Abstract
Fruit flies (Drosophila and its close relatives, or “drosophilids”) are a group that includes an important model organism, Drosophila melanogaster, and also very diverse species distributed worldwide. Many of these species have black or brown pigmentation patterns on their wings, and have been used as material for evo‐devo research. Pigmentation patterns are thought to have evolved rapidly compared with body plans or body shapes; hence they are advantageous model systems for studying evolutionary gains of traits and parallel evolution. Various groups of drosophilids, including genus Idiomyia (Hawaiian Drosophila), have a variety of pigmentations, ranging from simple black pigmentations around crossveins to a single antero‐distal spot and a more complex mottled pattern. Pigmentation patterns are sometimes obviously used for sexual displays; however, in some cases they may have other functions. The process of wing formation in Drosophila, the general mechanism of pigmentation formation, and the transport of substances necessary for pigmentation, including melanin precursors, through wing veins are summarized here. Lastly, the evolution of the expression of genes regulating pigmentation patterns, the role of cis‐regulatory regions, and the conditions required for the evolutionary emergence of pigmentation patterns are discussed. Future prospects for research on the evolution of wing pigmentation pattern formation in drosophilids are presented, particularly from the point of view of how they compare with other studies of the evolution of new traits.
Keywords: color pattern, Drosophila biarmipes, Drosophila guttifera, fruit fly, gene regulatory network
Fruit flies (Drosophila and its close relatives, or “drosophilids”) are a group that includes an important model organism, Drosophila melanogaster, and also very diverse species distributed worldwide. Many of these species have black or brown pigmentation patterns on their wings, and have been used as a material for evo‐devo research. The diversity, physiological regulation and cis‐regulatory evolution of Drosophila were summarized, and future prospects for research on the evolution of wing pigmentation pattern formation in drosophilids are presented.

1. INTRODUCTION
In evolutionary developmental biology, how patterning evolves is one of the major questions. The color pattern is easy to perceive visually, and we can trace evolutionary shifts or diversification of color patterns from their phenotypes. Moreover, because color patterns are developed in two dimensions, their measurement and analysis are straightforward and highly reproducible. Fruit flies of the genus Drosophila and their relatives (drosophilids) have been used as model systems, using which we can compare the mechanisms of pigmentation pattern formation among diverse species. Studies on various pigmentation patterns and powerful developmental genetic technologies have provided perspectives for elucidating mechanisms by which organismal phenotypic diversity is generated. The purpose of this review is to outline research on the evolution of Drosophila pigmentation patterns, including their diversity and physiological regulation, and the roles of cis‐regulatory evolution.
2. PIGMENTATION PATTERN DIVERSITY OF DROSOPHILIDS
Over 4,000 species in 72 genera have been described in family Drosophilidae, order Diptera (Toda, 2020; Yassin, 2013). There are two subfamilies, Drosophilinae and Steganinae, the former of which is larger and more extensively studied, and both of which have species with various wing pigmentations. Among the subfamily Drosophilinae, the largest but paraphyletic genus Drosophila includes more than 1,160 species (O'Grady & DeSalle, 2018a; Toda, 2020; Figure 1). Many species of Drosophilinae have patterns on their wings (Figure 2), although the most extensively used species in biological studies, Drosophila melanogaster, has no such wing pigmentation pattern (Figure 2a). In many species, pigmentation patterns are often related to veins, and with a few exceptions, vein patterns are fairly similar among species (Figure 2b).
Figure 1.
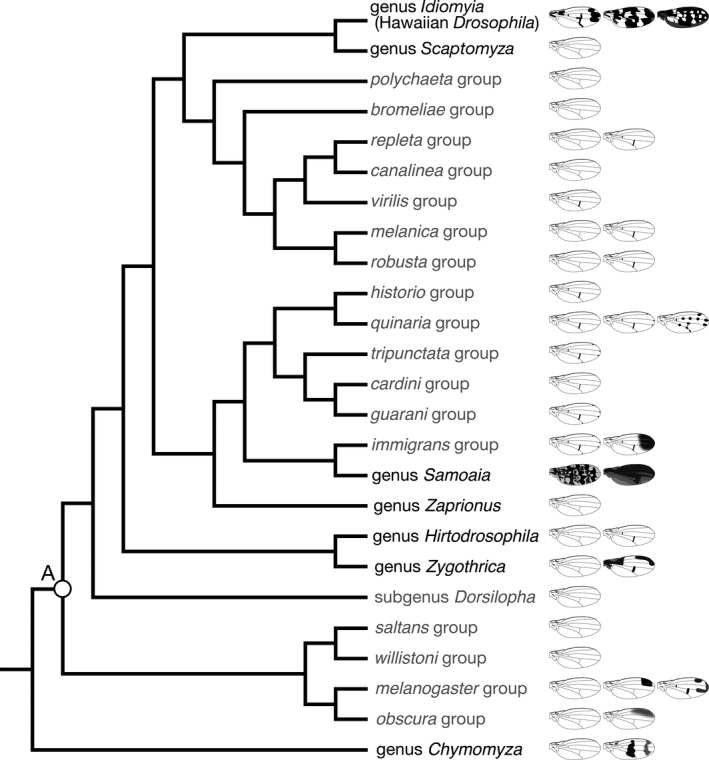
The phylogenetic relationships of representative genera, subgenera and species groups in subfamily Drosophilinae. The genus Drosophila is a paraphyletic group, and thus contains many other genera in the clade. Gray letters indicate subgenera and species groups that belong to the genus Drosophila. Wing illustrations indicate representative (but not all) pigmentation patterns of each operational taxonomic unit. The tree topology is based on Yassin (2013), except for the treatment of Drosophila guttifera (the rightmost illustration in the quinaria group), which is included in the quinaria group in this figure. The white circle (A) corresponds to the timepoint estimated as 62.9 ± 12.4 million years ago (Tamura, Subramanian, & Kumar, 2004)
Figure 2.
