Abstract
Objectives
Most early stage laryngeal squamous cell carcinomas (LSCC) are treated with radiotherapy. Discovery of new biomarkers are needed to improve prediction of outcome after radiotherapy and to identify potential targets for systemic targeted therapy. The ataxia telangiectasia mutated (ATM) gene plays a critical role in DNA damage response induced by ionizing radiation.
Methods
The prognostic value of immunohistochemical expression of pATM, pChk2, and p53 were investigated in 141 patients with T1‐T2 LSCC curatively treated with external beam radiotherapy. Uni‐ and multivariable Cox regression analyses were performed to examine the relation between expression levels of markers and local control.
Results
Local control was significantly worse in cases with high levels of pATM (HR 2.14; 95% CI, 1.08–4.24; P = .03). No significant associations with local control were found for pChk2 and p53 expression. The association of high pATM expression with poor local control was only found for supraglottic LSCC (HR 10.9; 95% CI, 1.40–84.4; P = .02).
Conclusion
Our findings suggest a potential role for ATM in response to radiotherapy in early stage supraglottic LSCC and imply ATM inhibition as a possibility to improve response to radiotherapy.
Level of Evidence
NA Laryngoscope, 130: 1954–1960, 2020
Keywords: Laryngeal squamous cell carcinoma, radiotherapy, local control, ataxia telangiectasia mutated (ATM)
INTRODUCTION
Early stage laryngeal squamous cell carcinoma (LSCC) is treated with radiotherapy, endoscopic surgery (mostly with CO2 laser) or external partial surgery.1, 2 Although a recent meta‐analysis suggests that surgery may result in less disease specific mortality compared to radiotherapy in early stage supraglottic LSCC,3 previously no differences were found in disease‐specific survival between these different treatment modalities.4, 5 Therefore, functional outcome, in particular voice quality, is an important factor in the choice of treatment. Hence, in the Netherlands most early stage LSCC patients are treated with radiotherapy as a single modality treatment, because of its ability to preserve laryngeal function.6 In the last 30 years the oncologic outcomes for early stage LSCC has hardly improved.7, 8 In the Netherlands, the 5‐year overall survival rates of stage I and II laryngeal cancer is 80% to 96% in glottic and 63% to 73% in supraglottic cancer,9 similar to results reported by others.5, 8 Survival is affected by the rate of local recurrences observed in approximately 25% of patients treated with radiotherapy.1, 6, 10, 11, 12, 13, 14 Patients who develop local recurrences after radiotherapy mostly require total laryngectomy with high morbidity. Prediction of patients who are likely to develop local recurrence after radiotherapy would therefore be useful since they might benefit from other treatment strategies. Discovery of new biomarkers are needed to improve prediction of outcomes after radiotherapy and to identify potential targets for altered treatment options. Radiotherapy affects cell growth by inducing DNA damage, including DNA double‐strand breaks (DSB), which lead to cell death by the activation of a complex DNA‐damage response (DDR) pathway that controls cell cycle checkpoints, DNA repair, and apoptosis.15, 16 Central in the DDR is the protein kinase ataxia telangiectasia mutated protein (ATM). DNA DSB induced by ionizing radiation leads to autophosphorylation of ATM (pATM) that subsequently phosphorylates a variety of substrates including the Checkpoint kinase 2 (pChk2).17, 18, 19 When activated, pChk2 is known to inhibit CDC25C phosphatase, preventing entry into mitosis. Activated pChk2 has also been shown to phosphorylate p53 resulting in cell cycle arrest and apoptosis.20, 21, 22 The tumor suppressor gene p53 can also be directly phosphorylated by ATM in response to DNA damage.23, 24
In the 1960s it was already reported that ataxia‐telangiectasia patients, who frequently carry mutations in the ATM gene, have a predisposition to malignancy and are hypersensitive to irradiation.25, 26 Consistent with this observation, down‐regulation of ATM was found to result in increased radiosensitivity in cervical cancer cells in vitro.27 Also in patients with cervical cancer treated with (chemo)radiation, high immunohistochemical expression levels of pATM were linked to poor loco‐regional disease free survival.27 The role of immunohistochemical expression of ATM in response to radiotherapy was reported in only one study in a series of 21 patients with early stage laryngeal cancer of the glottis without a correlation with local control.28
The aim of this study was to investigate whether local control after radiotherapy in laryngeal cancer is associated with the ATM‐associated DDR pathway activity. For this purpose, we tested the immunohistochemical expression of pATM, pCHK2, and p53 in 141 pre‐treatment biopsies in a well‐documented series of early stage laryngeal cancer patients, primary treated with radiotherapy.
MATERIALS AND METHODS
Patients
We selected a well‐defined homogenous group of 141 patients with stage T1‐T2 histologically confirmed LSCC treated with radiotherapy with curative intent. This group was composed of two previous reports29, 30 from a database covering 1,286 patients with LSCC diagnosed or treated at the University Medical Center Groningen between 1990 and 2008. Of all patients, clinical, histopathological, and follow‐up data were collected from our department archives. To attain a homogenous series and yet sufficient numbers of patients the inclusion criteria for this study were: histologically proven LSCC; stage T1 or T2; M0; curatively treated with radiotherapy; no prior treatment or pretreatment; formalin‐fixed and paraffin‐embedded tumor material available.
