Abstract
Background
Fluid accumulation frequently coexists with acute kidney injury (AKI) and is associated with increased risk for AKI progression and mortality. Among septic shock patients, restricted use of resuscitation fluid has been reported to reduce the risk of worsening of AKI. Restrictive fluid therapy, however, has not been studied in the setting of established AKI. Here, we present the protocol and statistical analysis plan of the REstricted fluid therapy VERsus Standard trEatment in Acute Kidney Injury—the REVERSE‐AKI trial that compares a restrictive fluid therapy regimen to standard therapy in critically ill patients with AKI.
Methods
REVERSE‐AKI is an investigator‐initiated, multinational, open‐label, randomized, controlled, feasibility pilot trial conducted in seven ICUs in five countries. We aim to randomize 100 critically ill patients with AKI to a restrictive fluid treatment regimen vs standard management. In the restrictive fluid therapy regimen, the daily fluid balance target is neutral or negative. The primary outcome is the cumulative fluid balance assessed after 72 hours from randomization. Secondary outcomes include safety, feasibility, duration, and severity of AKI, and outcome at 90 days (mortality and dialysis dependence).
Conclusions
This is the first multinational trial investigating the feasibility and safety of a restrictive fluid therapy regimen in critically ill patients with AKI.
Trial registration
clinical.trials.gov NCT03251131.
Keywords: acute kidney injury, critically ill, fluid balance, restrictive fluid therapy
1. INTRODUCTION
Acute kidney injury (AKI) is a frequent syndrome during critical illness affecting approximately 40%‐57% patients in the intensive care unit (ICU).1, 2 It carries an increased risk for adverse outcomes such as mortality, prolonged hospital stay, development of chronic kidney disease (CKD), and increased health‐care costs.1, 2, 3 Currently, the treatment of AKI is supportive, and approximately one fifth of patients with severe AKI require renal replacement therapy (RRT).1, 2
The rationale to administer fluid in AKI has been the desire to increase renal blood flow and perfusion pressure by increasing cardiac output and blood pressure.3, 4 However, patients with AKI are prone to develop fluid overload.5 Excessive fluid administration has also been associated with new development of AKI and progression of AKI.6, 7 The potential mechanisms include increased intra‐abdominal pressure, renal congestion, and renal subcapsular edema leading to raised intrarenal pressure.8 Multiple observational studies have also shown an association between fluid accumulation and increased mortality.5, 9, 10, 11
Restricting fluid input among patients with AKI might be beneficial in terms of reducing edema formation, which could potentially improve organ function and prevent further injury, and subsequently lead to increased survival. A feasibility study in septic shock patients comparing restrictive vs standard fluid resuscitation reported a lower risk of worsening of AKI in the restrictive group.12 Trials in other subgroups of critically ill patients have demonstrated that restricting fluid input after the initial resuscitation was safe.12, 13, 14 A large trial among patients undergoing major abdominal surgery comparing restrictive vs standard fluid therapy for 24 hours intra‐ and post‐operatively did not report difference in the disability‐free 1‐year survival, whereas the risk of AKI was increased among those with restrictive fluid therapy.15 The need for further studies specifically in critically ill patients with AKI has been identified as a research priority.16, 17
Our aim is to conduct a multinational, open‐label, randomized, controlled, feasibility pilot trial comparing a restrictive fluid treatment regimen to standard treatment among patients with AKI to study the feasibility of such intervention in terms of separation of groups, safety, and compliance. We hypothesize that the proposed restricted fluid treatment regimen will lead to 1200 mL lower cumulative fluid balance at 72 hours post‐randomization. Assessing the feasibility of this intervention is necessary before larger trials with patient‐centered outcomes as primary outcomes can be conducted.
2. METHODS
2.1. Trial design and setting
The REVERSE‐AKI trial is an investigator‐initiated, multicenter, open, randomized controlled pilot study. Helsinki University Hospital (in Helsinki Finland) is the coordinating center. Adult ICUs from Australia (Austin Hospital, Canberra Hospital), Belgium (University Hospital of Ghent), Switzerland (Lausanne University Hospital), and the UK (Guy's and St Thomas Hospital, The Royal London Hospital) will participate.
2.2. Trial registration
The REVERSE‐AKI trial was registered with clinicaltrials.gov (identifier NCT03251131) on 16 August 2017.
2.3. Trial conduct
The study protocol was prepared as outlined in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines.18 The study will be conducted according to the Declaration of Helsinki, the principles of Good Clinical Practice, and in accordance with local legislation in participating countries.