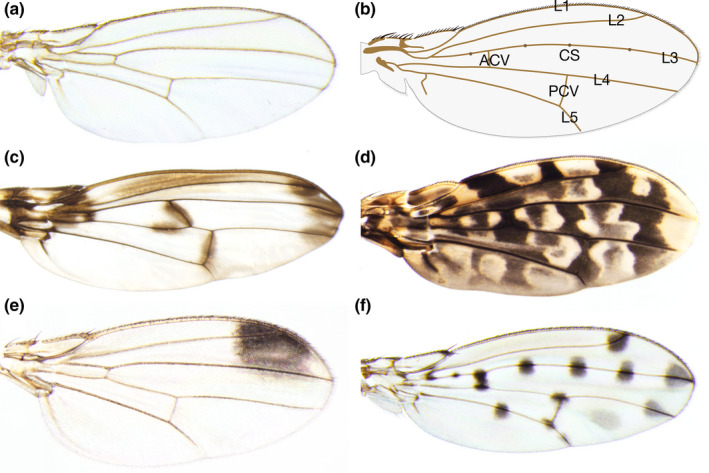
Wing morphology and pigmentation patterns of drosophilids. (a) A wing of Drosophila melanogaster (melanogaster group, subgenus Sophophora). (b) General morphology of a wing in drosophilids. L1: First longitudinal vein, L2: Second longitudinal vein, L3: Third longitudinal vein, L4: Fourth longitudinal vein, L5: Fifth longitudinal vein, ACV: Anterior crossvein, PCV: Posterior crossvein, CS: Campaniform sensilla. (c) A wing of Idiomyia heteroneura (picture wing group), which has pigmentations at the wing base, crossveins, and longitudinal vein tips, and some other locations. The crossvein connecting the middle of L3 and L4 is unique to some species of Idiomyia. This photo is courtesy of Cédric Finet. (d) A wing of Samoaia leonensis, which has a mottled pigmentation pattern. (e) A wing of Drosophila biarmipes (melanogaster group, subgenus Sophophora), which has an antero‐distal pigmentation spot. (f) A wing of Drosophila guttifera (quinaria group, subgenus Drosophila), which has pigmentations around crossveins, longitudinal vein tips, and campaniform sensilla
Among the Drosophilidae flies with wing pigmentation patterns, the most famous are the groups found in Hawaii. There are two major clades of drosophilids in Hawaii, genus Idiomyia (a group that is treated as a subgenus within genus Drosophila by some researchers, while some other researchers treat it as unspecified rank "Hawaiian Drosophila"), which is unique to Hawaii, and genus Scaptomyza, which includes members that are distributed in Hawaii. Notably, Idiomyia includes large‐sized species with various wing pigmentation patterns (Edwards, Doescher, Kaneshiro, & Yamamoto, 2007; O'Grady & DeSalle, 2018b; Figure 2c). Studies of Hawaiian drosophilids have greatly contributed to our understanding of speciation and adaptive radiation (Carson, Hardy, Spieth, & Stone, 1970; Carson & Kaneshiro, 1976; Hardy, 1965; O'Grady & DeSalle, 2018b). There is an ongoing debate about how the drosophilids (genus Idiomyia and genus Scaptomyza) immigrated to Hawaii; especially, there is debate about the number of immigration events from continents (Katoh, Izumitani, Yamashita, & Watada, 2017; Lapoint, O’Grady, & Whiteman, 2013).
Non‐Hawaiian drosophilids also include a large number of species with wing pigmentation (Patterson, 1943; Werner, Steenwinkel, & Jaenike, 2018; Wittkopp, Carroll, & Kopp, 2003), and some distinct groups of such species are briefly described below. A group endemic to the Samoan Islands, genus Samoaia, includes species with entirely black (or dark brown) wings and wings with mottled brown pigmentation (Figure 2d; Malloch, 1934; Wheeler & Kambysellis, 1966). The genus Zygothrica includes species with wing pigmentation in the wing base, the anterior part, crossveins and/or longitudinal vein tips (Grimaldi, 1987). The genus Chymomyza includes species with variously pigmented wings, such as wings with a wide band pattern (Okada, 1976). The genus Drosophila includes many species with unpigmented wings, but also includes many with pigmentation around crossveins and with various wing pigmentation patterns. For example, within the melanogaster species group of the genus Drosophila, species subgroups of elegans, takahashii, rophaloa and suzukii include species with antero‐distal wing pigmentations (Figure 2e, Kopp & True, 2002). The calloptera group includes species with complex wing pigmentation with combinations of pigmented crossveins, longitudinal vein tips and a band pattern (Patterson, 1943; this group was not included in Figure 1). The quinaria group includes species with no wing pigmentation, with only crossvein pigmentation, and with crossvein and longitudinal vein tip pigmentations. D. guttifera has pigmentation around the crossvein, longitudinal vein tips and campaniform sensilla (Figure 2f), and it was originally placed in the independent guttifera group (Sturtevant, 1942). However, it clearly resides in the clade of the quinaria group according to molecular phylogenetic evidence (Chialvo, White, Reed, & Dyer, 2019; Izumitani, Kusaka, Koshikawa, Toda, & Katoh, 2016; Markow & O'Grady, 2006). Comparisons of various groups in Drosophilidae have revealed that evolutionary elaborations of wing pigmentation patterns occurred multiple times in parallel.
3. FUNCTIONAL ASPECTS OF WING PIGMENTATION
The function of animal color patterns have not been clearly elucidated in general, and in particular, the functions of wing pigmentation in drosophilids have not been clearly elucidated. Males of the suzukii, elegans and rhopaloa subgroups (melanogaster group, subgenus Sophophora) with sex‐specific antero‐distal wing pigmentations extend their wings and display them to conspecific females, suggesting that pigmentation contributes to their mating success (Hegde, Chethan, & Krishna, 2005; Massey et al., 2019; Revadi et al., 2015; Setoguchi et al., 2014). The effect of pigmentation on mating success is not always strong, and how strongly pigmentation contributes to this success varies among studies (Fuyama, 1979; Roy & Gleason, 2019; Singh & Chatterjee, 1987; Yeh, Liou, & True, 2006). Hawaiian Idiomyia includes species with sexually dimorphic and monomorphic species, and species in both of these categories perform wing display during their courtships (Spieth, 1966).
Some non‐Hawaiian drosophilids have sexually monomorphic pigmentation, whose function has not been well studied. These pigmentations might have various functions, such as crypsis, aposematism or thermoregulation, but none of these possibilities have been well examined. In two species of Tephritids (Tephritidae, Diptera), wing pigmentation patterns were suggested to have the function of mimicry of jumping spiders, to repel other predatory jumping spiders (Greene, Orsak, & Whitman, 1987; Mather & Roitberg, 1987).
Shevtsova et al. found that some membranous wings of flies and wasps have wing interference patterns, which are particular types of structural color. These are colorful interference patterns in weakly pigmented membranous regions without black or brown color, and they are visible when the background is dark. The authors suggested the possibility that they function in visual communication, such as mating display, taking Drosophila as an example (Shevtsova, Hansson, Janzen, & Kjærandsen, 2011). Further studies will be needed to clarify the functions of color patterns and visual systems of drosophilids.
4. HOW DOES PIGMENTATION OCCUR? PHYSIOLOGICAL ASPECTS
The process of wing formation has been well studied in D. melanogaster (Blair, 2007; Johnson & Milner, 1987). The area within a wing disc called the "pouch", which is formed in the larva, extends and forms a bag‐like pupal wing that consists of two layers of epithelia. The epithelial cells proliferate, become folded, and secrete cuticles to prepare an adult wing. The wing extends after eclosion, forming the adult wing of full size. The epithelial cells detach from the cuticle, undergo apoptosis and are redirected to the body trunk by hemolymph flow (Kimura, Kodama, Hayashi, & Ohta, 2004; Link et al., 2007). These processes of wing formation are considered to be conserved among drosophilids.
The mechanisms of melanin biosynthesis and accumulation in the cuticle have been well studied using the abdominal epidermis of D. melanogaster (Gibert, Mouchel‐Vielh, & Peronnet, 2017), and the mechanisms in the wings are believed to be largely the same as those in the abdomen. One of the most famous body color mutations in D. melanogaster is the yellow mutant (Morgan & Bridges, 1916), which is now known to be due to an abnormality in the gene encoding the Yellow protein involved in melanin synthesis, although the precise molecular function of this protein is still unknown.
