Abstract
Piscine orthoreovirus genotype 1 (PRV‐1) is the causative agent of heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon (Salmo salar L.). The virus has also been found in Pacific salmonids in western North America, raising concerns about the risk to native salmon and trout. Here, we report the results of laboratory challenges using juvenile Chinook salmon, coho salmon and rainbow trout injected with tissue homogenates from Atlantic salmon testing positive for PRV‐1 or with control material. Fish were sampled at intervals to assess viral RNA transcript levels, haematocrit, erythrocytic inclusions and histopathology. While PRV‐1 replicated in all species, there was negligible mortality in any group. We observed a few erythrocytic inclusion bodies in fish from the PRV‐1‐infected groups. At a few time points, haematocrits were significantly lower in the PRV‐1‐infected groups relative to controls, but in no case was anaemia noted. The most common histopathological finding was mild, focal myocarditis in both the non‐infected controls and PRV‐1‐infected fish. All cardiac lesions were judged mild, and none were consistent with those of HSMI. Together, these results suggest all three species are susceptible to PRV‐1 infection, but in no case did infection cause notable disease in these experiments.
Keywords: Chinook salmon, coho salmon, Piscine orthoreovirus, rainbow trout
1. INTRODUCTION
The disease heart and skeletal muscle inflammation (HSMI) was first described in 1999 in farmed Atlantic salmon (Salmo salar L.) in Norway (Kongtorp, Kjerstad, Taksdal, Guttvik, & Falk, 2004). Outbreaks of HSMI are typically associated with aquaculture production stressors where HSMI‐associated morbidity can be high but direct mortality is typically low to moderate (Kongtorp, Halse, Taksdal, & Falk, 2006). The disease often manifests within the first year after seawater transfer of Atlantic salmon smolts and is diagnosed by observation of histopathological changes in the heart and skeletal muscle (Kongtorp, Taksdal, & Lyngoy, 2004). A viral aetiology for HSMI was suspected (Kongtorp, Kjerstad, et al., 2004; Watanabe et al., 2006), and deep sequencing technologies identified a novel virus, Piscine orthoreovirus (PRV), in tissues derived from HSMI‐affected fish (Palacios et al., 2010).
Molecular surveys showed that PRV was widespread throughout Norway in HSMI‐affected Atlantic salmon as well as in asymptomatic farmed and wild salmon in both marine and freshwater environments (Garseth, Fritsvold, Opheim, Skjerve, & Biering, 2013; Løvoll et al., 2012; Palacios et al., 2010; Wiik‐Nielsen, Ski, Aunsmo, & Lovoll, 2012). The high prevalence of PRV in asymptomatic fish created initial uncertainty about the relationship between PRV and HSMI leading to the hypothesis that additional host or environmental factors may be needed for HSMI to occur (Mikalsen, Haugland, Rode, Solbakk, & Evensen, 2012). However, studies demonstrated that tissues or first‐passage cell culture material containing PRV from HSMI‐diseased salmon could be used to recreate histopathological changes in naïve Atlantic salmon that were consistent with HSMI (Kongtorp, Kjerstad, et al., 2004; Kongtorp & Taksdal, 2009; Mikalsen et al., 2012) and challenges with highly purified virus confirmed PRV to be a causative agent of severe heart inflammation in farmed Atlantic salmon of Norway (Wessel et al., 2017).
Piscine orthoreovirus is a non‐enveloped reovirus with a genome comprised of 10 double‐stranded RNA segments (Palacios et al., 2010). Analysis of the PRV genome indicates that it is more similar to the orthoreoviruses of birds and mammals than it is to the fish aquareoviruses (Markussen et al., 2013). PRV was shown to preferentially replicate in red blood cells of Atlantic salmon and create cytoplasmic inclusion bodies reminiscent of those associated with erythrocytic inclusion body syndrome or EIBS (Finstad et al., 2014). The disease EIBS is associated with anaemia and heightened susceptibility to secondary infections in Pacific salmon that has been reported in fish from the west coast of North America, Japan and Europe (Piacentini, Rohovec, & Fryer, 1989; Rodger, 2007; Takahashi et al., 1992). Following an acute but transient peak of virus replication at approximately 4–6 weeks post‐infection (depending on the challenge dose and route of infection), the virus enters a long‐term persistent state in which it can be detected in blood cells and the erythroid progenitor cells of the kidney, but shedding and fish‐to‐fish transmission are greatly reduced (Garver, Johnson, et al., 2016; Haatveit et al., 2017; Malik et al., 2019; Polinski, Marty, Snyman, & Garver, 2019; Wessel et al., 2017).
Strains of PRV from Atlantic salmon in Northern Europe and the eastern coast of North America and from both Atlantic and Pacific salmon in western North America and South America have been shown to be genetically related and are now termed PRV genotype 1 (PRV‐1). A second genotype known as PRV‐2 has been detected in coho salmon (Oncorhynchus kisutch Walbaum) of Japan and reported to be the aetiological agent of EIBS in that country (Takano et al., 2016). A genotype known as PRV‐3 has been detected in Europe (Olsen, Hjortaas, Tengs, Hellberg, & Johansen, 2015) and South America (Cartagena, Tambley, Sandino, Spencer, & Tello, 2018; Godoy et al., 2016) and has been reported to cause HSMI‐like lesions in rainbow trout (Oncorhynchus mykiss Walbaum) in Europe (Dhamotharan et al., 2018; Hauge et al., 2017; Vendramin et al., 2019) as well as in coho salmon in Chile (Godoy et al., 2016). Neither PRV‐2 nor PRV‐3 has been reported to occur in North America, but there has been no extensive surveillance conducted for these viral strains.
Given the potential for pathogenicity demonstrated by each PRV genotype in either Europe or Asia, there were concerns about the impact of PRV on Pacific salmonids in western North America following reports of high PRV‐1 prevalence in farmed Atlantic salmon in British Columbia (BC), Canada (Kibenge et al., 2013). Based on molecular clock analysis of viral sequences, Kibenge et al. (2013) concluded that the strain of PRV‐1 found in BC was a recent introduction (ca. 2007), most likely from movement of infected eggs from Europe for Atlantic salmon farming. However, other retrospective studies have reported that PRV‐1 has been common in BC since 1987, with the first sequence‐verified detection occurring in archived samples collected in 1992 (Marty, Morrison, Bidulka, Joseph, & Siah, 2014). In addition to its presence in farmed Atlantic salmon, PRV‐1 has been detected in many species of farmed and free‐ranging Pacific salmon within the last decade including Chinook salmon (Oncorhynchus tshawytscha Walbaum), coho salmon, chum salmon (Oncorhynchus keta Walbaum), sockeye salmon (Oncorhynchus nerka Walbaum), pink salmon (Oncorhynchus gorbuscha Walbaum), steelhead trout (O. mykiss Walbaum) and cutthroat trout (Oncorhynchus clarkii Richardson) from BC, Canada, and the Puget Sound, Columbia River and coastal salmon populations of Alaska and Washington, USA (Kibenge et al., 2013; Marty et al., 2014; Purcell et al., 2018; Siah et al., 2015). The widespread finding of PRV‐1 on the west coast of North America has raised questions regarding the risk to Pacific salmon posed by PRV‐1 infections and whether PRV‐1 is associated with HSMI or any other disease conditions in these species. To address these issues for three species of Pacific salmon, we conducted controlled laboratory injection challenges to deliver a high dose of infectious material at a known time point. Tissues from Atlantic salmon infected with PRV‐1 were used to infect coho salmon, Chinook salmon and rainbow trout, collecting samples at intervals to assess viral RNA transcript levels, haematocrit, presence of inclusion bodies and histopathology.
