in the duration of CsA prophylaxis when the relapse risk was categorized as “high and low” (Fig. 2a, 74/75 responding centers). A comparison between malignant and nonmalignant diseases revealed a longer duration of CsA prophylaxis in patients with nonmalignant diseases (Fig. 2b 74/75 responding centers). GVHD prophylaxis containing immunosuppressive agents other than CsA varied considerably (Table 1B, 74/75 responding centers).
Figure 2.
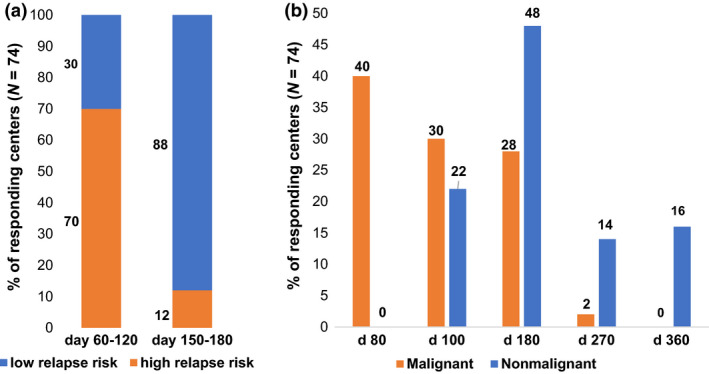
Duration of cyclosporine A (CsA) prophylaxis after myeloablative conditioning (MAC). (a) Duration of CsA prophylaxis regarding the relapse risk of malignant underlying disease (low relapse risk versus high relapse risk). (b) Duration of CsA prophylaxis regarding the underlying disease (malignant versus nonmalignant underlying disease). Responding centers (N = 74 of 75 participating centers).
GVHD prophylaxis in HCT with reduced‐intensity conditioning in mainly nonmalignant underlying diseases
While prophylactic regimens, mainly applied in nonmalignant HCT showed broad variety, the combination of CsA + MTX was most frequently used by the 69 respondents to this question (Fig. 3, 69/75 responding centers). More specifically, centers were asked about the details of the CsA regime: The majority of the centers reported no differences regarding donor and stem cell sources; regarding the targeted CsA blood levels, 68% (47/69) of the responding centers reported a CsA target level <200 ng/ml, similar to that of MAC. Of note, in our survey a concentration of >200 ng/ml was reported more often in RIC than in MAC regimens (16%, 11/69 vs 24%, 17/69 in MAC) and a concentration of ≤200 ng/ml was reported more often in MAC regimens (24%, 17/69 vs 11%, 8/69 in RIC, data not shown).
Figure 3.
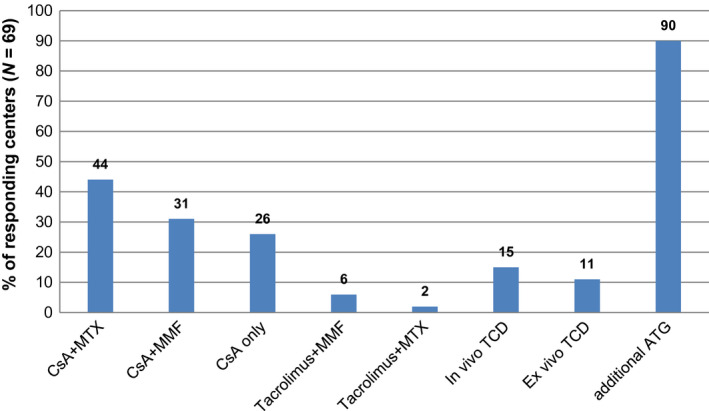
Graft‐versus‐host disease prophylaxis in transplantation with reduced‐intensity conditioning for mainly nonmalignant diseases. Responding centers (N = 69 of 75 participating centers).
Additional ATG was employed by 90% (62/69) of the centers, with the majority (73%, 45/62) of these using the same regimen as for MAC conditioning, although the median dose for grafalon was higher in RIC than MAC (20 mg/kg/day vs. 10 mg/kg/day).
