Abstract
As of 18 February 2020, the e‐cigarette or vaping product use‐associated lung injury (EVALI) epidemic has claimed the lives of 68 patients in the USA with the total number of reported cases standing at 2807 to date. We present the clinical and radiologic findings, course of illness, and treatment of EVALI in seven adolescent patients in Northeast Ohio. Five of our patients required supplemental oxygen with four requiring intensive care unit care for respiratory support during admission. Three patients were treated with systemic steroids while inpatient. Bilateral opacities were seen on radiographic imaging of all seven of our patients. All patients were discharged alive on room air. However, impaired diffusing capacity of the lungs for carbon monoxide (DLCO) with nonobstructive spirometry was seen in patients that were tested postdischarge. This suggests that although recovery from the acute illness process of EVALI is achieved, there may be long‐term impact on lung function in these patients. We recommend close follow‐up with a pediatric pulmonologist where spirometry and DLCO can be performed.
Keywords: acute respiratory distress syndrome (ARDS), cannabidiol (CBD), Centers for Disease Control and Prevention (CDC), diffusing capacity of the lungs for carbon monoxide (DLCO), e‐cigarette or vaping product use‐associated lung injury (EVALI), lung injury, nonnicotine substances such as tetrahydrocannabinol (THC), respiratory failure, vaping
Abbreviations
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- CBD
cannabidiol
- CDC
Centers for Disease Control and Prevention
- CPAP
continuous positive airway pressure
- CRP
C‐reactive protein
- CXR
chest X‐ray
- DLCO
diffusing capacity of the lung for carbon monoxide
- EVALI
e‐cigarette or vaping product use‐associated lung injury
- FEV1
forced expiratory volume in 1 second
- HFNC
high‐flow nasal cannula
- NC
nasal cannula
- PICU
pediatric intensive care unit
- RSV
respiratory syncytial virus
- RVP
respiratory viral panel
- THC
tetrahydrocannabinol
1. INTRODUCTION
E‐cigarettes are battery‐operated devices that heat a liquid and deliver an aerosolized product to the user via inhalation. The use of e‐cigarettes or vaping devices has seen a massive surge since their introduction to the U.S. market in 2007 as aids for smoking cessation. 1 Since 2007, the industry has dramatically expanded the types of e‐cigarette devices and the e‐liquid formulations to add numerous flavors, such as fruits, crèmes, and menthol, which appeal to the youth market. 2 Research conducted in 2018 found that young adults aged 18 to 24 were more likely to use flavored tobacco products than adults in the next age group 25 to 30. 3 In addition, it was reported that 3.15 million middle‐ and high‐school student tobacco product users had used flavored tobacco products in 2018. 3
While a vast array of vaping devices are available to users, the most popular device is the JUUL e‐cigarette. 4 , 5 The JUUL e‐cigarette is a “closed system” device with disposable pods containing e‐liquid. Refillable vaping devices termed “open system” devices are also available to users. 6 There has also been a rise in the vaping of nonnicotine substances such as tetrahydrocannabinol (THC) and cannabidiol among e‐cigarette users. 7
The first case report of respiratory failure secondary to e‐cigarette or vaping product use‐associated lung injury (EVALI), then known as hypersensitivity pneumonitis and acute respiratory distress syndrome (ARDS) related to e‐cigarette use, was reported by a group in Pittsburgh in June 2018. 8 Since then, several reports have demonstrated that vaping is rapidly growing across the U.S. 6 , 9 , 12 As of 18 February 2020, 2807 cases of EVALI from all 50 states, the District of Columbia, and two U.S. territories have been reported to the Centers for Disease Control and Prevention (CDC). 13 This number reflects hospitalized cases of EVALI in the U.S. Sixty‐eight deaths from EVALI have also been confirmed in 29 states and the District of Columbia. 13 While many chemical additives are present in e‐liquid and vape pods, vitamin E acetate is the first to be consistently linked with the site of injury. A recent study confirmed the detection of vitamin E acetate in bronchoalveolar lavage (BAL) samples from 48 out of 51 patients from 16 US states, which is the first consistent detection of a potential chemical involved in the pathology of EVALI. 13 , 15
The CDC defines a confirmed case of EVALI as the onset of respiratory symptoms within 90 days of using e‐cigarettes along with pulmonary infiltrate on radiographic or computerized tomography (CT) imaging plus the absence of pulmonary infection and other probable cause in the medical record. 14 The CDC further defines a probable case of EVALI as the onset of respiratory symptoms within 90 days of using e‐cigarettes along with pulmonary infiltrate on radiographic or CT imaging and evidence of infection on culture or polymerase chain reaction (PCR) that the primary medical team does not believe to be the cause of the underlying lung injury along with no other probable cause in the medical history. 14
Due to the relatively recent nature of this epidemic, the heterogeneity of substances and concentrations being vaped, the clinical presentation of EVALI remains quite variable, and there is as yet no consensus among pediatric pulmonologists on the best treatment approach. The vast number of different categories of vaping devices, ranging from e‐cigarettes to open and closed system devices to devices with adjustable wattage that are available on the market, adds a level of complexity to each EVALI case. Within each of these different categories of vaping devices, there are multiple combinations of battery size, e‐liquid capacity, variable temperature, and wattage control. Here, we report the presenting symptoms, examination findings, results of infectious studies, and radiological images along with the outcome of seven cases of EVALI in male and female adolescent patients aged 15 to 18 in Northeast Ohio from August to November 2019.