2. MATERIALS AND METHODS
2.1. Chinook salmon challenge
Kidney and spleen tissues were harvested from Atlantic salmon farmed in BC, Canada, from a population testing positive for PRV RNA with no diagnosis of cardiomyopathy or other farm‐level disease. All fish tissues were frozen at −80°C prior to being used for challenge. On the day of challenge, kidney and spleen tissues were thawed on ice and multiple individuals were combined to create a PRV + tissue pool (eight individuals pooled). The tissue pools were manually homogenized in minimal essential medium (MEM) supplemented with 50 µg/ml gentamicin and subjected to centrifugation at 2,500 g for 10 min at 4°C. The supernatants were collected and passed through a 5‐µm prefilter followed by a 0.2‐µm final filter. The final dilution of tissue to media was 1:6. Total RNA from the 140 µl of homogenate used for the challenge inoculum was isolated using the Viral RNA extraction kit (Qiagen) following the manufacturer's instruction. Total RNA was denatured with a 95°C pre‐incubation step required to efficiently detect both the dsRNA genome and mRNA (Polinski et al., 2019). Complementary DNA (cDNA) synthesis was initiated with 1.5 µg total RNA (in 20 µl final volume) by use of the High Capacity cDNA Kit following the manufacturer’s instructions (Invitrogen). The cDNA was diluted 1:5 in water and 5 µl was used for reverse transcriptase quantitative PCR (RT‐qPCR) targeting the L1 segment (as described in detail below). The PRV+ inoculum had an initial C T of 18.7 (~3 × 105 total PRV RNA copies in a reaction initiated with 75 ng RNA). An aliquot of the filtered supernatant (diluted to 1:40) was subjected to cell culture using the EPC, CHSE‐214 and GF‐1 cell lines and standard virology methods (AFS, 2016). The GF‐1 cells have been reported to replicate PRV‐1 but not to high titres (Mikalsen et al., 2012). The EPC and CHSE‐214 cells were used to screen for other potential virus agents in the inoculum. Cells were monitored for cytopathic effect (CPE) for 14 days and supernatant from cells with observable CPE were subjected to a second passage.
Spring Chinook salmon were obtained as eyed eggs from the Washington Department of Fish and Wildlife, Kendall Creek Hatchery (Deming, WA). Screening of the adult broodstock population for regulated pathogens and iodophor treatment of eggs followed protocols defined by the Fisheries Co‐Managers of Washington State (2006). Eyed eggs were surface‐disinfected upon receipt and reared in a quarantine laboratory on ambient temperature sand‐filtered, UV‐treated fresh water and fed a daily semi‐moist diet (Skretting). Chinook salmon fry tested specific pathogen free (SPF) by RT‐qPCR. At approximately 1 g size, the fish were acclimated to the challenge temperature 12°C, anaesthetized with buffered tricaine methanesulfonate (MS222; Argent Laboratories) and injected intraperitoneally (IP) with 25 µl of the filtered inoculate (N = 77). A mock group (N = 80) were injected with only MEM + gentamicin. Each group was held in a separate tank with flowing water and monitored daily for mortality. All fish experiments were performed under a protocol approved by the U.S. Geological Survey Western Fisheries Research Center (WFRC) Institutional Animal Care and Use Committee in compliance with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals.
Sampling of each group was conducted at 7, 14, 21, 30, 43, 63, 84 and 98 days post‐challenge (dpc). A total of eight fish were removed at each time point and killed by an overdose of MS222. For three of the fish, a small incision was made in the abdominal cavity and the whole body was submerged into Carson’s modified Millonig’s phosphate‐buffered formalin fixative (Carson, Martin, & Lynn, 1973) for histological analyses. Entire hearts were removed following fixation and processed using routine methods. The whole fish fixation method typically provides the highest quality tissue fixation when processing very small fish. For the remaining five fish, blood was collected in a capillary tube for haematocrit and blood smears made for analysis of inclusion bodies. The small size of fish resulted in a limited volume of whole blood and we did not always obtain a high enough blood volume for reliable RNA extraction. Since PRV‐1 infects the red blood cells at high loads, we targeted the blood‐rich kidney and spleen. The kidney/spleen tissues were preserved in RNAlater (Invitrogen) following the manufacturer’s recommendations and used for RT‐qPCR of viral RNA transcript levels.
2.2. Rainbow trout challenge
Research‐grade rainbow trout were obtained as 1 g fry from Clear Springs Foods, Inc. and confirmed SPF by RT‐qPCR. The challenge was conducted as described above for Chinook salmon but with a few exceptions. The most notable change was the use of a higher challenge water temperature (15°C) to mimic temperatures relevant to the Idaho aquaculture industry. The inoculum for this study was derived from the same Atlantic salmon frozen tissues described above but the homogenates included kidney, spleen, heart and the liver. The final filtered inoculum had a C T value of 24.2 (~8 × 103 total RNA copies in a reaction initiated with 75 ng RNA). Sampling procedures were as described above but four fish were taken for histology and six fish were taken for blood and tissue analyses at 7, 14, 21, 28, 42, 63 and 84 dpc.
2.3. Coho salmon challenge
Coho salmon were obtained as eyed eggs from the Quinault National Fish Hatchery (Humptulips, WA) and reared as described above until ~3 g size. Adult broodstock screening, egg disinfection and confirmation of SPF status were as described for Chinook salmon. The challenge was conducted as described above for Chinook salmon but with a few exceptions. Four different inoculum groups were used in this study: (a) mock (medium) control; (b) PRV‐ tissue inoculum; (c) PRV+ laboratory (lab) inoculum derived from Atlantic salmon infected in a laboratory with no diagnosis of HSMI; and (d) PRV+ field inoculum derived from farmed Atlantic salmon with HSMI‐like cardiomyopathy. The source of the PRV− material was Atlantic salmon held in sea water (DFO laboratory) and confirmed negative for PRV RNA by use of RT‐qPCR (Polinski et al., 2019). The source of the PRV+ lab material salmon was 10 Atlantic salmon that had been artificially infected with PRV in sea water; the virus had been subjected to three in vivo passages in Atlantic salmon in the laboratory with harvesting of the virus for each passage at ~3–4 weeks post‐infection (Zhang et al., 2019). The material for the PRV+ field inoculum was sourced from eight naturally infected Atlantic salmon diagnosed with heart inflammatory lesions suggestive of HSMI (Polinski et al., 2019). Kidney, spleen and heart tissues were pooled to create the PRV− and PRV+ lab inocula, while only heart tissue was used to create the PRV+ field inoculum. Tissues were diluted in L‐15 medium supplemented with gentamicin (50 µg/ml) at a final ratio of 1:10. Tissue was manually homogenized and sonicated eight times in 10‐s bursts with placement on ice for 30 s between each round of sonication. Homogenates were subjected to centrifugation for 2,000 g for 5 min at 4°C and a second round of centrifugation for 3,000 g for 5 min. Supernatant was transferred to a clean tube and subjected to a third round of centrifugation at 5,000 g for 5 min at 4°C. The unfiltered inocula were stored at −80°C, shipped from the DFO laboratory to the WFRC and maintained at −80°C upon receipt at the WFRC. The final inoculum value for PRV+ lab was C T 19.7 (~2 × 105 total PRV RNA copies) and for PRV+ field C T 20.3 (~1 × 105 total PRV RNA copies) in RT‐qPCR reactions initiated with 75 ng of RNA. Eighty coho salmon per treatment were IP injected with 50 µl of medium or one of the three homogenates (total fish = 320 fish). Sampling procedures were as described above but four fish were taken for histology and six fish were taken for blood and tissue analyses. Sampling occurred at 14, 21, 28, 42, 62 and 87 dpc.