The database contained 247 patients with T1–T2 LSCC. Ten patients were excluded because of prior regional radiotherapy, chemotherapy, or concurrent second primary malignancies. All pretreatment biopsy slides were revised and tumor percentages were estimated by an experienced pathologist. Of 237 remaining biopsy specimens, 141 contained sufficient tumor tissue for immunohistochemical staining.
The routine patient follow‐up ended after 5 years, with visits at the outpatient clinic every 3 months in the first 2 years and every 6 months in the third through fifth year.
Treatment
All patients included in this study were primarily treated with radiotherapy only in the University Medical Center Groningen or one of the affiliated hospitals, Isala in Zwolle or the Medical Center Leeuwarden. Radiotherapy was administered using 6MV linear accelerator equipment as previously described.29, 30 In short, until the year 2000 patients were treated with two opposing lateral fields with a median fraction dose of 2 Gy five times weekly with a total dose of 66 Gy to 70 Gy. From 2000, patients were generally treated with 6 fractions/week up to 70 Gy in 6 weeks. In case of lymph node metastasis, a total dose of 46 Gy was electively delivered to the primary planning target volume together with an additional boost of 70 Gy to the primary clinical target volume (CTV) of tumor and pathologic lymph nodes. In general CTV consisted of the gross tumor volume with or without pathological lymph nodes with 1‐cm margins. The boost volume consisted of the tumor and positive lymph nodes with 0.5‐cm margins. Before 2000, field arrangements were set by direct simulation. After 2000, contrast‐enhanced CT scans were used for planning. Most patients who developed local recurrence after radiotherapy were salvaged with total laryngectomy.
Immunohistochemistry
Through a series of ethanol dilutions and phosphate‐buffered saline (PBS), 4‐um paraffin sections of pretreatment tumor biopsies were deparaffinized and rehydrated. Antigen retrieval was achieved by heating Ethylene‐diamine‐tri‐acetic (EDTA) buffer pH 8.0 (for pATM) and Tris/EDTA buffer pH 9.0 (for p53, pChk2) in a microwave oven for 20 minutes. To block endogenous peroxidase, 0.3% hydrogen peroxidase was applied for 30 minutes at room temperature. The slides were incubated for 1 hour with pATM rabbit monoclonal antibody clone EP1890Y (Epitomics, Burlingame, CA, USA) dilution 1:50, p53 monoclonal mouse antibody clone DO‐7 (DakoCytomation, Glostrup, Denmark) dilution 1:1,000 at room temperature and with pChk2 rabbit monoclonal antibody clone C13C1 (Cell Signaling Technology, Leiden, The Netherlands) dilution 1:50 overnight at 4°C. This was followed by EnVision (Dako, Glostrup, Denmark) for pATM and p53. For pChk2, polyclonal goat anti‐rabbit immunoglobulins/horseradish peroxidase (HRP) (GARpo, Dako, Glostrup, Denmark) and polyclonal rabbit anti‐goat immunoglobulins/HRP conjugated (RAGpo, Dako, Glostrup, Denmark) dilution 1:100 in 1% bovine serum albumin/PBS complemented with 1% human AB serum was used as secondary and tertiary antibody, respectively. As quaternary step again GARpo dilution 1:100 was used. The peroxidase reaction was performed by applying 3.3′‐diaminobenzide tetrachloride (DAB) and the slides were counterstained with hematoxylin. Tissue specimens of human urinary bladder cancer for pATM, human testis for pChk2 and human oral squamous cell carcinoma for p53 were used as a positive control.31, 32
Evaluation of Staining
For the three different antibodies, scoring methods were set with an experienced pathologist based on existing literature. Only in malignancies other than LSCC, few studies performed immunohistochemistry with antibodies against pATM and pChk2.27, 33, 34, 35 In contrast, in hundreds of studies p53 immunohistochemistry was reported using different kinds of antibodies, procedures, and scoring criteria. For this study, we focused on those studies using immunostaining of p53 in relation with response to radiotherapy in LSCC and found clone DO‐07 was used in most studies36, 37, 38, 39, 40, 41, 42 For all three antibodies we assessed immunoreactivity only in the nucleus of neoplastic cells and nuclear staining above the level of any cytoplasmic background was considered as positive staining. The percentages of positively stained neoplastic cells in total neoplastic area were scored by two separated teams. The intensity for all three antibodies was relatively homogeneous and, therefore, was not incorporated into the scoring method. Differences in results were resolved in a consensus meeting.
Definitions for Expression Levels
Tumors with a percentage of positively stained neoplastic cells greater than a predetermined cut‐off value, were considered as high expression, and those with below the cut‐off as low expression. Because clone DO‐07 antibody against p53 recognizes expression of both wild type and mutant forms of the human p53 protein, many studies used cut‐off values for aberrant expression higher than 5%, 10%, or even 20% of positively stained neoplastic cells without reason.38, 39, 43 Nevertheless, none of these cut‐off values showed a relation with clinical outcome after radiotherapy36, 37, 38, 39, 40, 41, 42 The mutant p53 associated with very high percentages of strongly stained neoplastic cells was not taken into consideration in previous studies. Besides different staining protocols and cut‐off values, this may be one of the explanations for the divergent results when looking at the relationship with clinical outcome. Because no studies are available in LSCC showing the optimal cut‐off values for low and high expression for pATM, pChk2, and p53, cut‐off values of the percentages for dichotomization of the data were determined for each staining individually using receiver operating characteristic (ROC) curve analysis.44 The optimal cut‐off between sensitivity and specificity in predicting for local recurrence was chosen as the strongest deviation from the reference line. Tumors with a percentage of positive staining greater than the cut‐off level were considered to have high expression, and those with less than the cut‐off to have low expression. For ROC curve analysis of p53 expression, we excluded all mutant p53 cases defined as negative (<10%) and totally positive (>90%) staining. For the expression of ATM and Chk2, we deliberately chose the active, therefore phosphorylated, isotype of ATM (paTM) and Chk2 (pChk2). We have therefore assumed that the expression of pATM and pChk2 purely represents the active, non‐mutant form of ATM and Chk2.