One hypothesis proposes that Yellow is an enzyme involved in melanin synthesis (Wittkopp, True, & Carroll, 2002). Another hypothesis is that Yellow protein acts like a hormone or growth factor, an idea based on the fact that yellow mutation acts non‐cell‐autonomously for pigmentation and also affects male mating success, which is reminiscent of the actions of vertebrate pigmentation‐related hormones, such as α‐ melanocyte‐stimulating hormone and adrenocorticotropic hormone (Drapeau, 2003). The predicted enzymatic activity of Yellow as Dopa chrome converting enzyme (DCE) was not detected, and instead proteins belonging to the same protein family, Yellow‐f and Yellow‐f2, were found to have DCE activity (Han et al., 2002). The protein coding sequence of yellow suggested the presence of a signal peptide in the N terminus, and Yellow protein was observed to be extracellularly localized (Geyer, Spana, & Corces, 1986; Wittkopp et al., 2002). Taking all this evidence together, the hypothesis that appears most likely to be correct is that the Yellow protein serves as an anchoring pigment in the cuticle layer (Geyer et al., 1986; Hinaux et al., 2018).
The ebony gene has been identified as the causative gene of a mutant with darker body color. The Ebony protein enzymatically converts dopamine to N‐β‐alanyl dopamine (NBAD), and it thereby suppresses black pigmentation (Wittkopp et al., 2002; Wright, 1987). The tan gene has been identified from a mutation in body color, and Tan protein has been shown to enzymatically convert NBAD into dopamine, the reverse reaction to that of Ebony protein (True et al., 2005). Concerning possible functions in the pigmentation process and inter‐specific differences, these three genes (yellow, ebony and tan) are particularly well‐studied "effector genes" (Jeong et al., 2008; Rebeiz, Pool, Kassner, Aquadro, & Carroll, 2009). Regarding regulation of pigmentation in the abdomen, male‐specific pigmentation was shown to be controlled positively by Abdominal‐B and negatively by bric à brac genes, and pigmentation common to both sexes is positively controlled by optomotor‐blind (Kopp, Duncan, Godt, & Carroll, 2000). Many other genes, such as abdominal‐A, homothorax, and pou domain motif 3, are also known to affect body pigmentation (Dembeck, Huang, Carbone, & Mackay, 2015; Kalay et al., 2016; Kopp, 2009; Massey & Wittkopp, 2016; Mummery‐Widmer et al., 2009; Rogers et al., 2014; Yassin et al., 2016), and those genes might have functions in wings.
The black and brown pigmentation of the Drosophila wing is thought to be caused by the deposition of melanin pigment in the cuticle layer, as in the abdomen. True, Edwards, Yamamoto, and Carroll (1999) examined how pigmentation gene mutations and overexpression alter wing color of Drosophila melanogaster. Also, transport of melanin precursors (especially dopamine) through veins has been suggested to have important roles in pigmentation, based on experimental evidence from wing incubations in medium and wing vein amputations of D. biarmipes and Idiomyia grimshawi. Fukutomi, Matsumoto, Agata, Funayama, and Koshikawa (2017) showed that wing pigmentation of D. guttifera started in stage P11 (among the stages of pupal development defined as P1–P15; Fukutomi, Matsumoto, Funayama, & Koshikawa, 2018), and that the pigmentation process continued even after the epithelial cells detached from the wing and were retrieved by the body trunk in young adults. The importance of hemolymph circulation in veins was shown by vein amputation, a finding that was similar to that from a previous study by True et al. (1999).
5. THE LARGE PIGMENTATION PATCH IN THE ANTERO‐DISTAL PART OF THE WING: THE MECHANISM OF PIGMENTATION PATTERN IN D. biarmipes
Drosophila biarmipes is a frequently used model species of wing pigmentation formation. It belongs to the suzukii subgroup (melanogaster group, subgenus Sophophora), and typical of the species in this subgroup, it has male‐specific antero‐distal pigmentation in its wings. True et al. (1999) showed that addition of DOPA or dopamine can enhance pigmentation development in adult wings just after eclosion. Wittkopp et al. (2002) showed that Yellow protein was localized in the area of pigmentation, and Ebony protein was localized in the area lacking pigmentation, in pupal wings, as indicated by antibody staining.
Gompel, Prud'homme, Wittkopp, Kassner, and Carroll (2005) examined the enhancer activities of the cis‐regulatory region around the yellow gene of D. biarmipes. A DNA fragment of D. biarmipes, which was homologous to an enhancer that weakly drives yellow throughout the wing in D. melanogaster, was introduced into D. melanogaster together with EGFP gene to visualize transcriptional activity. Expression was strongly driven at the antero‐distal part of the wing, the region of D. biarmipes pigmentation. This suggested that the ancestral weak enhancer was elaborated in the lineage of D. biarmipes, in which it gained strong activity in the antero‐distal part of the wing. The absence of yellow expression in the posterior region was explained by repression by Engrailed through its binding to the enhancer region. Distal‐less was identified as a positive regulator of yellow by RNAi screening (Arnoult et al., 2013). In pupal wings of D. biarmipes, Distal‐less was expressed in the antero‐distal region. Ectopic expression of Distal‐less upregulated yellow (as visualized through enhancer activity) and downregulated ebony, and RNAi of Distal‐less caused downregulation of yellow and upregulation of ebony. Based on this evidence, it was concluded that Distal‐less regulates melanin synthesis through regulation of effector genes (Figure 3). Comparisons of these findings with those in related species led to the suggestion that the evolution of the Distal‐less expression pattern has contributed to the diversification of the pigmentation pattern.
Figure 3.
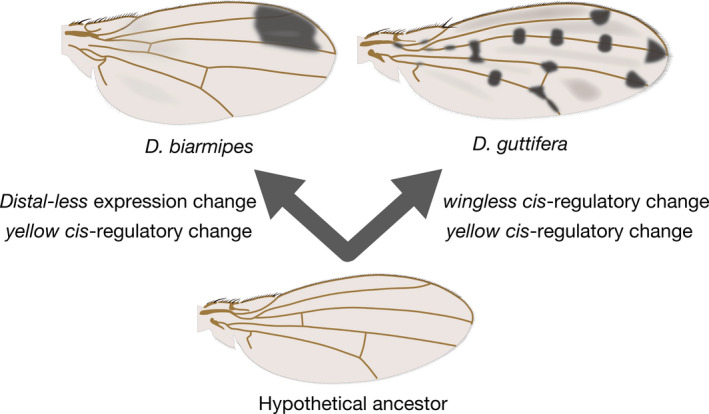
Evolutionary changes that enable pigmentation pattern formation. These events are a part of multiple evolutionary changes, and others await discovery
6. POLKA‐DOT PATTERN WITH MANY BLACK SPOTS: MECHANISM OF PIGMENTATION PATTERN FORMATION OF D. guttifera
D. guttifera belongs to the quinaria group (or to a group very closely related to the quinaria group) of subgenus Drosophila. It has black pigmentation around the crossveins, longitudinal vein tips and campaniform sensilla, making a polka‐dot pattern throughout the wings (Koshikawa, Matsumoto, & Fukutomi, 2017). The Yellow protein was localized in the places of prospective pigmentation spots of pupal wings (Gompel et al., 2005). Werner, Koshikawa, Williams, and Carroll (2010) observed that yellow mRNA was expressed in the polka‐dot pattern in pupal wings. The enhancer which drove the polka‐dot pattern (vein spot CRE) was identified within the cis‐regulatory region of the yellow gene using a transgenic reporter assay. The vein spot CRE that originated from D. guttifera, however, drove the expression in the wing outer margin and around crossveins when introduced to a different host species, D. melanogaster. This suggests that the trans environment is different between D. guttifera and D. melanogaster.