2. METHODS
Between August and November 2019, seven adolescent inpatients hospitalized for management of respiratory distress had reported using vape devices within 30 days before admission. Overall, four of the seven cases met the CDC criteria for EVALI, and the remaining met the CDC criteria for probable EVALI. In a follow‐up, spirometry was performed in all cases. The diffusing capacity of the lungs for carbon monoxide (DLCO) was performed in three of the seven cases. When available, DLCO was corrected for alveolar volume and hemoglobin. Table 1 shows patient demographics, admission, and treatment characteristics. Figure 1A,B shows a representation of the radiological findings.
Table 1.
Patient demographics, admission, and treatment characteristics nasal cannula (NC); high‐flow nasal cannula (HFNC), continuous positive airway pressure (CPAP)
| Patient characteristics, n = 7 | |
|---|---|
| Median age | 17 y (15‐18 y) |
| Race | 100% Caucasian |
| Sex | |
| Male | 4 (57%) |
| Female | 3 (43%) |
| Confirmed EVALI cases | 4 (57%) |
| Probable EVALI cases | 3 (43%) |
| Median length of stay | 6.5 d (1‐10 d) |
| Median length of PICU admission | 5 d (3‐7 d) |
| Patients requiring respiratory support | 5 (71%) |
| Median length of respiratory support | 8 d (5‐10 d) |
| Type of respiratory support | |
| NC | 5 (71%) |
| HFNC | 4 (57%) |
| CPAP | 1 (14%) |
| Patients treated with systemic steroids | 3 (42%) |
| Patients presenting with GI symptoms | 4 (57%) |
| Median CRP level | 34.9 mg/dL (0.2‐42.9 mg/dL) |
| Median postdischarge % predicted FEV1 | 105% |
Abbreviations: CRP, C‐reactive protein; EVALI, e‐cigarette or vaping product use‐associated lung injury; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; PICU, pediatric intensive care unit.
Figure 1.