Statistical Analysis
Follow‐up time was defined as time from diagnosis until last follow‐up with a maximum of 5 years or shorter when the patient died earlier or was lost to follow‐up. Local recurrence was defined as tumor recurrence at primary tumor site within 5 years. Local recurrence time was the time from diagnosis to local recurrence or last follow‐up. Local control was defined as having no local recurrence within 5 years after diagnosis.
To investigate the correlation between local control and expression of pATM, pChk2, p53, as well as age, gender, T‐stage, N‐stage, and sublocation of tumor uni‐ and multivariable Cox regression analysis were used. Kaplan–Meier survival analyses were performed for illustration. Age, gender, T‐stage, N‐stage, and sublocation of tumor were included in the multivariate Cox regression model to analyze the independent value of pATM expression. P values of <.05 were considered statistically significant. All statistical tests were performed using IBM SPSS Statistics version 23 (Armonk, NY, USA).
RESULTS
Patient Characteristics
Patient and tumor characteristics of the 141 included patients are presented in Table 1. Most patients were male in their seventh decade of life and the ratio between a glottic and supraglottic sublocation was about 2:1. The overall median follow‐up time was 60 months (range 1–60 months). Thirty‐four patients (24%) developed a local recurrence in the median follow‐up time of 12.5 months (range 2–46 months). Forty‐three (31%) patients died in the follow‐up period after a median time of 25 months (range 5–57 months) of which 18 patients deceased as a result of the original oncological disease. In 13 of them local recurrence was noted. Results did not change during the time span of this study.
Table 1.
Patient and Tumor Characteristics of All Patients at Baseline (n = 141).
| Characteristics | No. of Patients (%) |
|---|---|
| Age (y) | |
| Median (range) | 64 (33–95) |
| Gender | |
| Female | 21 (14.9) |
| Male | 120 (85.1) |
| Localization | |
| Glottic | 93 (66.0) |
| Supraglottic | 48 (34.0) |
| cT‐stadium | |
| T1 | 61 (43.3) |
| T2 | 80 (56.7) |
| cN‐stadium | |
| N0 | 125 (88.7) |
| N+ | 16 (11.3) |
N = node; T = tumor.
Cut‐Off Values for Low and High Expression for pATM, pChk2, and p53
Using the optimal sensitivity and specificity predicting local recurrence, for pATM the cut‐off was set at 92% and for pCHK2 at 69% positively stained tumor cells. For p53 we excluded all cases with negative (n = 60) and totally positive (n = 5) staining to analyze only the cases with functional p53 protein. For the remaining 76 cases the optimal cut‐off for low and high p53 expression was 26% positively stained tumor cells.
Expression of pATM, pChk2, and p53
Overall, we found a high percentage of pATM positively stained tumor cells (median 85%, range 0–98%), 38 patients having high expression above the threshold of 92% as set by the ROC analysis. The percentages of positive nuclei were considerably lower for pCHK2 (median 8.6% and five patients with high expression above 69%). For 53, the median percentage of positively stained tumor cells was 60.0% in 76 selected cases without mutant p53 expression. Of those, 65 patients showed high expression above 26%. Examples of typical staining patterns are illustrated in Figure 1.