The time to taper or withdraw immunosuppressive treatment was influenced by the chimeric status of patients in 90% (62/69) of the centers.
Treatment for aGVHD
First‐line treatment
All centers (75/75) indicated corticosteroids as first‐line treatment for aGVHD ≥grade 2 that was started using mainly intra‐intravenously methylprednisolone at the dose of 2 mg/kg/day in two doses. Specifics of first‐line treatment, which were reported by the majority of the 75 responding centers, are summarized in Fig. 4. More detailed, the overall severity of aGVHD influenced the initial dosing in only 35% (26/75) of the centers. A dose of 1–2 mg/kg/day was used for grade 1 or 2 involvement of the skin only. The duration of the initial treatment was 1 week in 40% (30/75), 5 days in 25% (19/75), and >10 days in 25% (19/75) of the centers (mean, 8 days; range, 2–15 days). Dose reduction was based on response (57%, 43/75) or preplanned schedule (43%, 32/75). Different tailing schedules were described, but a 25% dose reduction every 3–7 days was most common. A total of 74% (55/74) of the 74 responding centers reported the use of topical agents for aGVHD: For gastrointestinal GVHD the use of topical steroids, and for cutaneous GVHD the use of topical steroids and topical tacrolimus were reported without further details.
Figure 4.
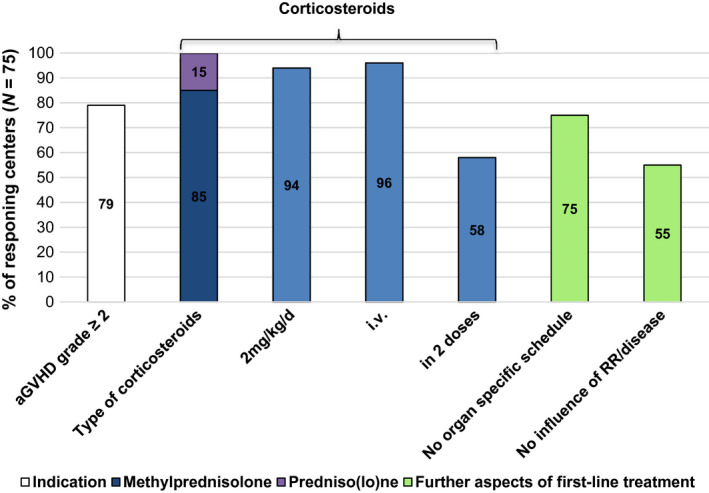
First‐line treatment for acute graft‐versus‐host disease. Responding centers (N = 75 of 75 participating centers). Relapse risk of underlying malignant disease.
Definitions of steroid resistance
Centers were questioned about the clinical criteria for definition of steroid resistance (SR), and time point of the assessment (duration of steroid treatment in days) and 68/75 centers responded. Sixty‐seven (45/68) percent of centers stated to diagnose SR of aGVHD within the first 5 days after treatment start. In greater detail, centers were asked whether the clinical diagnosis of SR was based on the progression of aGVHD in any organ, failure to improve in the individual organ, or failure to improve in the overall severity grading of aGVHD (Fig. 5). Failure to show improvement in the individual aGVHD organ was the main criterion and was used by 87% (59/68) of the responding centers. For each of the three clinical criteria of SR in use, we found distinct different time points of assessment. SR was diagnosed early when progression of aGVHD in any organ was the SR criterion (used by 71%, 48/68). Patients were allowed a longer period to respond before SR was diagnosed when failure to improve in an individual organ was the criterion. Importantly, up to 2 weeks was allowed by centers using failure to improve the overall severity grading of aGVHD (used by 56%, 38/68 of the responding centers).
Figure 5.
