Summary
Spinal cord stimulation at 10 kHz is a promising therapy for non‐surgical refractory back pain; however, published data are currently limited. We present a subanalysis of prospectively collected clinical outcome data for non‐surgical refractory back pain patients treated with 10 kHz spinal cord stimulation, from the independent cohorts of two previous studies (SENZA‐RCT and SENZA‐EU). Clinical outcomes were evaluated at pre‐implantation (baseline), 3 months, 6 months and 12 months following 10 kHz spinal cord stimulator implantation. These included: pain relief; responder rate (≥ 50% pain relief from baseline); remission rate (VAS ≤ 3.0 cm); disability (Oswestry Disability Index(ODI)); and opioid use. At 3 months, average back pain decreased by 70% in the combined cohort (60% in the SENZA‐RCT and 78% in the SENZA‐EU cohorts). This was sustained at 12 months, with a 73% back pain responder rate and 68% remission rate in the combined cohort. Leg pain relief results were generally comparable to those for back pain relief. At 12 months, the combined cohort had an average decrease in ODI scores of 15.7% points from baseline and opioid use more than halved. In conclusion, 10 kHz spinal cord stimulation reduced pain, disability and opioid consumption in non‐surgical refractory back pain subjects. Application of this therapy may improve the care of non‐surgical refractory back pain patients and reduce their opioid consumption.
Keywords: 10 kHz SCS, axial back pain, chronic pain, maiden back pain, non‐specific low back pain, non‐surgical back pain, opioids, VAS, virgin back pain
Introduction
The Global Burden of Disease Study currently ranks low back pain as the number one cause of disability among men and women worldwide 1. In 2017, the condition affected over 575 million people of all ages across the globe and resulted in the loss of 65 million years of productive life. A systematic review of 165 studies from 54 countries by Hoy et al. in 2008 reported a range of point prevalence from 1% to 50%, with a mean of 18% and lifetime prevalence estimated to be 39% 2. The associated direct and indirect costs are high, running into billions of dollars every year 3, 4, 5, 6, 7. In the vast majority of people (90%), a specific cause of the pain cannot be identified and low back pain is classified as non‐specific 7, 8, 9. Whereas around a third of people recover from a low back pain episode, pain persists in the remainder after 3 months 10. Overall, 65% of people with low back pain still report pain after 12 months, indicating that prognosis is poor after the onset of chronicity 10.
At present, treatment recommendations for low back pain vary between countries. However, they commonly emphasise non‐pharmacological modalities (for example: education; exercise; massage; acupuncture; spinal manipulation; and cognitive‐behavioural therapy) and limited use of analgesics 11. In the small number of patients with identifiable pathology, spinal surgery may be useful. However, while spinal surgery is indicated in a limited number of cases, national UK treatment guidelines for this patient population prohibit offering disc replacements or spinal fusion unless the latter is part of a randomised trial 12. There is a clear need for efficacious treatments to address the large population of chronic non‐specific low back pain patients ineligible for surgery. The descriptive terminologies for this indication include: non‐surgical refractory back pain; maiden back; and virgin back.
Spinal cord stimulation is a minimally invasive, reversible therapy used to treat various pain syndromes. New or persistent back and/or leg pain after spinal surgery is the most common reason for implantation (otherwise known as failed back surgery syndrome) 13, 14. Existing evidence has established efficacy of the therapy in failed back surgery syndrome for predominant radicular leg pain 15, 16, 17. However, for some people the feeling of paraesthesia, commonly associated with low‐frequency spinal cord stimulation, can be uncomfortable 18. It can also be a challenge to achieve adequate paraesthesia coverage of the lower back. This may be due to the depth, size and location of dorsal column fibres innervating the axial back, as well as the increased thickness of the dorsal cerebrospinal fluid in the thoracic region where stimulation is applied 19, 20.
10 kHz spinal cord stimulation is a novel, sub‐perception, stimulation paradigm that provides pain relief without paraesthesia (Senza® system, Nevro Corp., Redwood, CA, USA) 18, 21. A single‐arm, prospective study (SENZA‐EU) provided evidence to support its use in subjects with predominant chronic back pain 22, 23. A randomised, controlled trial (SENZA‐RCT) further established its long‐term efficacy and superiority over low‐frequency spinal cord stimulation in patients with chronic back and leg pain. The effectiveness of 10 kHz spinal cord stimulation has also been shown in a case‐controlled, single‐centre study and in a large multicentre, retrospective, real‐world study in patients with predominant back and/or leg pain 24, 25.