Figure 1.
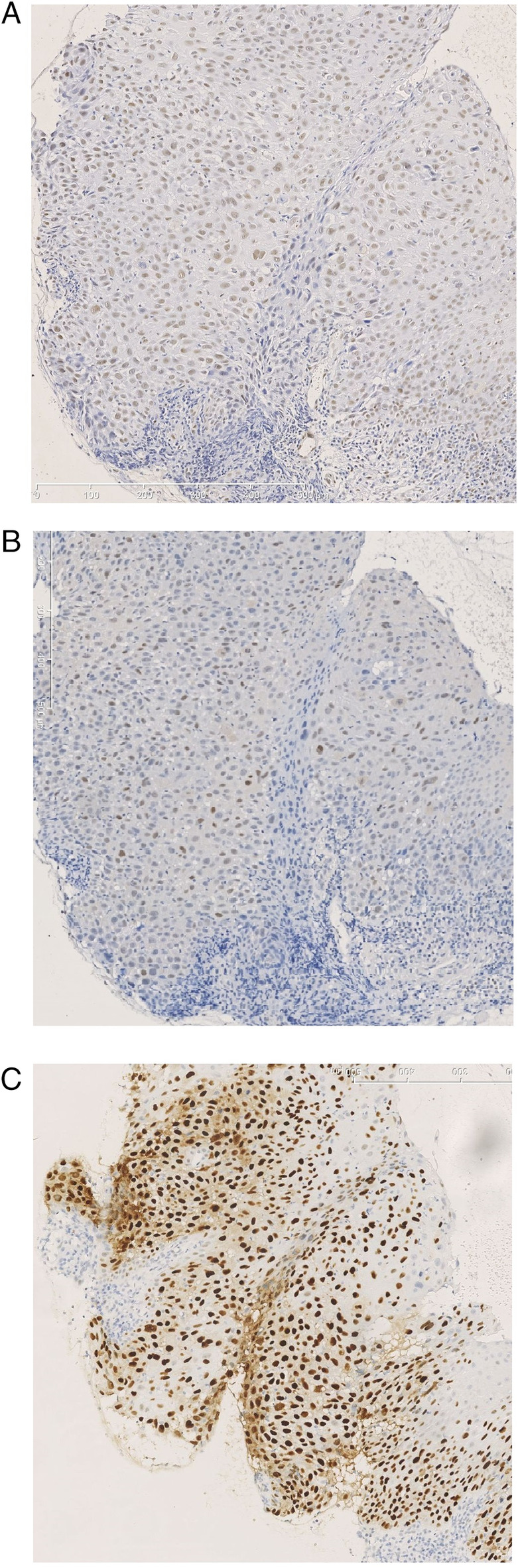
Example of a biopsy from a laryngeal tumor showing a high expression in immunohistochemical staining for (A) pATM, (B) pChk2, and (C) p53. Original magnification 200x. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
High Expression of pATM is Associated With Poor Response to Radiotherapy
Univariable Cox regression analysis (HR 2.14, 95% CI, 1.08–4.24, P = .03) as well as Kaplan–Meier survival analysis (long‐rank: P = .03) showed that high pATM expression was significantly associated with poor local control (Table 2 and Fig. 2A). Expression of pCHK2 (HR 3.16; 95% CI, 0.96–10.37; P = .06) and p53 (HR 1.31; 95% CI, 0.30–5.75; P = .72) as well as clinico‐pathological features as tumor size, lymph node status, gender, and age were not prognostic for local control (Table 2). Multivariable analysis showed that high pATM expression was independently associated with poor local control (HR 2.26; 95% CI, 1.05–4.88; P = .04).
Table 2.
Patient Characteristics, Tumor Characteristics, Immunohistochemical Expression in Relation to Local Recurrence (n = 34).
| Characteristics | No. of Patients With Local Recurrence (%) | Univariable HR (95% CI) | P |
|---|---|---|---|
| Age (y) | |||
| <65 | 18/74 (24.3) | 1.07 (0.55–2.11) | .84 |
| ≥65 | 16/67 (23.9) | 1 | |
| Gender | |||
| Female | 3/21 (14.3) | 1 | |
| Male | 31/120 (25.8) | 1.94 (0.59–6.34) | .27 |
| cT‐stadium | |||
| T1 | 11/61 (18.0) | 1 | |
| T2 | 23/80 (28.8) | 1.77 (0.86–3.62) | .12 |
| cN‐stadium | |||
| N0 | 28/125 (22.4) | 1 | |
| N+ | 6/16 (37.5) | 2.0 (0.83–4.85) | .12 |
| Location | |||
| Glottis | 22/93 (23.7) | 1 | |
| Supraglottis | 12/48 (25) | 1.04 (0.52–2.11) | .90 |
| pATM | |||
| Low | 20/103 (19.4) | 1 | |
| High | 14/38 (36.8) | 2.14 (1.08–4.24) | .03* |
| pChk2 | |||
| Low | 31/136 (22.8) | 1 | |
| High | 3/5 (60.0) | 3.16 (0.96–10.37) | .06 |
| p53 | |||
| Low | 2/11 (18.2) | 1 | |
| High | 14/65 (21.5) | 1.31 (0.30–5.75) | .72 |
CI = confidence interval; HR = hazard ratio; N = node; T = tumor.
Signifies statistically significant relation.
Figure 2.
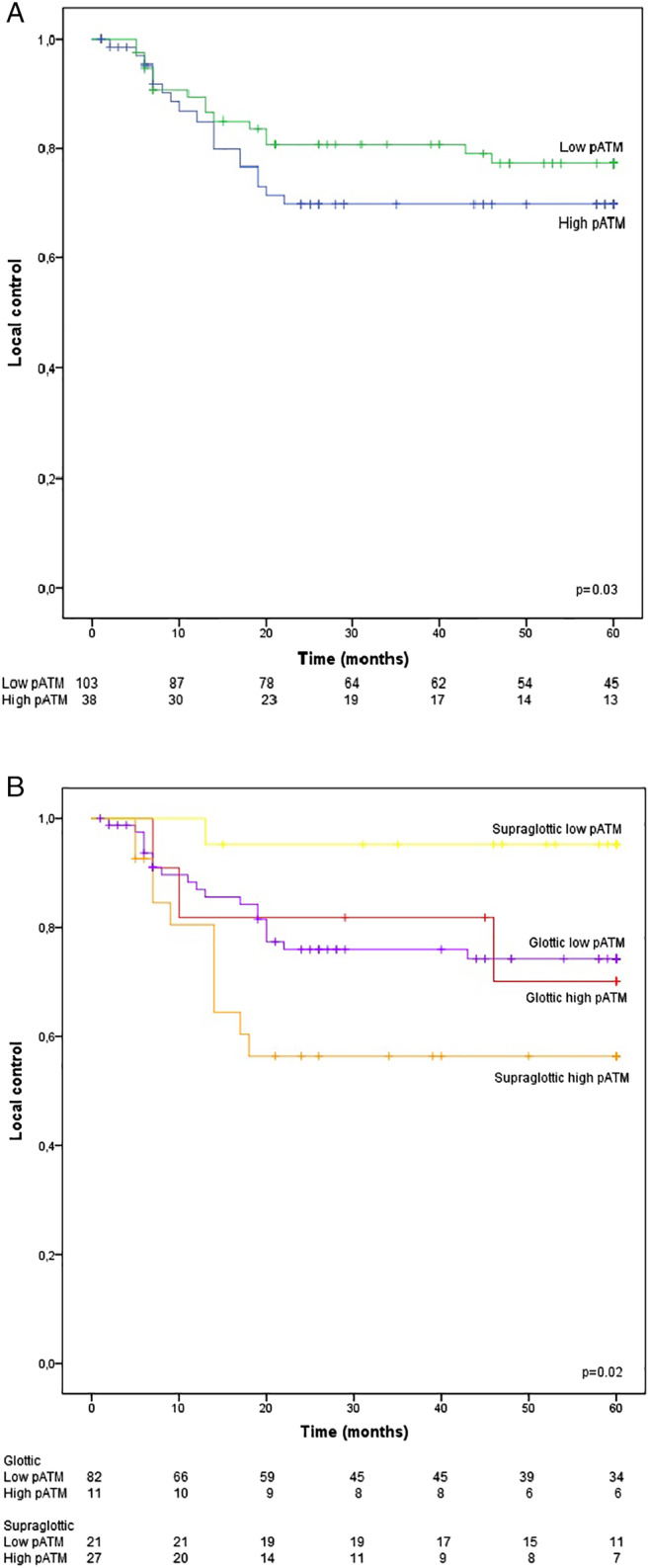
Local control rate as a function of (A) pATM and (B) pATM stratified for glottic/supraglottic location. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
pATM is Associated With Local Control Only in Supraglottic LSCC
Since tumors originating from the glottis and supraglottis have been suggested as different entities,31 we also evaluated the prognostic value of pATM, pCHk2, and p53 expression in these sublocations separately (Table 3). This analysis revealed that the association of high pATM with local control was restricted to the 48 supraglottic (HR 10.9; 95% CI, 1.40–84.4; P = .02) and not to the 93 glottic LSCC (HR 1.06; 95% CI, 0.31–3.57; P = .93) (Table 3 and Fig. 2B). Stratification by localization did not reveal a significant association between the expression of pChk2 and p53 and local control (Table 3).
Table 3.
Expression of pATM, pChk2, and p53 in Relation to Local Recurrence Separately for Glottic (n = 22) and Supraglottic (n = 12) Location.
| Glottic (n = 93) | Supraglottic (n = 48) | |||||