Because the wingless gene was known to be expressed in the wing outer margin and around crossveins in D. melanogaster, it was the first candidate of the putative trans factor giving input to the vein spot CRE. The wingless gene was expressed in centers of prospective pigmentation spots, and ectopic expression of wingless along longitudinal veins induced ectopic pigmentation, which supported the conclusion that wingless is the factor inducing pigmentation in wings (Figure 3; Werner et al., 2010). wingless is a homolog of vertebrate Wnt‐1, and encodes a signal ligand that is secreted from cells and transduces the signal to adjacent cells (Swarup & Verheyen, 2012). Because Wingless proteins were known to diffuse or be transported to a distance of only a few cell diameters in D. melanogaster (with the distance differing depending on the context and the particular study), it was thought that Wingless diffused from the source cells to certain areas and induced pigmentation there (Werner et al., 2010).
7. THE MECHANISM OF COLOR PATTERN EVOLUTION: ROLES OF CIS‐REGULATORY CHANGE AND CONSTRUCTION OF REGULATORY NETWORK
The expression pattern of wingless in D. guttifera was unique compared to its pattern in other species of the quinaria group, and therefore, this pattern was assumed to have evolved after the divergence of D. guttifera from other species. When they searched for enhancers around wingless, Koshikawa et al. (2015) found three different enhancer activities unique to D. guttifera. One drove expression in longitudinal vein tips, another did so in campaniform sensilla on wings, and the other did so in thoracic stripes. Gains of these enhancer activities were considered to be responsible for the evolution of new expression domains of wingless in D. guttifera. (Koshikawa, 2015; Koshikawa et al., 2015). Comparison between D. guttifera and D. melanogaster revealed that the function of the cis‐regulatory region of yellow had also diverged evolutionarily (Werner et al., 2010).
This inter‐specific comparison revealed the cis‐regulatory evolution of at least two genes, the upstream regulatory gene wingless and the downstream effector gene yellow. Prud'homme et al. (2006) compared the cis‐regulatory regions of yellow of D. elegans (elegans subgroup, subgenus Sophophora) and D. tristis (obscura group, subgenus Sophophora), and found that enhancers that drove antero‐distal pigmentations in wings had evolved independently in different species. In D. biarmipes, the function of the yellow regulatory region had evolved (Gompel et al., 2005), and the expression pattern of its regulator Distal‐less had also evolved in comparison with that in D. melanogaster (Arnoult et al., 2013). Thus, it was revealed that there were complex molecular levels of evolution behind the relatively simple‐looking Drosophila wing pigmentations. This series of studies provided examples not only of the evolution of wing pigmentation patterns, but of the evolution of gene expression patterns and biological traits by cis‐regulatory changes (Carroll, 2005, 2008).
8. FUTURE DIRECTION 1: HOW DID PIGMENTATION DIVERSITY OF DROSOPHILA EMERGE?
It is now clear that the Drosophila pigmentation patterns are very diverse, but not much is known about the mechanisms that generate the diversity. First, how many effector genes, such as genes encoding enzymes, are required for pigmentation formation is unknown. Gompel et al. (2005) observed that a D. melanogaster wing with overexpression of the yellow gene in an ebony mutant background showed "slight darkening", but not intense pigmentation. Riedel, Vorkel, and Eaton (2011) also showed that almost no pigmentation was induced by yellow overexpression in wings. These effects were different from that observed on the abdomen, where yellow overexpression induced visible pigmentation and the combination of this overexpression with mutation of ebony resulted in obvious black pigmentation (Wittkopp et al., 2002). Thus, multiple effector genes may need to work in concert to produce pigmentation patterns in wings.
Fukutomi, Kondo, Toyoda, Shigenobu and Koshikawa (2020) screened genes specifically expressed in pigmentation areas of pupal wings of D. guttifera. Analysis of a combination of multiple transcriptome data sets enabled identification of a set of genes expressed in the pigmentation area and also regulated by wingless, and this set is expected to include all of the genes required for pigmentation and expression at this developmental stage. As mentioned in the above section, in addition to genes expressed in wings, precursor(s) of melanin and/or signaling molecules transported through veins might also contribute to the inter‐specific differences of pigmentation.
9. FUTURE DIRECTION 2: HOW DO NEW TRAITS EMERGE?
Regarding the evolution of color patterns, there have been a great number of reports in recent years about how butterfly (Rhopalocera, Lepidoptera) color patterns are controlled and have evolved (Monteiro, 2015). It is now technically possible to identify genes that control color patterns using genomics, and to investigate gene functions by RNAi and genome editing (Kunte et al., 2014; Nishikawa et al., 2015; Zhang & Reed, 2016). Both in Drosophila and in butterflies, the yellow family and melanin synthesis genes contribute to pigmentation formation (Zhang, Martin, et al., 2017). Notably, some of the regulatory genes for color pattern, such as Distal‐less and Wnt ligand genes, have been shown to act both in Drosophila and in butterflies (Arnoult et al., 2013; Connahs et al., 2019; Martin et al., 2012; Martin & Reed, 2014; Mazo‐Vargas et al., 2017; Werner et al., 2010; Zhang & Reed, 2016).
On the other hand, it remains unknown whether or not some genes reported to regulate color patterns in butterflies, such as optix and cortex (Nadeau et al., 2016; Reed et al., 2011; Zhang, Mazo‐Vargas, & Reed, 2017b), have such functions in drosophilids. Based on the fact that drosophilids and butterflies are phylogenetically distant, and they have pigmentation on non‐homologous parts of the wings, i.e., that drosophilids have pigmentation on the membranous part of wings while butterflies mostly have pigmentation on scales, the mechanisms of color pattern formation are considered to have evolved independently in these two groups. Comparison of these independent systems would be fruitful for finding general rules of color pattern formation.
In addition, a number of studies revealed mechanisms of color pattern formation and pigmentation in other insect systems, such as caterpillars (larvae of butterflies), moths, and ladybeetles, and comparisons with them would be valuable (Ando et al., 2018; Futahashi, Banno, & Fujiwara, 2010; Gautier et al., 2018; Suzuki, Koshikawa, Kobayashi, Uchino, & Sezutsu, 2019; van’t Hof et al., 2016; Yamaguchi et al., 2013). Vertebrates have pigment cells specified for pigmentation formation, and these cells are known to migrate during development. Color pattern formation in vertebrates has been extensively studied, focusing on various aspects such as signal exchanges, cell–cell interaction and cell migration (Kaelin et al., 2012; Watanabe & Kondo, 2015). The mechanisms are largely different between vertebrates and insects; however, interesting comparisons would be possible, such as comparisons to examine whether there is a common logic for equidistant placement of pattern elements.