The efficacy of 10 kHz spinal cord stimulation in non‐surgical refractory back pain subjects was evaluated in a prospective, open‐label study by Al‐Kaisy et al. 26, 27. This reported significant improvements in pain, disability and quality of life, as well as reduced opioid consumption in subjects at 12 months post‐implantation. The clinical benefits were sustained after 3 years of stimulation. However, the cohort size of the study was relatively small (20 patients) and additional evidence demonstrating the efficacy of 10 kHz spinal cord stimulation for non‐surgical refractory back pain is needed. Therefore, all non‐surgical refractory back pain participants in the SENZA‐EU and SENZA‐RCT studies were selected and sub‐group analysis was performed to evaluate the efficacy of 10 kHz spinal cord stimulation in this patient population.
Methods
Data for all non‐surgical refractory back pain participants in the SENZA‐EU and SENZA‐RCT studies were extracted. Non‐surgical refractory back pain participants were defined as participants who were surgery‐naïve.
The SENZA‐EU trial was a prospective, multicentre, open‐label study conducted at two European centres between August 2009 and February 2011 22, 23. Each local ethics committee approved the study (Commissie voor Medische Ethiek AZ Nikolaas, Belgium; NRES Northern and Yorkshire REC, UK). It was conducted in compliance with local clinical research and data protection regulations, good clinical practice guidelines (ISO 14155) and the Declaration of Helsinki. Enrolled patients had chronic back pain (defined as lumbosacral pain) with or without leg pain that was refractory to conventional treatment for at least 6 months and had an average pain intensity of ≥ 5.0 cm on the visual analogue scale (VAS, a 0–10 cm scale anchored by ‘no pain’ to ‘worst pain’ imaginable) over the past 7 days. Outcome measures included: average back pain, leg pain and overall pain intensity as VAS scores over the past 7 days; disability score using the Oswestry Disability Index (ODI); and opioid medication dose (in morphine milligram equivalents (MME)). These were evaluated at baseline (pre‐implantation). Subjects then received a 10 kHz spinal cord stimulation therapy trial (Senza spinal cord stimulation system) for 14–30 days. Those who reported at least a 50% reduction in pain intensity from baseline at the end of the trial received a permanent spinal cord stimulator system. The study protocol allowed changes in pain medication and stimulation parameters during follow‐up.
The SENZA‐RCT was a multicentre randomised controlled trial carried out in 10 centres in the USA between June and December 2012 18, 21. The study was approved by the institutional review boards for each centre (Western Institutional Review Board, Puyallup, WA, USA; Forsyth Medical Center Institutional Review Board, Winston‐Salem, NC, USA) and was conducted in compliance with the US Code of Federal Regulations and the Declaration of Helsinki. Enrolled subjects had chronic, intractable back and/or leg pain refractory to conservative therapy for at least 3 months, average back pain and leg pain of ≥ 5 cm on a VAS over the past 7 days and ODI score of 41–80%, out of 100%. Outcome measures included: average back pain and leg pain intensity as a VAS score over the past 7 days; disability score using the ODI; and opioid medication dose in MME. These were evaluated pre‐implantation (baseline). Patients were then randomly allocated in a 1:1 ratio to receive stimulation with an investigational 10 kHz spinal cord stimulation therapy system (Senza System) or a commercially available low‐frequency spinal cord stimulation system (Precision Plus System; Boston Scientific, MA, USA). For each arm, the respective device manufacturer programmed the device under supervision of the investigator. Following a trial of the assigned system, lasting up to 14 days, a permanent system was implanted in those who reported at least a 40% reduction in back pain from baseline during the trial. From 28 days before enrolment, until activation of the implanted spinal cord stimulation system, oral analgesics (excluding allowances for peri‐operative analgesics) were stabilised in the subjects. Analgesic adjustments were allowed following activation of the implanted spinal cord stimulation system under the guidance of a study investigator as medically necessary. However, subjects who increased their opioid dose were considered as ‘non‐responders’ regardless of their pain relief.
In the SENZA‐EU and SENZA‐RCT studies, evident mechanical spinal instability was an exclusion criterion and all subjects signed informed consent, were 18 years or older and were candidates for spinal cord stimulation.
The SENZA‐EU and SENZA‐RCT studies assessed outcome measures at baseline (pre‐implantation), 3 months, 6 months and 12 months post‐implantation. These were: back pain intensity VAS scores over the past 7 days; leg pain intensity VAS scores over the past 7 days; disability score using the ODI; and opioid medication dose in MME. Responders were defined as participants with at least 50% pain reduction in VAS scores from baseline and those in remission were defined as having a VAS ≤ 3.0 cm 28. Disability was categorised using the ODI score (ODI: 0–20% = minimal disability; 21–40% = moderate disability; 41–60% = severe disability; 61–80% = crippled; or 81–100% = bed‐bound). Opioid dosage was categorised using MME as: 0 MME; 1–49 MME; 50–90 MME; or > 90 MME. Change in opioid use was also classified as: eliminated; decreased; no change; or increased.