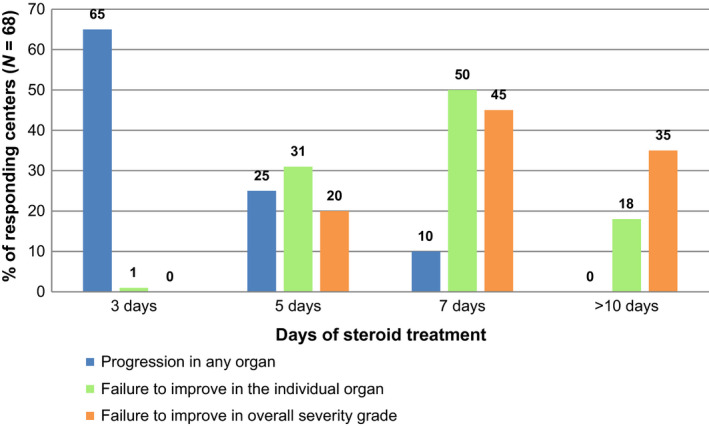
Clinical criteria for and timing of the diagnosis of steroid‐resistant acute graft‐versus‐host disease (SR‐aGVHD). Responding centers (N = 68 of 75 participating centers). Clinical criteria for SR‐aGVHD: (a) progression of aGVHD manifestations in any organ; (b) failure to improve manifestations in the individual organ of aGVHD; and (c) failure to improve in overall severity grade of aGVHD.
Second‐line treatment
A broad inter‐center variety has been reported by 74/75 centers regarding the choice of second‐line treatment for aGVHD (Fig. 6). However, a more detailed evaluation was beyond the scope of this survey. Second‐line treatments were combined with aGVHD prophylactic drugs by 98% (73/74) of the responding centers, but in 92% (68/74) of the centers, steroids were discontinued when new drugs were introduced. Similar to first‐line treatment, organ involvement of aGVHD did not affect the choice of second‐line treatment in 60% (44/73) of 73 responding centers.
Figure 6.
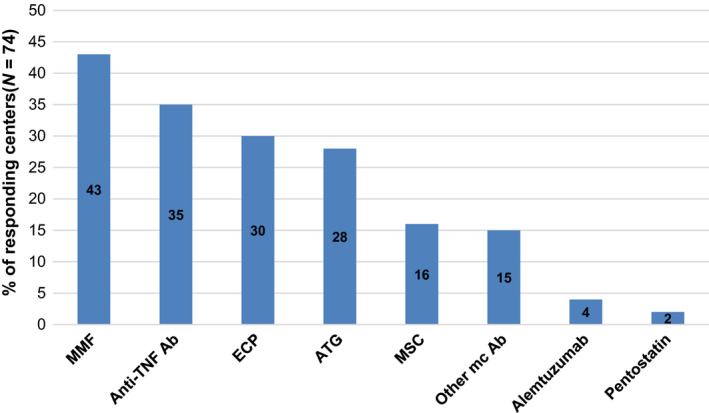
Second‐line treatment for acute graft‐versus‐host disease. Responding centers (N = 74 of 75 participating centers).
Discussion
The present survey aimed to study the real‐life approaches of prevention and treatment of aGVHD by indication and conditioning intensity (MAC for mainly malignant; RIC for mainly nonmalignant diseases) of pediatric HCT. The data have shown high agreement for aGVHD prophylaxis in MAC HCT for mainly malignancies which reveals that there are important dissimilarities when compared to published mainly adult recommendations [5, 6, 7]. We have summarized the results that have shown said agreement in more than two‐thirds of the 74 (74/75) responding centers in Table 1 to offer a platform for further optimization of aGVHD prophylaxis [10]. In contrast, our results show a broad variety regarding aGVHD prophylaxis in RIC HCT reflecting the complexity of HCT for nonmalignant pediatric diseases like inborn errors. We also aimed to collect details of the definition of SR‐aGVHD currently used in clinical practice, which is of interest to the GVHD community. The development of biomarkers for GVHD would expand the opportunity for clinical research in this field [12]. Since many biomarker studies combine adult and pediatric data, the findings of our study may aid the interpretation of those results. Differences between pediatric and adult practice patterns of prophylaxis and treatment may reflect (i) differences of immune reconstitution between adult and pediatric patients [12] and (ii) the heterogeneity of underlying diseases (particularly pediatric malignant and nonmalignant diseases). Our results highlight the need of a clear definition of SR in aGVHD within studies, since the reported earlier diagnosis of SR in pediatric aGVHD when compared to adult recommendations (≤5 days in 66% of responding centers) allows the earlier introduction of second‐line therapies. This may also impact the inclusion criteria of prospective studies.
