Abstract
Children with SCT have an increased risk of suboptimal neurodevelopment. Previous studies have shown an elevated risk for neurobehavioral problems in individuals with SCT. However, not much is known about neurobehavioral problems in very young children; knowledge that could help with early identification of children at risk for suboptimal development, and that could help establish targets for early intervention. This study addressed the question of what the behavioral profile of children with SCT aged 1–5 years looks like. In total, 182 children aged 1–5 years participated in this study (NSCT=87, Nnonclinical controls = 95). Recruitment and assessment took place in the Netherlands and the United States. The SCT group was recruited through prospective follow‐up (50%), information seeking parents (31%), and clinical referral (18%). Behavioral profiles were assessed with the child behavior checklist and the ages‐and‐stages social–emotional questionnaire.
Levels of parent‐rated problem behavior were higher in children with SCT. Difficulties with overall social–emotional functioning were already present in 1‐year‐olds, and elevated scores were persistent across the full age range. Affective and pervasive developmental behaviors were seen in late toddlerhood and prominent at preschool age. Anxiety, attention deficit, and oppositional defiant behaviors were seen in preschool‐aged children. Within this cross‐sectional study, the developmental trajectory of affective, pervasive developmental, and oppositional defiant behaviors seemed to be different for SCT children than nonclinical controls.
Collectively, these results demonstrate the importance of behavioral screening for behavioral problems in routine clinical care for children with SCT from a young age. Social–emotional problems may require special attention, as these problems seem most prominent, showing increased risk across the full age range, and with these problems occurring regardless of the timing of diagnosis, and across all three SCT karyotypes.
Keywords: behavioral development, behavioral problems, developmental impact, psychopathology, sex chromosome trisomy
1. INTRODUCTION
Sex chromosome trisomy (SCT; the presence of an extra X or Y chromosome) is one of the most common chromosomal duplications in humans, with an estimated prevalence from 1–650 to 1–1,000 live births (Bojesen, Juul, & Gravholt, 2003; Groth, Skakkebaek, Høst, Gravholt, & Bojesen, 2013; Morris, Alberman, Scott, & Jacobs, 2008). Children with SCT have an increased risk of suboptimal neurodevelopment, including problems with language development, social cognition, and executive functioning (for a review see Urbanus, van Rijn, & Swaab, 2020). An increased risk for neurodevelopmental disorders, such as autism spectrum disorder (ASD), and attention deficit hyperactivity disorder (ADHD) has been described in all subtypes of SCT (for a review see van Rijn, 2019). Although there is overlap in developmental phenotypes, some behavioral and emotional difficulties are found to be more common for specific karyotypes. Examples include high levels of anxiety in girls and boys with an extra X chromosome (Tartaglia, Howell, Sutherland, Wilson, & Wilson, 2010; Verri, Cremante, Clerici, Destefani, & Radicioni, 2010), and high levels of impulsivity and externalizing behavior in boys with an extra Y chromosome (Hong & Reiss, 2014).
Most studies on the impact of SCT on neurodevelopment have been conducted in school‐aged children, adolescents, and adults, and have shown that individuals with SCT have an elevated risk for serious behavioral dysfunctions. It is likely that early signs of these behavioral challenges emerge when children are younger. However, we have very little knowledge about the behavioral profile of young children with SCT and the impact of SCT on the neurodevelopment of toddlers and preschoolers. For that reason, this study aimed to describe the behavioral profile of children with SCT in a very early developmental stage.
It should be noted that while studies generally indicate increased risk for behavioral problems in SCT, it has also been indicated that the behavioral profile of individuals with SCT is highly variable (e.g., Ross et al., 2012; Samango‐Sprouse, Stapleton, Sadeghin, & Gropman, 2013; Tartaglia, Cordeiro, Howell, Wilson, & Janusz, 2010). Although SCT is associated with risk for behavioral problems and psychopathology, some individuals function without any problems. It is unknown which mechanisms modulate this variability. However, the developing brain could give more insight on when psychopathology emerges and how it unfolds (Andersen, 2003), and possibly the maturation of the brain could help explain the observed variability of outcomes in individuals with SCT.
It is also important to gain more knowledge about the behavioral profile, and possible early presentation of behavioral problems in young children with SCT, to allow for development of age‐specific screening (e.g., to identify children who are at risk for more serious neurodevelopmental disorders as early in life as possible), and for development of treatment recommendations (i.e., identifying targets for intervention and preventive support). Knowledge about the early behavioral profile of children with SCT can help reduce the risk of behavioral dysfunction later in life for children who are at risk for developing psychopathology.
Taken together, this study aimed to describe the social–emotional and behavioral profile of children aged 1 to 5 years with SCT. Since these early stages of childhood are characterized by substantial developmental changes in the brain, we expect high variability within this age group. For that reason, we will not merely focus on mean group findings, but also aim to describe the variability within this age group with risk assessment (i.e., how many of the children scored within borderline or clinical ranges). Our focus will be on age‐related presentation of the behavioral phenotype, to evaluate if the developmental impact can be found within this window of 1–5 years. Moreover, we were also interested to see if there is the stability of symptoms over time within this age range. Secondary to these research questions, differences in behavior problems between children with SCT and nonclinical controls were compared by karyotypes (XXX vs. XX, XXY vs. XY, XYY vs. XY). In addition, since problem behavior might be associated with the reason for the detection of the SCT, behavioral outcomes were compared between pre‐ and postnatally identified children, and the role of ascertainment was assessed.
2. METHOD
2.1. Participants
The present study is part of a larger ongoing project (the TRIXY Early Childhood Study ‐ Leiden the Netherlands), which includes children with SCT and nonclinical controls aged 1–7 years. The TRIXY Early Childhood Study is a longitudinal study that aims to identify neurodevelopmental risk in young children with an extra X or Y chromosome. For this study, only children aged 1 up to and including 5 years were included.
In total, 182 children participated in this study, 87 children with SCT and 95 nonclinical age‐matched children from the typical population. Ages ranged from 11 months to 5 years and 11 months (see Table 1 for mean ages per karyotype). Of the 87 children with SCT, 60 children received a prenatal diagnosis (i.e., because of [routine] prenatal screening or advanced maternal age). Of the 27 children who received a postnatal diagnosis, 13 received the diagnosis because of a developmental delay, 12 because of physical and/or growth problems, and 2 because of medical concerns.
TABLE 1.