2.4. Blood analysis
Haematocrit tubes were subjected to centrifugation and read within 2 hr of sampling. Blood smears were air‐dried, fixed with methanol and stained with pinacyanol chloride as previously described (Yasutake, 1987). A total of 100 blood cells were visually evaluated per individual blood smear by light microscopy at 100× magnification and the percentage of cells with inclusions recorded. Additionally, relative anomalies in the relative frequency of leucocytes, erythroblasts and “smudge cells” were noted.
2.5. Histopathology
Heart tissues were fixed and subjected to standard histological procedures including haematoxylin and eosin staining as previously described (Kurath et al., 2006). Heart tissues were examined blind to treatment by a pathologist with experience diagnosing HSMI (T. Taksdal). A subset of individual fish with heart lesions were reviewed by a second pathologist (D. Elliott).
2.6. Quantification of segment L1 viral RNA transcripts in tissue
RNA from pooled kidney and spleen tissues was extracted using the RNeasy Kit (Qiagen). Complementary DNA synthesis was initiated with 1.5 µg total RNA (in 20 µl final volume) by use of the High Capacity cDNA Kit following the manufacturer’s instructions (Invitrogen). The cDNA protocol utilized herein targets the ssRNA (mRNA transcripts) produced during active viral replication (a proxy of viral replication). Our protocol for kidney/spleen tissues did not include the 95°C pre‐incubation step required to efficiently detect the dsRNA genome (Polinski et al., 2019). The RT‐qPCR assay targeting the L1 segment used previously described primers and probe (Løvoll et al., 2012) and was performed using the ViiA 7 Real‐Time PCR instrument (Life Technologies). Gene primers were purchased from Integrated DNA Technology and MGB probes were purchased from Life Technologies. The standard cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Wells contained 12 µl of 1X ABI Universal PCR Master Mix with 900 nM concentration of forward and reverse primers, 200 nM concentration of probe and 5 µl of diluted (1:5) cDNA. Two technical replicates were run and amplification in two technical replicates was considered a positive test result. An eight‐step 10‐fold dilution series of artificial positive control (APC) encoding the L1 PRV target region was included in duplicate on each plate to ensure consistent PCR efficiency and provide quantitative reference. Final values are reported as PRV RNA transcript copies per 1.5 µg of RNA to account for dilutions made during cDNA synthesis. Utilization of the APC and the corresponding APC probe was as previously described (Purcell et al., 2013).
3. RESULTS
3.1. Chinook salmon challenge
The filtered supernatant of the tissue homogenate used for the PRV+ inoculum produced no observable CPE in cultures of EPC or CHSE‐214 cells. However, a modest, diffuse CPE was observed in the GF‐1 cell line starting approximately 13 days post‐inoculation but could not be reproduced upon second passage of cell culture supernatant to naïve GF‐1 cells. These results were consistent with previous unsuccessful attempts to replicate PRV in the GF‐1 cell line (Garver, Marty, et al., 2016), but did indicate a lack of other filterable, culturable agents in the inoculum.
Chinook salmon mortality was negligible in both treatment groups over the 98‐day study period. Specifically, we observed three mortalities in the mock control (one each at 17, 77 and 98 dpc) and two mortalities in the PRV+ group (both at 1 dpc, likely due to handling stress).
No amplification of PRV RNA by RT‐qPCR was observed in pooled kidney/spleen tissues of the control fish injected with medium (data not shown). All fish in the PRV+ group had copies of PRV ssRNA detectable in the kidney/spleen at all sampling dates except for one fish at 30 dpc. The PRV+ treatment groups showed a pattern of increasing viral RNA in kidney/spleen tissues with the peak levels observed 30–43 dpc (Figure 1a) The observed C T values (in a reaction initiated with 75 ng RNA) ranged from 18.5 to 19.7 and from 18.8 to 22.7 at 30 and 43 dpc, respectively (excluding the single fish that tested negative at 30 dpc). Viral RNA decreased at later time points but 100% of the fish still had detectable PRV RNA at 98 dpc.
FIGURE 1.
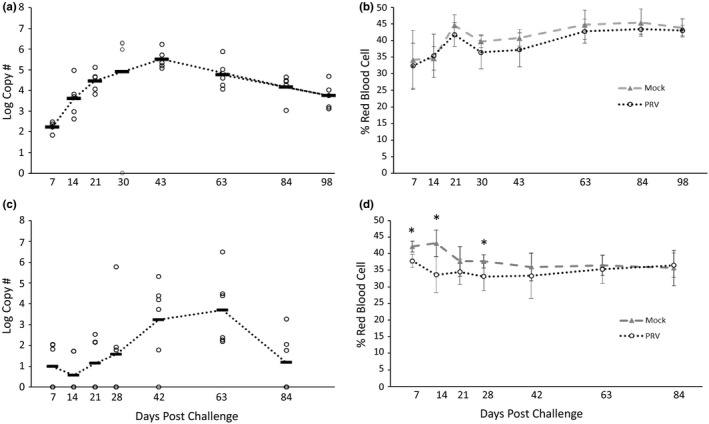
Piscine orthoreovirus (PRV) log viral RNA transcript levels (copy number per 1.5 µg RNA) in kidney/spleen tissues and corresponding haematocrit values (% red blood cells) from (a, b) Chinook salmon (Oncorhynchus tshawytscha Walbaum) injected with minimal essential medium (mock) or PRV+ homogenates, and (c, d) rainbow trout (Oncorhynchus mykiss Walbaum) injected with buffer or PRV+ homogenates. For viral RNA transcript graphs (a, c), each circle represents a single fish and the dark line indicates the mean of the group. All fish injected with medium tested negative for PRV viral RNA (data not visible on graphs a, c). For haematocrit graphs (b, d), each point represents the group mean (±SD). Asterisks denote significant differences between groups at a time point (t test; p < .05)
Haematocrit values were not significantly affected by PRV infection relative to the mock controls at any time point (p ≥ .05). There was a non‐significant trend towards slightly lower haematocrit levels relative to the control group at many of the time points (Figure 1b). Single, round, large, pink‐staining intracytoplasmic inclusion bodies (IBs) were observed in some erythrocytes in the PRV+ group at the 14–43 dpc time points, but not in the mock controls (Figure 2). The intensity of these IBs peaked at 30 dpc with an average of 7% of erythrocytes affected (data not shown). Few IBs were observed in the blood smears at the 63, 84 or 98 dpc time points and, when present, appeared as multiple, small, round, pink dots in the cytoplasm, perhaps indicative of degenerating inclusions from earlier time points. Some fish in the PRV+ treatment group sampled at day 7 and 14 also showed a higher percentage of leucocytes, erythroblasts and “smudge cells” (cell remnants lacking identifiable cytoplasmic membranes or nuclear structure) in the blood smears, likely as a result of the erythrocytic infection process.