In 1997 and 2012, Ruutu et al. [5, 6, 9] published surveys of aGVHD prophylaxis and treatment in adult HCT patients as well as recommendations on behalf of the EBMT and European LeukemiaNet (ELN WG). In 2000, the EBMT PDWP published a survey of aGVHD practices in children [7]. A recent pediatric study by Cuvelier et al. [11] underlined the importance of the prevention of grades 2–4 aGVHD, which was again shown to be a major risk factor for chronic GVHD.
The approach to aGVHD prophylaxis for children differs from that in adult practice essentially in MAC HCT for mainly malignant diseases. Children undergoing MSD HCT received CsA alone as GVHD prophylaxis in nearly half of all centers, whereas 87% of the adult centers used a combination of CsA + MTX. Weiss et al. [13] reported a superior outcome for relapse rate and 5‐year event‐free survival, with no increase in GVHD incidence for children undergoing MSD HCT for leukemia who had received CsA alone (versus CsA + MTX). The addition of ATG in MUD and MMD HCT was more frequently used in pediatric compared with adult practice (81% and 96% for MUD and MMD, respectively, compared with 57% for adults). EBMT and ELN (EBMT ELN) recommend the use of ATG at the clinician’s discretion, indicating the need for further studies to define the role of ATG [14].
Similarly, ex vivo TCD was more commonly used by pediatric centers (67% vs. 28% in adults) and typically in MMD HCT. TCD was not included in the adult recommendations [5, 6, 9]. Of note, the use of PBSC as the stem cell source in MAC HCT for malignancies did not change the choice of prophylaxis in the majority of the responding centers regardless of the donor type.
Our results suggest that clinical situations influence practice. The relapse risk of an underlying malignancy clearly shapes GVHD prophylaxis in pediatric centers, resulting in earlier withdrawal of CsA. While the influence of the persistence of minimal residual disease was not within the scope of this survey, it may impact future GVHD prophylaxis. In contrast, patients with nonmalignant diseases received prolonged CsA prophylaxis. The paucity of the detailed published data on GVHD prophylaxis may limit the interpretation of outcome data regarding GVHD incidence, disease course, and biomarkers.
A high inter‐center agreement has been reported among pediatric centers for CsA target levels; however, this approach differed from that of adult practice as the majority of the pediatric centers used a lower target level (<200 ng/ml) without a higher target level during the first weeks. This is in contrast to the recommendation by the EBMT ELN (200–300 ng/ml within the first 3–4 weeks post‐transplant). A lower incidence of grade II–IV aGVHD was reported when target CsA trough levels are achieved early in the post‐transplantation period, with the time to achieving the target CsA concentration being significant [15, 16]. CsA blood concentrations show high inter‐ and intra‐individual variability; therefore, age‐adjusted prospective studies with innovative approaches may offer a new insight into the optimal target and schedule [17].
The EBMT ELN recommendation of CsA prophylaxis for 6 months in adults is in striking contrast to our results that showed that around 70% of the centers stopped CsA prior to or at day +100 in patients with malignant diseases. The findings emphasize the requirement for different approaches to GVHD prophylaxis in children with malignant and nonmalignant diseases, since patients with nonmalignant diseases do not benefit from a graft‐versus‐leukemia/GVHD effect and require a more aggressive approach to GVHD prevention.
Our survey found that a less intensive approach to CsA prophylaxis undergoing MAC HCT for malignancies is likely to be related to a lower incidence and a milder clinical course of pediatric aGVHD when compared with those of adults [1, 4].