Mean ages per karyotype
| XXY | XXX | XYY | XY | XX | |
|---|---|---|---|---|---|
| N | 40 | 28 | 19 | 40 | 55 |
| Mean age in months (SD) | 33.48 (17.05) | 45.89 (18.74) | 37.47 (19.87) | 42.28 (18.32) | 42.38 (18.86) |
Recruitment and assessment took place on two sites: The Trisomy of the X and Y chromosomes (TRIXY) Expert Center the Netherlands, and the eXtraordinarY Kids Clinic in Developmental Pediatrics at Children's Hospital Colorado in the USA. Children in the SCT group were recruited with the help of clinical genetics departments (from the Netherlands, Colorado, and Belgium), pediatricians, and national advocacy or support groups for individuals with SCT with recruitment flyers and postings on the internet (e.g., TRIXY website and the eXtraordinarY Kids Facebook page). For the SCT group, ascertainment bias was assessed and three subgroups were identified: (a) “Active prospective follow‐up,” which included families who were actively followed after prenatal diagnosis (50% of the SCT group), (b) “Information seeking parents,” which included families who were actively looking for more information about SCT without having specific concerns about the behavior of their child (31% of the SCT group), and (c) “Clinically referred cases,” which included families seeking professional help based on specific concerns about their child's development (18% of the SCT group). Nonclinical controls were recruited from the western part of the Netherlands. Schools and daycare centers received information brochures that were distributed to parents with children of eligible age. Parents who were interested in participating contacted the researchers.
For all participants, inclusion criteria were Dutch or English‐speaking (child and parent). For the SCT group, SCT was defined by trisomy in at least 80% of the cells, which was confirmed in the study by standard karyotyping. Exclusion criteria for all participants included a history of traumatic brain injury, neurological illness, severely impaired hearing or sight, or colorblindness. For ethical reasons, children in the control group were not subjected to genetic screening, as these children were meant to be a representation of the general population. As the prevalence of SCT is ~1 in 1000, the risk of having one or more children with SCT in the control group was considered minimal and acceptable.
For all children, background information such as the presence of a second caregiver and marital status and age of the primary caregiver was assessed. Overall, 95.6% of the parents indicated that their child has a second caregiver, with no significant differences between the SCT and the nonclinical control group χ2 (1, N = 182) = .36, p = .55. Regarding the marital status of the primary caregiver, 92.9% indicated that they were (re)married, registered partners, or living with their partner. Of the remaining parents, 4.4% indicated that they were single and never married, 2.2% indicated that they were single and divorced, and 0.5% indicated that they were widowed. The distribution of marital status was similar for children in the SCT and children in the nonclinical control group χ2 (3, N = 182) = 2.37, p = .50. Finally, the age of the primary caregiver (93% female) ranged from 23 to 50 years. There was a significant difference between the research groups (p < .001); on average, the primary caregivers of the children in the SCT group were older (M = 38.51, SD = 4.71) than the primary caregivers of the children in the nonclinical control group (M = 35.06, SD = 5.18).
2.2. Instruments
2.2.1. Overall social–emotional functioning
Parents completed the age‐appropriate version of the ages‐and‐stages social–emotional questionnaire (ASQ‐SE‐2; Squires, Bricker, & Twombly, 2015). The ASQ‐SE‐2 is a parent‐report screening measure of social and emotional development, and can be used to assess children aged 1 to 72 months. Different forms are used, depending on the age of the child, with the number of questions ranging from 19 to 33. The items on the ASQ‐SE‐2 address seven behavioral constructs: (a) Self‐regulation, (b) compliance, (c) adaptive functioning, (d) autonomy, (e) affect, (f) social‐communication, and (g) interaction. Parents can respond to each item with “rarely or never", “sometimes”, or “most of the time”. In addition, parents can indicate if the behavior is a concern for each item. Answers on the seven constructs add up to a total score, with higher scores indicating increased risk for social–emotional deficits or delays.
2.2.2. Behavioral functioning
Parents were asked to complete the child behavior checklist (CBCL; Achenbach & Ruffle, 2000) for children aged 1–5 years. The CBCL is a standardized measure of behavioral problems and is used to assess competencies and psychopathology. The CBCL contains 100 items, which assess emotional and behavioral problems that occurred in the past 6 months. Parents can answer each item with one of the following answers: (0) not true, (1) somewhat or sometimes true, (2) very true or often true, with higher scores indicating more problems. Answers on the items yield empirical syndrome scales and DSM‐oriented scales. For this study, the DSM‐Oriented scales were used to assess behavioral functioning, since these are based on profiles more than on individual behavioral items. The DSM‐oriented scales consist of five different profiles: (a) Affective problems (as an indication for mood disorders), (b) anxiety problems, (c) pervasive developmental problems (as an indication of disorders on the autism spectrum), (d) attention‐deficit/hyperactivity problems, and (e) oppositional defiant problems. These five scales overlap with the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000)
2.3. Procedure
This study was approved by the Ethical Committee of Leiden University Medical Center, the Netherlands, and the Colorado Multiple Institutional Review Board (COMIRB) in Colorado, USA. After describing the study to the parent(s) of the child, written informed consent according to the declaration of Helsinki was obtained. The primary caregiving parent (93% mother) of the child completed both questionnaires, either in Dutch or in English.
The assessment took place at different sites (Colorado USA, the Netherlands, Belgium). Researchers from Leiden University were responsible for project and data‐management (i.e., training and supervision of researchers, processing, and scoring of data).
2.4. Statistical analyses
2.4.1. Raw scores versus standardized scores
For both measurements, two types of scores were used. First, raw scores were used to compare the children with SCT and the nonclinical controls. As the ASQ‐SE‐2 has different items depending on age, ASQ raw scores were corrected for the maximum possible score (which depended on the form used). Secondly, normed or cutoff scores were used for risk assessment. For the CBCL standardized T‐scores (M = 50, SD = 10) were used, where T < 65 was classified as “nonclinical”, 65 < T < 70 as “borderline”, and T > 70 as “clinical”. For the ASQ‐SE‐2, cutoff scores were used (depending on the form used) where children were categorized as “below risk/below cutoff”, “borderline/monitoring area”, or “at risk/above cutoff”.
2.4.2. Age groups
Participants were divided into age groups; resulting in three groups (a) aged 11–23 months (labeled as the 1‐year‐old group or early toddlerhood; N SCT = 31, N controls = 29), (b) 24–47 months (labeled as the 2–3‐year‐old group, or late toddlerhood; N SCT = 27, N controls = 23), and (c) 48–71months (labeled as the 4–5‐year‐old group, or preschool‐age; N SCT = 29, N controls = 43). With a one‐way analysis of variance (ANOVA), we tested if there were differences between SCT and nonclinical control group within each age group. There were no statistically significant differences, F(1,180) = 1.83, p = .178.