2.4. Randomization
Eligible patients who fulfill all inclusion criteria and have no exclusion criteria will be randomized using web‐based allocation concealment based on a computer‐based algorithm created by an independent statistician. Randomization will be stratified according to (a) the presence of clinical signs of fluid accumulation (defined by peripheral pitting edema and/or positive fluid balance with P/F ratio less than 200 mmHg) and (b) severity of AKI (stage 1 vs stage 2 or 3 as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria16; detailed definition presented in the Supplement Data S1). Permutated blocks of varying sizes from 2 to 4 will be used.
2.5. Blinding
The trial intervention will not be blinded for investigators, ICU personnel, or patients due to its nature. The statistician conducting the data analysis will be masked for the group allocation. We will compile an abstract of the study results prior to becoming aware of the group allocation, which will be included in the appendix of the original publication.
2.6. Inclusion and exclusion criteria
All admissions to ICU will be initially screened. The detailed definitions of inclusion and exclusion criteria are presented in the Supplement Data S1.
2.6.1. Inclusion criteria
18 years or older and admitted to critical care with an arterial line in place
The patient has been in critical care for at least 12 hours but no more than 72 hours
-
The patient has AKI but is not receiving acute RRT:
AKI is defined by the following criteria:
-
Increase in serum creatinine over 1.5 times above baseline without a decline of 27 µmol/L or more from the last preceding measurement (at least 12 hours apart)
AND/OR
Overall urine output less than 0.5 mL kg−1 h−1 (or 6 mL/kg) for the previous 12 hours (with urine catheter in place for the period)
-
The patient is judged by the treating clinician not to be intravascularly hypovolemic
The patient is likely to remain in critical care for 48 hours after randomization
2.6.2. Exclusion criteria
Active bleeding necessitating transfusion
Maintenance fluid therapy is necessary due to diabetic ketoacidosis, non‐ketotic coma, severe burns, or other clinical reason determined by the medical staff
Need for RRT due to intoxication of a dialyzable toxin
Commencement of RRT is expected in the next 6 hours
On chronic RRT (maintenance dialysis or renal transplant)
Presence or a strong clinical suspicion of parenchymal AKI (eg, glomerulonephritis, vasculitis, acute interstitial nephritis) or post‐renal obstruction
Severe hyponatremia (Na <125 mmol/L) or hypernatremia (Na >155 mmol/L)
Need for extracorporeal membrane oxygenation or molecular absorbent recirculating system
Pregnant or lactating
Patients who are not to receive full active treatment
No baseline creatinine available
Lack of consent
The patient has been enrolled in another trial where co‐enrollment is not feasible
2.7. Trial interventions
Table 1 presents the timeline of eligibility, randomization, and study interventions. The intervention period relates to the first 7 days after randomization as long as the patient is still in the critical care unit.
Table 1.
Overview of the schedule of enrollment, interventions, and assessments according to the SPIRIT 2013 statement
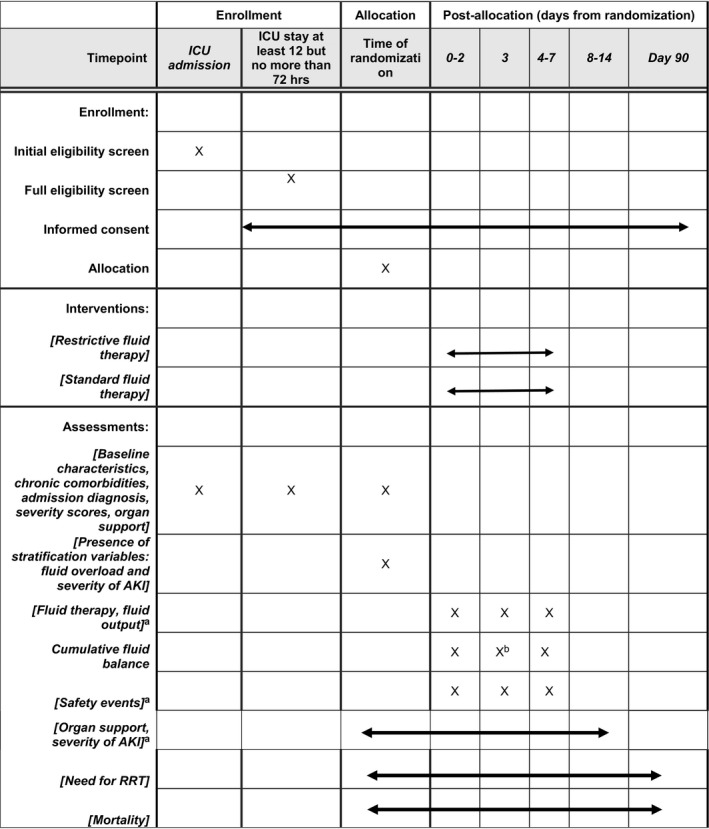
Abbreviations: AKI; acute kidney injury, RRT; renal replacement therapy.