|---|---|---|---|---|---|---|
| Characteristics | No. of Patients With Local Recurrence (%) | Univariable HR (95% CI) | P | No. of Patients With Local Recurrence (%) | Univariable HR (95% CI) | P |
| pATM | ||||||
| Low | 19/82 (23.2) | 1 | 1/21 (4.8) | 1 | ||
| High | 3/11 (27.3) | 1.06 (0.31–3.57) | .93 | 11/27 (40.7) | 10.9 (1.40–84.4) | .02* |
| pChk2 | ||||||
| Low | 22/93 (23.7) | — | — | 9/43(20.9) | 1 | |
| High | 0/0 | — | 3/5 (60) | 3.13 (0.85–11.6) | .09 | |
| p53 | ||||||
| Low | 2/9 (22.2) | 1 | 0/2 (0) | 1 | ||
| High | 10/44 (22.7) | 1.07 (0.24–4.90) | .93 | 4/21 (19.0) | 23.55 (<0.001–>1,000) | .67 |
CI = confidence interval; HR = hazard ratio; N = node; T = tumor.
Signifies statistically significant relation.
DISCUSSION
We investigated the prognostic value of the expression of proteins involved in the ATM‐associated DDR pathway in a well‐defined homogeneous series of T1‐T2 laryngeal cancer patients treated with radiotherapy with curative intent. High pATM expression showed a correlation with poor local control, exclusively in supraglottic tumors. No associations were found between pChk2 and p53 expression with local control.
To our knowledge this is the first time that the phosphorylated isoform of ATM (pATM) was assessed with immunohistochemistry in a well‐defined series of T1‐T2 laryngeal cancer patients primarily treated with radiotherapy to validate the prognostic value for local control. Only one study has investigated the immunohistochemical expression of non‐phosphorylated ATM in correlation with radioresponse in a very small series of 21 glottic laryngeal cancers but no correlation was found.28 Despite of the small size of the study, their results could be in agreement with our findings that high pATM expression is not associated with local control in patients with glottic but solely in patients with supraglottic LSCC. Another explanation is that they studied the expression of non‐phosphorylated ATM. Bartkova et al. found that most human tissues contain the non‐phosphorylated isoform of ATM and the phosphorylated isoform is normally absent.31 In normal cells, nuclear staining of pATM was only detected in bone‐marrow lymphoblasts and primary spermatocytes, cell types in which DSB are generated during physiology. In contrast, in various malignancies expression of pATM was already detected in the early phase of carcinogenesis.32
In this study, we found only a predictive value of pATM in supraglottic tumors. Although the supraglottic LSCC demonstrated a similar recurrence rate as the glottic LSCC in this study, there were differences as well. In the supraglottic LSCC, significantly more T2‐staged tumors (P = .005) and more N+ cases were present (P < .001). These differences between glottic and supraglottic LSCC have been known for years,45 suggesting they might represent different entities. On an embryological basis, the supraglottis develops from the buccopharyngeal sac, whereas the (sub)glottis develops from the tracheopulmonary sac. Moreover, exposure of the different sublocations to carcinogens, such as tobacco and alcohol, cannot be considered identical and also might explain the variation in clinical outcome, genomic alterations, and protein expression levels.