FIGURE 2.
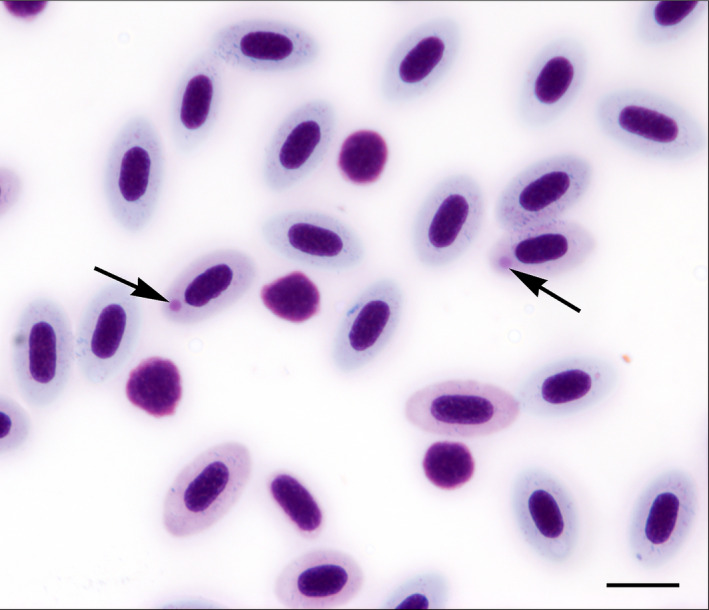
Blood smear from a Chinook salmon (Oncorhynchus tshawytscha Walbaum) at 30 days post‐infection with Piscine orthoreovirus genotype 1 (PRV‐1). Round, pink, medium‐sized cytoplasmic inclusion bodies (arrows) can be seen in two erythrocytes. The smear also shows an increase in the ratio of leucocytes and erythroblasts consistent with an intraerythrocytic infection. Scale bar equals 10 µm [Colour figure can be viewed at wileyonlinelibrary.com]
Histopathological evaluation of heart tissue was unremarkable for all mock control fish analysed (N = 16; Table 1; Figure 1a,b). In the PRV+ group, two of 20 fish analysed had a single small focus of mild lymphohistiocytic myocarditis in the atrium (43 dpc) and ventricle (63 dpc) (Table 1; Figure S1c,d); heart tissue from the remaining PRV + group was unremarkable.
Table 1.
Chinook salmon Oncorhynchus tshawytscha Walbaum and rainbow trout Oncorhynchus mykiss Walbaum injected with medium (mock) or PRV+ homogenate were examined for histopathological lesions in the heart (number of individuals with lesions/number of individuals examined). Images of heart lesions observed are presented in Figures S1 and S2
| Species | Day | Mock | PRV+ |
|---|---|---|---|
| Chinook salmon | 14 | 0/2 | 0/3 |
| 21 | 0/3 | 0/3 | |
| 30 | 0/1 | 0/3 | |
| 43 | 0/3 | 1/3 | |
| 63 | 0/2 | 1/3 | |
| 84 | 0/3 | 0/2 | |
| 98 | 0/2 | 0/3 | |
| Total | 0/16 | 2/20 | |
| Rainbow trout | 14 | 0/4 | 0/4 |
| 21 | 0/4 | 0/4 | |
| 28 | 1/4 | 1/4 | |
| 42 | 0/4 | 0/4 | |
| 63 | 0/4 | 0/4 | |
| 84 | 0/4 | 0/4 | |
| Total | 1/24 | 1/24 |
3.2. Rainbow trout challenge
Over the 84‐day holding period, no mortality was observed in the negative control fish while two mortalities were observed in the PRV+ treatment group at 2 and 26 dpc. No detectable amplification of PRV RNA was observed in the pooled kidney/spleen tissues of negative control fish (data not shown). PRV RNA was only detectable in kidney/spleen tissues of a few fish in the PRV+ treatment at 7 and 14 dpc (Figure 1c). Increasing viral RNA transcript levels started at 21 dpc and peaked at 63 dpc (C T range 20.3–34.6 per reaction) when kidney/spleen tissues tested positive for 100% of the fish. Viral transcript levels decreased at the last time point (84 dpc) with approximately half the fish testing positive.
The PRV+ injected group had significantly lower mean haematocrit at 7 and 14 dpc, but not at later time points (Figure 1d). This transient, early reduction in haematocrit in the PRV+ treatment group occurred when kidney/spleen PRV RNA transcript levels were still relatively low and may be due to an unrelated cause. Blood smears revealed small, round, single blue IBs in a very low percentage of erythrocytes of both the control and PRV+ treatment groups at various time points; however, beginning at day 42, there was an increase in the presence of leucocytes, erythroblasts, “smudge cells” and an occasional red, round intracytoplasmic IB in some fish in the PRV+ treatment group.
Histopathological evaluation of heart tissue was unremarkable for all 24 mock control fish analysed except 1 fish with focal myocardial necrosis from 28 dpc (Table 1; Figure S2a). In the PRV+ group, 1 of 24 fish analysed had a single small focus of mild lymphohistiocytic myocarditis in the ventricle (28 dpc) (Table 1; Figure S2); heart tissue from the remaining PRV+ group was unremarkable.
3.3. Coho salmon challenge
We observed 2–3 mortalities in each of the groups at <5 dpc, likely from handling stress. Over the remainder of the 83‐day holding period, no mortality was observed in the mock control, PRV− tissue or PRV+ field treatment groups, while 1 mortality was observed in the PRV+ lab treatment group at 59 dpc. No detectable amplification of PRV RNA was observed in the kidney/spleen tissues of mock or PRV− control fish (data not shown). PRV RNA transcripts were detectable in 100% of the kidney/spleen tissues of both the PRV+ treatments at all time points tested. The viral RNA transcript levels peaked at 42 dpc, declining thereafter (Figure 3a); the C T ranges per reaction at 42 dpc were 22.6–25.9 and 21.6–26.7 in the PRV+ lab and PRV+ field groups, respectively.
FIGURE 3.