While in the ICU.
Primary outcome.
2.7.1. Experimental arm (restricted fluid management)
Targets from the time of randomization.
Total daily fluid input is restricted to only medications and nutritional fluids (enteral or parenteral) and blood products (if clinically necessary). No maintenance IV fluids will be administered. Fluid bolus therapy can be given if clinically deemed necessary.
Maintenance fluid is only acceptable if enteral nutrition is not tolerated and parenteral nutrition is contraindicated.
Fluid output (with unrestricted use of diuretics) to match input whenever possible and to achieve a fluid balance which is preferably negative and always less than +300 mL/d
If such a fluid balance target cannot be achieved, RRT is considered to remove the required fluid. Commencing RRT is not mandated in the trial.
If continuous RRT is not considered clinically desirable, acceptance of a less than targeted fluid balance temporarily up to +900 mL provided it lasts only 24 hours.
Fluid balance is calculated by subtracting total fluid output (urine output, losses to drains, losses from gastrointestinal tract, and ultrafiltration by RRT) from total fluid input (intravenous and per oral). Insensible losses are not considered.
2.7.2. Standard group
All aspects of fluid therapy will be at the discretion of the treating clinical team.
2.7.3. Concomitant treatment
All other aspects of care will be according to the local practices and treating clinicians’ judgment. Fluid removal (either using diuretics or ultrafiltration) will not be controlled. Initiation of RRT will be according to the clinician's judgment.
2.8. Outcome measures
2.8.1. Primary outcome measure
Cumulative fluid balance at first 72 hours after randomization is the primary outcome of the study. The time point chosen to assess the outcome was justified by data from two large cohort studies1, 2 that have reported the median ICU stay of AKI patients to be 3.7‐6 days.
2.8.2. Secondary outcome measures
Duration of AKI in days defined by the KDIGO creatinine and urine output criteria (truncated at ICU discharge or 14 days, whichever comes first)
Number of patients requiring RRT (truncated at 14 days)
-
Cumulative fluid balance at
24 hours after randomization
at ICU discharge (or truncated at 7 days if ICU stay exceeds 7 days)
Cumulative dose of diuretics during the intervention period (while in the ICU, maximum of 7 days) adjusted for the duration of observation period
Duration of AKI is a secondary outcome of the trial. We will test the feasibility of recording it, the potential challenges as a result of using creatinine‐based diagnostics (possible dilution and subsequently lower incidence in the standard group), and the potentially increased use of diuretics in the interventional arm that could confound the assessment of urine output criterion.19
2.8.3. Exploratory outcomes
Mechanical ventilation‐free and days alive (truncated at 14 days)
Vasopressor‐free days and alive (truncated at 14 days)
ICU‐free days and alive (truncated at 14 days)
RRT‐free days and alive (90 days)
90‐day dialysis dependence
90‐day mortality
2.8.4. Safety and feasibility outcomes
-
Number of patients with one or more (serious) adverse events and reactions in both arms (detailed definitions are provided in the Supplement Data S1)
Ventricular tachycardia/fibrillation
New onset of atrial fibrillation requiring medication/defibrillation
Ischemic events
Radiologically diagnosed pulmonary edema
Adverse events related to RRT and diuretics use
Frequency of hypokalemia (serum K <3.5 mmol/L)
Frequency of hypomagnesemia (serum Mg <0.8 mmol/L)
Frequency of serum pH >7.5
Other
Screened vs recruited patients’ ratio
Recruitment rate (patients/center/month)
Protocol compliance (number of patients with protocol violations in both arms)
2.9. Data collection
Trained research personnel will perform the data collection using an electronic platform (Absolute imaginary Software Ltd). Data about baseline characteristics, ICU severity scores and admission diagnoses, daily fluid input and output, and need for organ supportive therapies will be collected. Trial follow‐up regarding need for RRT and vital status will last until 90 days. The detailed list of collected data items and schedule for collected plasma samples are provided in the Supplement Data S1.
2.10. Statistical analysis
2.10.1. General analytical principles
Statistical analyses will be performed on the intention‐to‐treat (ITT) population defined as all randomized subjects with consent to use data in the analysis. The conclusions of the analysis will be based on the ITT analysis.