2.4.3. Analyses
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Version 25. Level of significance was set at p ≤ .05, two‐tailed. Multivariate analysis of variance (MANOVA) was used to test for differences, with the ASQ‐SE‐2 and the CBCL‐DSM Scales (affective, anxiety, pervasive developmental, attention deficit, oppositional defiant) as dependent variables and research group and age groups as independent variables. When unequal variance–covariance was indicated (i.e., Box's M test p < .05), Pillai's trace was used to assess the multivariate effect. Significant multivariate effects were then further analyzed with univariate ANOVAs and simple effect analyses to determine the locus of the statistically significant multivariate effect. Risk assessment was done with cross‐tabulation analysis. Post hoc analyses were used to identify significant group effects. Effect sizes were calculated with Cohen's d when applicable.
3. RESULTS
First, we addressed the question what the behavioral profile of children ages 1–5 with SCT looks like. As different behaviors are expected at different ages, the focus is on differences within age groups (SCT vs. nonclinical controls) and between age groups within the SCT group (to assess developmental stability). Lastly, the behavioral profile of boys (with vs. without SCT) and of girls (with vs. without SCT) aged 1–5 years was compared, and the effect of time of diagnosis and ascertainment was assessed.
3.1. Social–emotional functioning and behavioral difficulties: SCT versus nonclinical controls
There was a significant effect of research group on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .262, F(6,175) = 10.37, p < .001, partial ƞ2 = .262.
Univariate ANOVAs for the social–emotional scale and the five DSM scales indicated that on average, children with SCT showed more problems in overall social–emotional functioning, and more behavioral symptoms of affective and pervasive developmental problems compared with the nonclinical control group. Cohen's d effect sizes (see Table 2) indicate moderate to high clinical significance. For the anxiety, attention deficit, and oppositional defiant scales, there was no significant difference in the behavioral symptoms.
TABLE 2.
Behavioral differences SCT versus control groups
| SCT N = 87 | Nonclinical controls N = 95 | p‐value | Cohen's d | |
|---|---|---|---|---|
| ASQ‐SE‐2 a | Mean (SD) | Mean (SD) | ||
| Social–emotional functioning | 11.48 (10.14) | 5.37 (3.79) | < .001 | .80 |
| CBCL DSM scales a | ||||
| Affective | 2.72 (2.13) | 1.49 (1.49) | < .001 | .67 |
| Anxiety | 3.33 (3.32) | 2.52 (2.30) | .053 | .28 |
| Pervasive developmental | 5.05 (4.23) | 2.79 (2.23) | < .001 | .67 |
| Attention deficit | 4.57 (2.72) | 4.05 (2.50) | .179 | .20 |
| Oppositional defiant | 3.53 (3.08) | 3.59 (2.43) | .882 | .02 |
Higher scores denote more problems.
In addition to average outcomes, we were also interested how many of the children in each group scored around or above clinical cutoff. The cross‐tabulation analysis was used for risk assessment; that is, how many of the children in each group scored in the nonclinical, borderline, and clinical range. As the CBCL provides normed scores for children aged 18 months and above, children younger than 18 months were excluded from the cross‐tabulation analyses with CBCL DSM scores.
All children were included in the analysis with ASQ social–emotional scores. The numbers were divided by the total number of participants in each group and shown in Table 3 as percentages per group. Pearson Chi‐square indicates significant group differences for overall social–emotional functioning, and affective problems, anxiety problems, and pervasive developmental problems, indicating differences in distribution between groups (see Figure 1 for a visual representation).
TABLE 3.
Percentages of children at risk for behavioral problems
| Research group | Risk assessment | χ2 significance | |||
|---|---|---|---|---|---|
| ASQ‐SE‐2 a | Below risk | Monitoring area | At risk | ||
| Social–emotional functioning | SCT | 59.8% | 18.4% | 21.8% | <.001 |
| Control | 95.8% | 2.1% | 2.1% | ||
| CBCL DSM Scales a | Nonclinical range T < 65 | Borderline range 65 < T < 70 | Clinical range T > 70 | ||
| Affective | SCT | 88.4% | 4.3% | 7.2% | .018 |
| Control | 98.8% | 1.2% | 0% | ||
| Anxiety | SCT | 84.1% | 1.4% | 14.5% | .019 |
| Control | 95.3% | 2.4% | 2.4% | ||
| Pervasive developmental | SCT | 62.3% | 14.5% | 23.2% | <.001 |
| Control | 94.1% | 3.5% | 2.4% | ||
| Attention deficit | SCT | 95.7% | 0% | 4.3% | .316 |
| Control | 97.6% | 1.2% | 1.2% | ||
| Oppositional defiant | SCT | 85.5% | 7.2% | 7.2% | .189 |
| Control | 94.1% | 2.4% | 3.5% | ||
Higher scores denote more problems.
FIGURE 1.
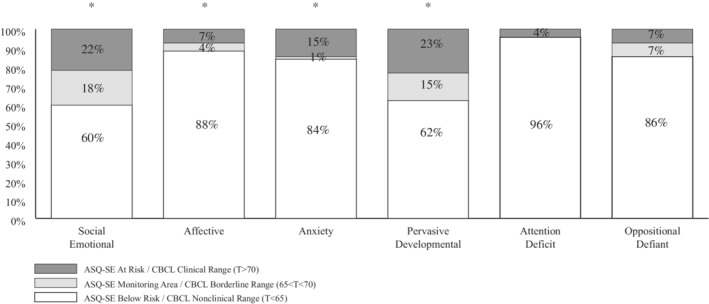
Proportion of children with SCT with ASQ‐SE and CBCL DSM cutoff score in the below risk/nonclinical, monitoring/borderline, and at risk/clinical ranges. *Distribution significantly different when compared to nonclinical controls (p < .05); Nsct DSM scales = 69, Nsct ASQ‐SE = 87
3.2. Social–emotional functioning and behavioral difficulties across ages
Within each age group, differences in the behavioral outcomes between the SCT and nonclinical control group were analyzed with three separate MANOVAs. Descriptive statistics for all MANOVAs can be found in Table 4.
TABLE 4.