In response to the difference in results between glottic and supraglottic LSCC, we also looked into ROC‐based cut‐off values for each sub‐location separately (data not shown). Interestingly, for sub‐analysis of the supraglottic LSCC, exactly the same cut‐off value of 92% emerged. The sub‐analyses did not change the results of pChk2 and p53 predictive value, emphasizing that the groups have become so small that the power is missing.
Previously, we investigated whether pATM expression was associated with response to (chemo)radiation and found that high levels of pATM were related to poor locoregional disease‐free survival in a cohort of 375 patients with cervical carcinoma.27 In addition, in cervical carcinoma cell lines we showed that high levels of active ATM prior to irradiation were related with increased radioresistance in vitro. Clonogenic survival analysis revealed that ATM inhibition strongly radiosensitized cervical cancer cells but not non‐transformed epithelial cells and fibroblasts. In one study using head and cancer cells, inhibition of ATM resulted also in radio‐sensitization.46 In line with these data, inhibition of ATM by targeted drug application resulted in enhanced sensitivity to radiotherapy in other malignancies as well.47, 48, 49, 50
In early stage LSCC we observed similar associations with local control as reported in the cohort of cervical carcinomas treated with (chemo)radiation, in the present study. These data suggest that LSCC patients with high pATM expression might also benefit from treatment with ATM‐inhibitors. Two ATM inhibitors are currently being tested in phase I trials, one combined with fractionated palliative radiotherapy in patients with solid tumors and in the other as a monotherapy and in combination with olaparib or 5‐fluorouracil, folinic acid, and irinotecan, in patients with advanced‐stage solid cancers.16 This current study is the first study analyzing the association between high expression of pATM in relation to local control in early stage LSCC (using a cut‐off of 92%). Compared to our cervical cancer cohort (using a cut‐off of 75%) the optimal cut‐off differed for both of these very different cancer entities. Therefore, it is important to confirm our conclusions using our cut‐off in a similar independent cohort of early staged LSCC primarily treated with radiotherapy.
For the downstream protein pChk2, no significant association with local control was found in this study. Because in our series of 141 patients with LSCC, only five cases (3%) revealed high pChk2 expression, no firm conclusions can be drawn regarding a possible role in radio response. On the other hand, our findings revealed that pChk2 expression is not very common in LSCC and therefore not expected to be of relevance as a prognostic marker for local control. To our knowledge there are no other studies evaluating the prognostic value of pChk2 expression for local control in laryngeal cancer. However, pChk2 expression was previously found in oral squamous cell carcinoma showing a higher incidence of high pChk2 expression (25–77%). In these studies, associations with clinical outcomes were not investigated.33, 34 In esophageal carcinoma treated with neoadjuvant chemoradiotherapy, the predictive value of pChk2 was reported but no correlation was found.35 Inhibitors of Chk2 have been tested in phase I studies with different compounds.51, 52 Many of these inhibitors inhibit both CHK1 and CHK2. In addition to low efficacy, severe cardiac toxicities were observed.51, 52
In this cohort of LSCC, no correlation was found between p53 and local control. This is in line with many other studies reporting no correlation between p53 expression and clinical outcome after radiotherapy in mostly early stage LSCC36, 37, 38, 39, 40, 41, 42 Only one study composed of solely T1 glottic carcinomas reported a relationship with clinical outcomes.40 To exclude that the association is restricted to T1 carcinomas, we evaluated in our cohort whether the association with p53 expression was linked to tumor size. However, we also did not observe an association between p53 expression and local control when analyzing T1 and T2 tumors separately (data not shown). The relatively high expression of p53 found in our cohort corresponds to other studies in LSCC.37, 41, 42, 53, 54 Though data about p53 expression is highly dependent on different staining protocols, scoring methods and cut‐off values used. We believe it is only useful to look at p53 as predictive marker when looking at the functional p53 protein. Nevertheless, similar to previous studies our findings suggest that p53 is no relevant prognostic marker for radiotherapy response in LSCC.
CONCLUSION
Our results presented in this study suggests that reduced levels of pATM may be associated with significantly better local control in early stage supraglottic cancers in the larynx treated with radiotherapy. Therefore, targeting ATM kinase activity could be an interesting therapeutic option to sensitize tumor cells for radiotherapy in supraglottic LSCC patients with high levels of active ATM before start of radiotherapy. More studies are required to confirm our findings on the association between pATM expression and local control in supraglottic LSCC.
Editor's Note: This Manuscript was accepted for publication on March 2, 2020.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
We thank B.A.C. van Dijk, Comprehensive Cancer Center, Department of Research, The Netherlands for providing data from the cancer registry.