Data were analysed separately for each study cohort (SENZA‐EU and SENZA‐RCT) and as a combined cohort for: mean pain VAS scores; percentage pain relief from baseline at 3 months, 6 months and 12 months post‐implantation; 12‐month responder rates; and 12‐month remission rates. Disability scores and opioid consumption were analysed in the combined cohort at baseline and 12 months. All outcome analyses were conducted using data from subjects completing their 12‐month follow‐up assessment while excluding the missing subject from the analyses.
Results
The SENZA‐RCT had 12‐month data available for 89 subjects permanently implanted with a 10 kHz spinal cord stimulation system (Fig. 1). Of these, 12 were surgery naïve. In the SENZA‐EU study, 15 of the 67 subjects permanently implanted with a 10 kHz spinal cord stimulation system were surgery naïve (Fig. 1). The combined sub‐group comprised all 27 subjects at baseline (Fig. 1). One subject was lost to follow‐up in the SENZA‐EU study and therefore, data from 26 subjects were used for analysis.
Figure 1.
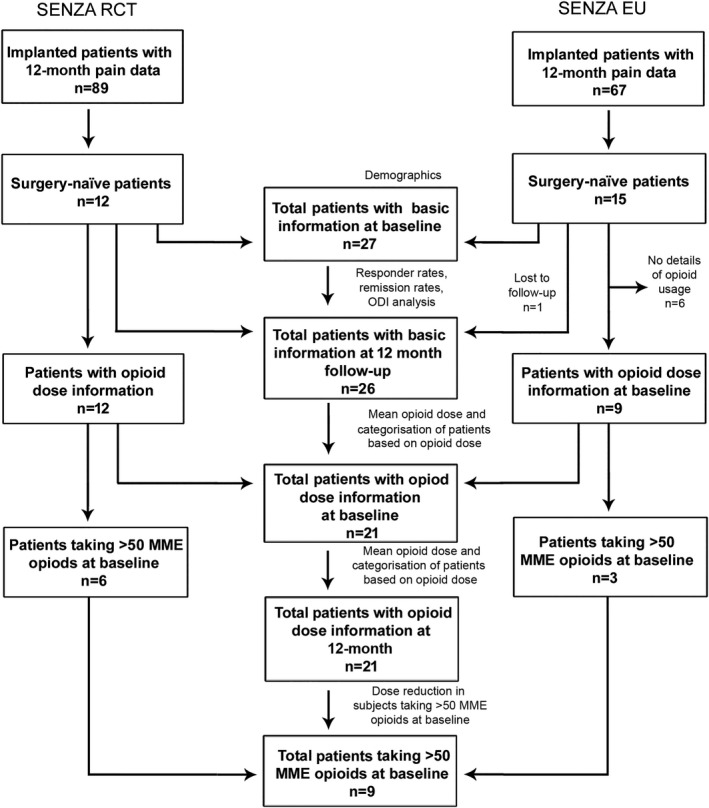
Subject datasets used for analysis. MME, morphione milligram equivalents.
Table 1 presents baseline characteristics for the SENZA‐RCT and SENZA‐EU sub‐groups and combined cohort. The two sub‐groups were broadly the same for: age; years since diagnosis; back and leg pain score; and disability score. The SENZA‐EU sub‐group comprised of more women. Daily opioid dose was higher among the SENZA‐RCT sub‐group than the SENZA‐EU sub‐group, and a higher proportion of subjects in the SENZA‐RCT sub‐group reported a dosage of ≥ 50 MME. The majority of the SENZA‐EU sub‐group had pain due to degenerative back disease or other reasons such as facet degeneration, spinal stenosis or scoliosis. The majority of the SENZA‐RCT sub‐group had pain due to radiculopathy, spondylosis and/or degenerative disc disease.
Table 1.