Behavioral problems across age groups
| 1‐year olds | 2–3 year‐olds | 4–5 year‐olds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SCTN = 31 | Controls N = 29 | SCT N = 27 | Controls N = 23 | SCT N = 30 | Controls N = 43 | ||||
| ASQ‐SE‐2 a | Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value |
| Social–emotional functioning | 8.74 (4.95) | 5.60 (3.09) | .005 | 11.70 (7.16) | 4.87 (3.51) | <.001 | 14.20 (15.04) | 5.49 (4.37) | .001 |
| CBCL DSM scales a | |||||||||
| Affective | 1.71 (1.37) | 1.28 (1.60) | n.s. | 2.89 (2.23) | 1.26 (1.42) | .004 | 3.66 (2.29) | 1.77 (1.45) | <.001 |
| Anxiety | 1.94 (1.79) | 2.07 (1.71) | n.s. | 3.07 (2.56) | 2.26 (1.86) | n.s. | 5.07 (4.36) | 2.95 (2.77) | .014 |
| Pervasive developmental | 2.19 (2.34) | 1.83 (1.71) | n.s. | 5.37 (3.33) | 2.78 (1.68) | .001 | 7.79 (4.66) | 3.44 (2.58) | <.001 |
| Attention deficit | 3.87 (2.63) | 4.17 (2.27) | n.s. | 4.22 (2.21) | 3.78 (2.35) | n.s. | 5.66 (3.02) | 4.12 (2.76) | .029 |
| Oppositional defiant | 1.65 (1.89) | 3.24 (2.34) | .005 | 3.85 (2.60) | 4.00 (2.11) | n.s. | 5.24 (3.46) | 3.60 (2.67) | .027 |
Abbreviation: n.s., not significant.
Higher scores denote more problems.
3.2.1. 1‐year‐old children: Early toddlerhood
There was a significant effect of research group on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .292, F(6,53) = 3.64, p = .004, partial ƞ2 = .292. Univariate ANOVAs for the social–emotional scale and the five DSM scales indicated significant differences for oppositional defiant behavior and overall social–emotional functioning. On average, children with SCT showed more problems in overall social–emotional functioning than nonclinical controls (see Table 4 for descriptives, and Figure 2). Conversely, for oppositional defiant behavior, children with SCT on average showed fewer problems than nonclinical controls. No significant group differences were found for affective problems, anxiety problems, pervasive developmental problems, and attention deficit problems, indicating that in 1‐year‐olds, children with SCT showed similar amounts of these behaviors to nonclinical controls.
FIGURE 2.
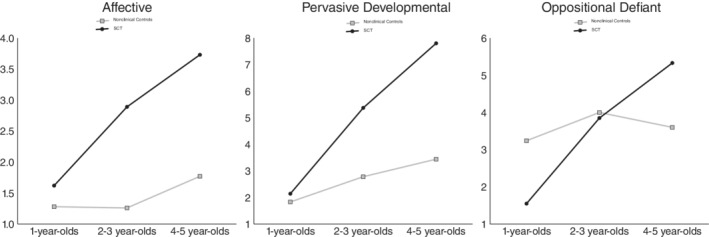
Mean scores for affective problems, pervasive developmental problems, and oppositional defiant problems at different ages: SCT versus nonclinical controls
3.2.2. 2–3‐Year‐old children: Late toddlerhood
There was a significant effect of the research group on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .369, F(6,43) = 4.19, p = .002, partial ƞ2 = .369. Univariate ANOVAs for the social–emotional scale and the five DSM scales indicated significant differences for overall social–emotional functioning, and affective and pervasive developmental problems. On average, children with SCT showed more problems in overall social–emotional functioning, and more behavioral symptoms of affective problems and pervasive developmental problems than nonclinical controls (see Table 4 for descriptives, and Figure 2). No significant group differences were found for anxiety problems, attention deficit problems, or oppositional defiant problems, indicating that in 2–3‐year‐olds, children with SCT group showed similar amounts of these behaviors to nonclinical controls.
3.2.3. 4–5‐year‐old children: Preschool‐age
There was a significant effect of the research group on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .346, F(6,65) = 5.72, p < .001, partial ƞ2 = .346.
Univariate ANOVAs for the social–emotional scale and the five DSM scales indicated significant differences for all scales (see Table 4 for descriptives, and Figure 2). On average, children with SCT showed more problems in overall social–emotional functioning and more, behavioral symptoms of affective problems, anxiety problems, and pervasive developmental problems. In addition, children with SCT also showed more behavioral symptoms of attention‐deficit problems and oppositional defiant problems than nonclinical controls.
3.2.4. Developmental stability
To assess whether there is developmental stability or variability of problem behavior, a MANOVA was used to test for significant differences, with the social–emotional scale and the DSM Scales (affective, anxiety, pervasive developmental, attention deficit, oppositional defiant) as dependent variables and research group and age groups as independent variables. Only the outcomes of the research group × age group interaction will be reported.
There was no significant research group × age group interaction effect on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .111, F(12,344) = 1.69, p = .068, partial ƞ2 = .056. Univariate effects, however, showed significant research group × age group interactions for affective problems (F[2,176] = 3.04, p = .050, partial ƞ2 = .033), pervasive developmental problems (F[2,176] = 7.57, p = .001, partial ƞ2 = .079), and oppositional defiant problems (F[2,176] = 6.38, p = .002, partial ƞ2 = .068). Significant effects were further analyzed with simple effect analyses, relevant means can be found in Table 4.
3.2.5. Affective problems
The statistically significant effect was produced by the 2–3‐year‐old, and the 4–5‐year‐old SCT children, who showed significantly more affective problems than the 2–3‐year‐old, and 4–5‐year‐old nonclinical controls. Conversely, in the 1‐year‐old group, both the SCT children and the nonclinical controls showed similar amounts of affective problems. These results collectively indicate that it is possible that—in this cross‐sectional sample—the developmental trajectory is different for SCT children and nonclinical controls (see Figure 2).
3.2.6. Pervasive developmental problems
The statistically significant effect was produced by the 2–3‐year‐old, and the 4–5‐year‐old SCT children, who showed significantly more pervasive developmental problems than the 2–3‐year‐old, and 4–5‐year‐old nonclinical controls. Conversely, in the 1‐year‐old group, both the SCT children and the nonclinical controls showed similar amounts of pervasive developmental problems. These results collectively indicate that possibly—in this cross‐sectional sample—the developmental trajectory is different for SCT children and nonclinical controls (see Figure 2).