Relative to D. melanogaster, both D. biarmipes and D. guttifera seem to have experienced evolution of multiple genes, including the downstream yellow gene. How the gene expression of multiple genes has evolved in concert during the process of evolution of one particular trait would be a good point of view from which to explore the evolution of novel traits. In particular, some critical questions are how existing gene regulatory networks that specify pre‐patterns and networks of effector genes required for pigmentations acquire new connections, and how many genetic mutations are required for acquiring such connections (Prud'homme, Gompel, & Carroll, 2007; Rebeiz & Tsiantis, 2017). Recently, in addition to the various color patterns discussed above, the genetic basis of evolution of new traits and character states, such as trichome patterns of Drosophila larvae, Drosophila adult terminaria, early development and skeletogenesis of sea urchins, beetle horns, and fans of water striders, have been studied (Gao & Davidson, 2008; Glassford et al., 2015; Moczek & Rose, 2009; Rebeiz & Williams, 2017; Santos, Le Bouquin, Crumière, & Khila, 2017; Stern & Frankel, 2013). Together with these phenomena, Drosophila wing pigmentation will continue to be an attractive system for exploring which regulatory networks need to be modified to enable the evolution of new traits.
ACKNOWLEDGEMENTS
I thank Cédric Finet for a wing image, Yuichi Fukutomi for comments, and Elizabeth Nakajima for English editing. A part of this work was supported by KAKENHI (17K19427, 18H02486) and Yamada Science Foundation.
Koshikawa S. Evolution of wing pigmentation in Drosophila: Diversity, physiological regulation, and cis‐regulatory evolution. Develop Growth Differ. 2020;62:269–278. 10.1111/dgd.12661
Funding information
MEXT/JSPS KAKENHI (17K19427, 18H02486). Yamada Science Foundation.
[The copyright line for this article was changed on 29 June 2020 after original online publication]
REFERENCES
- Ando, T. , Matsuda, T. , Goto, K. , Hara, K. , Ito, A. , Hirata, J. , … Niimi, T. (2018). Repeated inversions within a pannier intron drive diversification of intraspecific colour patterns of ladybird beetles. Nature Communications, 9, 3843 10.1038/s41467-018-06116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult, L. , Su, K. F. , Manoel, D. , Minervino, C. , Magriña, J. , Gompel, N. , & Prud'homme, B. (2013). Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science, 339, 1423–1426. 10.1126/science.1233749 [DOI] [PubMed] [Google Scholar]
- Blair, S. S. (2007). Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annual Review of Cell and Developmental Biology, 23, 293–319. 10.1146/annurev.cellbio.23.090506.123606 [DOI] [PubMed] [Google Scholar]
- Carroll, S. B. (2005). Evolution at two levels: On genes and form. PLoS Biology, 3, e245 10.1371/journal.pbio.0030245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, S. B. (2008). Evo‐devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell, 134, 25–36. 10.1016/j.cell.2008.06.030 [DOI] [PubMed] [Google Scholar]
- Carson, H. L. , Hardy, D. E. , Spieth, H. T. , & Stone, W. S. (1970). The evolutionary biology of the Hawaiian Drosophilidae In Hecht M. K., & Steere W. C. (Eds.), Essays in evolution and genetics in honor of Theodosius Dobzhansky (pp. 437–543). New York, NY: Springer; (Meredith Corporation). 10.1007/978-1-4615-9585-4_15 [DOI] [Google Scholar]
- Carson, H. L. , & Kaneshiro, K. Y. (1976). Drosophila of Hawaii: Systematics and ecological genetics. Annual Review of Ecology and Systematics, 7, 311–345. 10.1146/annurev.es.07.110176.001523 [DOI] [Google Scholar]
- Chialvo, C. H. S. , White, B. E. , Reed, L. K. , & Dyer, K. A. (2019). A phylogenetic examination of host use evolution in the quinaria and testacea groups of Drosophila . Molecular Phylogenetics and Evolution, 130, 233–243. 10.1016/j.ympev.2018.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connahs, H. , Tlili, S. , van Creij, J. , Loo, T. Y. , Banerjee, T. D. , Saunders, T. E. , & Monteiro, A. (2019). Activation of butterfly eyespots by Distal‐less is consistent with a reaction‐diffusion process. Development, 146, dev169367 10.1242/dev.169367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembeck, L. M. , Huang, W. , Carbone, M. A. , & Mackay, T. F. (2015). Genetic basis of natural variation in body pigmentation in Drosophila melanogaster . Fly (Austin), 9, 75–81. 10.1080/19336934.2015.1102807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau, M. D. (2003). A novel hypothesis on the biochemical role of the Drosophila Yellow protein. Biochemical and Biophysical Research Communications, 311, 1–3. 10.1016/j.bbrc.2003.09.106 [DOI] [PubMed] [Google Scholar]
- Edwards, K. A. , Doescher, L. T. , Kaneshiro, K. Y. , & Yamamoto, D. (2007). A database of wing diversity in the Hawaiian Drosophila . PLoS ONE, 2, 10.1371/journal.pone.0000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi, Y. , Kondo, S. , Toyoda, A. , Shigenobu, S. , & Koshikawa, S. (2020). Transcriptome analysis reveals wingless regulates neural development and signaling genes in the region of wing pigmentation of a polka‐dotted fruit fly. bioRxiv, 10.1101/2020.01.09.899864 [DOI] [PubMed] [Google Scholar]
- Fukutomi, Y. , Matsumoto, K. , Agata, K. , Funayama, N. , & Koshikawa, S. (2017). Pupal development and pigmentation process of a polka‐dotted fruit fly, Drosophila guttifera (Insecta, Diptera). Development Genes and Evolution, 227, 171–180. 10.1007/s00427-017-0578-3 [DOI] [PubMed] [Google Scholar]
- Fukutomi, Y. , Matsumoto, K. , Funayama, N. , & Koshikawa, S. (2018). Methods for staging pupal periods and measurement of wing pigmentation of Drosophila guttifera . Journal of Visualized Experiments, 131, e56935 10.3791/56935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futahashi, R. , Banno, Y. , & Fujiwara, H. (2010). Caterpillar color patterns are determined by a two‐phase melanin gene prepatterning process: New evidence from tan and laccase2 . Evolution and Development, 12, 157–167. 10.1111/j.1525-142X.2010.00401.x [DOI] [PubMed] [Google Scholar]
- Fuyama, Y. (1979). A visual stimulus in the courtship of Drosophila suzukii . Experientia, 35, 1327–1328. 10.1007/BF01963987 [DOI] [Google Scholar]