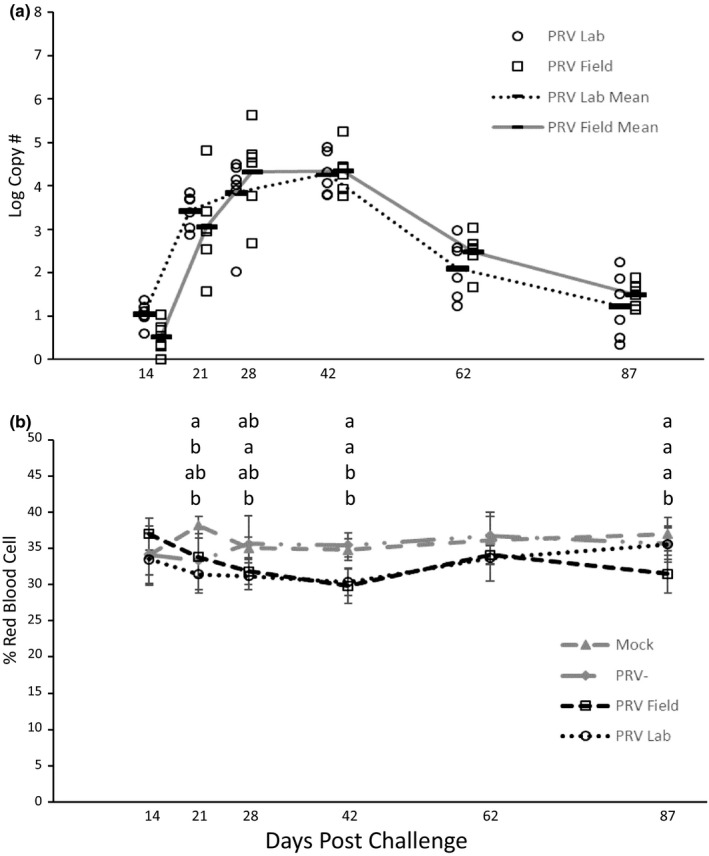
(a) Piscine orthoreovirus (PRV) log viral RNA transcript levels (copy number per 1.5 µg RNA) in kidney/spleen tissues and (b) corresponding haematocrit values (% red blood cells) in Coho salmon (Oncorhynchus kisutch Walbaum) injected with buffer, PRV− homogenates, PRV + homogenates derived from field material and PRV+ homogenates derived from a laboratory challenge. For viral RNA transcript graphs, each circle or square represents a single fish and the dark line indicates the mean of the group. All fish injected with medium or PRV− homogenates tested negative for PRV viral RNA (data not visible on graph a). For haematocrit graphs, each point represents the group mean (±SD). Letters denote significant differences among groups and the order of the groups corresponds to the order shown in the figure legend (ANOVA; Tukey’s post hoc; p < .05)
The mean haematocrits of the two PRV+ treatment groups were lower than controls at some time points. However, only at 42 dpc were mean haematocrits in both the PRV+ lab and PRV+ field groups significantly lower than those in the PRV− group (Figure 3b). At 87 dpc, the mean haematocrit in the PRV+ field group was significantly lower than that in the PRV− group. Inclusion bodies were not common, but we did observe a few small, round blue IBs in the erythrocytes of both the control and PRV+ treatment groups at several time points and a few, single, round, pink‐staining IBs in only the PRV+ treatments at 28 dpi and later. Higher percentages of erythroblasts and “smudge cells” were observed in blood smears from fish in both PRV+ treatments at 42 dpi and later, suggesting an increase in erythrocyte fragility that may account for the lower haematocrits observed for fish in the PRV+ treatment groups at that time point.
Histopathological lesions were observed in all treatment groups starting at 21–28 dpc (Table 2). The highest frequency of lesions was observed in the mock‐treated group (8 of 24 fish). Lesions in the mock/PRV− groups were not qualitatively different from the PRV+ groups with most common finding being mild lymphohistiocytic myocarditis (single focus or small number of foci) in either the atrium or ventricle (see representative images in Figure S3). An example of an unremarkable heart is shown in Figure S3a).
Table 2.
Coho salmon Oncorhynchus kisutch Walbaum injected with medium (mock), PRV‐ homogenate, PRV + homogenate (derived from a laboratory challenge of Atlantic salmon Salmo salar L.) and PRV + homogenate (derived from a naturally infected Atlantic salmon) were examined for histopathological lesions in the heart (number of individuals with lesions/number of individuals examined). Representative images of heart lesions observed are presented in Figure S3
| Day | Mock | PRV− | PRV+ lab | PRV+ field |
|---|---|---|---|---|
| 14 | 0/4 | 0/4 | 0/4 | 0/4 |
| 21 | 0/4 | 0/4 | 0/3 | 1/4 |
| 28 | 1/4 | 0/4 | 1/4 | 1/4 |
| 42 | 2/4 | 1/4 | 0/4 | 0/4 |
| 62 | 4/4 | 2/4 | 1/4 | 2/4 |
| 87 | 1/4 | 1/4 | 1/4 | 1/4 |
| Total | 8/24 | 4/24 | 3/23 | 5/24 |
4. DISCUSSION
The initial discovery of PRV‐1 in farmed Atlantic salmon in BC, Canada, and the assumption that it represented a novel introduction (Kibenge et al., 2013) raised concerns about the impact of PRV‐1 infections in Pacific salmon species that are of high economic and cultural value in western North America. These concerns were further heightened by suggestions that PRV‐1 infections in Pacific salmon might cause reduced fitness (Morton, Routledge, Hrushowy, Kibenge, & Kibenge, 2017) or anaemia and jaundice (Di Cicco et al., 2018). However, these latter studies were correlative and lacked paired controls or may have been confounded by the presence of multiple infections. Here, we report the results of controlled laboratory injection challenges of Chinook and coho salmon and rainbow trout using a strain of PRV‐1 from Pacific Canada that was derived from tissue homogenates of farmed Atlantic salmon testing positive for the virus.
Standard virological procedures using three cell lines suggested that PRV‐1 was likely the only filterable agent in the inoculum. While PRV‐1 RNA transcripts were detected in essentially 100% of recipients at the peak time points, the kinetics of virus replication differed among the species. Peak virus transcript levels for Chinook and coho salmon were observed at 4–6 weeks post‐infection, which was generally similar to Atlantic salmon challenged by injection where a rapid rise in viral RNA copy number was observed with peak levels around 4–6 weeks (depending on challenge route, dose and method of virus quantification) with a progressive decline over the following weeks, but without evidence of virus clearance (Haatveit et al., 2017; Polinski et al., 2019; Wessel et al., 2018; Wessel, Krasnov, Timmerhaus, Rimstad, & Dahle, 2019). In contrast, rainbow trout had slower viral replication, lower overall viral transcript levels that peaked around 9 weeks post‐injection and evidence of clearance at the final time point. The difference observed for rainbow trout may be due to differences in species susceptibility, water temperature, challenge dose or multiple factors combined.
In our laboratory challenge model, PRV‐1 infections in the three species tested were not associated with any notable mortality. Histopathological examination found no lesions in any of the three species at any time point that were consistent with a diagnosis of HSMI. An early indicator of HSMI in Atlantic salmon challenge models is mononuclear cell infiltration in the epicardium, followed by spread of this inflammation from the epicardium to the compact layer of the myocardium (Mikalsen et al., 2012) and to the spongy layer of the myocardium (Kongtorp, Taksdal, et al., 2004). In our study, the most frequent histopathological observation was mild, focal lymphohistiocytic myocarditis in the atrium or ventricle and no observations of epicarditis. In coho salmon, a higher frequency of myocarditis was observed in the mock controls relative to the PRV+ treated group.
Our results are in agreement with other controlled laboratory challenge studies using strains of PRV‐1 from Pacific Canada in which sockeye and Atlantic salmon were challenged with PRV‐1 in an effort to reproduce HSMI (Garver, Johnson, et al., 2016) or in which Chinook, sockeye and Atlantic salmon were challenged in an effort to reproduce Jaundice Syndrome (Garver, Marty, et al., 2016). In those studies, the Pacific salmon species showed evidence of extensive replication of PRV‐1, but no significant mortality or pathology was recorded in the challenged fish. More recent studies by Polinski et al. (2019) have further confirmed that strains of PRV‐1 from Pacific Canada are of lower virulence for stocks of Atlantic salmon reared in BC, Canada, than that reported for Norwegian strains of PRV‐1 in Atlantic salmon reared in Norway, possibly due to genetic differences in strains of PRV‐1 or in differences in the innate resistance of the local stocks of Atlantic salmon. In this regard, phylogenetic and sequence analyses of 31 PRV genomes from fish with and without HSMI collected over a 30‐year period has shown that Norwegian strains of PRV‐1 associated with HSMI cluster separately from low‐virulent strains originating from fish prior to the emergence of HSMI in Norway or from fish without evidence of HSMI, including strains from western North America (Dhamotharan et al., 2019). The low virulence of the Pacific Canada strain of PRV‐1 for a Pacific‐adapted stock of Atlantic salmon was also confirmed by Zhang et al. (2019) who demonstrated high viral RNA copy number in infected Atlantic salmon without evidence of physiological impairment. In contrast, exposure of Norwegian‐reared Atlantic salmon stock to the higher virulence Norwegian strain of PRV‐1 resulted in similar viral RNA copy numbers, more severe (moderate) heart inflammation and transient impairment of some but not all measures of cardiac performance (Lund et al., 2017).