3.2.7. Oppositional defiant problems
The statistically significant effect was produced by the 4–5‐year‐old SCT children, who showed significantly more oppositional defiant problems than the nonclinical controls. Conversely, in the 1‐year‐olds, the children with SCT showed significantly fewer oppositional defiant problems than nonclinical controls. Finally, in the 2–3‐year‐old group, both the SCT children and the nonclinical controls showed similar amounts of oppositional defiant problems. These results collectively indicate that it is possible that—in this cross‐sectional sample—the developmental trajectory is different for SCT children and nonclinical controls (see Figure 2).
3.3. Social–emotional and behavioral differences between groups: Gender/karyotype differences, time of diagnosis, and ascertainment
As we were also interested in the specific behavioral profile of boys and girls, and the individual karyotype group, we compared boys and girls separately (i.e., girls with/without +1X, boys with/without +1X, and boys with/without +1Y). In addition, the effect time of diagnosis and the reason for enrollment (i.e., ascertainment) were assessed separately. It should be noted that the factor age was left out of these analyses; results are shown as averages across the whole age range (1–6 years).
3.3.1. Social–emotional and behavioral differences between gender/karyotype
Three one‐way between‐subjects MANOVA were conducted on six dependent variables (CBCL‐DSM scales; affective, anxiety, pervasive developmental, attention deficit, oppositional defiant, and the ASQ social–emotional scale). The independent variables were Karyotype (XXX, XX), (XXY, XY), and (XYY, XY).
There was a significant effect of karyotypes on behavioral phenotype (social–emotional functioning and behavioral difficulties) (XXX Pillai's trace = .345, F(6,76) = 6.67, p < .001, partial ƞ2 = .345; XXY Pillai's trace = .320, F(6,73) = 5.72, p < .001, partial ƞ2 = .320; XYY Pillai's trace = .351, F(6,52) = 4.69, p = .001, partial ƞ2 = .351). Univariate ANOVAs for the social–emotional scale and the five DSM scales were conducted on each dependent measure separately for each karyotype to determine the locus of the statistically significant multivariate effect. Results are shown in Table 5.
TABLE 5.
Behavioral differences between groups: Gender differences
| XXX N = 29 | XX N = 55 | XXY N = 40 | XY N = 40 | XYY N = 19 | XY N = 40 | ||||
|---|---|---|---|---|---|---|---|---|---|
| ASQ‐SE‐2 a | Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value | Mean (SD) | Mean (SD) | p‐value |
| Social–emotional functioning | 11.00 (9.64) | 5.36 (3.40) | <.001 | 9.56 (6.32) | 5.39 (4.32) | .001 | 16.21 (15.27) | 5.39 (4.32) | <.001 |
| CBCL DSM scales a | |||||||||
| Affective | 2.93 (1.86) | 1.49 (1.35) | <.001 | 2.43 (2.18) | 1.50 (1.70) | .037 | 3.05 (2.39) | 1.50 (1.70) | .006 |
| Anxiety | 4.89 (4.09) | 2.64 (2.45) | .002 | 2.38 (2.15) | 2.35 (2.10) | n.s. | 3.05 (3.42) | 2.35 (2.10) | n.s. |
| Pervasive developmental | 6.18 (3.90) | 2.71 (2.27) | <.001 | 3.85 (3.16) | 2.90 (2.21) | n.s. | 5.89 (5.92) | 2.90 (2.21) | .006 |
| Attention deficit | 5.11 (2.90) | 4.25 (2.53) | n.s. | 3.93 (2.46) | 3.78 (2.47) | n.s. | 5.16 (2.79) | 3.78 (2.47) | n.s. |
| Oppositional defiant | 4.29 (3.09) | 3.51 (2.64) | n.s. | 2.85 (3.03) | 3.70 (2.15) | n.s. | 3.84 (3.01) | 3.70 (2.15) | n.s. |
Abbreviation: n.s., not significant.
Higher scores denote more problems.
3.3.2. Time of diagnosis: Prenatal versus postnatal diagnosis
A one‐way between‐subjects MANOVA was conducted on six dependent variables (CBCL‐DSM scales; affective, anxiety, pervasive developmental, attention deficit, oppositional defiant, and the ASQ social–emotional scale). The independent variable was the time of diagnosis (prenatal, postnatal, controls).
There was a significant effect of time of diagnosis on behavioral phenotype (social–emotional functioning and behavioral difficulties), Pillai's trace = .460, F(12,350) = 8.70, p < .001, partial ƞ2 = .230.
Univariate ANOVAs for the social–emotional scale and the five DSM scales indicated significant differences for all scales except for attention deficit problems, which was not significant. Post‐hoc analyses were used to determine which group differences were significantly different (see Table 6). For overall social–emotional functioning, children with SCT, regardless of the time of diagnosis, showed more problems than controls. For affective problems, pervasive developmental problems, and oppositional defiant problems, children who were diagnosed postnatally showed significantly more of these behavioral problems than children with prenatal diagnosis and controls, with the latter not significantly differing. For anxiety problems, although there was a significant group effect, post hoc analysis failed to reach significance.
TABLE 6.
Differences in behavioral problems: Time of diagnosis
| Prenatal N = 60 | Postnatal N = 27 | Controls N = 95 | p‐value | Post hoc | |
|---|---|---|---|---|---|
| ASQ‐SE‐2 a | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Social–emotional functioning | 9.83 (6.20) | 15.13 (15.26) | 5.37 (3.79) | <.001 | C < pre = post |
| CBCL DSM scales a | |||||
| Affective | 2.12 (1.72) | 4.07 (2.69) | 1.49 (1.49) | < .001 | C = pre < post |
| Anxiety | 2.78 (2.69) | 4.56 (4.21) | 2.52 (2.30) | .004 | n.s. |
| Pervasive developmental | 3.78 (3.30) | 7.85 (4.75) | 2.79 (2.23) | < .001 | C = pre < post |
| Attention deficit | 4.33 (2.69) | 5.11 (2.75) | 4.05 (2.50) | n.s. | n/a |
| Oppositional defiant | 2.78 (2.62) | 5.11 (3.42) | 3.59 (2.43) | .001 | C = pre < post |
Abbreviations: c, nonclinical controls; n.s., not significant; pre, prenatal diagnosis of SCT; post, postnatal diagnosis of SCT.
Higher scores denote more problems.
3.3.3. Ascertainment bias
Within the SCT group, we tested for differences on the behavioral outcomes between the three ascertainment groups with MANOVA. There were no significant differences for the behavioral outcomes (see Table 7); how children enrolled in the study did not appear to affect the data on behavioral outcomes.
TABLE 7.