BIBLIOGRAPHY
- 1. Hartl DM, Ferlito A, Brasnu DF, et al. Evidence‐based review of treatment options for patients with glottic cancer. Head Neck 2011;33:1638–1648. [DOI] [PubMed] [Google Scholar]
- 2. Steuer CE, El‐Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin 2017;67:31–50. [DOI] [PubMed] [Google Scholar]
- 3. Patel KB, Nichols AC, Fung K, Yoo J, MacNeil SD. Treatment of early stage supraglottic squamous cell carcinoma: meta‐analysis comparing primary surgery versus primary radiotherapy. J Otolaryngol Head Neck Surg 2018;47:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrijvers ML, van Riel EL, Langendijk JA, et al. Higher laryngeal preservation rate after CO2 laser surgery compared with radiotherapy in T1a glottic laryngeal carcinoma. Head Neck 2009;31:759–764. [DOI] [PubMed] [Google Scholar]
- 5. Gioacchini FM, Tulli M, Kaleci S, Bondi S, Bussi M, Re M. Therapeutic modalities and oncologic outcomes in the treatment of T1b glottic squamous cell carcinoma: a systematic review. Eur Arch Otorhinolaryngol 2017;274:4091–4102. [DOI] [PubMed] [Google Scholar]
- 6. Jones AS, Fish B, Fenton JE, Husband DJ. The treatment of early laryngeal cancers (T1‐T2 N0): surgery or irradiation? Head Neck 2004;26:127–135. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 2006;116:1–13. [DOI] [PubMed] [Google Scholar]
- 8. Chen AY, Fedewa S, Zhu J. Temporal trends in the treatment of early‐ and advanced‐stage laryngeal cancer in the United States, 1985‐2007. Arch Otolaryngol Head Neck Surg 2011;137:1017–1024. [DOI] [PubMed] [Google Scholar]
- 9. www.iknl.nl.
- 10. Colasanto JM, Haffty BG, Wilson LD. Evaluation of local recurrence and second malignancy in patients with T1 and T2 squamous cell carcinoma of the larynx. Cancer J 2004;10:61–66. [DOI] [PubMed] [Google Scholar]
- 11. Spector JG, Sessions DG, Chao KS, Hanson JM, Simpson JR, Perez CA. Management of stage II (T2N0M0) glottic carcinoma by radiotherapy and conservation surgery. Head Neck 1999;21:116–123. [DOI] [PubMed] [Google Scholar]
- 12. Lefebvre JL. Laryngeal preservation in head and neck cancer: multidisciplinary approach. Lancet Oncol 2006;7:747–755. [DOI] [PubMed] [Google Scholar]
- 13. Chera BS, Amdur RJ, Morris CG, Kirwan JM, Mendenhall WM. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:461–466. [DOI] [PubMed] [Google Scholar]
- 14. Lyhne NM, Johansen J, Kristensen CA, et al. Pattern of failure in 5001 patients treated for glottic squamous cell carcinoma with curative intent ‐ a population based study from the DAHANCA group. Radiother Oncol 2016;118:257–266. [DOI] [PubMed] [Google Scholar]
- 15. Thoms J, Bristow RG. DNA repair targeting and radiotherapy: a focus on the therapeutic ratio. Semin Radiat Oncol 2010;20:217–222. [DOI] [PubMed] [Google Scholar]
- 16. Pilie PG, Tang C, Mills GB, Yap TA. State‐of‐the‐art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol 2019;16:81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421:499–506. [DOI] [PubMed] [Google Scholar]
- 18. Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998;282:1893–1897. [DOI] [PubMed] [Google Scholar]
- 19. Ahn JY, Schwarz JK, Piwnica‐Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 2000;60:5934–5936. [PubMed] [Google Scholar]
- 20. Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 21. Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage‐inducible sites. Genes Dev 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 22. Hirao A, Kong YY, Matsuoka S, et al. DNA damage‐induced activation of p53 by the checkpoint kinase Chk2. Science 2000;287:1824–1827. [DOI] [PubMed] [Google Scholar]
- 23. Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 1998;281:1674–1677. [DOI] [PubMed] [Google Scholar]
- 24. Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998;281:1677–1679. [DOI] [PubMed] [Google Scholar]
- 25. Gotoff SP, Amirmokri E, Liebner EJ. Ataxia telangiectasia. Neoplasia, untoward response to x‐irradiation, and tuberous sclerosis. Am J Dis Child 1967;114:617–625. [DOI] [PubMed] [Google Scholar]
- 26. Savitsky K, Bar‐Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI‐3 kinase. Science 1995;268:1749–1753. [DOI] [PubMed] [Google Scholar]
- 27. Roossink F, Wieringa HW, Noordhuis MG, et al. The role of ATM and 53BP1 as predictive markers in cervical cancer. Int J Cancer 2012;131:2056–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Condon LT, Ashman JN, Ell SR, Stafford ND, Greenman J, Cawkwell L. Overexpression of bcl‐2 in squamous cell carcinoma of the larynx: a marker of radioresistance. Int J Cancer 2002;100:472–475. [DOI] [PubMed] [Google Scholar]
- 29. Schrijvers ML, van der Laan BF, de Bock GH, et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA‐IX predict for local recurrence in stage T1‐T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:161–169. [DOI] [PubMed] [Google Scholar]
- 30. Wachters JE, Schrijvers ML, Slagter‐Menkema L, et al. Prognostic significance of HIF‐1a, CA‐IX, and OPN in T1‐T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope 2013;123:2154–2160. [DOI] [PubMed] [Google Scholar]
- 31. Bartkova J, Bakkenist CJ, Rajpert‐De Meyts E, et al. ATM activation in normal human tissues and testicular cancer. Cell Cycle 2005;4:838–845. [DOI] [PubMed] [Google Scholar]