While the use of MTX was similar in pediatric and adult practices, leucovorin rescue was more commonly used in children (85% vs. 49%). Tacrolimus was more commonly used in pediatric compared with adult centers (19% vs. 7%). European experience with tacrolimus seems to be more limited than that of the United States [18, 19, 20]. Pediatric centers employed mycophenolate mofetil (MMF) in GVHD prophylaxis in MAC HCT more frequently than adult centers (43% vs. 12%), but details are lacking. No adult consensus recommendations are available.
Regarding the RIC setting, fewer pediatric centers used CsA + MMF (30% vs. 69%), and TCD was more common (15% vs. 3%). The EBMT ELN recommendations for RIC conditioning endorse CsA + MMF plus ATG in MUD HCT. Beside the great disparity in pediatric RIC prophylaxis, higher planned CsA target levels were reported. Only pediatric patients with nonmalignant diseases – mainly form the RIC cohort in which GVHD offers no benefit – were targeted for higher CsA blood levels >200 ng/m. Our survey confirmed the wide variety of RIC regimens reported in a previous EBMT PDWP survey [21]. Since the mid‐90s multiple RIC regimens for HCT in nonmalignant diseases have been developed in a variety of pediatric HCT centers but randomized studies comparing RIC HCT with conventional intensity conditioning in children are scarce [30]. This reflects the clinical practice of pediatricians who face an enormous variety of transplant indications that require a patient‐adapted strategy, particularly for the huge variety of immunodeficiency syndromes [21].
First‐line treatment for aGVHD in children shows good consensus with most recommendations [2, 9, 22, 23]. Histological confirmation of aGVHD was required more often by pediatric compared with adult centers (24% vs. 18%) but occurred less often than previously reported [23].
We have focused our survey on aspects which apply for the most common pediatric HCT settings, which is why several important evaluable parameters are missing in this survey, particularly ATG schedules, GVHD prophylaxis in cord blood HCT, and details of steroid taper. Another limitation of our survey is the response rate of 39% (75/193) which is about or even slightly above average [31]. It seems probable that response rates from those centers with more interest in GVHD were higher, but we have limited ability to explore this response bias.
Our results suggest that the majority of pediatric centers consider patients to be SR for aGVHD after a shorter period of time compared with adult practice. Unsurprisingly, when the criterion for SR was progression in any organ, SR was diagnosed early, with the majority of the centers (62%) diagnosing first‐line treatment failure after 3 days. The EBMT ELN recommendation is diagnosis of SR after 5 days. SR was most commonly defined as failure to improve in the individual organ. The time point for evaluation was mainly between 5 and 7 days after starting steroid treatment. There are no pediatric data to support the recommendation that initiating second‐line treatment on day five or earlier improves outcome, and a prospective study to evaluate this is required. Nevertheless, a more precise definition of SR should be considered to facilitate prospective GVHD and biomarker studies [8].
Our survey showed second‐line treatment to be as variable in children as it is in adults, with two exceptions: MMF (43% vs. 33%) and extracorporeal photopheresis (ECP; 30% vs. 17%). Pediatric preference for ECP may reflect the published recommendations and data [24, 25, 26]. The pediatric preference for MMF may reflect daily clinical experience [23], although the published results are less encouraging [22, 27].
Our survey results showing that pediatric approaches are divergent in some aspects of aGVHD prophylaxis and treatment has been included in recent consensus recommendations of the EBMT about the prophylaxis and management of graft‐versus‐host disease after HCT for hematological malignancies [32]. Another impact of our study results is that a harmonized recommendation for the CsA blood levels has been included into the study protocol of the ALL SCTped 2012 FORUM study. Regarding SR of aGVHD treatment, we are aiming to implement the two following definitions: either (i) progression of aGVHD in any organ within 3 days or (ii) failure to show improvement in the individual aGVHD organ within 7 days in a prospective second‐line treatment study for aGVHD in pediatric and adolescent patients.
Adult practice and recommendations often form the basis of pediatric practice; therefore, our results comparing pediatric real‐life approaches with published adult surveys and recommendations provide useful information on which to judge the appropriateness of this approach. The benefit of standardization of prophylaxis and treatment for aGVHD is obvious [28]; however, our findings highlight the need for different approaches for children with malignant and various nonmalignant underlying diseases.