Differences in behavioral profiles across ascertainment groups
| Prospective follow‐up N = 44 | Information seeking parents N = 27 | Clinically referred cases N = 16 | p‐value | |
|---|---|---|---|---|
| ASQ‐SE‐2 a | Mean (SD) | Mean (SD) | Mean (SD) | |
| Social–emotional functioning | 10.70 (10.96) | 11.60 (10.38) | 13.40 (7.28) | .682 |
| CBCL DSM scales a | ||||
| Affective | 2.36 (2.18) | 2.74 (1.87) | 3.69 (2.21) | .137 |
| Anxiety | 2.98 (2.81) | 3.81 (4.04) | 3.50 (3.39) | .571 |
| Pervasive developmental | 4.18 (4.33) | 5.78 (4.15) | 6.19 (3.78) | .173 |
| Attention deficit | 4.61 (2.70) | 4.30 (3.06) | 4.94 (2.24) | .726 |
| Oppositional defiant | 3.39 (3.32) | 3.26 (3.11) | 4.38 (2.25) | .507 |
Higher scores denote more problems.
4. DISCUSSION
This study aimed to describe the early behavioral profile of toddlers and preschoolers with SCT, and more specifically to identify if the presentation of the behavioral phenotype is age‐dependent in a large group of children with SCT aged 1–5 years. First, we addressed the question whether behavioral problems could already be found in very young children; between the ages of 1–5 years. Results indicated that children with SCT showed more problems with overall social–emotional functioning, and more behavioral symptoms of affective and pervasive developmental problems than children without SCT. Effect sizes indicated moderate to high clinical significance, indicating that these behaviors are important to monitor during development. When we look at risk assessment, much variability within the SCT group was found, with some children showing no (behavioral) problems, and other children showing (behavioral) problems at a clinical level. Overall, the majority of children with SCT scored within the nonclinical range on the CBCL and ASQ‐SE‐2 (Table 3). However, there were significantly more children in the SCT group than the control group in the borderline or clinical range for overall social–emotional functioning, and for affective, anxiety, and pervasive developmental behavioral problems, with overall social–emotional functioning and pervasive developmental behaviors seeming to be affected the most. These findings are in concordance with results of similar studies evaluating categorical results of behavioral findings such as Ross et al. (2012), and Tartaglia, Cordeiro, et al. (2010). In sum, these results show that in some children with SCT differences in overall social–emotional functioning can be identified even at a very young age (as early as in 1‐year‐old children) and that when problems are present they are highest in the domains of affective and pervasive developmental behaviors.
Key to our research question, we further explored the question whether differences in behavioral problems between children with and without SCT were age‐dependent. Already in 1‐year olds, there were significant differences between the SCT and control group in overall social–emotional functioning; children with SCT showed more difficulties with overall social–emotional functioning than the nonclinical controls. Oppositional defiant problems, however, were less frequent in the SCT group compared to the control group. In the 2–3‐year‐old group, the children with SCT also showed more problems in overall social–emotional functioning, in addition to more affective and pervasive developmental problems. Finally, in the 4–5 year‐olds, the children with SCT showed more problems across all domains. Taken together, these results show that already in toddlerhood, children with SCT are at risk for suboptimal behavioral development, and this risk increases and expands across behavioral domains as children get older. From a developmental perspective, it is possible that a subset of challenging behaviors will not emerge until later in development, depending on brain maturation. For example, in our study, only the 4–5 year‐olds with SCT showed increased levels of ADHD symptoms, which fits with ADHD typically being diagnosed later in development (i.e., around 7–9 years; Kessler et al., 2007), when attentional expectations increase. These findings deserve additional study with a longitudinal study design, and with consideration of other factors that may contribute to behavioral differences, such as cognitive or language skills.
In addition, we addressed the question whether there was developmental stability or variability of problem behavior; that is, is the developmental path—within this cross‐sectional sample—the same in the SCT group as it is in the control group. Results indicated that there was developmental variability for affective behavior, pervasive developmental behavior, and oppositional defiant behavior. Although children with SCT did not differ from nonclinical controls (or in the case of oppositional defiant behavior, showed even fewer problems) in early toddlerhood, children with SCT showed more problem behaviors in late toddlerhood and preschool age. While this is a cross‐sectional sample, these findings suggest that the developmental path may be different for controls and children with SCT, and that the impact of behavior problems between children with and without SCT increases as children get older. It should also be noted that for example overall social–emotional functioning did not show this developmental variability, but a more stable development, which fits with our other findings that children with SCT scored differently than controls on all ages; problems with overall social–emotional functioning are persistent over time.
When exploring differences of each karyotype compared with sex‐matched controls, results showed that social–emotional and affective domains were higher for all SCTkaryotypes. However, anxiety symptoms were more significant only in the XXX group, and pervasive developmental problems only in XXX and XYY. This pattern is interesting and consistent with previous studies evaluating ASD symptoms in older male children with SCT, where males with XYY have been shown to have a higher risk for pervasive developmental and autism symptoms compared to XXY (Cordeiro, Tartaglia, Roeltgen, & Ross, 2012; Ross et al., 2012; Tartaglia et al., 2017). Further, anxiety symptoms and anxiety disorders are recognized as risks in XXX in later childhood and adulthood (Freilinger et al., 2018; van Rijn & Swaab, 2015; Wigby et al., 2016), and these findings suggest symptoms of anxiety may be detected in some very young girls with XXX, which gives promise for early detection and intervention opportunities. Pervasive developmental and autism symptoms have also been identified in other older cohorts with XXX (Bishop et al., 2011; van Rijn et al., 2014), and further study of the prevalence and profile of clinical autism diagnosed is needed for girls with XXX.
When we look at the time of diagnosis, it appears that even children with a prenatal diagnosis on average display more difficulties with overall social–emotional functioning than controls; indicating that difficulties with social–emotional functioning can be very persistent. In addition, children with a postnatal diagnosis, often show more behavior problems compared with both controls and prenatally diagnosed children with SCT. This has been shown consistently in other studies (Bardsley et al., 2013; Bishop et al., 2011; Samango‐Sprouse et al., 2018), and is very important in counseling families with a prenatal diagnosis. This finding is not surprising, as a postnatal diagnosis is often made because of behavioral and/or physical problems. In addition, it is possible that parents who receive the diagnosis before birth are more aware of the possibilities of (behavioral) outcomes, and for that reason possibly already participate in interventions and preventive support, such as psycho‐education or behavioral interventions at a young age. These outcomes stress the need for early identification and monitoring, and for more comprehensive evaluation of the longitudinal behavioral profiles in a prenatally identified cohort.