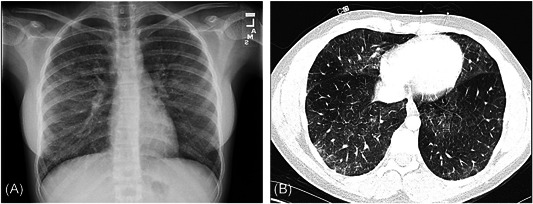
Examples of radiographic findings in two of our patients showing bilateral interstitial opacities. A, Anteroposterior radiograph; B, axial pulmonary CT angiography. CT, computed tomography
3. CASE REPORTS
3.1. Case 1
A 15‐year‐old female with a past medical history of anxiety, depression, and attention deficit hyperactivity disorder (ADHD) presented to her primary care physician with a history of 4 days of nausea, vomiting, diarrhea, and mild intermittent dry cough. Upon inquiry, the patient reported vaping for the past 6 months, two to three times per week, about five “hits” during each vape session. However, she denied vaping for 1 week before the onset of symptoms. She reported using both open and closed system devices. She reported vaping nicotine pods and using an open system device to vape a THC‐containing product. Her vital signs were significant for heart rate (HR) of 116, respiratory rate (RR) of 34, oxygen saturation (SpO2) of 78%, and temperature of 100.5 Fahrenheit (F). She was admitted to the pediatric nursing unit on 3 L of supplemental oxygen via nasal cannula (NC). Initial chest X‐ray (CXR) showed findings of bilateral interstitial reticular opacities with trace bilateral pleural effusions. A Chest CT scan showed dependent lung consolidation and bilateral ground‐glass opacities with intralobular septal thickening along with small bilateral pleural effusions. C‐reactive protein (CRP) was noted to be highly elevated at 42 mg/dL. Immediately after admission, her respiratory status deteriorated. She was transferred to the pediatric intensive care unit (PICU) and was placed on 30 liters per minute (LPM) with a fraction of inspired oxygen (FiO2) of 93% high‐flow nasal cannula (HFNC). She was treated empirically with intravenous (IV) ceftriaxone and azithromycin for 48 hours. On day 2 of her admission, her respiratory support required further escalation to continuous positive airway pressure (CPAP) of 8 cmH2O and FiO2 of 60% due to progression in dyspnea and hypoxia, and IV methylprednisolone was added to her treatment regimen. Her infectious workup, including respiratory viral panel (RVP), legionella urine antigen, HIV antibodies, and fungitell assay for 1,3 D‐B‐glucan, blood culture urine culture, and stool culture were negative. She remained on CPAP for 4 days before transitioning back to NC and finally to room air before discharge. The methylprednisolone was switched to oral prednisone and slowly tapered over 9 days. Postdischarge spirometry was nonobstructive with a forced expiratory volume over 1 second/forced vital capacity (FEV1/FVC) of 96%; however, DLCO was mildly reduced (72% predicted, also 79% when corrected for alveolar volume, 72% when corrected for hemoglobin). Per CDC guidelines, this case is defined as a confirmed case of EVALI.
3.2. Case 2
A 17‐year‐old male with a past medical history of depression presented to the emergency department (ED) with 3 days of nausea, cough, chest pain, and dyspnea. The patient reported a 4‐year history of daily vaping of nicotine pods and a 1‐week history of THC‐containing product use via an open system device. He stated that symptoms started directly after THC vaping. He was noted to be tachypneic on the exam and unable to speak in full sentences. His vital signs were significant for HR 110, RR 32, SpO2 88%, and temperature of 101.3 F. A chest CT scan showed diffuse peripheral and basilar airspace disease. He continued to be tachypneic with hypoxemia on 3 LPM oxygen via NC. He was admitted to the PICU and was started on 25 LPM at FiO2 of 45% HFNC with the improvement of his pulse oximetry. Ceftriaxone, azithromycin, and IV methylprednisolone were started on day 1 of admission. However, ceftriaxone was discontinued after 3 days. CRP was noted to be highly elevated at 39 mg/dL. Infectious workup, including RVP, mycoplasma antibodies, HIV antibodies, streptococcus pneumonia antigen, legionella urinary antigen, and blood culture, were all negative. He was gradually weaned off HFNC, and on day 8 of admission was placed on NC. He completed a 5‐day course of oral azithromycin and prednisone and was discharged home on room air on day 10. His postdischarge spirometry was nonobstructive with FEV1/FVC of 100%, but DLCO was moderately reduced (64% predicted uncorrected; 70% when corrected for alveolar volume, 69% when corrected for hemoglobin). Per CDC guidelines, this case is defined as a confirmed case of EVALI.
3.3. Case 3
A 17‐year‐old male with a history of hypertension presented to the ED with 4 days of nausea, vomiting, cough, fever, and dyspnea. The patient reported 2 years of daily vaping of nicotine pods in a closed system device as recently as the day of presentation to the ED. However, he denied using any THC products. On exam, he was noted to be tachypneic with crackles noted in the right lower lobe. His vital signs were significant for HR 111, RR 38, and SpO2 85%. He was started on 3 L of oxygen via NC, which was quickly advanced to 30 LPM FiO2 45% HFNC due to continued hypoxemia. His CXR showed bilateral confluent and patchy airspace opacities. He was admitted directly to the PICU and was started on IV ceftriaxone and azithromycin for 5 days. CRP was noted to be highly elevated at 43 mg/dL. RVP was shown to be positive for rhino/enterovirus. He was weaned from HFNC to NC on day 2 of admission. He was gradually weaned off supplemental oxygen and discharged home on room air on day 6. No steroids were prescribed during his hospitalization. Spirometry on the day of discharge was nonobstructive with FEV1/FVC of 100%. He will follow up in the pediatric pulmonary clinic for spirometry and DLCO testing. Per CDC guidelines, this case is defined as a probable case of EVALI.