As reported by others, PRV‐1 is associated with inclusion body formation in red blood cells of Atlantic salmon similar to those present in salmonids affected by erythrocytic inclusion body syndrome (Finstad et al., 2014). We observed the presence of IBs in the erythrocytes of all three species of Pacific salmon, but their prevalence was very low in coho salmon and rainbow trout. Chinook salmon demonstrated a higher percentage of erythrocytes with large, single, pink‐staining IBs at 30 dpi (7%), with clusters of smaller IBs being observed at later time points similar to those in Atlantic salmon described by Polinski et al. (2019). Future studies with Pacific salmon would benefit from the inclusion of specific immune staining to confirm PRV‐1 virions are present in the IBs. While the haematocrits of PRV‐infected and control Chinook salmon did not differ significantly at any time point, we did note a modest, but significant, decline in the haematocrits of coho salmon at 42 dpi that occurred during the period of peak PRV‐1 replication. The transient haematocrit decline in rainbow trout was not temporally associated with increasing PRV‐1 RNA copy number. In no case did PRV‐1 infections recreate the steep haematocrit declines and severe anaemia typical of EIBS (Piacentini et al., 1989).
Di Cicco et al. (2018) has proposed a multi‐step model involving PRV‐1 infection for an anaemia/jaundice condition that occurs in farmed Chinook salmon in British Columbia that requires empirical validation. However, controlled transmission trials (Garver, Marty, et al., 2016) demonstrated that PRV infection alone is not sufficient to cause jaundice in Chinook salmon, but it does not exclude the hypothesis that PRV may have some role in jaundice along with other environmental, host or pathogen factors that are yet to be identified. We likewise observed no overt evidence of jaundice, such as yellowing of the skin or internal organs, or anaemia in any of the fish from any of the treatment groups.
Heart and skeletal muscle inflammation in farmed Atlantic salmon and jaundice syndrome in farmed BC Chinook salmon are most commonly observed during the saltwater phase (Garver, Marty, et al., 2016; Kongtorp, Taksdal, et al., 2004), whereas EIBS in North America is associated with freshwater hatchery rearing (Piacentini et al., 1989). It should be noted that our study was conducted using small juveniles held in fresh water, while nearly all previous challenge studies of PRV‐1 have used seawater‐adapted salmon. This key difference should be considered when comparing our results to other studies. Generally, viral diseases of fish may show some size differences in disease potential, such that younger fish are more susceptible to infection and show greater levels of disease. However, physiological transitions associated with the anadromous life cycle of anadromous salmon can alter disease susceptibility. Notably, Atlantic salmon parr mounted an earlier immunological response to PRV‐1 and had reduced viral loads relative to smolts in a controlled laboratory study, indicating the potential for better outcomes for salmon exposed to PRV‐1 at the freshwater stage (Johansen et al., 2016).
It is important to note that our study only addresses the risk of infections with a Pacific Canadian strain of PRV‐1 in three species of juvenile Pacific salmonids and caution should be used when extrapolating results beyond these three species and this PRV strain type. While PRV‐1 is currently the only strain of the virus known to be present in western North America, other strains of the virus are being identified as agents of disease in Pacific salmon and trout in other areas of the world. In Japan, PRV‐2 has been reported to be the causative agent of EIBS in farmed coho salmon (Takano et al., 2016), while coho salmon in Chile (Godoy et al., 2016) and rainbow trout in Europe (Dhamotharan et al., 2018; Hauge et al., 2017; Olsen et al., 2015) have been reported to suffer disease from PRV‐3. Thus, controlled laboratory infection studies using PRV‐2, PRV‐3 or even HSMI‐associated strains of PRV‐1 from Norway in various species of Pacific salmonids would be warranted but will require a higher level of containment for the experimental fish as these strains of the virus are currently believed exotic to North America. Meanwhile, it would be prudent for management agencies to take efforts to prevent the introduction of these exotic strains of PRV into western North America.
CONFLICT OF INTEREST
The authors declare no competing interests. The fish used for the study were provided in kind by Washington Department of Fish and Wildlife, US Fish and Wildlife Service and Clear Springs Foods.
Supporting information
FigureS1
FigureS2
FigureS3
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the Ecosystems Mission Area of the US Geological Survey (USGS), the USGS–US Fish and Wildlife Service (USFWS) Science Support Partnership and Quick Response Program, and Department of Fisheries and Oceans Canada Partnership Fund. The authors thank the USFWS Quinault National Fish Hatchery, Washington Department Fish and Wildlife Kendall Creek Hatchery and Clear Springs Food Inc. for the in‐kind donation of fish used for this study. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Purcell MK, Powers RL, Taksdal T, et al. Consequences of Piscine orthoreovirus genotype 1 (PRV‐1) infections in Chinook salmon (Oncorhynchus tshawytscha), coho salmon (O. kisutch) and rainbow trout (O. mykiss). J Fish Dis. 2020;43:719–728. 10.1111/jfd.13182
DATA AVAILABILITY STATEMENT
Raw data and machine‐readable metadata are accessible through a US Geological Survey data release: Purcell, M. (2020) Laboratory exposure of Chinook salmon (Oncorhynchus tshawytscha), coho salmon (O. kisutch) and rainbow trout (O. mykiss) to a Pacific Canadian strain of piscine orthoreovirus genotype 1 (PRV‐1): U.S. Geological Survey data release, https://doi.org/10.5066/P9HAD6D0.