Authorship
AL and CP: designed the study. AD and AL: analyzed the data. AL: wrote the manuscript. AB, DB, and BG: critically revised the manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors have declared no conflicts of interest.
Acknowledgements
We thank the data managers and pediatricians from all EBMT centers that participated in this study.
References
- 1. Jacobsohn DA. Acute graft‐versus‐host disease in children. Bone Marrow Transplant 2008; 41: 215. [DOI] [PubMed] [Google Scholar]
- 2. Dignan FL, Clark A, Amrolia P, et al Diagnosis and management of acute graft‐versus‐host disease. Br J Haematol 2012; 158: 30. [DOI] [PubMed] [Google Scholar]
- 3. Davies SM, Wang D, Wang T, et al Recent decrease in acute graft‐versus‐host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant 2009; 15: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faraci M, Caviglia I, Biral E, et al Acute graft‐versus‐host disease in pediatric allogeneic hematopoietic stem cell transplantation. Single‐center experience during 10 yr. Pediatr Transplant 2012; 16: 887. [DOI] [PubMed] [Google Scholar]
- 5. Ruutu T, Niederwieser D, Gratwohl A, Apperley JF. A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow, Transplantation (EBMT). Chronic Leukaemia Working Party of the EBMT. Bone Marrow Transplant 1997; 19: 759. [DOI] [PubMed] [Google Scholar]
- 6. Ruutu T, van Biezen A, Hertenstein B, et al Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2012; 47: 1459. [DOI] [PubMed] [Google Scholar]
- 7. Peters C, Minkov M, Gadner H, et al Statement of current majority practices in graft‐versus‐host disease prophylaxis and treatment in children. Bone Marrow Transplant 2000; 26: 405. [DOI] [PubMed] [Google Scholar]
- 8. Schoemans HM, Lee SJ, Ferrara JL, et al EBMT‐NIH‐CIBMTR Task Force position statement on standardized terminology & guidance for graft‐versus‐host disease assessment. Bone Marrow Transplant 2018; 53: 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruutu T, Gratwohl A, de Witte T, et al Prophylaxis and treatment of GVHD: EBMT‐ELN working group recommendations for a standardized practice. Bone Marrow Transplant 2014; 49: 168. [DOI] [PubMed] [Google Scholar]
- 10. Rezvani AR, Storb RF. Prevention of graft‐vs.‐host disease. Expert Opin Pharmacother 2012; 13: 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuvelier GDE, Nemecek ER, Wahlstrom JT, et al Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood 2019; 134: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolff D, Greinix H, Lee SJ, et al Biomarkers in chronic graft‐versus‐host disease: quo vadis? Bone Marrow Transplant 2018; 53: 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiss M, Steinbach D, Zintl F, Beck J, Gruhn B. Superior outcome using cyclosporin A alone versus cyclosporin A plus methotrexate for post‐transplant immunosuppression in children with acute leukemia undergoing sibling hematopoietic stem cell transplantation. J Cancer Res Clin Oncol 2015; 141: 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ram R, Storb R. Pharmacologic prophylaxis regimens for acute graft‐versus‐host disease: past, present and future. Leuk Lymphoma 2013; 54: 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin P, Bleyzac N, Souillet G, et al Clinical and pharmacological risk factors for acute graft‐versus‐host disease after paediatric bone marrow transplantation from matched‐sibling or unrelated donors. Bone Marrow Transplant 2003; 32: 881. [DOI] [PubMed] [Google Scholar]
- 16. Punnett A, Sung L, Price V, et al Achievement of target cyclosporine concentrations as a predictor of severe acute graft versus host disease in children undergoing hematopoietic stem cell transplantation and receiving cyclosporine and methotrexate prophylaxis. Ther Drug Monit 2007; 29: 750. [DOI] [PubMed] [Google Scholar]