Lastly, we looked at ascertainment bias, and found no significant differences between the prospective follow‐up group, information seeking parents group, or clinically referred cases group. It is important to note however, that bias within the research sample will always be present. Although it is expected that more individuals will be diagnosed with the introduction of less invasive methods during pregnancy (Samango‐Sprouse, Keen, Sadeghin, & Gropman, 2017), two decades ago, only around 25% of individuals with SCT was diagnosed (Abramsky & Chapple, 1997). As noninvasive prenatal screening is not part of routine screening in all countries, the percentage of individuals who will be diagnosed is variable, and results of the research will not be generalizable to all individuals with SCT. However, it is possible to generalize our results to children who are diagnosed with SCT.
This study has both strengths and limitations. One of the limitations of this study is its design, with a cross‐sectional rather than a longitudinal perspective. It is important that future studies will follow children over time, to monitor the behavioral pattern across ages. It should be noted, however, that (to our knowledge) this is one of the first studies to research the behavioral profile of very young children with SCT. In addition, with our relatively large sample size, we were able to look for behavioral differences at specific ages (i.e., early toddlerhood, late toddlerhood, preschool age); our results highlight the importance of early identification of children at risk, and show that already when a child is 1‐year‐old problem behaviors, especially with overall social–emotional functioning, can occur. Future research could focus on neurocognitive and environmental factors (e.g., SES and services received) that could serve as risk‐ or protective factors in the development of behavior, as there is a complex relation between genetics, environmental factors and neuro(behavioral) development (Karmiloff‐Smith, 2009).
Social–emotional and behavioral problems have been negatively associated with a child's daily functioning. Social competence, school performance, and peer acceptance, for example, can be affected because a child experiences behavioral problems (de Lijster et al., 2019). The presence of behavioral problems during early childhood could be predictive of later psychopathology and severity of behavioral problems at a later age (Goodwin, Fergusson, & Horwood, 2004; Ormel et al., 2015; Roza, Hofstra, van der Ende, & Verhulst, 2003). Even though both the CBCL and the ASQ‐SE‐2 are screening instruments rather than diagnostic evaluations, results on these screeners clearly demonstrate higher risks for psychopathology for some children with SCT, and the need for early monitoring and implementation of intervention, especially in the domain of social–emotional functioning.
In conclusion, our findings give some important implications for clinical care. First of all, with the broad behavioral phenotype, it is important to include behavioral screening in routine clinical care for children with SCT, and to monitor the developmental trajectory. Difficulties with social–emotional development seem most prominent, as there is an increased risk already when children are 1‐year‐old, and elevated scores were persistent across the full 1–5 year age range, regardless of time of diagnosis, and across all three karyotypes. While each child with SCT is different, our results suggest a pattern of affective and pervasive developmental problems emerging in the late‐toddler stage, and finally anxiety, attention deficits, and oppositional defiant problems emerging during the preschool years. It is important to monitor the behavioral development closely, with a focus on these specific domains on specific ages, so interventions and preventive support can be administered as early as possible, to optimize outcomes. Routine screenings should be done from an early age onwards, as behaviors can already be clinically relevant from a very young age, and without early assessment, opportunities for early intervention could be missed. In addition, it is important that parents who receive the diagnosis are aware of the wide variability of outcomes, and receive psycho‐education on the possible behavioral problems, in particular affective problems, pervasive developmental problems, and social–emotional development, as our results show that these difficulties already arise at a very young age, and problems possibly could intensify over time. Knowledge about these early neurobehavioral risks should ideally fuel implementation of early interventions and psycho‐education, optimizing outcomes of children with SCT.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by a grant from the Dutch Organization for Scientific Research (NWO funding # 016.165.397 to S. R.). Work in Colorado was partially supported by infrastructure of NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors' sole responsibility and do not necessarily represent official NIH views. The authors thank the families that participated in our study, and the research assistants and students for their help with data collection and processing.
Urbanus E, Swaab H, Tartaglia N, Cordeiro L, van Rijn S. The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). Am J Med Genet Part C. 2020;184C:444–455. 10.1002/ajmg.c.31788
REFERENCES
- Abramsky, L. , & Chapple, J. (1997). 47,XXY (Klinefelter syndrome) and 47,XYY: Estimated rates of inidcation for postnatal diagnosis with implications for prenatal counselling. Prenatal Diagnosis, 17(4), 363–368. [DOI] [PubMed] [Google Scholar]
- Achenbach, T. , & Ruffle, T. (2000). The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review, 21, 265–271. 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Andersen, S. L. (2003). Trajectories of brain development: Point of vulnerability of window of opportunity? Neuroscience and Biobehavioral Reviews, 27, 3–18. 10.1016/S0149-7634(03)00005-8 [DOI] [PubMed] [Google Scholar]
- Bardsley, M. Z. , Kowal, K. , Levy, C. , Gosek, A. , Ayari, N. , Tartaglia, N. , … Ross, J. L. (2013). 47,XYY syndrome: Clinical phenotype and timing of ascertainment. The Journal of Pediatrics, 163(4), 1085–1094. 10.1016/j.jpeds.2013.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D. V. M. , Jacobs, P. A. , Lachlan, K. , Wellesley, D. , Barnicoat, A. , Boyd, P. A. , … Scerif, G. (2011). Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood, 96(10), 954–959. 10.1136/adc.2009.179747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen, A. , Juul, S. , & Gravholt, C. H. (2003). Prenatal and postnatal prevelance of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology & Metabolism, 88(2), 622–626. 10.1210/jc.2002-021491 [DOI] [PubMed] [Google Scholar]