Characteristics and baseline clinical outcomes for subjects recruited to the SENZA‐RCT and SENZA‐EU trials. Values are mean (SD), mean (SEM) or number (proportion)
| SENZA‐RCT | SENZA‐EU | Combined | |
|---|---|---|---|
| Number of subjects | 12 | 15 | 27 |
| Age | 47.8 (11.5) | 52.3 (9.1) | 50.3 (10.3) |
| Female gender | 6 (50.0%) | 12 (80%) | 18 (67%) |
| Years since diagnosis | 6.8 (5.3) | 9.1 (8.3) | 8.0 (7.1) |
| Aetiology (subjects could have more than one) | |||
| Degenerative disc/back disease | 9 (75%) | 8 (53%) | 17 (67%) |
| Radiculopathy | 11 (91%) | 1 (7%) | 12 (46%) |
| Spondylosis | 10 (83%) | 0 | 10 (39%) |
| Sacroiliac dysfunction | 7 (58%) | 0 | 7 (27%) |
| Others | 5 (42%) | 7 (47%) | 12 (46%) |
| Pain location | |||
| Back and leg | 12 (100%) | 11 (73%) | 23 (85%) |
| Back only | 0 | 4 (27%) | 4 (15%) |
| Leg only | 0 | 0 | 0 |
| Pain intensity | |||
| Baseline back pain VAS in cm | 7.2 (0.3) | 8.1 (0.2) | 7.7 (0.2) |
| Baseline leg pain VAS in cm | 7.2 (0.3) | 7.4 (0.5) | 7.3 (0.3) |
| Disability | |||
| Baseline ODI score in % | 51.7 (2.4) | 52.9 (2.4) | 52.3 (1.7) |
| Opioid use | |||
| Number of subjects using opioids at baseline | 10 (83%) | 9 (60%) | 19 (70%) |
| Average dose of opioids at baseline in MME | 111.1 (31.4) | 50.9 (26.8) | 85.3 (21.9) |
| Subjects taking opioids at doses ≥ 50 MME | 7 (58%) | 2 (13%) | 9 (33%) |
| Opioid dose in subjects taking ≥ 50 MME | 176.9 (36.6) | 160.0 (100.0) | 173.2 (32.6) |
MME, morphine milligram equivalents; ODI, Oswestry Disability Index; VAS, visual analogue scale.
Figure 2 shows back and leg pain intensity VAS and pain relief from baseline at 3 months, 6 months and 12 months following implantation of the 10 kHz spinal cord stimulation system. At 3 months, both sub‐groups reported an decrease in back pain and leg pain from baseline.
Figure 2.
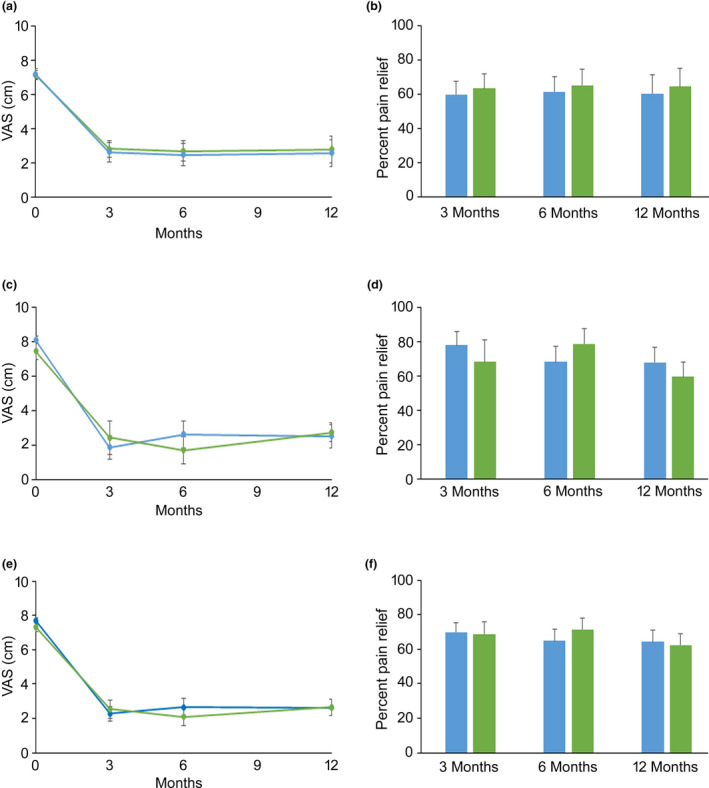
Back and leg pain intensity VAS (a, c, e) and pain relief (b, d, f) for the SENZA‐RCT sub‐group (top row) for the SENZA‐EU sub‐group (middle row) for the combined cohort (bottom row) at 3 months, 6 months and 12 months following implantation of the 10 kHz spinal cord stimulation system. Data are mean with error bars showing SEM. Back pain (blue) and leg pain (green).
In the SENZA‐RCT sub‐group, 75% of subjects (9/12) were responders for both back pain and leg pain. The corresponding rates of responders in the SENZU‐EU sub‐group were 71% (10/14) and 70% (7/10), respectively. The combined cohort yielded a responder rate of 73% for back (19/26) and leg pain (16/22), and 42% (11/26) of subjects reported back pain reduction from baseline exceeding 80%. Sixty‐seven percent (8/12) and 75% (9/12) of the SENZA‐RCT sub‐group met the criteria for remission of back pain and leg pain at 12 months, respectively. In the SENZA‐EU sub‐group the corresponding rates were 57% (8/14) and 60% (6/10), respectively. The combined cohort remission rates were 62% (16/26) for back pain and 68% (15/22) for leg pain.