- 17. Leclerc V, Ducher M, Bleyzac N. Bayesian networks: a new approach to predict therapeutic range achievement of initial cyclosporine blood concentration after pediatric hematopoietic stem cell transplantation. Drugs R&D 2018; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osunkwo I, Bessmertny O, Harrison L, et al A pilot study of tacrolimus and mycophenolate mofetil graft‐versus‐host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant 2004; 10: 246. [DOI] [PubMed] [Google Scholar]
- 19. Offer K, Kolb M, Jin Z, et al Efficacy of tacrolimus/mycophenolate mofetil as acute graft‐versus‐host disease prophylaxis and the impact of subtherapeutic tacrolimus levels in children after matched sibling donor allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2015; 21: 496. [DOI] [PubMed] [Google Scholar]
- 20. Ziakas PD, Zervou FN, Zacharioudakis IM, Mylonakis E. Graft‐versus‐host disease prophylaxis after transplantation: a network meta‐analysis. PLoS One 2014; 9: e114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawitschka A, Faraci M, Yaniv I, et al Paediatric reduced intensity conditioning: analysis of centre strategies on regimens and definitions by the EBMT Paediatric Diseases and Complications and Quality of Life WP. Bone Marrow Transplant 2015; 50: 592. [DOI] [PubMed] [Google Scholar]
- 22. Martin PJ, Rizzo JD, Wingard JR, et al First‐ and second‐line systemic treatment of acute graft‐versus‐host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012; 18: 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolff D, Ayuk F, Elmaagacli A, et al Current practice in diagnosis and treatment of acute graft‐versus‐host disease: results from a survey among German‐Austrian‐Swiss hematopoietic stem cell transplant centers. Biol Blood Marrow Transplant 2013; 19: 767. [DOI] [PubMed] [Google Scholar]
- 24. Das‐Gupta E, Dignan F, Shaw B, et al Extracorporeal photopheresis for treatment of adults and children with acute GVHD: UK consensus statement and review of published literature. Bone Marrow Transplant 2014; 49: 1251. [DOI] [PubMed] [Google Scholar]
- 25. Rutella S, Valentini CG, Ceccarelli S, et al Extracorporeal photopheresis for paediatric patients experiencing graft‐versus‐host disease (GVHD). Transfus Apher Sci 2014; 50: 340. [DOI] [PubMed] [Google Scholar]
- 26. Calore E, Marson P, Pillon M, et al Treatment of acute graft‐versus‐host disease in childhood with extracorporeal photochemotherapy/photopheresis: The padova experience. Biol Blood Marrow Transplant 2015; 21: 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inagaki J, Kodama Y, Fukano R, Noguchi M, Okamura J. Mycophenolate mofetil for treatment of steroid‐refractory acute graft‐versus‐host disease after pediatric hematopoietic stem cell transplantation. Pediatr Transplant 2015; 19: 652. [DOI] [PubMed] [Google Scholar]
- 28. Choi SW, Reddy P. Current and emerging strategies for the prevention of graft‐versus‐host disease. Nat Rev Clin Oncol 2014; 11: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Novitzky N, Thomas V, Hale G, Waldmann H. Ex vivo depletion of T cells from bone marrow grafts with CAMPATH‐1 in acute leukemia: graft‐versus‐host disease and graft‐versus‐leukemia effect. Biol Blood Marrow Transplant 1999; 67: 620. [DOI] [PubMed] [Google Scholar]
- 30. Satwani P, Cooper N, Rao K, et al Reduced intensity conditioning and allogeneic stem cell transplant in childhood malignant and nonmalignant diseases. Bone Marrow Transplant 2008; 41: 173. [DOI] [PubMed] [Google Scholar]
- 31. Cook DA, Wittich CM, Daniels WL, et al Incentive and reminder strategies to improve response rate for internet‐based physician surveys: a randomized experiment. J Med Internet Res 2016; 16: e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penack O, Marchetti M, Ruutu T, et al Prophylaxis and management of graft versus host disease after stem‐cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol 2020; 7: e157. [DOI] [PubMed] [Google Scholar]