3.4. Case 4
A 15‐year‐old male with a history of anxiety and ADHD presented to the ED with dyspnea in the setting of cough and intermittent fevers for 5 days. Upon further inquiry, the patient reported a 3‐month history of vaping nicotine pods and THC‐containing products via an open system device up to the day before the onset of symptoms. He was noted to be tachypneic and appeared anxious. His vital signs were significant for HR 91, RR 36, SpO2 88%, and temperature of 98.6 F. He was placed on 2 LPM oxygen via NC on the ED with ongoing tachypnea and anxiety. He was admitted to the PICU and started on 25 LPM at 50% FiO2 HFNC with an improvement in respiratory status. CXR showed bilateral opacities consolidated in the bases. CRP was noted to be elevated at 28 mg/dL. RVP was negative, but acute mycoplasma lower respiratory tract infection was suspected based on the serology. He was started on oral azithromycin for 5 days. He was weaned off HFNC to NC on day 5 of admission and finally to room air before discharge on day 7. Postdischarge spirometry showed mild airway obstruction with FEV1/FVC of 75%. DLCO carried out on this patient was also mildly reduced (75% predicted uncorrected; 72% when corrected for alveolar volume). DLCO was not corrected for hemoglobin for this patient. Per CDC guidelines, this case is defined as a probable case of EVALI.
3.5. Case 5
A 17‐year‐old female with a past medical history of gastroesophageal reflux disease and anxiety presented to the ED with a history of fever, cough, nausea, vomiting, and abdominal pain of 5 days duration. She was tachycardic and exhibited bilateral costovertebral angle, and right lower quadrant tenderness on exam. Her vital signs were significant for HR 120, RR 18, SpO2 94%, and temperature of 99 F. The patient reported several months of daily use of a THC vape pen device up to 1 week before the onset of illness. An abdomen and pelvis CT scan was performed in the ED. No intra‐abdominal pathology was identified; however, lung bases displayed bilateral interstitial changes. A CXR was performed, which confirmed interstitial and airspace opacities bilaterally, most notably in the left lower lobe. She was admitted to the pediatric inpatient floor and was started on IV ceftriaxone and azithromycin. CRP was elevated at 35 mg/dL. As her RVP was negative, and she remained afebrile, both antibiotics were discontinued on day 1 of admission. She was started on 60 mg of oral prednisone once a day and continued to improve on steroids alone with no further respiratory symptoms. She did not require any supplemental oxygen during her admission. She completed a 5‐day course of oral prednisone and was discharged home. In follow‐up at the pediatric pulmonary clinic, her symptoms had resolved. She had a suboptimal technique during spirometry maneuvers, and we were unable to interpret the study. She will return for further spirometry and DLCO at her next follow‐up. Per CDC guidelines, this case is defined as a confirmed case of EVALI.
3.6. Case 6
A 16‐ year‐old female with a past medical history of intermittent asthma, irritable bowel syndrome, and anxiety presented to the ED with 2 days of chest pain, fever, and dyspnea. The patient reported a 1‐year history of daily vaping of nicotine pods and a THC‐containing product via an open system device. Her vital signs were significant for HR 132, RR 20, and SpO2 99%. CRP was noted to be elevated at 35 mg/dL. IV ceftriaxone was started on admission. Extensive workup, including blood cultures, urine cultures, RVP, cytomegalovirus, Epstein‐Barr virus, HIV, Bartonella, legionella urine PCR, syphilis, creatinine kinase, ferritin, electrocardiogram, and echocardiogram showed normal findings. Chest CT scan revealed bilateral diffuse interstitial infiltrates, and CXR showed diffuse fine reticular interstitial opacities. Despite the lack of hypoxemia, the patient requested oxygen via NC for relief of dyspnea and was placed on 0.5 LPM. A flexible fiberoptic bronchoscopy was performed, which showed no evidence of tracheobronchitis such as edema or erythema of airway mucosa. Bronchoalveolar lavage was negative for bacterial culture, pneumocystis jirovecii by quantitative PCR, and acid‐fast bacilli. She was gradually weaned off supplemental oxygen as symptoms of dyspnea and chest pain resolved and discharged home on day 8. In a follow‐up, spirometry carried out 2 weeks postdischarge was nonobstructive with an FEV1/FVC of 95%. Per CDC guidelines, this case is defined as a confirmed case of EVALI.