- Gao, F. , & Davidson, E. H. (2008). Transfer of a large gene regulatory apparatus to a new developmental address in echinoid evolution. Proceedings of the National Academy of Sciences of the United States of America, 105, 6091–6096. 10.1073/pnas.0801201105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier, M. , Yamaguchi, J. , Foucaud, J. , Loiseau, A. , Ausset, A. , Facon, B. , … Prud’homme, B. (2018). The genomic basis of color pattern polymorphism in the harlequin ladybird. Current Biology, 28, 3296–3302.e7. 10.1016/j.cub.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer, P. K. , Spana, C. , & Corces, V. G. (1986). On the molecular mechanism of gypsy‐induced mutations at the yellow locus of Drosophila melanogaster . The EMBO Journal, 5, 2657–2662. 10.1002/j.1460-2075.1986.tb04548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert, J. M. , Mouchel‐Vielh, E. , & Peronnet, F. (2017). Modulation of yellow expression contributes to thermal plasticity of female abdominal pigmentation in Drosophila melanogaster . Scientific Reports, 7, 43370 10.1038/srep43370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassford, W. J. , Johnson, W. C. , Dall, N. R. , Smith, S. J. , Liu, Y. , Boll, W. , … Rebeiz, M. (2015). Co‐option of an ancestral Hox‐regulated network underlies a recently evolved morphological novelty. Developmental Cell, 34, 520–531. 10.1016/j.devcel.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompel, N. , Prud'homme, B. , Wittkopp, P. J. , Kassner, V. A. , & Carroll, S. B. (2005). Chance caught on the wing: C is‐regulatory evolution and the origin of pigment patterns in Drosophila . Nature, 433, 481–487. 10.1038/nature03235 [DOI] [PubMed] [Google Scholar]
- Greene, E. , Orsak, L. J. , & Whitman, D. W. (1987). A tephritid fly mimics the territorial displays of its jumping spider predators. Science, 236, 310–312. 10.1126/science.236.4799.310 [DOI] [PubMed] [Google Scholar]
- Grimaldi, D. A. (1987). Phylogenetics and taxonomy of Zygothrica (Diptera: Drosophilidae). Bulletin of the American Museum Natural History, 186, 104–268. [Google Scholar]
- Han, Q. , Fang, J. , Ding, H. , Johnson, J. K. , Christensen, B. M. , & Li, J. (2002). Identification of Drosophila melanogaster yellow‐f and yellow‐f2 proteins as dopachrome‐conversion enzymes. Biochemical Journal, 368, 333–340. 10.1042/bj20020272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, E. D. (1965). Diptera: Cyclorrhapha II. Series schizophora, section acalypterae i, family drosophilidae. Insects of Hawaii (Vol. 12). Honolulu, HI: University of Hawai'i Press. [Google Scholar]
- Hegde, S. N. , Chethan, B. K. , & Krishna, M. S. (2005). Mating success of males with and without wing patch in Drosophila biarmipes. Indian Journal of Experimental Biology, 43, 902–909. [PubMed] [Google Scholar]
- Hinaux, H. , Bachem, K. , Battistara, M. , Rossi, M. , Xin, Y. , Jaenichen, R. , … Gompel, N. (2018). Revisiting the developmental and cellular role of the pigmentation gene yellow in Drosophila using a tagged allele. Developmental Biology, 438, 111–123. 10.1016/j.ydbio.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Izumitani, H. F. , Kusaka, Y. , Koshikawa, S. , Toda, M. J. , & Katoh, T. (2016). Phylogeography of the subgenus Drosophila (Diptera: Drosophilidae): Evolutionary history of faunal divergence between the Old and the New Worlds. PLoS ONE, 11, e0160051 10.1371/journal.pone.0160051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, S. , Rebeiz, M. , Andolfatto, P. , Werner, T. , True, J. , & Carroll, S. B. (2008). The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell, 132, 783–793. 10.1016/j.cell.2008.01.014 [DOI] [PubMed] [Google Scholar]
- Johnson, S. A. , & Milner, M. J. (1987). The final stages of wing development in Drosophila melanogaster . Tissue and Cell, 19, 505–513. 10.1016/0040-8166(87)90044-9 [DOI] [PubMed] [Google Scholar]
- Kaelin, C. B. , Xu, X. , Hong, L. Z. , David, V. A. , McGowan, K. A. , Schmidt‐Kuntzel, A. , … Menotti‐Raymond, M. (2012). Specifying and sustaining pigmentation patterns in domestic and wild cats. Science, 337, 1536–1541. 10.1126/science.1220893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay, G. , Lusk, R. , Dome, M. , Hens, K. , Deplancke, B. , & Wittkopp, P. J. (2016). Potential direct regulators of the Drosophila yellow gene identified by yeast one‐hybrid and RNAi screens. G3: Genes Genomes, Genetics, 6, 3419–3430. 10.1534/g3.116.032607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, T. , Izumitani, H. F. , Yamashita, S. , & Watada, M. (2017). Multiple origins of Hawaiian drosophilids: Phylogeography of Scaptomyza Hardy (Diptera: Drosophilidae). Entomological Science, 20, 33–44. 10.1111/ens.12222 [DOI] [Google Scholar]
- Kimura, K. , Kodama, A. , Hayashi, Y. , & Ohta, T. (2004). Activation of the cAMP/PKA signaling pathway is required for post‐ecdysial cell death in wing epidermal cells of Drosophila melanogaster . Development, 131, 1597–1606. 10.1242/dev.01049 [DOI] [PubMed] [Google Scholar]
- Kopp, A. (2009). Metamodels and phylogenetic replication: A systematic approach to the evolution of developmental pathways. Evolution, 63, 2771–2789. 10.1111/j.1558-5646.2009.00761.x [DOI] [PubMed] [Google Scholar]
- Kopp, A. , Duncan, I. , Godt, D. , & Carroll, S. B. (2000). Genetic control and evolution of sexually dimorphic characters in Drosophila . Nature, 408, 553–559. 10.1038/35046017 [DOI] [PubMed] [Google Scholar]
- Kopp, A. , & True, J. R. (2002). Evolution of male sexual characters in the oriental Drosophila melanogaster species group. Evolution & Development, 4, 278–291. 10.1046/j.1525-142X.2002.02017.x [DOI] [PubMed] [Google Scholar]
- Koshikawa, S. (2015). Enhancer modularity and the evolution of new traits. Fly (Austin), 9, 155–159. 10.1080/19336934.2016.1151129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa, S. , Giorgianni, M. W. , Vaccaro, K. , Kassner, V. A. , Yoder, J. H. , Werner, T. , & Carroll, S. B. (2015). Gain of cis‐regulatory activities underlies novel domains of wingless gene expression in Drosophila . Proceedings of the National Academy of Sciences of the United States of America, 112, 7524–7529. 10.1073/pnas.1509022112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa, S. , Matsumoto, K. , & Fukutomi, Y. (2017).Drosophila guttifera as a model system for unraveling color pattern formation In Sekimura T. & Nijhout H. F. (Eds.), Diversity and evolution of butterfly wing patterns (pp. 287–301). Singapore: Springer; 10.1007/978-981-10-4956-9_16 [DOI] [Google Scholar]
- Kunte, K. , Zhang, W. , Tenger‐Trolander, A. , Palmer, D. H. , Martin, A. , Reed, R. D. , … Kronforst, M. R. (2014). doublesex is a mimicry supergene. Nature, 507, 229–232. [DOI] [PubMed] [Google Scholar]