REFERENCES
- AFS (2016) American Fisheries Society, Fish Health Section. Suggested procedures for the detection and identification of certain finfish and shellfish pathogens. Retrieved from https://units.fisheries.org/fhs/fish‐health‐section‐blue‐book‐2016/ [Google Scholar]
- Carson, F. L. , Martin, J. H. , & Lynn, J. A. (1973). Formalin fixation for electron microscopy: A re‐evaluation. American Journal of Clinical Pathology, 59, 365–373. 10.1093/ajcp/59.3.365 [DOI] [PubMed] [Google Scholar]
- Cartagena, J. , Tambley, C. , Sandino, A. M. , Spencer, E. , & Tello, M. (2018). Detection of piscine orthoreovirus in farmed rainbow trout from Chile. Aquaculture, 493, 79–84. 10.1016/j.aquaculture.2018.04.044 [DOI] [Google Scholar]
- Dhamotharan, K. , Tengs, T. , Wessel, Ø. , Braaen, S. , Nyman, I. B. , Hansen, E. F. , … Markussen, T. (2019). Evolution of the Piscine orthoreovirus genome linked to emergence of heart and skeletal muscle inflammation in farmed Atlantic salmon. Viruses, 11, 465 10.3390/v11050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamotharan, K. , Vendramin, N. , Markussen, T. , Wessel, O. , Cuenca, A. , Nyman, I. B. , … Rimstad, E. (2018). Molecular and antigenic characterization of Piscine orthoreovirus (PRV) from rainbow trout (Oncorhynchus mykiss) Viruses, 10, 170 10.3390/v10040170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cicco, E. , Ferguson, H. W. , Kaukinen, K. H. , Schulze, A. D. , Li, S. , Tabata, A. , … Miller, K. M. (2018). The same strain of Piscine orthoreovirus (PRV‐1) is involved in the development of different, but related, diseases in Atlantic and Pacific Salmon in British Columbia. FACETS, 3, 599–641. [Google Scholar]
- Finstad, Ø. S. , Dahle, M. K. , Lindholm, T. H. , Nyman, I. B. , Løvoll, M. , Wallace, C. , … Rimstad, E. (2014). Piscine orthoreovirus (PRV) infects Atlantic salmon erythrocytes. Veterinary Research, 45, 35 10.1186/1297-9716-45-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisheries Co‐Managers of Washington State (2006). The salmonid disease control policy. Retrieved from https://nwifc.org/about‐us/enhancement/fish‐health‐program/documents. [Google Scholar]
- Garseth, A. H. , Fritsvold, C. , Opheim, M. , Skjerve, E. , & Biering, E. (2013). Piscine reovirus (PRV) in wild Atlantic salmon, Salmo salar L., and sea‐trout, Salmo trutta L., in Norway. Journal of Fish Diseases, 36, 483–493. [DOI] [PubMed] [Google Scholar]
- Garver, K. A. , Johnson, S. C. , Polinski, M. P. , Bradshaw, J. C. , Marty, G. D. , Snyman, H. N. , … Richard, J. (2016). Piscine orthoreovirus from western North America is transmissible to Atlantic salmon and sockeye salmon but fails to cause heart and skeletal muscle inflammation. PLoS One, 11, e0146229 10.1371/journal.pone.0146229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver, K. A. , Marty, G. D. , Cockburn, S. N. , Richard, J. , Hawley, L. M. , Mueller, A. , … Saksida, S. (2016). Piscine reovirus, but not jaundice syndrome, was transmissible to Chinook salmon, Oncorhynchus tshawytscha (Walbaum), sockeye salmon, Oncorhynchus nerka (Walbaum), and Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 39, 117–128. [DOI] [PubMed] [Google Scholar]
- Godoy, M. G. , Kibenge, M. J. T. , Wang, Y. , Suarez, R. , Leiva, C. , Vallejos, F. , & Kibenge, F. S. B. (2016). First description of clinical presentation of Piscine orthoreovirus (PRV) infections in salmonid aquaculture in Chile and identification of a second genotype (Genotype II) of PRV. Virology Journal, 13, 98 10.1186/s12985-016-0554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haatveit, H. M. , Wessel, O. , Markussen, T. , Lund, M. , Thiede, B. , Nyman, I. B. , … Rimstad, E. (2017). Viral protein kinetics of piscine orthoreovirus infection in Atlantic salmon blood cells. Viruses, 9, 49 10.3390/v9030049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge, H. , Vendramin, N. , Taksdal, T. , Olsen, A. B. , Wessel, O. , Mikkelsen, S. S. , … Dahle, M. K. (2017). Infection experiments with novel Piscine orthoreovirus from rainbow trout (Oncorhynchus mykiss) in salmonids. PLoS One, 12, e0180293 10.1371/journal.pone.0180293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, L. , Dahle, M. , Wessel, Ø. , Timmerhaus, G. , Løvoll, M. , Røsæg, M. , … Krasnov, A. (2016). Differences in gene expression in Atlantic salmon parr and smolt after challenge with Piscine orthoreovirus (PRV). Molecular Immunology, 73, 138–150. 10.1016/j.molimm.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Kibenge, M. J. , Iwamoto, T. , Wang, Y. , Morton, A. , Godoy, M. G. , & Kibenge, F. S. (2013). Whole‐genome analysis of piscine reovirus (PRV) shows PRV represents a new genus in family Reoviridae and its genome segment S1 sequences group it into two separate sub‐genotypes. Virology Journal, 10, 230 10.1186/1743-422X-10-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongtorp, R. T. , Halse, M. , Taksdal, T. , & Falk, K. (2006). Longitudinal study of a natural outbreak of heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 29, 233–244. 10.1111/j.1365-2761.2006.00710.x [DOI] [PubMed] [Google Scholar]
- Kongtorp, R. T. , Kjerstad, A. , Taksdal, T. , Guttvik, A. , & Falk, K. (2004). Heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L: A new infectious disease. Journal of Fish Disease, 27, 351–358. 10.1111/j.1365-2761.2004.00549.x [DOI] [PubMed] [Google Scholar]
- Kongtorp, R. T. , & Taksdal, T. (2009). Studies with experimental transmission of heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 32, 253–262. [DOI] [PubMed] [Google Scholar]
- Kongtorp, R. T. , Taksdal, T. , & Lyngoy, A. (2004). Pathology of heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon Salmo salar . Diseases of Aquatic Organisms, 59, 217–224. 10.3354/dao059217 [DOI] [PubMed] [Google Scholar]
- Kurath, G. , Garver, K. A. , Corbeil, S. , Elliott, D. G. , Anderson, E. D. , & LaPatra, S. E. (2006). Protective immunity and lack of histopathological damage two years after DNA vaccination against infectious hematopoietic necrosis virus in trout. Vaccine, 16, 345–354. 10.1016/j.vaccine.2005.07.068 [DOI] [PubMed] [Google Scholar]
- Løvoll, M. , Alarcon, M. , Bang Jensen, B. , Taksdal, T. , Kristoffersen, A. B. , & Tengs, T. (2012). Quantification of piscine reovirus (PRV) at different stages of Atlantic salmon Salmo salar production. Diseases of Aquatic Organisms, 99, 7–12. 10.3354/dao02451 [DOI] [PubMed] [Google Scholar]