3.7. Case 7
An 18‐year‐old previously healthy male presented to ED with 4 days of productive cough and dyspnea. His vital signs were significant for HR 84, RR 18, SpO2 98%, and temperature of 100.4 F. The patient reported a 1‐year history of daily vaping of nicotine pods and a THC‐containing product via an open system device. Rapid flu and respiratory syncytial virus were negative. A chest CT scan showed peribronchial thickening, centrilobular nodules, and tree‐in‐bud pattern noted bilaterally. The patient had stable vital signs throughout the ED stay and did not require any supplemental oxygen. He was discharged on oral doxycycline for 10 days. The patient was ultimately lost to follow‐up. In this patient, no infectious cause was found, however, the workup was not as robust as the previous cases. As such, we have classed this case as a probable EVALI case per CDC guidelines.
4. DISCUSSION
We have reported seven adolescent patients with recent vaping history who presented with respiratory distress and/or failure along with gastrointestinal and constitutional symptoms whom we diagnosed with EVALI per CDC criteria. 14 All of our patients had bilateral opacities and interstitial disease on chest X‐ray, which was redemonstrated on those who underwent chest CT. The median length of stay was 6.5 days, and all seven were discharged home on room air.
Of those that have had a follow‐up with a pediatric pulmonologist, spirometry has been documented as nonobstructive with the median postdischarge percent predicted FEV1 at 105%. However, three of the patients also underwent DLCO at postdischarge pulmonary follow‐up, which showed diminished DLCO. The long‐term follow‐up of EVALI patients is not well documented owing to the recent nature of EVALI itself. Persistent airflow obstruction was described in one EVALI case from Canada, but no DLCO was carried out on this patient. 16 Another group reported two adult cases with reduced DLCO, but normal lung volumes and nonobstructive pattern in spirometry. 17 The other study showed low DCLO in five of the six patients that had abnormal pulmonary function test. 10 Taken together, these reports, along with our data, highlight the importance of close follow‐up following EVALI. Spirometry and DLCO should be carried out to adequately follow any potential sequelae that may be present in the absence of overt respiratory symptoms.
The presentation of our seven patients mirrored those reported in case reports nationwide. 10 , 12 , 18 Both respiratory failure and an exaggerated inflammatory response seem to be the cornerstones of the EVALI syndrome, which are both evident in our cases. 19 , 20 Our patient population had a high prevalence of ADHD, anxiety, and depression as comorbid conditions. These conditions have been long associated with increased rates of substance abuse among adolescents. 21 , 22 As our patient population is small, further studies are required to investigate this apparent common thread.
Little is known about the exact pathophysiology of this condition. To date, the symptom presentation and laboratory findings, including histopathologic patterns of EVALI are largely nonspecific. Some authors have suggested staining for lipid‐laden macrophages as a diagnostic test for EVALI in BAL samples but the frequency of detection has varied from case to case. 23 , 25 It is also noted that in adolescents, lipid‐laden macrophages have been found in numerous lung pathologies ranging from cystic fibrosis to bronchiolitis obliterans. 26 Lipoid pneumonia was a popular thought initially in the vaping epidemic and EVALI crisis that was not supported by additional pathology reports. Notable classic findings such as areas of low attenuation on CT imaging and macrophages with coarse intracytoplasmic vacuoles on histology, along with the involvement of the interstitium by lipid vacuoles surrounded by the foreign body‐type giant cells have been absent in multiple case reports, including those where lung biopsy was performed. 25 , 27 In addition, vitamin E acetate was identified in 48 of 51 BAL samples from EVALI patients from 16 states, 13 , 15 which is the first detection of a chemical of concern in biological samples from the primary site of injury. The detection of vitamin E acetate in these BAL samples provides a potential target for investigation in those patients who require bronchoscopy during workup. Interestingly, a mouse model of inhaled vitamin E acetate also showed evidence of lung injuries, such as lung epithelial damage and increased lipid‐laden macrophages. 28
With the rapid expansion of the e‐cigarette and vaping market including the multitude of youth‐friendly flavors such as watermelon, strawberry milk, cherry, mint, strawberry lemonade, 29 chocolate, vanilla, coffee, and cookies, pediatricians must be vigilant in gathering history about the use of vaping products during all office visits with adolescents. From the 12‐year‐old well‐child visit onward, adolescents should not only be asked about their personal use of vaping and e‐cigarettes but also about friends' and classmates' use of these products. Pediatricians should also inquire if the adolescent has any questions about vaping or e‐cigarettes, and stress the dangers associated with vaping. Parents should be educated on different types of vaping devices. The most popular has been designed to look like a USB flash drive and is rechargeable via a USB port. This design enables users to use their e‐cigarette covertly. 5
Similarly, hospitalists and pediatric pulmonologists must keep EVALI in the list of differential diagnoses when assessing any adolescent presenting not only with respiratory distress but gastrointestinal and constitutional symptoms. As such, thorough and detailed vaping history in all adolescent admissions is essential. Vaping devices are more popular as they are cheap and easy to obtain without regulation, which has allowed the EVALI epidemic to unfold rapidly. Open system vaping devices have made the addition of illicit substances easy and increased the likelihood of experimentation among adolescents.