- Lapoint, R. T. , O’Grady, P. M. , & Whiteman, N. K. (2013). Diversification and dispersal of the Hawaiian Drosophilidae: The evolution of Scaptomyza . Molecular Phylogenetics and Evolution, 69, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, N. , Chen, P. O. , Lu, W.‐J. , Pogue, K. , Chuong, A. , Mata, M. , … Abrams, J. M. (2007). A collective form of cell death requires homeodomain interacting protein kinase. Journal of Cell Biology, 178, 567–574. 10.1083/jcb.200702125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloch, J. R. (1934). Part VI. Diptera. Drosophilidae. Insects of Samoa and other Samoan terrestrial Arthropoda, British Museum (Natural History). Pp. 267–312. [Google Scholar]
- Markow, T. A. , & O’Grady, P. (2006). Drosophila: A guide to species identification and use. New York, NY: Academic Press. [Google Scholar]
- Martin, A. , Papa, R. , Nadeau, N. J. , Hill, R. I. , Counterman, B. A. , Halder, G. , … Reed, R. D. (2012). Diversification of complex butterfly wing patterns by repeated regulatory evolution of a Wnt ligand. Proceedings of the National Academy of Sciences of the United States of America, 109, 12632–12637. 10.1073/pnas.1204800109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. , & Reed, R. D. (2014). Wnt signaling underlies evolution and development of the butterfly wing pattern symmetry systems. Developmental Biology, 395, 367–378. 10.1016/j.ydbio.2014.08.031 [DOI] [PubMed] [Google Scholar]
- Massey, J. H. , Gavin, R. , Rice, G. R. , Firdaus, A. , Chen, C. Y. , Yeh, S. D. , Wittkopp, P. J. (2019). Co‐evolving wing spots and mating displays are genetically separable traits in Drosophila. bioRxiv, 10.1101/869016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey, J. H. , & Wittkopp, P. J. (2016). The genetic basis of pigmentation differences within and between Drosophila species. Current Topics in Developmental Biology, 119, 27–61. 10.1016/bs.ctdb.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather, M. H. , & Roitberg, B. D. (1987). A sheep in wolf's clothing: Tephritid flies mimic spider predators. Science, 236, 308–310. 10.1126/science.236.4799.308 [DOI] [PubMed] [Google Scholar]
- Mazo‐Vargas, A. , Concha, C. , Livraghi, L. , Massardo, D. , Wallbank, R. W. R. , Zhang, L. , … Martin, A. (2017). Macroevolutionary shifts of WntA function potentiate butterfly wing‐pattern diversity. Proceedings of the National Academy of Sciences of the United States of America, 114, 10701–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczek, A. P. , & Rose, D. J. (2009). Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proceedings of the National Academy of Sciences of the United States of America, 106, 8992–8997. 10.1073/pnas.0809668106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, A. (2015). Origin, development, and evolution of butterfly eyespots. Annual Review of Entomology, 60, 253–271. 10.1146/annurev-ento-010814-020942 [DOI] [PubMed] [Google Scholar]
- Morgan, T. H. , & Bridges, C. B. (1916). Sex‐linked inheritance in Drosophila (p. 237). Washington, DC: Carnegie Institution of Washington Publication. [Google Scholar]
- Mummery‐Widmer, J. L. , Yamazaki, M. , Stoeger, T. , Novatchkova, M. , Bhalerao, S. , Chen, D. , … Knoblich, J. A. (2009). Genome‐wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature, 458, 987–992. 10.1038/nature07936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, N. J. , Pardo‐Diaz, C. , Whibley, A. , Supple, M. A. , Saenko, S. V. , Wallbank, R. W. R. , … Jiggins, C. D. (2016). The gene cortex controls mimicry and crypsis in butterflies and moths. Nature, 534, 106–110. 10.1038/nature17961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, H. , Iijima, T. , Kajitani, R. , Yamaguchi, J. , Ando, T. , Suzuki, Y. , … Fujiwara, H. (2015). A genetic mechanism for female‐limited Batesian mimicry in Papilio butterfly. Nature Genetics, 47, 405–409. [DOI] [PubMed] [Google Scholar]
- O’Grady, P. M. , & DeSalle, R. (2018a). Phylogeny of the genus Drosophila . Genetics, 209, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady, P. , & DeSalle, R. (2018b). Hawaiian Drosophila as an evolutionary model clade: Days of future past. BioEssays, 40, 1700246 10.1002/bies.201700246 [DOI] [PubMed] [Google Scholar]
- Okada, T. (1976). Subdivision of the genus Chymomyza Czeryny (Diptera, Drosophilidae), with description of three new species. Kontyû, 44, 496–511. [Google Scholar]
- Patterson, J. T. (1943). The Drosophilidae of the Southwest. The University of Texas Publication, 4313, 7–216. [Google Scholar]
- Prud'homme, B. , Gompel, N. , & Carroll, S. B. (2007). Emerging principles of regulatory evolution. Proceedings of the National Academy of Sciences, 104, 8605–8612. 10.1073/pnas.0700488104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme, B. , Gompel, N. , Rokas, A. , Kassner, V. A. , Williams, T. M. , Yeh, S.‐D. , … Carroll, S. B. (2006). Repeated morphological evolution through cis‐regulatory changes in a pleiotropic gene. Nature, 440, 1050–1053. 10.1038/nature04597 [DOI] [PubMed] [Google Scholar]
- Rebeiz, M. , Pool, J. E. , Kassner, V. A. , Aquadro, C. F. , & Carroll, S. B. (2009). Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science, 326, 1663–1667. 10.1126/science.1178357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz, M. , & Tsiantis, M. (2017). Enhancer evolution and the origins of morphological novelty. Current Opinion in Genetics & Development, 45, 115–123. 10.1016/j.gde.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz, M. , & Williams, T. M. (2017). Using Drosophila pigmentation traits to study the mechanisms of cis‐regulatory evolution. Current Opinion in Insect Science, 19, 1–7. 10.1016/j.cois.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, R. D. , Papa, R. , Martin, A. , Hines, H. M. , Counterman, B. A. , Pardo‐Diaz, C. , … McMillan, W. O. (2011). optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science, 333, 1137–1141. 10.1126/science.1208227 [DOI] [PubMed] [Google Scholar]
- Revadi, S. , Lebreton, S. , Witzgall, P. , Anfora, G. , Dekker, T. , & Becher, P. (2015). Sexual behavior of Drosophila Suzukii. Insects, 6, 183–196. 10.3390/insects6010183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel, F. , Vorkel, D. , & Eaton, S. (2011). Megalin‐dependent Yellow endocytosis restricts melanization in the Drosophila cuticle. Development, 138, 149–158. 10.1242/dev.056309 [DOI] [PubMed] [Google Scholar]