- 32. Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti‐cancer barrier in early human tumorigenesis. Nature 2005;434:864–870. [DOI] [PubMed] [Google Scholar]
- 33. Yutori H, Semba S, Komori T, Yokozaki H. Restoration of fragile histidine triad expression restores Chk2 activity in response to ionizing radiation in oral squamous cell carcinoma cells. Cancer Sci 2008;99:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chou SC, Azuma Y, Varia MA, Raleigh JA. Evidence that involucrin, a marker for differentiation, is oxygen regulated in human squamous cell carcinomas. Br J Cancer 2004;90:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarbia M, Ott N, Puhringer‐Oppermann F, Brucher BL. The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the oesophagus. Br J Cancer 2007;97:1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wildeman MA, Gibcus JH, Hauptmann M, et al. Radiotherapy in laryngeal carcinoma: can a panel of 13 markers predict response? Laryngoscope 2009;119:316–322. [DOI] [PubMed] [Google Scholar]
- 37. Ahmed WA, Suzuki K, Imaeda Y, Horibe Y. Ki‐67, p53 and epidermal growth factor receptor expression in early glottic cancer involving the anterior commissure treated with radiotherapy. Auris Nasus Larynx 2008;35:213–219. [DOI] [PubMed] [Google Scholar]
- 38. Parikh RR, Yang Q, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in T1‐2 N0 laryngeal cancer treated with primary radiation therapy. Cancer 2007;109:566–573. [DOI] [PubMed] [Google Scholar]
- 39. Cho EI, Kowalski DP, Sasaki CT, Haffty BG. Tissue microarray analysis reveals prognostic significance of COX‐2 expression for local relapse in T1‐2N0 larynx cancer treated with primary radiation therapy. Laryngoscope 2004;114:2001–2008. [DOI] [PubMed] [Google Scholar]
- 40. Narayana A, Vaughan AT, Kathuria S, Fisher SG, Walter SA, Reddy SP. P53 overexpression is associated with bulky tumor and poor local control in T1 glottic cancer. Int J Radiat Oncol Biol Phys 2000;46:21–26. [DOI] [PubMed] [Google Scholar]
- 41. Pai HH, Rochon L, Clark B, Black M, Shenouda G. Overexpression of p53 protein does not predict local‐regional control or survival in patients with early‐stage squamous cell carcinoma of the glottic larynx treated with radiotherapy. Int J Radiat Oncol Biol Phys 1998;41:37–42. [DOI] [PubMed] [Google Scholar]
- 42. Saunders ME, MacKenzie R, Shipman R, Fransen E, Gilbert R, Jordan RC. Patterns of p53 gene mutations in head and neck cancer: full‐length gene sequencing and results of primary radiotherapy. Clin Cancer Res 1999;5:2455–2463. [PubMed] [Google Scholar]
- 43. Jiang H, Reinhardt HC, Bartkova J, et al. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev 2009;23:1895–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soreide K. Receiver‐operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol 2009;62:1–5. [DOI] [PubMed] [Google Scholar]
- 45. Raitiola H, Pukander J, Laippala P. Glottic and supraglottic laryngeal carcinoma: differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol 1999;119:847–851. [DOI] [PubMed] [Google Scholar]
- 46. Zou J, Qiao X, Ye H, et al. Inhibition of ataxia‐telangiectasia mutated by antisense oligonucleotide nanoparticles induces radiosensitization of head and neck squamous‐cell carcinoma in mice. Cancer Biother Radiopharm 2009;24:339–346. [DOI] [PubMed] [Google Scholar]
- 47. Hickson I, Zhao Y, Richardson CJ, et al. Identification and characterization of a novel and specific inhibitor of the ataxia‐telangiectasia mutated kinase ATM. Cancer Res 2004;64:9152–9159. [DOI] [PubMed] [Google Scholar]
- 48. Fuhrman CB, Kilgore J, LaCoursiere YD, et al. Radiosensitization of cervical cancer cells via double‐strand DNA break repair inhibition. Gynecol Oncol 2008;110:93–98. [DOI] [PubMed] [Google Scholar]
- 49. Li W, Jian W, Xiaoping X, Yingfeng L, Tao X, Xiaoyan X. Enhanced radiation‐mediated cell killing of human cervical cancer cells by small interference RNA silencing of ataxia telangiectasia‐mutated protein. Int J Gynecol Cancer 2006;16:1620–1630. [DOI] [PubMed] [Google Scholar]
- 50. Zeng YC, Xing R, Zeng J, et al. Sodium glycididazole enhances the radiosensitivity of laryngeal cancer cells through downregulation of ATM signaling pathway. Tumour Biol 2016;37:5869–5878. [DOI] [PubMed] [Google Scholar]
- 51. Sausville E, Lorusso P, Carducci M, et al. Phase I dose‐escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharmacol 2014;73:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seto T, Esaki T, Hirai F, et al. Phase I, dose‐escalation study of AZD7762 alone and in combination with gemcitabine in japanese patients with advanced solid tumours. Cancer Chemother Pharmacol 2013;72:619–627. [DOI] [PubMed] [Google Scholar]
- 53. Kokoska MS, Piccirillo JF, El‐Mofty SK, Emami B, Haughey BH, Schoinick SB. Prognostic significance of clinical factors and p53 expression in patients with glottic carcinoma treated with radiation therapy. Cancer 1996;78:1693–1700. [PubMed] [Google Scholar]
- 54. Kropveld A, Slootweg PJ, van Mansfeld AD, Blankenstein MA, Hordijk GJ. Radioresistance and p53 status of T2 laryngeal carcinoma. Analysis by immunohistochemistry and denaturing gradient gel electrophoresis. Cancer 1996;78:991–997. [DOI] [PubMed] [Google Scholar]