5. CONCLUSION
Our report highlights the clinical and radiological presentation of the EVALI on seven adolescent patients in Northeast Ohio. This patient population is especially vulnerable to EVALI as vaping becomes more and more popular and easily accessible among today's youth. Pediatricians' must be vigilant to the dangers of vaping, and their promotion of awareness of EVALI is vital. Our small patient population had a high prevalence of ADHD, anxiety, and depression as comorbid conditions. These patients may represent a group within the adolescent patient population for which EVALI is potentially higher risk. These patients may benefit from a further study on the best tactics for reducing overall substance abuse, including vaping, given the danger posed by EVALI. Pediatricians must keep EVALI in their list of differential diagnoses when evaluating any unwell adolescent patient presenting to their office. The initial presenting symptoms of EVALI may not be solely respiratory in nature, as seen in several of our patients. Post‐EVALI follow‐up is equally as important. We recommend close follow‐up with a pediatric pulmonologist, including spirometry and DLCO following discharge, to monitor the recovery process of this relatively new entity. We have seen abnormalities in DLCO at the initial follow‐up appointment of three of our adolescent patients despite the resolution of symptoms. This suggests that following EVALI, special attention should be paid to the pulmonary function testing to recognize any long‐term sequelae. Our report, while highlighting important clinical aspects of EVALI, is limited by a small number of cases, lack of uniformity in the infectious workup, and lack of uniform follow‐up with pulmonary function testing amongst our patients. Additional studies and longitudinal observations will be needed to determine the extent of the clinical outcome and effect on pulmonary function.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENT
This study was supported by the National Heart, Lung, and Blood Institute—R01‐HL‐148057 (FR).
Corcoran A, Carl JC, Rezaee F. The importance of anti‐vaping vigilance—EVALI in seven adolescent pediatric patients in Northeast Ohio. Pediatric Pulmonology. 2020;55:1719–1724. 10.1002/ppul.24872
Abstract submitted to the Pediatric Academic Societies Meeting 2020 and Region V Academic Pediatric Association Meeting 2020.