- Rogers, W. A. , Grover, S. , Stringer, S. J. , Parks, J. , Rebeiz, M. , & Williams, T. M. (2014). A survey of the trans‐regulatory landscape for Drosophila melanogaster abdominal pigmentation. Developmental Biology, 385, 417–432. 10.1016/j.ydbio.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Roy, P. R. , & Gleason, J. M. (2019). Assessing the use of wing ornamentation and visual display in female choice sexual selection. Behavioural Processes, 158, 89–96. 10.1016/j.beproc.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Santos, M. E. , Le Bouquin, A. , Crumière, A. J. , & Khila, A. (2017). Taxon‐restricted genes at the origin of a novel trait allowing access to a new environment. Science, 358, 386–390. 10.1126/science.aan2748 [DOI] [PubMed] [Google Scholar]
- Setoguchi, S. , Takamori, H. , Aotsuka, T. , Sese, J. , Ishikawa, Y. , & Matsuo, T. (2014). Sexual dimorphism and courtship behavior in Drosophila prolongata . Journal of Ethology, 32, 91–102. 10.1007/s10164-014-0399-z [DOI] [Google Scholar]
- Shevtsova, E. , Hansson, C. , Janzen, D. H. , & Kjærandsen, J. (2011). Stable structural color patterns displayed on transparent insect wings. Proceedings of the National Academy of Sciences of the United States of America, 108, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. N. , & Chatterjee, S. (1987). Greater mating success of Drosophila biarmipes males possessing an apical dark black wing patch. Ethology, 75, 81–83. [Google Scholar]
- Spieth, H. T. (1966). Courtship behavior of endemic Hawaiian Drosophila. The University of Texas Publication, 6615, 245–313. [Google Scholar]
- Stern, D. L. , & Frankel, N. (2013). The structure and evolution of cis‐regulatory regions: The shavenbaby story. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20130028 10.1098/rstb.2013.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant, A. H. (1942). The classification of the genus Drosophila, with the description of nine new species. The University of Texas Publication, 4213, 5–51. [Google Scholar]
- Suzuki, T. K. , Koshikawa, S. , Kobayashi, I. , Uchino, K. , & Sezutsu, H. (2019). Modular cis‐regulatory logic of yellow gene expression in silkmoth larvae. Insect Molecular Biology, 28, 568–577. 10.1111/imb.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, S. , & Verheyen, E. M. (2012). Wnt/wingless signaling in Drosophila . Cold Spring Harbor Perspectives in Biology, 4, a007930 10.1101/cshperspect.a007930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Subramanian, S. , & Kumar, S. (2004). Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Molecular Biology and Evolution, 21, 36–44. [DOI] [PubMed] [Google Scholar]
- Toda, M. J. (2020). DrosWLD‐Species: Taxonomic information database for world species of Drosophilidae. Retrieved from https://bioinfo.museum.hokudai.ac.jp/db/index.php [Google Scholar]
- True, J. R. , Edwards, K. A. , Yamamoto, D. , & Carroll, S. B. (1999). Drosophila wing melanin patterns form by vein‐dependent elaboration of enzymatic prepatterns. Current Biology, 9, 1382–1391. 10.1016/S0960-9822(00)80083-4 [DOI] [PubMed] [Google Scholar]
- True, J. R. , Yeh, S.‐D. , Hovemann, B. T. , Kemme, T. , Meinertzhagen, I. A. , Edwards, T. N. , … Li, J. (2005). Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genetics, 1, e63 10.1371/journal.pgen.0010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hof, A. E. , Campagne, P. , Rigden, D. J. , Yung, C. J. , Lingley, J. , Quail, M. A. , … Saccheri, I. J. (2016). The industrial melanism mutation in British peppered moths is a transposable element. Nature, 534, 102–105. 10.1038/nature17951 [DOI] [PubMed] [Google Scholar]
- Watanabe, M. , & Kondo, S. (2015). Is pigment patterning in fish skin determined by the Turing mechanism? Trends in Genetics, 31, 88–96. 10.1016/j.tig.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Werner, T. , Koshikawa, S. , Williams, T. M. , & Carroll, S. B. (2010). Generation of a novel wing colour pattern by the Wingless morphogen. Nature, 464, 1143–1148. 10.1038/nature08896 [DOI] [PubMed] [Google Scholar]
- Werner, T. , Steenwinkel, T. , & Jaenike, J. (2018). Drosophilids of the Midwest and Northeast. Version 2. Open Access Books (Vol. 1). Houghton, MI: Michigan Technological University. [Google Scholar]
- Wheeler, M. R. , & Kambysellis, M. P. (1966). Notes on the Drosophilidae of Samoa. The University of Texas Publication, 6615, 533–565. [Google Scholar]
- Wittkopp, P. J. , Carroll, S. B. , & Kopp, A. (2003). Evolution in black and white: Genetic control of pigment patterns in Drosophila . Trends in Genetics, 19, 495–504. 10.1016/S0168-9525(03)00194-X [DOI] [PubMed] [Google Scholar]
- Wittkopp, P. J. , True, J. R. , & Carroll, S. B. (2002). Reciprocal functions of the Drosophila Yellow and Ebony proteins in the development and evolution of pigment patterns. Development, 129, 1849–1858. [DOI] [PubMed] [Google Scholar]
- Wright, T. R. (1987). The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster . Advances in Genetics, 24, 127–222. 10.1016/S0065-2660(08)60008-5 [DOI] [PubMed] [Google Scholar]
- Yamaguchi, J. , Banno, Y. , Mita, K. , Yamamoto, K. , Ando, T. , & Fujiwara, H. (2013). Periodic Wnt1 expression in response to ecdysteroid generates twin‐spot markings on caterpillars. Nature Communications, 4, 1857 10.1038/ncomms2778 [DOI] [PubMed] [Google Scholar]
- Yassin, A. (2013). Phylogenetic classification of the Drosophilidae Rondani (Diptera): The role of morphology in the postgenomic era. Systematic Entomology, 38, 349–364. 10.1111/j.1365-3113.2012.00665.x [DOI] [Google Scholar]
- Yassin, A. , Delaney, E. K. , Reddiex, A. J. , Seher, T. D. , Bastide, H. , Appleton, N. C. , … Kopp, A. (2016). The pdm3 locus is a hotspot for recurrent evolution of female‐limited color dimorphism in Drosophila . Current Biology, 26, 2412–2422. 10.1016/j.cub.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, S. D. , Liou, S. R. , & True, J. R. (2006). Genetics of divergence in male wing pigmentation and courtship behavior between Drosophila elegans and D. gunungcola . Heredity, 96, 383–395. 10.1038/sj.hdy.6800814 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Martin, A. , Perry, M. W. , van der Burg, K. R. , Matsuoka, Y. , Monteiro, A. , & Reed, R. D. (2017a). Genetic basis of melanin pigmentation in butterfly wings. Genetics, 205, 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Mazo‐Vargas, A. , & Reed, R. D. (2017b). Single master regulatory gene coordinates the evolution and development of butterfly color and iridescence. Proceedings of the National Academy of Sciences of the United States of America, 114, 10707–10712. 10.1073/pnas.1709058114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , & Reed, R. D. (2016). Genome editing in butterflies reveals that spalt promotes and Distal‐less represses eyespot colour patterns. Nature Communications, 15, 11769 10.1038/ncomms11769 [DOI] [PMC free article] [PubMed] [Google Scholar]