- Lund, M. , Dahle, M. , Timmerhaus, G. , Alarcon, M. , Powell, M. , Aspehaug, V. , … Jørgensen, S. M. (2017). Hypoxia tolerance and responses to hypoxic stress during heart and skeletal muscle inflammation in Atlantic salmon (Salmo salar). PLoS One, 12, e0181109 10.1371/journal.pone.0181109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, M. S. , Bjørgen, H. , Dhamotharan, K. , Wessel, Ø. , Koppang, E. O. , Di Cicco, E. , … Rimstad, E. (2019). Erythroid progenitor cells in Atlantic salmon (Salmo salar) may be persistently and productively infected with Piscine Orthoreovirus (PRV). Viruses, 11, E824 10.3390/v11090824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markussen, T. , Dahle, M. K. , Tengs, T. , Lovoll, M. , Finstad, O. W. , Wiik‐Nielsen, C. R. , … Rimstad, E. (2013). Sequence analysis of the genome of piscine orthoreovirus (PRV) associated with heart and skeletal muscle inflammation (HSMI) in Atlantic salmon (Salmo salar). PLoS One, 8, e70075 10.1371/journal.pone.0070075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty, G. D. , Morrison, D. B. , Bidulka, J. , Joseph, T. , & Siah, A. (2014). Piscine reovirus in wild and farmed salmonids in British Columbia, Canada: 1974–2013. Journal of Fish Diseases, 38, 713–728. 10.1111/jfd.12285 [DOI] [PubMed] [Google Scholar]
- Mikalsen, A. B. , Haugland, O. , Rode, M. , Solbakk, I. T. , & Evensen, O. (2012). Atlantic salmon reovirus infection causes a CD8 T cell myocarditis in Atlantic salmon (Salmo salar L.). PLoS One, 7, e37269 10.1371/journal.pone.0037269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, A. , Routledge, R. , Hrushowy, S. , Kibenge, M. , & Kibenge, F. (2017). The effect of exposure to farmed salmon on piscine orthoreovirus infection and fitness in wild Pacific salmon in British Columbia, Canada. PLoS One, 12, e188793 10.1371/journal.pone.0188793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, A. B. , Hjortaas, M. , Tengs, T. , Hellberg, H. , & Johansen, R. (2015). First description of a new disease in rainbow trout (Oncorhynchus mykiss (Walbaum)) similar to heart and skeletal muscle inflammation (HSMI) and detection of a gene sequence related to piscine orthoreovirus (PRV). PLoS One, 10, e0131638 10.1371/journal.pone.0131638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, G. , Løvoll, M. , Tengs, T. , Hornig, M. , Hutchison, S. , Hui, J. , … Lipkin, W. I. (2010). Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS One, 5, e11487 10.1371/journal.pone.0011487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini, S. C. , Rohovec, J. S. , & Fryer, J. L. (1989). Epizootiology of erythrocytic inclusion body syndrome. Journal of Aquatic Animal Health, 1, 173–179. [DOI] [Google Scholar]
- Polinski, M. P. , Marty, G. D. , Snyman, H. N. , & Garver, K. A. (2019). Piscine orthoreovirus demonstrates high infectivity but low virulence in Atlantic salmon of Pacific Canada. Nature Scientific Reports, 9, 3297 10.1038/s41598-019-40025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, M. K. , Powers, R. L. , Evered, J. , Kerwin, J. , Meyers, T. R. , Stewart, B. , & Winton, J. R. (2018). Molecular testing of adult Pacific salmon and trout (Oncorhynchus spp.) for several RNA viruses demonstrates widespread distribution of piscine orthoreovirus in Alaska and Washington. Journal of Fish Diseases, 41, 347–355. [DOI] [PubMed] [Google Scholar]
- Purcell, M. K. , Thompson, R. L. , Garver, K. A. , Hawley, L. M. , Batts, W. N. , Sprague, L. , … Winton, J. R. (2013). Development and validation of a universal reverse‐transcriptase real‐time PCR for infectious hematopoietic necrosis virus (IHNV). Diseases of Aquatic Organisms, 106, 103–115. 10.3354/dao02644 [DOI] [PubMed] [Google Scholar]
- Rodger, H. D. (2007). Erythrocytic inclusion body syndrome virus in wild Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 30, 411–418. 10.1111/j.1365-2761.2007.00831.x [DOI] [PubMed] [Google Scholar]
- Siah, A. , Morrison, D. B. , Fringuelli, E. , Savage, O. , Richmond, Z. , Johns, R. , … Saksida, S. M. (2015). Piscine reovirus: Genomic and molecular phylogenetic analysis from farmed and wild salmonids collected on the Canada/US Pacific coast. PLoS One, 10, e0141475 10.1371/journal.pone.0141475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Okamoto, N. , Kumagai, A. , Maita, M. , Ikeda, Y. , & Rohovec, J. S. (1992). Epizootics of erythrocytic inclusion body syndrome in coho salmon cultured in seawater in Japan. Journal of Aquatic Animal Health, 4, 174–181. [DOI] [Google Scholar]
- Takano, T. , Nawata, A. , Sakai, T. , Matsuyama, T. , Ito, T. , Kurita, J. , … Nakayasu, C. (2016). Full‐genome sequencing and confirmation of the causative agent of erythrocytic inclusion body syndrome in coho salmon identifies a new type of piscine orthoreovirus. PLoS One, 11, e0165424 10.1371/journal.pone.0165424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendramin, N. , Dhamotharan, K. , Olsen, A. , Cuenca, A. , Teige, L. , Wessel, Ø. , … Olesen, N. (2019). Piscine orthoreovirus subtype 3 (PRV‐3) causes heart inflammation in rainbow trout (Oncorhynchus mykiss). Veterinary Research, 50, 14 10.1186/s13567-019-0632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. , Karlsen, M. , Devold, M. , Isdal, E. , Litlabo, A. , & Nylund, A. (2006). Virus‐like particles associated with heart and skeletal muscle inflammation (HSMI). Diseases of Aquatic Organisms, 70, 183–192. 10.3354/dao070183 [DOI] [PubMed] [Google Scholar]
- Wessel, O. , Braaen, S. , Alarcon, M. , Haatveit, H. , Roos, N. , Markussen, T. , … Rimstad, E. (2017). Infection with purified Piscine orthoreovirus demonstrates a causal relationship with heart and skeletal muscle inflammation in Atlantic salmon. PLoS One, 12, e0183781 10.1371/journal.pone.0183781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel, O. , Krasnov, A. , Timmerhaus, G. , Rimstad, E. , & Dahle, M. K. (2019). Antiviral responses and biological consequences of Piscine orthoreovirus infection in salmonid erythrocytes. Frontiers in Immunology, 9, 3182 10.3389/fimmu.2018.03182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel, Ø. , Haugland, Ø. , Rode, M. , Fredriksen, B.N. , Dahle, M.K. , & Rimstad, E. (2018). Inactivated Piscine orthoreovirus vaccine protects against heart and skeletal muscle inflammation in Atlantic salmon. J Fish Dis, 41, 1411–1419. 10.1111/jfd.12835 [DOI] [PubMed] [Google Scholar]
- Wiik‐Nielsen, C. R. , Ski, P. M. , Aunsmo, A. , & Lovoll, M. (2012). Prevalence of viral RNA from piscine reovirus and piscine myocarditis virus in Atlantic salmon, Salmo salar L., broodfish and progeny. Journal of Fish Diseases, 35, 169–171. 10.1111/j.1365-2761.2011.01328.x [DOI] [PubMed] [Google Scholar]
- Yasutake, W. (1987). Standardization of stain used for diagnosing erythrocytic inclusion body syndrome (EIBS). Fish Health Section AFS Newsletter, 15, 7. [Google Scholar]
- Zhang, Y. , Polinski, M. P. , Morrison, P. R. , Brauner, C. J. , Farrewll, A. P. , & Garver, K. A. (2019). High‐load reovirus infections do not imply physiological impairment in salmon. Frontiers in Physiology, 10, 114 10.3389/fphys.2019.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1
FigureS2
FigureS3
Supplementary Material
Data Availability Statement
Raw data and machine‐readable metadata are accessible through a US Geological Survey data release: Purcell, M. (2020) Laboratory exposure of Chinook salmon (Oncorhynchus tshawytscha), coho salmon (O. kisutch) and rainbow trout (O. mykiss) to a Pacific Canadian strain of piscine orthoreovirus genotype 1 (PRV‐1): U.S. Geological Survey data release, https://doi.org/10.5066/P9HAD6D0.