REFERENCES
- 1. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e‐cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3‐iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E‐cigarette market trends in traditional U.S. Retail channels, 2012‐2013. Nicotine Tob Res. 2015;17(10):1279‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cullen KA, Liu ST, Bernat JK, et al. Flavored tobacco product use among middle and high school students — United States, 2014–2018. Morb Mortal Wkly Rep. 2019;68(39):839‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nardone N, Helen GS, Addo N, Meighan S, Benowitz NL. Juul electronic cigarettes: nicotine exposure and the user experience. Drug Alcohol Depend. 2019;203:83‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and Juul. Pediatrics. 2019;143(6):e20182741. [DOI] [PubMed] [Google Scholar]
- 6. Abeles M, Popofsky S, Wen A, et al. Vaping‐associated lung injury caused by inhalation of cannabis oil. Pediatr Pulmonol. 2020;55(1):226‐228. [DOI] [PubMed] [Google Scholar]
- 7. Trivers KF, Phillips E, Gentzke AS, Tynan MA, Neff LJ. Prevalence of cannabis use in electronic cigarettes among us youth. Jama Pediatr. 2018;172(11):1097‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e‐cigarette use. Pediatrics. 2018;141:e20163927. [DOI] [PubMed] [Google Scholar]
- 9. Ali M, Khan K, Buch M, et al. A case series of vaping‐induced lung injury in a community hospital setting. Case Rep Pulmonol. 2020;2020:9631916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short‐term outcomes of lung injury associated with e‐cigarettes or vaping: a prospective observational cohort study. Lancet. 2019;394(10214):2073‐2083. [DOI] [PubMed] [Google Scholar]
- 11. Sakla NM, Gattu R, Singh G, Sadler M. Vaping‐associated acute respiratory distress syndrome. Emerg Radiol. 2020;27(1):103‐106. [DOI] [PubMed] [Google Scholar]
- 12. Schier JG, Meiman JG, Layden J, et al. Severe pulmonary disease associated with electronic‐cigarette‐product use ‐ interim guidance. Morb Mortal Wkly Rep. 2019;68(36):787‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Outbreak of lung injury associated with the use of e‐cigarette, or vaping, products. 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed February 25, 2020.
- 14. For healthcare providers | electronic cigarettes | smoking & tobacco use | CDC. 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/healthcare-providers/index.html#what-is-new. Accessed December 4, 2019.
- 15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin e acetate in bronchoalveolar‐lavage fluid associated with evali. N Engl J Med. 2020;382(8):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landman ST, Dhaliwal I, Mackenzie CA, Martinu T, Steele A, Bosma KJ. Life‐threatening bronchiolitis related to electronic cigarette use in a canadian youth. CMAJ. 2019;191(48):E1321‐E1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmad M, Aftab G, Rehman S, Frenia D. Long‐term impact of e‐cigarette and vaping product use‐associated lung injury on diffusing capacity for carbon monoxide values: A case series. Cureus. 2020;12(2):e7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Triantafyllou GA, Tiberio PJ, Zou RH, et al. Vaping‐associated acute lung injury: a case series. Am J Respir Crit Care Med. 2019;200(11):1430‐1431. [DOI] [PubMed] [Google Scholar]
- 19. Artunduaga M, Rao D, Friedman J, et al. Pediatric chest radiographic and ct findings of electronic cigarette or vaping product use‐associated lung injury (EVALI). Radiology. 2020;295:430‐438. [DOI] [PubMed] [Google Scholar]
- 20. Henry TS, Kligerman SJ, Raptis CA, Mann H, Sechrist JW, Kanne JP. Imaging findings of vaping‐associated lung injury. AJR Am J Roentgenol. 2020;214(3):498‐505. [DOI] [PubMed] [Google Scholar]
- 21. Harstad E, Levy S, Committee on Substance A. Attention‐deficit/hyperactivity disorder and substance abuse. Pediatrics. 2014;134(1):e293‐e301. [DOI] [PubMed] [Google Scholar]
- 22. McCauley Ohannessian C. Anxiety and substance use during adolescence. Subst Abus. 2014;35(4):418‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boland JM, Aesif SW. Vaping‐associated lung injury. Am J Clin Pathol. 2020;153(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 24. Larsen BT, Butt YM, Smith ML. More on the pathology of vaping‐associated lung injury. Reply. N Engl J Med. 2020;382(4):388‐390. [DOI] [PubMed] [Google Scholar]
- 25. Mukhopadhyay S, Mehrad M, Dammert P, et al. Lung biopsy findings in severe pulmonary illness associated with e‐cigarette use (vaping). Am J Clin Pathol. 2020;153(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 26. Pambuccian SE. Testing for lipid‐laden macrophages in bronchoalveolar lavage fluid to diagnose vaping‐associated pulmonary injury. Are we there yet? J Am Soc Cytopathol. 2020;9(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 27. Dicpinigaitis PV, Trachuk P, Fakier F, Teka M, Suhrland MJ. Vaping‐associated acute respiratory failure due to acute lipoid pneumonia. Lung. 2020;198(1):31‐33. [DOI] [PubMed] [Google Scholar]
- 28. Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled vitamin e acetate and EVALI‐like lung injury. N Engl J Med. 2020;382(12):1175‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. 2018. https://vapecraftinc.com/e-liquid/120ml-e-liquid.html. Accessed December 4, 2019.