Twenty‐six subjects from the combined cohort had complete ODI data available at baseline and 12 months (Fig. 3). At baseline ODI scores categorised: 5/26 subjects as ‘crippled’; 20/26 subjects as ‘severe’; and 1/26 subjects as ‘moderate’. At 12 months ODI scores categorised: 1/26 subjects as ‘crippled’; 10/26 subjects as ‘severe’; 13/26 subjects as ‘moderate’; and 2/26 subjects as ‘minimal’. At baseline, 96% (25/26) of the combined cohort were classified by the ODI scores as crippled or severely disabled. At 12 months this was 42% (11/26), at which time 81% (21/26) had moved into a lower disability category and disability had improved by an average of 15.7 percentage points (baseline mean (SD) 52.3 (1.7)%; 12 months: 36.6 (2.7)%).
Figure 3.
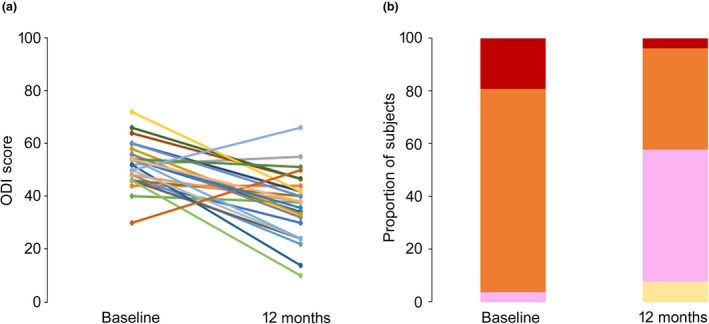
Oswestry Disability Index (a) for individual subjects and (b) proportion of the combined cohort in each category of disability, at baseline and 12 months following 10 kHz spinal cord stimulation treatment. Crippled (red), severe (orange), moderate (pink), minimal (cream).
Opioid use data at baseline and 12 months were available for 21 subjects in the combined cohort (Fig. 4). At baseline: two were categorised as 0 MME; ten as 1–49 MME; three as 50–90 MME; and six as > 90 MME. At 12 months: eight were categorised as 0 MME; seven as 1–49 MME; three as 50–90 MME; and three as > 90 MME. During this period, average opioid consumption more than halved from mean (SD) baseline values of 85.3 (21.9) MME to 39.8 (12.9) MME at 12 months. Almost one third of subjects stopped taking opioids (29%, 6/21), 24% (5/21) decreased their intake, 33% (7/21) had no change in intake and 14% (3/21) increased their intake (mean (SD) increase was 26.7 (7.3) MME). In total, more than half of the cohort reduced or eliminated their opioids (11/21). Among the nine subjects with ≥ 50 MME baseline dosage, mean (SD) opioid consumption more than halved from 173.2 (32.6) MME at baseline to 72.6 (26.0) MME at 12 months. Of these, two thirds (6/9) reduced or eliminated their opioid intake. Mean (SD) opioid consumption reduced among the 12 subjects with < 50 MME baseline dosage from 19.4 (4.3) MME at baseline to 15.2 (5.9) MME at 12 months. Almost half of this group (5/12) reduced or eliminated their opioid intake.
Figure 4.
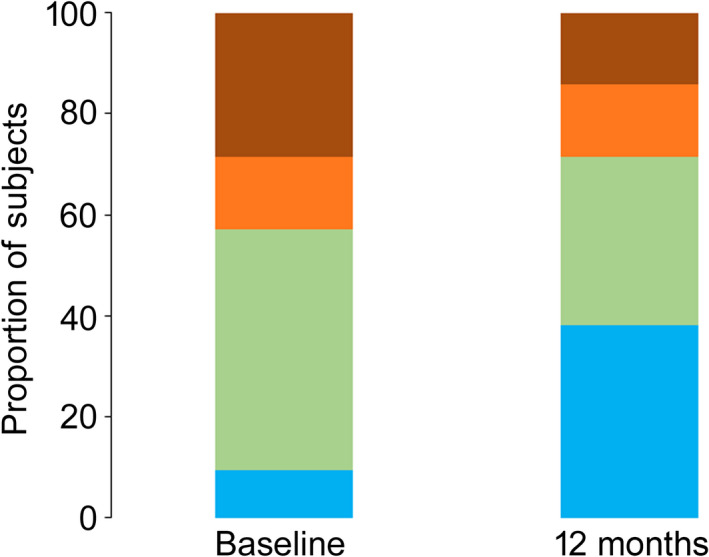
Opioid use categorised in morphine milligram equivalents (MME) at baseline and 12 months following 10 kHz spinal cord stimulation treatment. 0 MME (blue) 1–49 MME (green) 59–90 MME (orange) > 90 MME (brown).