A sensitivity analysis will be conducted in the per‐protocol population, defined as the ITT population after exclusion of subjects who experienced one or several protocol violations or stayed less than 48 hours in the ICU post‐randomization.
Assumption of normality will be checked by Shapiro‐Wilk test for continuous variables. Statistical significance will be set to 0.05 and two‐sided P‐values will be reported.
2.10.2. Primary outcome
The primary outcome between restrictive fluid treatment regimen and standard groups will be adjusted for the two stratification variables (presence of fluid accumulation and severity of AKI) using two‐tailed general linear or median regression and depending on distribution, estimated means with standard deviation (SD) or medians with interquartile range (IQR) with a measure of distribution will be reported. Additionally, we will calculate the difference with 95% confidence intervals (CIs) between the groups using mean or median regression. We will report the crude cumulative fluid balance in both groups and compare them using appropriate test depending on the variable distribution.
2.10.3. Secondary and exploratory outcomes
All secondary and exploratory outcome variables will be adjusted for the two stratification variables either with two‐tailed logistic regression (dichotomous outcomes, given that the number of observed outcomes will permit adjustment) or a linear model (continuous outcome variables, mean or median regression model). Risk ratios with 95% CIs or difference in means/median with 95% CIs will be reported. Additionally, we will report crude outcomes with risk ratios for dichotomous outcomes and absolute differences with 95% CIs for continuous outcomes.
2.10.4. Other variables
All categorical data will be compared using Chi‐square test and continuous variables using Mann‐Whitney U test or Student's t test depending on normal distribution.
2.10.5. Missing data
Some patients may be discharged from the ICU before completing the 72 hours post‐randomization phase in full. For these patients, the balance available at ICU discharge will be used in the analyses. Regarding other outcome variables, we expect the number of missing data to be low and therefore, no imputation is planned. Regarding baseline characteristics and data for daily SOFA scores, if the number of missing observations is less than 5%, we will not impute data. In case the number of missing data exceeds 5%, an appropriate multiple imputation strategy (ie, missing at random or missing completely at random) will be used.
2.11. Sample size
Due to the lack of previous interventional trials in the field, it is difficult to estimate the meaningful difference in the primary outcome. Using unpublished data from the FINNAKI study1 cohort including 480 patients with AKI defined as the trial inclusion criteria and excluding patients who commenced RRT within 24 hours of ICU admission, we found the median cumulative fluid balance at 72 hours to be 2653 mL (interquartile range from 427 mL to 5918 mL). Cumulative fluid balance (in liters) associated with an increased risk for 90‐day mortality with an odds ratio of 1.09 (95% CI 1.04‐1.13), P < .001. Thus, we assume that a decrease in fluid balance of 1.2 L could ultimately translate into meaningful differences in patient‐centered outcomes.
Therefore, we aim to randomize 100 patients (50 in each group) to have a >80% power to detect a difference in fluid balance at 72 hours after randomization of +2700 mL in the standard group vs +1500 ml in the fluid restrictive group (both with a SD of 2000 mL) at an alpha error of 0.05. This would represent a difference of 400 mL/d, which appears achievable.
Power calculations for secondary or exploratory outcomes were not done.
2.12. Pre‐planned subgroup analyses
No subgroup analyses will be conducted.
2.13. Trial profile
We will report the flow of trial participants according to the CONSORT statement.20
2.14. Data monitoring and safety committee
No data and safety monitoring committee (DSMB) will be formed as the trial is a low‐risk pilot trial. Informed consents, inclusion and exclusion criteria, and collected data will be monitored.
2.15. Interim analyses
No interim analyses will be conducted.
3. DISCUSSION
International expert panels identified the investigation of restricting fluid therapy among patients with AKI as a research priority.16, 17 Trials in acute respiratory distress syndome (ARDS) and septic shock patients using various protocols to study restrictive fluid therapy in comparison to standard12 or liberal fluid therapy13, 14 have shown that it is safe in critically ill patients. However, data in critically ill patients with AKI are missing. Our trial will provide data about the safety, feasibility, and efficacy of a restrictive fluid regimen in patients with AKI. The results will help to plan larger randomized controlled trials studying patient‐centered outcomes as primary endpoints.
The strengths of this trial include its multinational design. Moreover, the restrictive fluid regimen is planned to target the fluid balance, and not just specifically restricting fluid input. This flexible approach allows the treating clinicians to tailor the fluid therapy individually and this strategy will probably lead to a more clinically meaningful approach than solely restricting only fluid input or enhancing fluid output. Finally, we will enroll patients with AKI of different severity (excluding only those already receiving RRT or in whom commencing RRT is imminent), which will increase the generalizability of the results.