- Cordeiro, L. , Tartaglia, N. , Roeltgen, D. , & Ross, J. (2012). Social deficits in male children and adolescents with sex chromosome aneuploidy: A comparison of XXY, XYY, and XXYY syndromes. Research in Developmental Disabilities, 33(4), 1254–1263. 10.1016/j.ridd.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lijster, J. M. , van den Dries, M. A. , van der Ende, J. , Utens, E. , Jaddoe, V. W. , Dieleman, G. C. , … Legerstee, J. S. (2019). Developmental trajectories of anxiety and depression symptoms from early to middle childhood: A population‐based cohort study in The Netherlands. Journal of Abnormal Child Psychology, 47(11), 1785–1798. 10.1007/s10802-019-00550-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilinger, P. , Kliegel, D. , Hanig, S. , Oehl‐Jaschkowitz, B. , Henn, W. , & Meyer, J. (2018). Behavioral and psychological features in girls and women with triple‐X syndrome. American Journal of Medical Genetics Part A, 176(11), 2284–2291. 10.1002/ajmg.a.40477 [DOI] [PubMed] [Google Scholar]
- Goodwin, R. D. , Fergusson, D. M. , & Horwood, L. J. (2004). Early anxious/withdrawn behaviours predict later internalising disorders. Journal of Child Psychology and Psychiatry, 45(4), 874–883. 10.1111/j.1469-7610.2004.00279.x [DOI] [PubMed] [Google Scholar]
- Groth, K. A. , Skakkebaek, A. , Høst, C. , Gravholt, C. H. , & Bojesen, A. (2013). Klinefelter syndrome–A clinical update. The Journal of Clinical Endocrinology & Metabolism, 98(1), 20–30. 10.1210/jc.2012-2382 [DOI] [PubMed] [Google Scholar]
- Hong, D. S. , & Reiss, A. L. (2014). Cognitive and neurological aspects of sex chromosome aneuploidies. The Lancet Neurology, 13(3), 306–318. 10.1016/s1474-4422(13)70302-8 [DOI] [PubMed] [Google Scholar]
- Karmiloff‐Smith, A. (2009). Nativism versus neuroconstructivism: Rethinking the study of developmental disorders. Developmental Psychology, 45(1), 56–63. 10.1037/a0014506 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Amminger, G. P. , Aguilar‐Gaxiola, S. , Alonso, J. , Lee, S. , & Üstün, T. B. (2007). Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry, 20(4), 359–364. 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. K. , Alberman, E. , Scott, C. , & Jacobs, P. (2008). Is the prevalence of Klinefelter syndrome increasing? European Journal of Human Genetics, 16, 163–170. 10.1038/sj.ejhg.5201956 [DOI] [PubMed] [Google Scholar]
- Ormel, J. , Raven, D. , van Oort, F. , Hartman, C. A. , Reijneveld, S. A. , Veenstra, R. , … Oldehinkel, A. J. (2015). Mental health in Dutch adolescents: A TRAILS report on prevalence, severity, age of onset, continuity and co‐morbidity of DSM disorders. Psychological Medicine, 45(2), 345–360. 10.1017/S0033291714001469 [DOI] [PubMed] [Google Scholar]
- Ross, J. L. , Roeltgen, D. P. , Kushner, H. , Zinn, A. R. , Reiss, A. , Bardsley, M. Z. , … Tartaglia, N. (2012). Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. 10.1542/peds.2011-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza, S. J. , Hofstra, M. B. , van der Ende, J. , & Verhulst, F. C. (2003). Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: A 14‐year follow‐up during childhood, adolescence, and young adulthood. American Journal of Psychiatry, 160, 2116–2121. 10.1176/appi.ajp.160.12.2116 [DOI] [PubMed] [Google Scholar]
- Samango‐Sprouse, C. A. , Keen, C. , Sadeghin, T. , & Gropman, A. L. (2017). The benefits and limitations of cell‐free DNA screening for 47,XXY (Klinefelter syndrome). Prenatal Diagnosis, 37(5), 497–501. 10.1002/pd.5044 [DOI] [PubMed] [Google Scholar]
- Samango‐Sprouse, C. A. , Stapleton, E. , Chea, S. , Lawson, P. , Sadeghin, T. , Cappello, C. , … van Rijn, S. (2018). International investigation of neurocognitive and behavioral phenotype in 47,XXY (Klinefelter syndrome): Predicting individual differences. American Journal of Medical Genetics Part A, 176(4), 877–885. 10.1002/ajmg.a.38621 [DOI] [PubMed] [Google Scholar]
- Samango‐Sprouse, C. A. , Stapleton, E. , Sadeghin, T. , & Gropman, A. L. (2013). Is it all the X: Familial learning dysfunction and the impact of behavioral aspects of the phenotypic presentation of XXY? American Journal of Medical Genetics Part C (Seminars in Medical Genetics), 163(C), 27–34. 10.1002/ajmc.31353 [DOI] [PubMed] [Google Scholar]
- Squires, J. , Bricker, D. , & Twombly, E. (2015). Ages & stages questionnaires®: Social‐emotional, second edition (ASQ®:SE‐2): A parent‐completed child monitoring system for social‐emotional behaviors. Baltimore: Paul H. Brookes Publishing Co., Inc. [Google Scholar]
- Tartaglia, N. R. , Cordeiro, L. , Howell, S. , Wilson, R. , & Janusz, J. (2010). The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews, 8, 151–159. [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, N. R. , Howell, S. , Sutherland, A. , Wilson, R. , & Wilson, L. (2010). A review of trisomy X (47,XXX). Orphanet Journal of Rare Diseases, 5, 8 10.1186/1750-1172-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia, N. R. , Wilson, R. , Miller, J. S. , Rafalko, J. , Cordeiro, L. , Davis, S. , … Ross, J. (2017). Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics, 38(3), 197–207. 10.1097/dbp.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus, E. , van Rijn, S. , & Swaab, H. (2020). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics, 97(1), 156–167. 10.1111/cge.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn, S. (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47,XYY). Current Opinion in Psychiatry, 32(2), 79–84. 10.1097/yco.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn, S. , Stockmann, L. , Borghgraef, M. , Bruining, H. , van Ravenswaaij‐Arts, C. , Govaerts, L. , … Swaab, H. (2014). The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and trisomy X): A comparison with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(2), 310–320. 10.1007/s10803-013-1860-5 [DOI] [PubMed] [Google Scholar]
- van Rijn, S. , & Swaab, H. (2015). Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes, Brain, and Behavior, 14(2), 200–208. 10.1111/gbb.12203 [DOI] [PubMed] [Google Scholar]
- Verri, A. , Cremante, A. , Clerici, F. , Destefani, V. , & Radicioni, A. (2010). Klinefelter's syndrome and psychoneurologic function. Molecular Human Reproduction, 16(6), 425–433. 10.1093/molehr/gaq018 [DOI] [PubMed] [Google Scholar]
- Wigby, K. , D'Epagnier, C. , Howell, S. , Reicks, A. , Wilson, R. , Cordeiro, L. , & Tartaglia, N. (2016). Expanding the phenotype of triple X syndrome: A comparison of prenatal versus postnatal diagnosis. American Journal of Medical Genetics Part A, 170(11), 2870–2881. 10.1002/ajmg.a.37688 [DOI] [PMC free article] [PubMed] [Google Scholar]