Discussion
There is a large unmet clinical need for efficacious treatment modalities to address the pervasive complaint of non‐surgical refractory back pain. One potential modality is 10 kHz spinal cord stimulation. Our analysis of non‐surgical refractory back pain subjects treated with this therapy revealed reduced pain, disability and opioid consumption up to 1 year after implantation. Data were drawn from two independent studies spanning three countries and two continents that included a randomised clinical trial population (SENZA‐RCT) and a prospective, single‐arm study population (SENZA‐EU). Our combined cohort results compare favourably with those reported by the SENZA‐RCT and SENZA‐EU studies in which the majority of subjects had undergone previous spinal surgery 18, 22. In the SENZA‐RCT study at 12 months, 79% of subjects were deemed responders to the therapy for both back and leg pain and average baseline pain intensity VAS scores reduced by approximately 5 cm. Similarly, in the SENZA‐EU study at 12 months, the responder rate was 70% for back pain, 65% for leg pain and average baseline pain VAS scores reduced by 5.6 cm for back pain and 3.4 cm for leg pain.
A prospective, open‐label, proof‐of‐concept study by Al‐Kaisy et al. evaluated treatment outcomes in a non‐surgical refractory back pain cohort with predominant low back pain persistent for at least 6 months (back VAS ≥ 5 cm and if leg pain was present, back pain VAS was greater than leg pain VAS by at least 2 cm) 26. Of the 21 enrolled subjects, 20 completed a successful trial and received a permanent system. At 12 months, 90% (19/20) were back pain responders while baseline back pain reduced by an average of 5.6 cm (p < 0.0001). Overall, current analysis showed that responder rates and pain relief in the non‐surgical refractory back pain sub‐group were comparable to the complete cohorts of the SENZA‐RCT and SENZA‐EU studies and the study by Al‐Kaisy et al.
The SENZA‐RCT and SENZA‐EU studies showed disability measured using the ODI scale decreased (−16.5% points) in subjects treated with 10 kHz spinal cord stimulation and 70% of subjects had an improved ODI score that allowed reclassification into a lower disability category 29. In the current analysis of the non‐surgical refractory back pain combined cohort, 81% of subjects were reclassified into a lower disability category based on their ODI score at 12 months. Furthermore the proportion of patients in the ‘minimal’ to ‘moderate’ disability category increased from 4% at baseline to 58% at 12 months, indicating the benefits of 10 kHz spinal cord stimulation for non‐surgical refractory back pain patients.
Reduction in the use of opioids following 10 kHz spinal cord stimulation treatment was reported in the SENZA‐RCT and SENZA‐EU studies (in which the majority of subjects had prior spinal surgery), as well as in the prospective non‐surgical refractory back pain study by Al‐Kaisy et al. In the SENZA‐RCT study, about one third of subjects reduced or ceased their opioid intake at 12 months and the average opioid dose was decreased by 24.8 MME. In the SENZA‐EU study, 70% of the subjects reduced or ceased their opioid intake at 12 months and the average opioid dose was decreased by 64.2 MME. The prospective study by Al‐Kaisy et al. reported a reduction in 64% (−72.3 MME) in average opioid dose at the 12‐month follow‐up. Analysis of combined opioid data from the SENZA‐RCT and SENZA‐EU studies found that 53% of subjects reduced or ceased their opioid intake and the average opioid dose reduced by 48.2 MME 30. The analysis also found that the proportion of subjects taking safe doses of opioids (0–49 MME/day) increased from 36% to 59% and the proportion of subjects taking ‘high‐risk’ doses of opioids (> 90 MME/day) decreased from 40% to 23%. In our analysis, 67% of the combined cohort subjects taking ≥ 50 MME opioids at baseline reduced or eliminated their opioid dose by 12 months. These are in line with previous reports demonstrating opioid reduction following 10 kHz spinal cord stimulation treatment 31. We did, however, observe a notable difference in baseline opioid usage between our two sub‐groups. The average daily opioid dose among the SENZA‐RCT sub‐group was more than double that of the SENZA‐EU sub‐group (111 vs. 51 MME, respectively) while more than twice as many SENZA‐RCT subjects reported a baseline dosage of ≥ 50 MME (58% vs. 22%, respectively). This variation in opioid usage may arise from small sample sizes and differences in prescribing practice between the USA and the EU.