Our trial has some limitations, too. First, due to the nature of the intervention, the patient, clinical team, or the investigators are not blinded. However, the trial statistician will be blinded to group allocation. Second, management of fluid balance in standard therapy arm will be according to the local practices of each participating center, and potentially these may vary between geographical regions. Additionally, we will enroll patients after the initial fluid resuscitation has been completed. Thus, after stabilization, the clinical treatment goals typically include fluid removal, which means targeting negative fluid balances also in the standard arm.21 Moreover, the sample size calculation was based on data from a Finnish observational study, during which rather liberal fluid practices were in place.1 Therefore, it is possible that large variation in fluid treatment practices across centers and negative balance targets also in the standard arm hinder observing a significant separation between groups in the primary outcome.
In conclusion, the multinational REVERSE‐AKI pilot trial will investigate the safety, feasibility, and efficacy of a restrictive fluid therapy regimen compared to standard therapy. Its results will help to plan larger randomized trials to be conducted in the future.
4. ETHICAL CONSIDERATIONS AND CONSENT TO PARTICIPATE
The approval of local ethics committee has been obtained prior to commencing study screening in all participating countries. The consent policy follows local requirements. In some settings, a deferred consent has been approved with an informed, written consent obtained as soon as possible.
5. DATA SHARING STATEMENT
The de‐identified trial data set will be published as a supplement to the original publication.
6. DISSEMINATION
Results of the study will be submitted in a peer‐reviewed medical journal regardless of the results.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We are grateful for the study patients and their relatives for their willingness to participate in the trial. We warmly thank the clinical and research staff at participating ICUs and acknowledge that the study would not be possible without them.
Vaara ST, Ostermann M, Selander T, et al. Protocol and statistical analysis plan for the REstricted fluid therapy VERsus Standard trEatment in Acute Kidney Injury—REVERSE‐AKI randomized controlled pilot trial. Acta Anaesthesiol Scand. 2020;64:831–838. 10.1111/aas.13557
Funding information
The trial has received support from the Academy of Finland (317061) and Orion Research Foundation. STV has received a Fellowship grant from the Sigrid Juselius Foundation. The funders have no role in the trial design, conduction, or interpretation of the results.
REFERENCES
- 1. Nisula S, Kaukonen KM, Vaara ST, et al. Incidence, risk factors and 90‐day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420‐428. [DOI] [PubMed] [Google Scholar]
- 2. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI‐EPI study. Intensive Care Med. 2015;41:1411‐1423. [DOI] [PubMed] [Google Scholar]
- 3. Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37‐47. [DOI] [PubMed] [Google Scholar]
- 4. Ostermann M, Liu K, Kashani K. Fluid management in acute kidney injury. Chest. 2019;156:594‐603. [DOI] [PubMed] [Google Scholar]
- 5. RENAL T . observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial*. Crit Care Med. 2012;40:1753‐1760. [DOI] [PubMed] [Google Scholar]
- 6. Raimundo M, Crichton S, Martin JR, et al. Increased fluid administration after early acute kidney injury is associated with less renal recovery. Shock (Augusta, Ga). 2015;44:431‐437. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Crichton S, Dixon A, Seylanova N, Peng ZY, Ostermann M. Cumulative fluid accumulation is associated with the development of acute kidney injury and non‐recovery of renal function: a retrospective analysis. Crit Care. 2019;23:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107‐115. [DOI] [PubMed] [Google Scholar]
- 9. Vaara ST, Korhonen AM, Kaukonen KM, et al. Fluid overload is associated with an increased risk for 90‐day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16:R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422‐427. [DOI] [PubMed] [Google Scholar]
- 12. Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel‐group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695‐1705. [DOI] [PubMed] [Google Scholar]
- 13. Chen C, Kollef MH. Targeted fluid minimization following initial resuscitation in septic shock: A pilot study. Chest. 2015;148:1462‐1469. [DOI] [PubMed] [Google Scholar]
- 14. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid‐management strategies in acute lung injury. N Engl J Med. 2006;354:2564‐2575. [DOI] [PubMed] [Google Scholar]
- 15. Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263‐2274. [DOI] [PubMed] [Google Scholar]
- 16. Kellum JA, Lameire N, Aspelin P, et al. Kidney diseases: improving global outcomes (KDIGO) acute kidney Injury workgroup: KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl. 2012;1‐138. [Google Scholar]
- 17. Pickkers P, Ostermann M, Joannidis M, et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med. 2017;43(9):1198‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665‐2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726‐732. [DOI] [PubMed] [Google Scholar]
- 21. Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113:740‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