Results from Al‐Kaisy et al. suggest that benefits from 10 kHz spinal cord stimulation (reduced chronic pain, improved disability and reduced opioid consumption) are sustained long‐term (at 36 months), along with improved quality of life and employment status. The positive impact on opioid consumption is important in the context of the current opioid crisis and the rising toll of harms associated with long‐term, high‐dose consumption as more than half of long‐term opioid users have back pain 32, 33. Our analysis show non‐surgical refractory back pain patients may derive much the same benefit from 10 kHz spinal cord stimulation as those with failed back surgery syndrome; this represents an exciting opportunity in this difficult‐to‐treat population. It may also bring cost benefits to healthcare providers 34.
Although we derived our independent sub‐groups from two prospective studies designed to assess the safety and efficacy of 10 kHz spinal cord stimulation in patients with chronic back and leg pain, our post‐hoc analysis is subject to inherent pitfalls such as: lack of pre‐specification; small sample size; and the absence of a comparative placebo‐control group (e.g. sham stimulation). Placebo‐control trial design has not historically been possible in spinal cord stimulation due to the necessity of paraesthesia. Paraesthesia‐independent modalities can theoretically allow placebo‐control trials; however, there are practical impediments that risk unblinding of investigators or participants to their treatment arm. These include the need for programming of devices to achieve satisfactory pain relief and possible differences in device battery recharge requirements between placebo and test. Furthermore, there are ethical issues surrounding risk without benefit when using an ‘invasive’ placebo. Despite similar inclusion and exclusion criteria between SENZA‐EU and SENZA‐RCT, there were some study differences. SENZA‐RCT required both back and leg pain VAS scores to be ≥ 5 cm, whereas SENZA‐EU required back pain VAS to be ≥ 5 cm regardless of leg pain. Secondly, whereas SENZA‐EU was a single‐arm study designed to assess efficacy, SENZA‐RCT was designed to compare 10 kHz spinal cord stimulation, with traditional spinal cord stimulation and focused on pain management rather than opioid reduction. SENZA‐RCT had 10 participating centres whereas SENZA‐EU had 2 participating centres. This introduces heterogeneity in study populations and variation in opioid use and healthcare utilisation due to differences in healthcare systems. Furthermore, other external variables not measured in either study, may also have influenced outcomes. Given the limitations described, our results should be interpreted with caution. Our goal was to provide a clinically valuable insight into the benefits of 10 kHz spinal cord stimulation for non‐surgical refractory back pain while we await results from an ongoing randomised controlled trial comparing the therapy with conventional medical management (NCT03680846).
In conclusion, our analysis showed that 10 kHz spinal cord stimulation reduced pain, disability and opioid consumption in those living with non‐surgical refractory back pain. Given the high prevalence of the condition and its soaring economic burden, potential improvements in the care of this patient population are an essential consideration for clinicians and healthcare providers. The application of this modality may mitigate the rising use of opioids for coping with non‐surgical refractory back pains.
Acknowledgements
The authors thank M. Bhandaru Ph.D. for preparing the illustrations for the manuscript. AA‐K, J‐PVB, KA and LK are consultants to Nevro Corp. DC, BG, JS and AR are employees of Nevro Corp. DE is a consultant medical writer for Nevro Corp., Redwood City, CA, USA. No other competing interests declared.
Presented in part at the North American Neuromodulation Society Annual Meeting, Las Vegas, NV, USA, January 2020.
Contributor Information
K. Amirdelfan, @KasraAmirdelfan.
J. Subbaroyan, @JeySubbaroyan.
A. Rotte, Email: anand.rotte@nevro.com, @AnandRotte.
References
- 1. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis and Rheumatology 2012; 64: 2028–37. [DOI] [PubMed] [Google Scholar]
- 3. Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000; 84: 95–103. [DOI] [PubMed] [Google Scholar]
- 4. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine Journal 2008; 8: 8–20. [DOI] [PubMed] [Google Scholar]
- 5. Lambeek LC, van Tulder MW, Swinkels IC, Koppes LL, Anema JR, van Mechelen W. The trend in total cost of back pain in The Netherlands in the period 2002 to 2007. Spine 2011; 36: 1050–8. [DOI] [PubMed] [Google Scholar]
- 6. Walker BF, Muller R, Grant WD. Low back pain in Australian adults: the economic burden. Asia Pacific Journal of Public Health 2003; 15: 79–87. [DOI] [PubMed] [Google Scholar]
- 7. Maher C, Underwood M, Buchbinder R. Non‐specific low back pain. Lancet 2017; 389: 736–47. [DOI] [PubMed] [Google Scholar]
- 8. Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. British Medical Journal 2006; 332: 1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet 2018; 391: 2356–67. [DOI] [PubMed] [Google Scholar]
- 10. Itz CJ, Geurts JW, van Kleef M, Nelemans P. Clinical course of non‐specific low back pain: a systematic review of prospective cohort studies set in primary care. European Journal of Pain 2013; 17: 5–15. [DOI] [PubMed] [Google Scholar]
- 11. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2017; 166: 514–30. [DOI] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence (NICE) . Low back pain and sciatica in over 16s: assessment and management. NICE Guideline [NG59], London, 2016. www.nice.org.uk/guidance/ng59 [PubMed]
- 13. Moore DM, McCrory C. Spinal cord stimulation. British Journal of Anaesthesia Education 2016; 16: 258–63. [Google Scholar]
- 14. Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the field survey: spinal cord stimulator use by academic pain medicine practices. Pain Medicine 2014; 15: 188–95. [DOI] [PubMed] [Google Scholar]
- 15. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005; 56: 98–106. discussion 7 [DOI] [PubMed] [Google Scholar]
- 16. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007; 132: 179–88. [DOI] [PubMed] [Google Scholar]
- 17. Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta‐regression analysis. Pain Practice 2014; 14: 489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kapural L, Yu C, Doust MW, et al. Novel 10 kHz High‐frequency therapy (HF10 Therapy) is superior to traditional low‐frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA‐RCT randomized controlled trial. Anesthesiology 2015; 123: 851–60. [DOI] [PubMed] [Google Scholar]
- 19. Deer T, Pope J, Hayek S, et al. Neurostimulation for the treatment of axial back pain: a review of mechanisms, techniques, outcomes, and future advances. Neuromodulation 2014; 17(Suppl. 2): 52–68. [DOI] [PubMed] [Google Scholar]
- 20. Taghva A, Karst E, Underwood P. Clinical paresthesia atlas illustrates likelihood of coverage based on spinal cord stimulator electrode location. Neuromodulation 2017; 20: 582–8. [DOI] [PubMed] [Google Scholar]
- 21. Kapural L, Yu C, Doust MW, et al. Comparison of 10 kHz High‐Frequency and traditional low‐frequency Spinal Cord Stimulation for the treatment of chronic back and leg pain: 24‐month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016; 79: 667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al‐Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high‐frequency spinal cord stimulation for patients with chronic, low back pain: 24‐month results of a prospective multicenter study. Pain Medicine 2014; 15: 347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Buyten JP, Al‐Kaisy A, Smet I, Palmisani S, Smith T. High‐frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 2013; 16: 59–65; discussion ‐6. [DOI] [PubMed] [Google Scholar]
- 24. Stauss T, El Majdoub F, Sayed D, et al. A multicenter real‐world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Annals of Clinical and Translational Neurology 2019; 6: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 kHz spinal cord stimulation: a retrospective analysis of real‐world data from a community‐based, interdisciplinary pain facility. Journal of Pain Research 2018; 11: 2929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al‐Kaisy A, Palmisani S, Smith TE, et al. 10 kHz High‐Frequency Spinal Cord Stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof‐of‐concept study. Neuromodulation 2017; 20: 63–70. [DOI] [PubMed] [Google Scholar]
- 27. Al‐Kaisy A, Palmisani S, Smith TE, et al. Long‐term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10 kHz high‐frequency spinal cord stimulation over 36 months. Pain Medicine 2018; 19: 1219–26. [DOI] [PubMed] [Google Scholar]
- 28. Amirdelfan K, Gliner BE, Kapural L, et al. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24‐month outcomes with spinal cord stimulation. Postgraduate Medicine 2019; 131: 278–86. [DOI] [PubMed] [Google Scholar]
- 29. Amirdelfan K, Yu C, Doust MW, et al. Long‐term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12‐month results from the SENZA‐RCT. Quality of Life Research 2018; 27: 2035–44. [DOI] [PubMed] [Google Scholar]
- 30. Al‐Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: Post hoc analysis of data from two prospective studies. Scientific Reports 2019; 9: 11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al‐Kaisy A, Van Buyten JP, Amirdelfan K, et al. Opioid‐sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Annals of New York Academy of Sciences 2020; 1462: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donroe JH, Socias ME, Marshall BDL. The deepening opioid crisis in North America: historical context and current solutions. Current Addiction Reports 2018; 5: 454–63. [Google Scholar]
- 33. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 2018; 391: 2368–83. [DOI] [PubMed] [Google Scholar]
- 34. Annemans L, Van Buyten J‐P, Smith T, Al‐Kaisy A. Cost effectiveness of a novel 10 kHz high‐frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). Journal of Long‐Term Effects of Medical Implants 2014; 24: 173–83. [DOI] [PubMed] [Google Scholar]


